Effects of Tenofovir Disoproxil Fumarate on Bone Quality beyond Bone Density—A Scoping Review of the Literature
Abstract
:1. Introduction
2. Results
3. Discussion
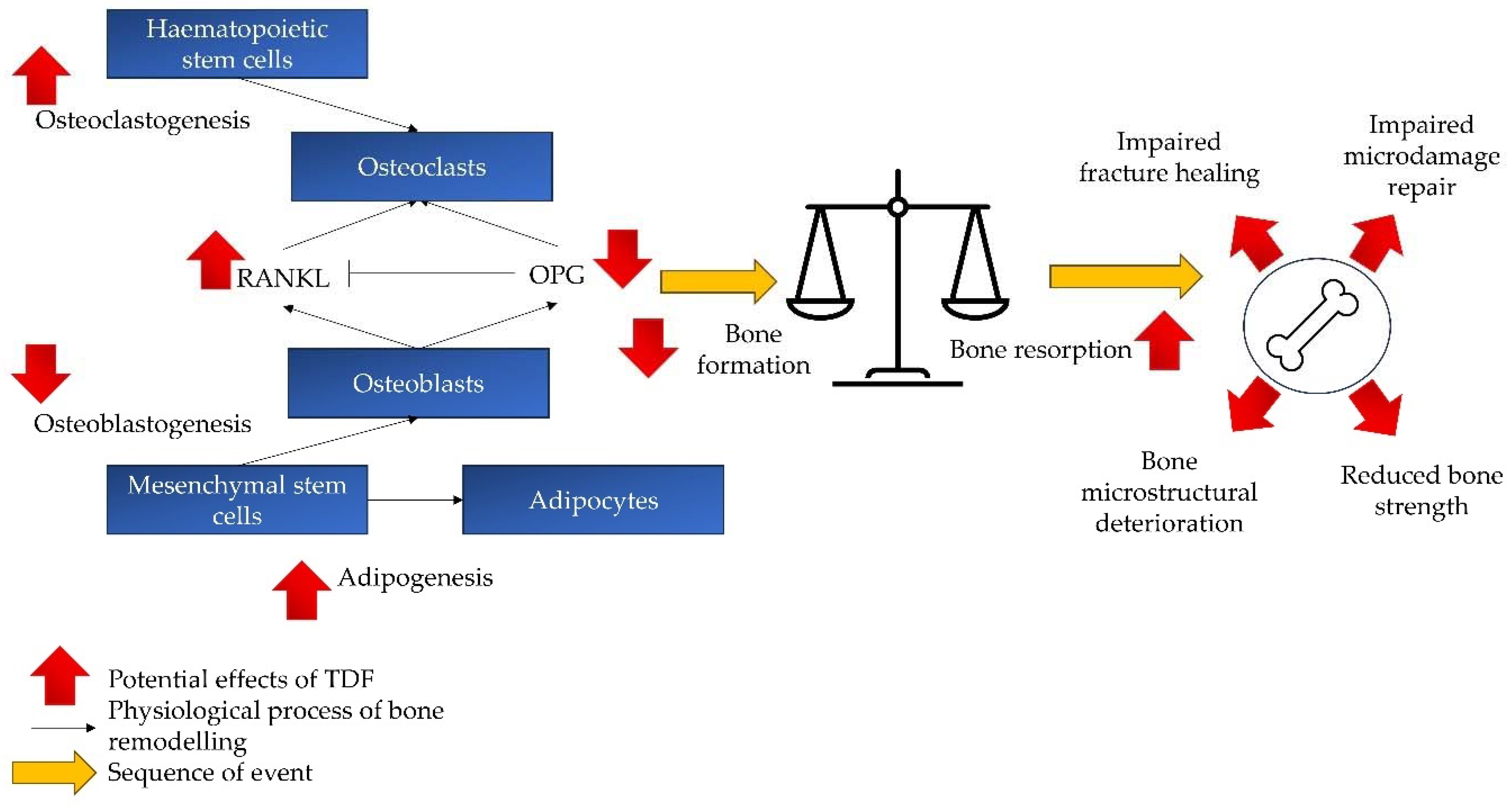
3.1. Effects on Osteoblasts
3.2. Effects on Osteoclasts
3.3. Effects on Bone Remodelling and Quality
3.4. Limitations
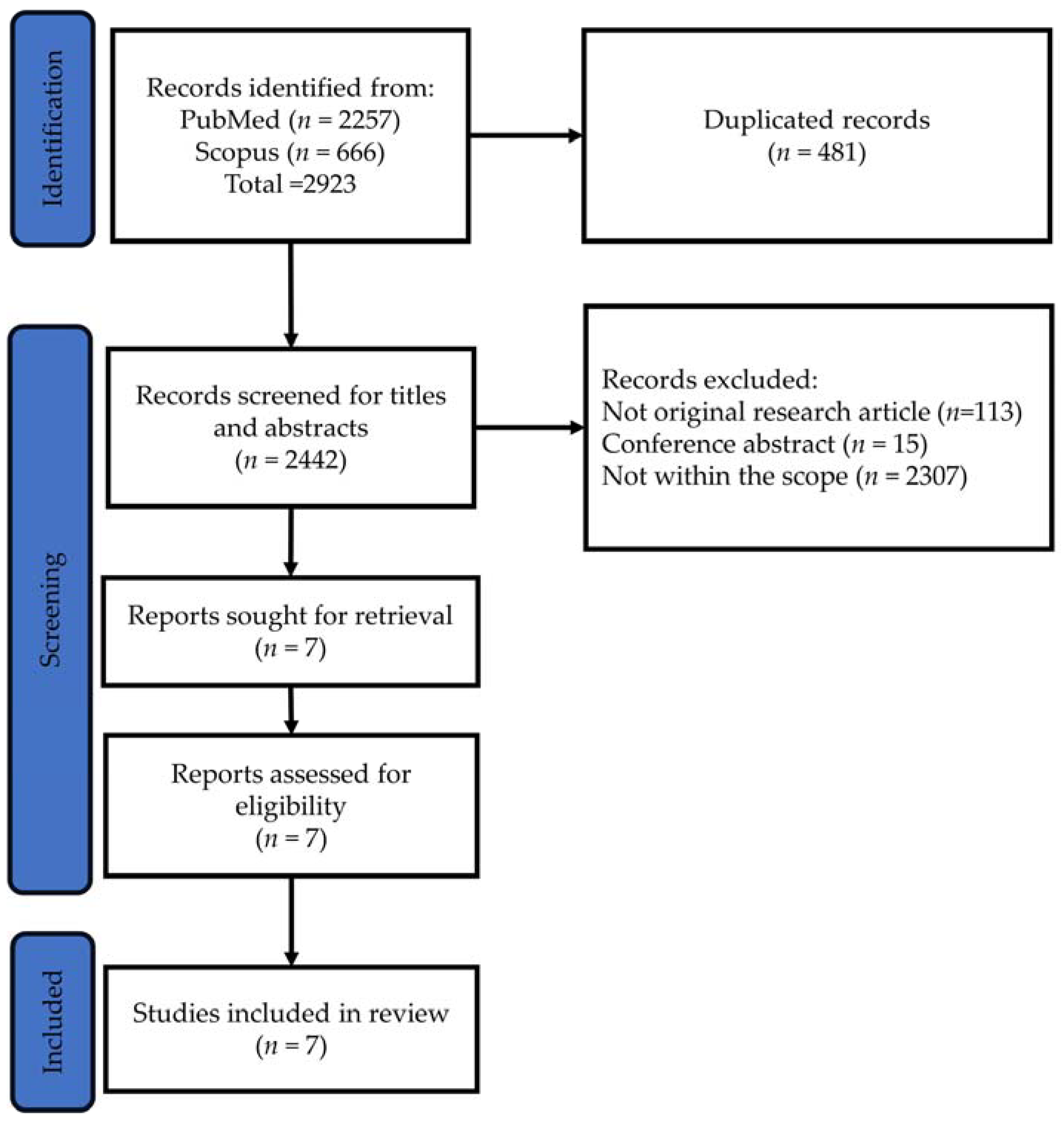
4. Methodology
4.1. Identifying the Research Question
4.2. Identifying Relevant Studies
4.3. Study Selection
4.4. Charting the Data
4.5. Collating, Summarising and Reporting the Results
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erlandson, K.M.; Allshouse, A.A.; Jankowski, C.M.; MaWhinney, S.; Kohrt, W.M.; Campbell, T.B. Functional impairment is associated with low bone and muscle mass among persons aging with HIV infection. J. Acquir. Immune Defic. Syndr. 2013, 63, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Chen, J.; Wang, X.; Xie, Y.; Zhang, X.; Han, D.; Fu, H.; Yin, W.; Wu, N. Global, regional, and national HIV/AIDS disease burden levels and trends in 1990–2019: A systematic analysis for the global burden of disease 2019 study. Front. Public Health 2023, 11, 1068664. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Allergy and Infectious Disease. Antiretroviral Drug Discovery and Development. Available online: https://www.niaid.nih.gov/diseases-conditions/antiretroviral-drug-development (accessed on 28 December 2023).
- Pau, A.K.; George, J.M. Antiretroviral therapy: Current drugs. Infect. Dis. Clin. N. Am. 2014, 28, 371–402. [Google Scholar] [CrossRef] [PubMed]
- Clinicalinfo.HIV.gov. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Available online: https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-arv/what-start-initial-combination-regimens (accessed on 28 December 2023).
- Atta, M.G.; De Seigneux, S.; Lucas, G.M. Clinical Pharmacology in HIV Therapy. Clin. J. Am. Soc. Nephrol. 2019, 14, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Kearney, B.P.; Flaherty, J.F.; Shah, J. Tenofovir disoproxil fumarate: Clinical pharmacology and pharmacokinetics. Clin. Pharmacokinet. 2004, 43, 595–612. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Hepatitis B. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed on 16 January 2024).
- Liborio, A.B.; Andrade, L.; Pereira, L.V.; Sanches, T.R.; Shimizu, M.H.; Seguro, A.C. Rosiglitazone reverses tenofovir-induced nephrotoxicity. Kidney Int. 2008, 74, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Kohler, J.J.; Hosseini, S.H.; Green, E.; Abuin, A.; Ludaway, T.; Russ, R.; Santoianni, R.; Lewis, W. Tenofovir renal proximal tubular toxicity is regulated by OAT1 and MRP4 transporters. Lab. Invest. 2011, 91, 852–858. [Google Scholar] [CrossRef]
- Gallant, J.E.; Staszewski, S.; Pozniak, A.L.; DeJesus, E.; Suleiman, J.M.; Miller, M.D.; Coakley, D.F.; Lu, B.; Toole, J.J.; Cheng, A.K.; et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: A 3-year randomized trial. JAMA 2004, 292, 191–201. [Google Scholar] [CrossRef]
- Stellbrink, H.J.; Orkin, C.; Arribas, J.R.; Compston, J.; Gerstoft, J.; Van Wijngaerden, E.; Lazzarin, A.; Rizzardini, G.; Sprenger, H.G.; Lambert, J.; et al. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin. Infect. Dis. 2010, 51, 963–972. [Google Scholar] [CrossRef]
- McComsey, G.A.; Kitch, D.; Daar, E.S.; Tierney, C.; Jahed, N.C.; Tebas, P.; Myers, L.; Melbourne, K.; Ha, B.; Sax, P.E. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: Aids Clinical Trials Group A5224s, a substudy of ACTG A5202. J. Infect. Dis. 2011, 203, 1791–1801. [Google Scholar] [CrossRef]
- Aurpibul, L.; Puthanakit, T. Review of tenofovir use in HIV-infected children. Pediatr. Infect. Dis. J. 2015, 34, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Bloch, M.; Tong, W.W.; Hoy, J.; Baker, D.; Lee, F.J.; Richardson, R.; Carr, A.; for the TROP (Switch from Tenofovir to Raltegravir for Low Bone Density) study team. Switch from tenofovir to raltegravir increases low bone mineral density and decreases markers of bone turnover over 48 weeks. HIV Med. 2014, 15, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Maggiolo, F.; Rizzardini, G.; Raffi, F.; Pulido, F.; Mateo-Garcia, M.G.; Molina, J.M.; Ong, E.; Shao, Y.; Piontkowsky, D.; Das, M.; et al. Bone mineral density in virologically suppressed people aged 60 years or older with HIV-1 switching from a regimen containing tenofovir disoproxil fumarate to an elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide single-tablet regimen: A multicentre, open-label, phase 3b, randomised trial. Lancet HIV 2019, 6, e655–e666. [Google Scholar] [CrossRef] [PubMed]
- Negredo, E.; Domingo, P.; Perez-Alvarez, N.; Gutierrez, M.; Mateo, G.; Puig, J.; Escrig, R.; Echeverria, P.; Bonjoch, A.; Clotet, B. Improvement in bone mineral density after switching from tenofovir to abacavir in HIV-1-infected patients with low bone mineral density: Two-centre randomized pilot study (OsteoTDF study). J. Antimicrob. Chemother. 2014, 69, 3368–3371. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, K.; Glidden, D.V.; Anderson, P.L.; Liu, A.; McMahan, V.; Gonzales, P.; Ramirez-Cardich, M.E.; Namwongprom, S.; Chodacki, P.; de Mendonca, L.M.; et al. Effects of Emtricitabine/Tenofovir on Bone Mineral Density in HIV-Negative Persons in a Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Infect. Dis. 2015, 61, 572–580. [Google Scholar] [CrossRef]
- Havens, P.L.; Stephensen, C.B.; Van Loan, M.D.; Schuster, G.U.; Woodhouse, L.R.; Flynn, P.M.; Gordon, C.M.; Pan, C.G.; Rutledge, B.; Liu, N.; et al. Decline in Bone Mass with Tenofovir Disoproxil Fumarate/Emtricitabine Is Associated with Hormonal Changes in the Absence of Renal Impairment When Used by HIV-Uninfected Adolescent Boys and Young Men for HIV Preexposure Prophylaxis. Clin. Infect. Dis. 2017, 64, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, R.; Sahin, A.; Ozturk, O.; Calhan, T.; Sayar, S.; Kanat, E.; Doganay, L.; Ozdil, K. Effects of Long-Term Tenofovir and Entecavir Treatment on Bone Mineral Density in Patients with Chronic Hepatitis B. Turk. J. Gastroenterol. 2022, 33, 35–43. [Google Scholar] [CrossRef]
- Gill, U.S.; Zissimopoulos, A.; Al-Shamma, S.; Burke, K.; McPhail, M.J.; Barr, D.A.; Kallis, Y.N.; Marley, R.T.; Kooner, P.; Foster, G.R.; et al. Assessment of bone mineral density in tenofovir-treated patients with chronic hepatitis B: Can the fracture risk assessment tool identify those at greatest risk? J. Infect. Dis. 2015, 211, 374–382. [Google Scholar] [CrossRef]
- Da Wang, F.; Zhou, J.; Li, L.Q.; Li, Y.J.; Wang, M.L.; Tao, Y.C.; Zhang, D.M.; Wang, Y.H.; Chen, E.Q. Improved bone and renal safety in younger tenofovir disoproxil fumarate experienced chronic hepatitis B patients after switching to tenofovir alafenamide or entecavir. Ann. Hepatol. 2023, 28, 101119. [Google Scholar] [CrossRef]
- Shiau, S.; Arpadi, S.M.; Yin, M.T. Bone Update: Is It Still an Issue without Tenofovir Disoproxil Fumarate? Curr. HIV/AIDS Rep. 2020, 17, 1–5. [Google Scholar] [CrossRef]
- Carnovali, M.; Banfi, G.; Mora, S.; Mariotti, M. Tenofovir and bone: Age-dependent effects in a zebrafish animal model. Antivir. Ther. 2016, 21, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Conesa-Buendía, F.M.; Llamas-Granda, P.; Larrañaga-Vera, A.; Wilder, T.; Largo, R.; Herrero-Beaumont, G.; Cronstein, B.; Mediero, A. Tenofovir Causes Bone Loss via Decreased Bone Formation and Increased Bone Resorption, Which Can Be Counteracted by Dipyridamole in Mice. J. Bone Miner. Res. 2019, 34, 923–938. [Google Scholar] [CrossRef] [PubMed]
- Conradie, M.M.; van de Vyver, M.; Andrag, E.; Conradie, M.; Ferris, W.F. A Direct Comparison of the Effects of the Antiretroviral Drugs Stavudine, Tenofovir and the Combination Lopinavir/Ritonavir on Bone Metabolism in a Rat Model. Calcif. Tissue Int. 2017, 101, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Matuszewska, A.; Nowak, B.; Nikodem, A.; Jedrzejuk, D.; Szkudlarek, D.; Zduniak, K.; Filipiak, J.; Sznadruk-Bender, M.; Tomkalski, T.; Ceremuga, I.; et al. Effects of efavirenz and tenofovir on bone tissue in Wistar rats. Adv. Clin. Exp. Med. 2020, 29, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Castillo, A.B.; Tarantal, A.F.; Watnik, M.R.; Martin, R.B. Tenofovir treatment at 30 mg/kg/day can inhibit cortical bone mineralization in growing rhesus monkeys (Macaca mulatta). J. Orthop. Res. 2002, 20, 1185–1189. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.M.; Jalal, M.M.K.; Lalloo, D.G.; Hamish, R.W.S.A. The effect of anti-retroviral therapy on fracture healing: An in vivo animal model. Bone Jt. Res. 2022, 11, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, J.; Martins, C.S.W.; Galvao, J.; Furukawa, L.N.; Domingues, W.V.; Oliveira, I.B.; Dos Reis, L.M.; Pereira, R.M.; Nickolas, T.L.; Yin, M.T.; et al. Treatment of Human Immunodeficiency Virus Infection with Tenofovir Disoproxil Fumarate-Containing Antiretrovirals Maintains Low Bone Formation Rate, but Increases Osteoid Volume on Bone Histomorphometry. J. Bone Miner. Res. 2019, 34, 1574–1584. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 2008, 3 (Suppl. S3), S131–S139. [Google Scholar] [CrossRef]
- Feng, X.; McDonald, J.M. Disorders of bone remodeling. Annu. Rev. Pathol. 2011, 6, 121–145. [Google Scholar] [CrossRef]
- Kim, J.M.; Lin, C.; Stavre, Z.; Greenblatt, M.B.; Shim, J.H. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells 2020, 9, 2073. [Google Scholar] [CrossRef]
- Katagiri, T.; Takahashi, N. Regulatory mechanisms of osteoblast and osteoclast differentiation. Oral. Dis. 2002, 8, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Okagu, I.U.; Ezeorba, T.P.C.; Aguchem, R.N.; Ohanenye, I.C.; Aham, E.C.; Okafor, S.N.; Bollati, C.; Lammi, C. A Review on the Molecular Mechanisms of Action of Natural Products in Preventing Bone Diseases. Int. J. Mol. Sci. 2022, 23, 8468. [Google Scholar] [CrossRef] [PubMed]
- Devlin, M.J.; Rosen, C.J. The bone-fat interface: Basic and clinical implications of marrow adiposity. Lancet Diabetes Endocrinol. 2015, 3, 141–147. [Google Scholar] [CrossRef]
- Grigsby, I.F.; Pham, L.; Mansky, L.M.; Gopalakrishnan, R.; Carlson, A.E.; Mansky, K.C. Tenofovir treatment of primary osteoblasts alters gene expression profiles: Implications for bone mineral density loss. Biochem. Biophys. Res. Commun. 2010, 394, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Fukuchi, M.; Fukai, Y.; Masuda, N.; Miyazaki, T.; Nakajima, M.; Sohda, M.; Manda, R.; Tsukada, K.; Kato, H.; Kuwano, H. High-level expression of the Smad ubiquitin ligase Smurf2 correlates with poor prognosis in patients with esophageal squamous cell carcinoma. Cancer Res. 2002, 62, 7162–7165. [Google Scholar] [PubMed]
- Boyce, B.F.; Yao, Z.; Xing, L. Osteoclasts have multiple roles in bone in addition to bone resorption. Crit. Rev. Eukaryot. Gene Expr. 2009, 19, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H. Osteoimmunology: Shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 2007, 7, 292–304. [Google Scholar] [CrossRef]
- Komatsu, N.; Okamoto, K.; Sawa, S.; Nakashima, T.; Oh-hora, M.; Kodama, T.; Tanaka, S.; Bluestone, J.A.; Takayanagi, H. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat. Med. 2014, 20, 62–68. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Kodama, J.; Kaito, T. Osteoclast Multinucleation: Review of Current Literature. Int. J. Mol. Sci. 2020, 21, 5685. [Google Scholar] [CrossRef]
- Chin, K.-Y.; Ng, B.N.; Rostam, M.K.I.; Muhammad Fadzil, N.F.D.; Raman, V.; Mohamed Yunus, F.; Syed Hashim, S.A.; Ekeuku, S.O. A Mini Review on Osteoporosis: From Biology to Pharmacological Management of Bone Loss. J. Clin. Med. 2022, 11, 6434. [Google Scholar] [CrossRef] [PubMed]
- Foger-Samwald, U.; Dovjak, P.; Azizi-Semrad, U.; Kerschan-Schindl, K.; Pietschmann, P. Osteoporosis: Pathophysiology and therapeutic options. EXCLI J. 2020, 19, 1017–1037. [Google Scholar] [CrossRef] [PubMed]
- Osterhoff, G.; Morgan, E.F.; Shefelbine, S.J.; Karim, L.; McNamara, L.M.; Augat, P. Bone mechanical properties and changes with osteoporosis. Injury 2016, 47 (Suppl. S2), S11–S20. [Google Scholar] [CrossRef] [PubMed]
- Vieth, R. Weaker bones and white skin as adaptions to improve anthropological “fitness” for northern environments. Osteoporos. Int. 2020, 31, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, S.; Ozono, K.; Michigami, T.; Minagawa, M.; Okazaki, R.; Sugimoto, T.; Takeuchi, Y.; Matsumoto, T. Pathogenesis and diagnostic criteria for rickets and osteomalacia—Proposal by an expert panel supported by Ministry of Health, Labour and Welfare, Japan, the Japanese Society for Bone and Mineral Research and the Japan Endocrine Society. Endocr. J. 2015, 62, 665–671. [Google Scholar] [CrossRef]
- Zimmerman, L.; McKeon, B. Osteomalacia. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551616/ (accessed on 28 December 2023).
- Motosuneya, T.; Asazuma, T.; Yasuoka, H.; Tsuji, T.; Fujikawa, K. Severe kyphoscoliosis associated with osteomalacia. Spine J. 2006, 6, 587–590. [Google Scholar] [CrossRef]
- Mohamad, N.V.; Ima-Nirwana, S.; Chin, K.Y. Are Oxidative Stress and Inflammation Mediators of Bone Loss Due to Estrogen Deficiency? A Review of Current Evidence. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 1478–1487. [Google Scholar] [CrossRef]
- Mohamad, N.V.; Soelaiman, I.N.; Chin, K.Y. A concise review of testosterone and bone health. Clin. Interv. Aging 2016, 11, 1317–1324. [Google Scholar] [CrossRef]
- Abraham, P.; Ramamoorthy, H.; Isaac, B. Depletion of the cellular antioxidant system contributes to tenofovir disoproxil fumarate—Induced mitochondrial damage and increased oxido-nitrosative stress in the kidney. J. Biomed. Sci. 2013, 20, 61. [Google Scholar] [CrossRef]
- Canale, D.; de Bragança, A.C.; Gonçalves, J.G.; Shimizu, M.H.; Sanches, T.R.; Andrade, L.; Volpini, R.A.; Seguro, A.C. Vitamin D deficiency aggravates nephrotoxicity, hypertension and dyslipidemia caused by tenofovir: Role of oxidative stress and renin-angiotensin system. PLoS ONE 2014, 9, e103055. [Google Scholar] [CrossRef]
- Sun, Y.X.; Xu, A.H.; Yang, Y.; Li, J. Role of Nrf2 in bone metabolism. J. Biomed. Sci. 2015, 22, 101. [Google Scholar] [CrossRef]
- Dessordi, R.; Watanabe, L.M.; Guimaraes, M.P.; Romao, E.A.; de Lourdes Candolo Martinelli, A.; de Carvalho Santana, R.; Navarro, A.M. Bone loss in hepatitis B virus-infected patients can be associated with greater osteoclastic activity independently of the retroviral use. Sci. Rep. 2021, 11, 10162. [Google Scholar] [CrossRef]
- Titanji, K.; Vunnava, A.; Foster, A.; Sheth, A.N.; Lennox, J.L.; Knezevic, A.; Shenvi, N.; Easley, K.A.; Ofotokun, I.; Weitzmann, M.N. T-cell receptor activator of nuclear factor-kappaB ligand/osteoprotegerin imbalance is associated with HIV-induced bone loss in patients with higher CD4+ T-cell counts. AIDS 2018, 32, 885–894. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Ott, S.M. Bone strength: More than just bone density. Kidney Int. 2016, 89, 16–19. [Google Scholar] [CrossRef]
| Researchers | Study Design | Findings |
|---|---|---|
| Carnovali et al. (2016) [24] | Animals: 3-, 6- and 12-month-old Danio rerio AB male zebrafish of similar weight and length Study design: TDF in various concentrations (50 nM, 100 nM and 1 mM) | Osteogenesis in embryos: No significant effect on mineralisation rate of embryos. No significant skeletal or growth deformity or vitality impairment of embryos. Remodelling of scale: ↓ ALP activity in 6-month-old fish exposed to TDF (50 nM, 100 nM and 1 mM). At 100 nM, the reduction in ALP became more significant with age (12 > 6 > 3 months old). ↑ TRAP activity at 100 nM TDF exposure. At 100 nM, the increase in ALP became more significant with age (12 > 6 months old). Phenotype of scales: TDF completely blocked growing circle formation of scale in 6- and 12-month-old fish. TDF ↑ resorption area along the scale’s edge. |
| Conesa-Buendía et al. (2019) [25] | In vitro study: Bone marrow cells from female C57BL/6 mice were exposed to TDF (10 mM to 1 nM) in the presence of RANKL (osteoclast assays) or osteogenic medium (osteoblast assays) In vivo study: Animals: Male C57BL/6 mice and female C57BL/6 mice Study design: Male C57BL/6 mice: n = 10/group Control: saline TDF: 75 mg/kg/day s.c. Dipyridamole: 25 mg/kg/day i.p. Combination: TDF 75 mg/kg/day s.c. + dipyridamole 25 mg/kg/day i.p. Period: 4 weeks Female C57BI/6 mice: n = 10/group Sham: not OVX Control: OVX + saline TDF: OVX + 75 mg/kg/day s.c. Dipyridamole: OVX + 25 mg/kg/day i.p. Combination: OVX + TDF 75 mg/kg/day s.c. + dipyridamole 25 mg/kg/day i.p. Period: 5 weeks | In vitro study: Tenofovir ↑ RANKL-induced TRAP-positive cell formation. Tenofovir ↑ cathepsin K, NFATc1 and osteopontin mRNA expression. Knocking down pannexin-1 or connexin-43 ablated the effects of tenofovir on osteoclast and resorption pit formation. A2AR silencing did not reduce the effects of tenofovir on pit formation. Tenofovir activated ERK1/2, p38 and NFκB nuclear translocation during osteoclast differentiation. Tenofovir (IC50: 0.4 mM) ↓ osteoblast differentiation in a dose-dependent manner Tenofovir ↑ RANKL mRNA expression and ↓ OPG mRNA expression Tenofovir ↓ nuclear translocation of beta-catenin In vivo study: Whole-body BMD and BMC: ↓ in tenofovir vs. normal control Bone dynamic histomorphometry: ↓ mineral apposition in tenofovir vs. normal control Bone static histomorphometry: ↑ osteoclast number and ↓ osteoblast number in tenofovir vs. normal control µCT: Cortical bone: ↓ BV/TV Trabecular bone: ↓ BV/TV, Tb.N and ↑ Tb.Sp in tenofovir vs. normal control IHC: ↑ TRAP-positive osteoclasts ↑ cathepsin K ↑ macrophages (CD68 + cells) ↑ RANKL-positive cells ↓ OPG-positive cells ↑ sclerostin-expressing osteocytes in tenofovir vs. normal control ALP-positive cells, collagens or I and III: no change with tenofovir Changes in OVX mice were parallel with male mice. |
| Conradie et al. (2017) [26] | Animals: 12- to 14-week-old male Wistar rats Treatment (10 rats/group): p.o. daily Normal control 1.5 mL water/day LPV/r 70.8 mg/kg/day Stavudine 6.2 mg/kg/day TDF 26.6 mg/kg/day Period: 9 weeks | BMD: no significant difference between TDF and control at the lumbar and femur (average of left and right). Bone mechanical strength: bending stress at max deflection of TDF ↓ vs. control. No other significant difference. Bone dynamic histomorphometry: MS/BS ↓ in TDF and stavudine vs. normal control. BFR/BS ↓ marginally (p = 0.06) in TDF vs. normal control. Bone static histomorphometry: ES/BS, Oc.S/BS, N.Oc/TA/mm2: no significant increase in TDF vs. normal control. Stavudine was significant vs. normal control. Ob.S/BS and OS/BS: no significant reduction in TDF vs. normal control. Stavudine was significant vs. normal control. Bone marrow adiposity: Number of lipid droplets ↑ in TDF and stavudine vs. normal control. |
| Matuszewska et al. (2020) [27] | Animals: 8-week-old male albino Wistar rats Treatment (n = 12/group): p.o. daily Control group: saline solution (4 mL/kg) EF group: 25 mg/kg of efavirenz and T group: 15 mg/kg of tenofovir disoproxil. Period: 24 weeks | Bone macrometric measurements: Femoral indices, femoral weight and mid-femoral diameter ↓ in TDF vs. normal control. The mid-tibial diameter ↓ in TDF vs. normal control. BMD: total BMD ↓ in TDF vs. normal control after 24 weeks but not 12 weeks. No significant changes in lumbar, tibial and femoral BMD. IHC: ALP and TRAP expression at lumbar 2 did not change in TDF vs. normal control. Bone structural histomorphometry: Femoral TbN ↓ and Tb.Sp ↑ in TDF vs. normal control. No significant changes in other structural indices at femur, tibia and L2. Bone remodelling markers: Serum IGF-1, osteocalcin, TRAP, CTX, ALP, vitamin D, calcium and phosphate—no significant change between TDF and normal control. Bone mechanical strength: Young’s modulus ↓ in TDF vs. normal control. No change in flexural strength and rigidity. |
| Graham et al. (2022) [29] | Animals: Adult male Wistar rats (450 to 550 g) Study design: n = 8/group Group 1: daily oral ART therapy (TDF 30 mg + lamivudine 30 mg + efavirenz 60 mg) Group 2: control Bone fracture: 3 weeks after therapy On one tibial shaft under anaesthesia and fixed with intramedullary nailing Period: 3 weeks pre-fracture + 8 weeks post-fracture | Fracture healing: At week 4, union rate ↓ in the ART group vs. control group. At week 8, no significant difference µCT: At week 8, some fractures of the ART group showed non-union with a gap with two separated fracture ends. Histological fracture: The gap at the fracture ends filled with fibrous tissue. No woven bone or cartilaginous callus in the inter-fragmentary area. Biomechanical strength: No significant difference between ART and normal control. However, biomechanical strength of contralateral tibia ↑ marginally in ART vs. normal control. |
| Castillo et al. (2002) [28] | Animals: Growing rhesus monkeys (Macaca mulatta) Study design: Control (untreated/uninfected, n = 4) TDF-treated/uninfected (n = 4) Untreated/SIV-infected (n = 12) TDF-treated/SIV-infected (n = 13) Dose of TDF: 30 mg/kg; prenatal by transplacental transfer and postnatal | Tenofovir ↑ tibial osteoid seam (marker of bone microdamage) width regardless of SIV infection. SIV ↑ resorption cavity density regardless of tenofovir treatment. |
| Ramalho et al. (2019) [30] | Patients: 26 ART-naive males with human immunodeficiency virus aged 18 to 40 years. Treatment: TDF + lamivudine + efavirenz | ↓ BMD at total hip, femoral neck and lumbar spine post-ART. ↑ osteocalcin and RANKL post-ART. No significant changes in CTX, P1NP, sclerostin or OPG. ↑ intact parathyroid and vitamin D, ↓ FGF-23 post-ART. ↓ TNFα post-ART. IL-6 ↓ but not significant. ↑ cortical thickness post-ART. ↓ cortical porosity but not significant. ↑ OV/BV and Ob.S/BS post-ART. ↑ OcS/BS post-ART. No significant changes in bone dynamic parameters (MS/BS, MAR, BFR, mineralisation lag time). No significant changes in bone protein expression of TNFα, IL-6, IL-1β, RANKL, OPG, FGF-23, sclerostin. IHC: ↑ OPG+ osteoblast lining cells. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashwin Singh, T.S.; Jashwin Singh, T.S.; Chin, K.-Y. Effects of Tenofovir Disoproxil Fumarate on Bone Quality beyond Bone Density—A Scoping Review of the Literature. Pharmaceuticals 2024, 17, 146. https://doi.org/10.3390/ph17020146
Hashwin Singh TS, Jashwin Singh TS, Chin K-Y. Effects of Tenofovir Disoproxil Fumarate on Bone Quality beyond Bone Density—A Scoping Review of the Literature. Pharmaceuticals. 2024; 17(2):146. https://doi.org/10.3390/ph17020146
Chicago/Turabian StyleHashwin Singh, Tejpal Singh, Tejpal Singh Jashwin Singh, and Kok-Yong Chin. 2024. "Effects of Tenofovir Disoproxil Fumarate on Bone Quality beyond Bone Density—A Scoping Review of the Literature" Pharmaceuticals 17, no. 2: 146. https://doi.org/10.3390/ph17020146
APA StyleHashwin Singh, T. S., Jashwin Singh, T. S., & Chin, K.-Y. (2024). Effects of Tenofovir Disoproxil Fumarate on Bone Quality beyond Bone Density—A Scoping Review of the Literature. Pharmaceuticals, 17(2), 146. https://doi.org/10.3390/ph17020146







