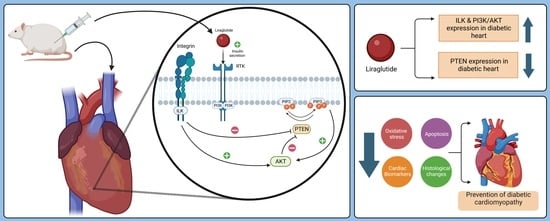
Liraglutide Attenuates Diabetic Cardiomyopathy via the ILK/PI3K/AKT/PTEN Signaling Pathway in Rats with Streptozotocin-Induced Type 2 Diabetes Mellitus
Abstract
:1. Introduction
2. Results
2.1. Liraglutide Treatment’s Effects on Body Weight, Blood Glucose, and Heart Weight-to-Body Weight Ratio in Rats with Diabetic
2.2. Effect of Liraglutide on Cardiac Marker Enzymes in the Serum
2.3. Effect of Liraglutide on the Heart of Diabetic Rats Evaluated Using H&E-Stained Heart Sections
2.4. Liraglutide Treatment Effect on Cardiac Oxidative Stress Biomarkers in Rats with Diabetes
2.5. Liraglutide Treatment’s Effects on Cardiomyocyte Apoptosis in Rats with Diabetes
2.6. Effect of Liraglutide on Cardiac Cell Apoptosis in Diabetic Rats Evaluated Using the TUNEL Assay
2.7. Mitigation of Diabetic Cardiomyopathy by Liraglutide through Restoration of the Expression of PI3K/AKT in the Heart of Diabetic Rats
3. Discussion
Study Limitations
4. Materials and Methods
4.1. Drugs, Chemicals, and Antibodies
4.2. Experimental Animals
4.3. Induction of Diabetes
4.4. Experimental Design
- Group 1: Normal saline (0.9% NaCl; drug vehicle) was administered to nondiabetic controls via oral gavage for the entire six-week period.
- Group 2: Normal saline (0.9% NaCl) was administered to diabetic untreated rats via oral gavage for six weeks.
- Group 3: Normal saline was administered to nondiabetic rats for the initial 3 weeks before they were injected with 150 μg/kg liraglutide subcutaneously (SC) twice daily for the subsequent 3 weeks. The dose was chosen based on previous studies demonstrating its cardioprotective effects in diabetic rats [71]. Notably, the volume of liraglutide stock solution (6 mg/mL) injected was dependent on each rat’s weight.
- Group 4: Normal saline was administered to diabetic rats for the initial 3 weeks, and they were subsequently injected with 150 μg/kg liraglutide SC twice daily [71] for the remaining 3 weeks.
4.5. Biochemical and Molecular Analyses
4.5.1. Determination of Serum Glucose Levels
4.5.2. Determination of Diabetic Cardiomyopathy Biomarkers
4.5.3. Estimation of Oxidative Stress Biomarkers
4.5.4. Western Blot Analysis
4.5.5. Histological Examination
4.5.6. Immunohistochemistry
4.5.7. Detection of Apoptotic Cardiomyocytes
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, S.; Wang, J.; Zhang, B.; Li, X.; Liu, Y. Diabetes Mellitus and Cause-Specific Mortality: A Population-Based Study. Diabetes Metab. J. 2019, 43, 319–341. [Google Scholar] [CrossRef]
- World Health Organization. Global Health Estimates: Leading Causes of Death. 2019. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death (accessed on 9 December 2020).
- Gulsin, G.S.; Athithan, L.; McCann, G.P. Diabetic cardiomyopathy: Prevalence, determinants and potential treatments. Ther. Adv. Endocrinol. Metab. 2019, 10, 2042018819834869. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Whaley-Connell, A.; Sowers, J.R. Diabetic cardiomyopathy: A hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia 2018, 61, 21–28. [Google Scholar] [CrossRef]
- Jia, G.; DeMarco, V.G.; Sowers, J.R. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat. Rev. Endocrinol. 2016, 12, 144–153. [Google Scholar] [CrossRef]
- Ramachandran, V.; Saravanan, R. Glucose uptake through translocation and activation of GLUT4 in PI3K/Akt signaling pathway by asiatic acid in diabetic rats. Hum. Exp. Toxicol. 2015, 34, 884–893. [Google Scholar] [CrossRef]
- Hannigan, G.E.; Leung-Hagesteijn, C.; Fitz-Gibbon, L.; Coppolino, M.G.; Radeva, G.; Filmus, J.; Bell, J.C.; Dedhar, S. Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin-linked protein kinase. Nature 1996, 379, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-C.; Lee, W.-C.; Wang, C.-C.; Yeh, S.-A.; Chen, W.-H.; Chen, P.-J. Targeting PI3K/AKT/mTOR Signaling Pathway as a Radiosensitization in Head and Neck Squamous Cell Carcinomas. Int. J. Mol. Sci. 2022, 23, 15749. [Google Scholar] [CrossRef] [PubMed]
- Walkowski, B.; Kleibert, M.; Majka, M.; Wojciechowska, M. Insight into the Role of the PI3K/Akt Pathway in Ischemic Injury and Post-Infarct Left Ventricular Remodeling in Normal and Diabetic Heart. Cells 2022, 11, 1553. [Google Scholar] [CrossRef]
- Xu, T.J.; Liu, Y.; Yuan, B. Effect of insulin in combination with selenium on Irs/PI3K-mediated GLUT4 expression in cardiac muscle of diabetic rats. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 1452–1460. [Google Scholar]
- Obara, S.; Nakata, M.; Takeshima, H.; Katagiri, H.; Asano, T.; Oka, Y.; Maruyama, I.; Kuratsu, J. Integrin-linked kinase (ILK) regulation of the cell viability in PTEN mutant glioblastoma and in vitro inhibition by the specific COX-2 inhibitor NS-398. Cancer Lett. 2004, 208, 115–122. [Google Scholar] [CrossRef]
- Kitamura, Y.; Koide, M.; Akakabe, Y.; Matsuo, K.; Shimoda, Y.; Soma, Y.; Ogata, T.; Ueyama, T.; Matoba, S.; Yamada, H.; et al. Manipulation of cardiac phosphatidylinositol 3-kinase (PI3K)/Akt signaling by apoptosis regulator through modulating IAP expression (ARIA) regulates cardiomyocyte death during doxorubicin-induced cardiomyopathy. J. Biol. Chem. 2014, 289, 2788–2800. [Google Scholar] [CrossRef] [PubMed]
- Damilano, F.; Perino, A.; Hirsch, E. PI3K kinase and scaffold functions in heart. Ann. N. Y. Acad. Sci. 2010, 1188, 39–45. [Google Scholar] [CrossRef]
- Hannigan, G.E.; Coles, J.G.; Dedhar, S. Integrin-linked kinase at the heart of cardiac contractility, repair, and disease. Circ. Res. 2007, 100, 1408–1414. [Google Scholar] [CrossRef]
- Bock-Marquette, I.; Saxena, A.; White, M.D.; Dimaio, J.M.; Srivastava, D. Thymosin beta4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature 2004, 432, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.C.; Hu, H.F.; Hong, P.; Zhang, Q.H.; Xu, W.W.; He, Q.Y.; Li, B. Significance of integrin-linked kinase (ILK) in tumorigenesis and its potential implication as a biomarker and therapeutic target for human cancer. Am. J. Cancer Res. 2019, 9, 186–197. [Google Scholar]
- Brishti, M.A.; Raghavan, S.; Lamar, K.; Singh, U.P.; Collier, D.M.; Leo, M.D. Diabetic Endothelial Cell Glycogen Synthase Kinase 3β Activation Induces VCAM1 Ectodomain Shedding. Int. J. Mol. Sci. 2023, 24, 14105. [Google Scholar] [PubMed]
- White, D.E.; Coutu, P.; Shi, Y.F.; Tardif, J.C.; Nattel, S.; St Arnaud, R.; Dedhar, S.; Muller, W.J. Targeted ablation of ILK from the murine heart results in dilated cardiomyopathy and spontaneous heart failure. Genes Dev. 2006, 20, 2355–2360. [Google Scholar] [CrossRef]
- Zelniker, T.A.; Wiviott, S.D.; Raz, I.; Im, K.; Goodrich, E.L.; Furtado, R.H.M.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; et al. Comparison of the Effects of Glucagon-Like Peptide Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors for Prevention of Major Adverse Cardiovascular and Renal Outcomes in Type 2 Diabetes Mellitus. Circulation 2019, 139, 2022–2031. [Google Scholar] [CrossRef]
- Sharma, D.; Verma, S.; Vaidya, S.; Kalia, K.; Tiwari, V. Recent updates on GLP-1 agonists: Current advancements & challenges. Biomed. Pharmacother. 2018, 108, 952–962. [Google Scholar] [CrossRef]
- Bernardini, F.; Nusca, A.; Coletti, F.; La Porta, Y.; Piscione, M.; Vespasiano, F.; Mangiacapra, F.; Ricottini, E.; Melfi, R.; Cavallari, I.; et al. Incretins-Based Therapies and Their Cardiovascular Effects: New Game-Changers for the Management of Patients with Diabetes and Cardiovascular Disease. Pharmaceutics 2023, 15, 1858. [Google Scholar] [CrossRef]
- Canu, M.; Carabelli, A.; Debiossat, M.; Broisat, A.; Desvignes, M.; Ghezzi, C.; Barone-Rochette, G.; Riou, L. Effects of liraglutide on heart function and myocardial perfusion entropy in a type-2 diabetic rat model. Arch. Cardiovasc. Dis. Suppl. 2020, 12, 212. [Google Scholar] [CrossRef]
- Almutairi, M.; Gopal, K.; Greenwell, A.A.; Young, A.; Gill, R.; Aburasayn, H.; Al Batran, R.; Chahade, J.J.; Gandhi, M.; Eaton, F.; et al. The GLP-1 Receptor Agonist Liraglutide Increases Myocardial Glucose Oxidation Rates via Indirect Mechanisms and Mitigates Experimental Diabetic Cardiomyopathy. Can. J. Cardiol. 2021, 37, 140–150. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiao, X.; Zheng, J.; Li, M.; Yu, M.; Ping, F.; Wang, T.; Wang, X. Liraglutide protects cardiac function in diabetic rats through the PPARα pathway. Biosci. Rep. 2018, 38, BSR20180059. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Bain, S.C.; Atkin, S.L.; Rossing, P.; Scott, D.; Shamkhalova, M.S.; Bosch-Traberg, H.; Syrén, A.; Umpierrez, G.E. Efficacy and Safety of Liraglutide Versus Placebo as Add-on to Glucose-Lowering Therapy in Patients With Type 2 Diabetes and Moderate Renal Impairment (LIRA-RENAL): A Randomized Clinical Trial. Diabetes Care 2016, 39, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Anderson, C.; Broyde, A.; Polizzi, C.; Fernandez, R.; Baron, A.; Parkes, D.G. Glucagon-like peptide-1 and the exenatide analogue AC3174 improve cardiac function, cardiac remodeling, and survival in rats with chronic heart failure. Cardiovasc. Diabetol. 2010, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fang, H.; Xu, G.; Zhen, Y.; Zhang, Y.; Tian, J.; Zhang, D.; Zhang, G.; Xu, J. Liraglutide improves cognitive impairment via the AMPK and PI3K/Akt signaling pathways in type 2 diabetic rats. Mol. Med. Rep. 2018, 18, 2449–2457. [Google Scholar] [CrossRef]
- Wu, X.M.; Ou, Q.Y.; Zhao, W.; Liu, J.; Zhang, H. The GLP-1 analogue liraglutide protects cardiomyocytes from high glucose-induced apoptosis by activating the Epac-1/Akt pathway. Exp. Clin. Endocrinol. Diabetes 2014, 122, 608–614. [Google Scholar] [CrossRef]
- Nakamura, K.; Miyoshi, T.; Yoshida, M.; Akagi, S.; Saito, Y.; Ejiri, K.; Matsuo, N.; Ichikawa, K.; Iwasaki, K.; Naito, T.; et al. Pathophysiology and Treatment of Diabetic Cardiomyopathy and Heart Failure in Patients with Diabetes Mellitus. Int. J. Mol. Sci. 2022, 23, 3587. [Google Scholar] [CrossRef]
- Skovsø, S. Modeling type 2 diabetes in rats using high fat diet and streptozotocin. J. Diabetes Investig. 2014, 5, 349–358. [Google Scholar] [CrossRef]
- Andonova, M.; Dzhelebov, P.; Trifonova, K.; Yonkova, P.; Kostadinov, N.; Nancheva, K.; Ivanov, V.; Gospodinova, K.; Nizamov, N.; Tsachev, I.; et al. Metabolic Markers Associated with Progression of Type 2 Diabetes Induced by High-Fat Diet and Single Low Dose Streptozotocin in Rats. Vet. Sci. 2023, 10, 431. [Google Scholar] [CrossRef]
- Lee, W.S.; Kim, J. Application of Animal Models in Diabetic Cardiomyopathy. Diabetes Metab. J. 2021, 45, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Grubić Rotkvić, P.; Planinić, Z.; Liberati Pršo, A.M.; Šikić, J.; Galić, E.; Rotkvić, L. The Mystery of Diabetic Cardiomyopathy: From Early Concepts and Underlying Mechanisms to Novel Therapeutic Possibilities. Int. J. Mol. Sci. 2021, 22, 5973. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, S.; Cai, L. Diabetic cardiomyopathy and its mechanisms: Role of oxidative stress and damage. J. Diabetes Investig. 2014, 5, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Mohan, M.; Dihoum, A.; Mordi, I.R.; Choy, A.M.; Rena, G.; Lang, C.C. Left Ventricular Hypertrophy in Diabetic Cardiomyopathy: A Target for Intervention. Front. Cardiovasc. Med. 2021, 8, 746382. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.J.; Wulff, E.M.; Gotfredsen, C.F.; Brand, C.L.; Sturis, J.; Vrang, N.; Knudsen, L.B.; Lykkegaard, K. Combination of the insulin sensitizer, pioglitazone, and the long-acting GLP-1 human analog, liraglutide, exerts potent synergistic glucose-lowering efficacy in severely diabetic ZDF rats. Diabetes Obes. Metab. 2008, 10, 301–311. [Google Scholar] [CrossRef]
- Bajic, Z.; Sobot, T.; Uletilovic, S.; Mandic-Kovacevic, N.; Cvjetkovic, T.; Malicevic, U.; Djukanovic, D.; Duran, M.; Vesic, N.; Avram, S.; et al. Cardioprotective effects of liraglutide pretreatment on isoprenaline-induced myocardial injury in rats. Can. J. Physiol. Pharmacol. 2023, 101, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Margulies, K.B.; Hernandez, A.F.; Redfield, M.M.; Givertz, M.M.; Oliveira, G.H.; Cole, R.; Mann, D.L.; Whellan, D.J.; Kiernan, M.S.; Felker, G.M.; et al. Effects of Liraglutide on Clinical Stability Among Patients With Advanced Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. JAMA 2016, 316, 500–508. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Yang, L.; Yang, L.; Ma, H. Liraglutide ameliorates myocardial damage in experimental diabetic rats by inhibiting pyroptosis via Sirt1/AMPK signaling. Iran. J. Basic Med. Sci. 2021, 24, 1358–1365. [Google Scholar] [CrossRef]
- Wu, H.; Sheng, Z.Q.; Xie, J.; Li, R.; Chen, L.; Li, G.N.; Wang, L.; Xu, B. Reduced HMGB 1-Mediated Pathway and Oxidative Stress in Resveratrol-Treated Diabetic Mice: A Possible Mechanism of Cardioprotection of Resveratrol in Diabetes Mellitus. Oxid. Med. Cell. Longev. 2016, 2016, 9836860. [Google Scholar] [CrossRef]
- Suzuki, H.; Kayama, Y.; Sakamoto, M.; Iuchi, H.; Shimizu, I.; Yoshino, T.; Katoh, D.; Nagoshi, T.; Tojo, K.; Minamino, T.; et al. Arachidonate 12/15-Lipoxygenase–Induced Inflammation and Oxidative Stress Are Involved in the Development of Diabetic Cardiomyopathy. Diabetes 2014, 64, 618–630. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H. Oxidative Stress in Pancreatic Beta Cell Regeneration. Oxid. Med. Cell. Longev. 2017, 2017, 1930261. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, L.; Chen, S.; Feng, B.; Lu, X.; Bai, Y.; Liang, G.; Tan, Y.; Shao, M.; Skibba, M.; et al. The prevention of diabetic cardiomyopathy by non-mitogenic acidic fibroblast growth factor is probably mediated by the suppression of oxidative stress and damage. PLoS ONE 2013, 8, e82287. [Google Scholar] [CrossRef] [PubMed]
- Teringova, E.; Tousek, P. Apoptosis in ischemic heart disease. J. Transl. Med. 2017, 15, 87. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Zha, W.; Guo, S.; Cheng, H.; Wu, J.; Liu, C. Flos Puerariae extract prevents myocardial apoptosis via attenuation oxidative stress in streptozotocin-induced diabetic mice. PLoS ONE 2014, 9, e98044. [Google Scholar] [CrossRef]
- Wu, M.P.; Zhang, Y.S.; Zhou, Q.M.; Xiong, J.; Dong, Y.R.; Yan, C. Higenamine protects ischemia/reperfusion induced cardiac injury and myocyte apoptosis through activation of β2-AR/PI3K/AKT signaling pathway. Pharmacol. Res. 2016, 104, 115–123. [Google Scholar] [CrossRef]
- Chu, D.; Zhang, Z. Trichosanthis Pericarpium Aqueous Extract Protects H9c2 Cardiomyocytes from Hypoxia/Reoxygenation Injury by Regulating PI3K/Akt/NO Pathway. Molecules 2018, 23, 2409. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Chen, L.; Wang, Y.; Li, J. Glucagon-Like Peptide-1 Analog Liraglutide Protects against Diabetic Cardiomyopathy by the Inhibition of the Endoplasmic Reticulum Stress Pathway. J. Diabetes Res. 2013, 2013, 630537. [Google Scholar] [CrossRef]
- Urschel, K.; Tauchi, M.; Achenbach, S.; Dietel, B. Investigation of Wall Shear Stress in Cardiovascular Research and in Clinical Practice—From Bench to Bedside. Int. J. Mol. Sci. 2021, 22, 5635. [Google Scholar] [CrossRef]
- Godoy-Parejo, C.; Deng, C.; Liu, W.; Chen, G. Insulin Stimulates PI3K/AKT and Cell Adhesion to Promote the Survival of Individualized Human Embryonic Stem Cells. Stem Cells 2019, 37, 1030–1041. [Google Scholar] [CrossRef]
- Hatem-Vaquero, M.; Griera, M.; García-Jerez, A.; Luengo, A.; Álvarez, J.; Rubio, J.A.; Calleros, L.; Rodríguez-Puyol, D.; Rodríguez-Puyol, M.; De Frutos, S. Peripheral insulin resistance in ILK-depleted mice by reduction of GLUT4 expression. J. Endocrinol. 2017, 234, 115–128. [Google Scholar] [CrossRef]
- Bin Dayel, A.F.; Alonazi, A.S.; Alrasheed, N.M.; Alamin, M.A.; Sarawi, W.S.; Alharbi, A.O.; Alabbad, N.A.; Albuaijan, D.A.; Alassiri, D.N.; Aljarbua, A.F.; et al. Role of the integrin-linked kinase/TGF-β/SMAD pathway in sitagliptin-mediated cardioprotective effects in a rat model of diabetic cardiomyopathy. J. Pharm. Pharmacol. 2024, 76, 64–73. [Google Scholar] [CrossRef]
- Georgescu, M.M. PTEN Tumor Suppressor Network in PI3K-Akt Pathway Control. Genes Cancer 2010, 1, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Ferhatbegović, L.; Mršić, D.; Macić-Džanković, A. The benefits of GLP1 receptors in cardiovascular diseases. Front. Clin. Diabetes Healthc. 2023, 4, 1293926. [Google Scholar] [CrossRef]
- Baggio, L.L.; Yusta, B.; Mulvihill, E.E.; Cao, X.; Streutker, C.J.; Butany, J.; Cappola, T.P.; Margulies, K.B.; Drucker, D.J. GLP-1 Receptor Expression Within the Human Heart. Endocrinology 2018, 159, 1570–1584. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef] [PubMed]
- McLean, B.A.; Wong, C.K.; Kabir, M.G.; Drucker, D.J. Glucagon-like Peptide-1 receptor Tie2+ cells are essential for the cardioprotective actions of liraglutide in mice with experimental myocardial infarction. Mol. Metab. 2022, 66, 101641. [Google Scholar] [CrossRef] [PubMed]
- Trang, N.N.; Chung, C.C.; Lee, T.W.; Cheng, W.L.; Kao, Y.H.; Huang, S.Y.; Lee, T.I.; Chen, Y.J. Empagliflozin and Liraglutide Differentially Modulate Cardiac Metabolism in Diabetic Cardiomyopathy in Rats. Int. J. Mol. Sci. 2021, 22, 1177. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tang, Y.; He, H.; Wei, W. Liraglutide restores late cardioprotective effects of remote preconditioning in diabetic rats via activation of hydrogen sulfide and nuclear factor erythroid 2-related factor 2 signaling pathway. Acta Cir. Bras. 2021, 36, e360207. [Google Scholar] [CrossRef]
- Bułdak, Ł.; Machnik, G.; Bułdak, R.J.; Łabuzek, K.; Bołdys, A.; Belowski, D.; Basiak, M.; Okopień, B. Exenatide (a GLP-1 agonist) expresses anti-inflammatory properties in cultured human monocytes/macrophages in a protein kinase A and B/Akt manner. Pharmacol. Rep. 2016, 68, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Elekofehinti, O.O.; Oyedokun, V.O.; Iwaloye, O.; Lawal, A.O.; Ejelonu, O.C. Momordica charantia silver nanoparticles modulate SOCS/JAK/STAT and PI3K/Akt/PTEN signalling pathways in the kidney of streptozotocin-induced diabetic rats. J. Diabetes Metab. Disord. 2021, 20, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Madhu, D.; Hammad, M.; Kavalakatt, S.; Khadir, A.; Tiss, A. GLP-1 Analogue, Exendin-4, Modulates MAPKs Activity but not the Heat Shock Response in Human HepG2 Cells. Proteom. Clin. Appl. 2018, 12, 1600169. [Google Scholar] [CrossRef] [PubMed]
- Trefts, E. Integrin-Linked Kinase Is Critical for Hepatic Cellular Organization, Metabolism, and Glucoregulation. Ph.D. Thesis, Vanderbilt University, Nashville, TN, USA, 2019. [Google Scholar]
- Cai, C.; Wu, F.; He, J.; Zhang, Y.; Shi, N.; Peng, X.; Ou, Q.; Li, Z.; Jiang, X.; Zhong, J.; et al. Mitochondrial quality control in diabetic cardiomyopathy: From molecular mechanisms to therapeutic strategies. Int. J. Biol. Sci. 2022, 18, 5276–5290. [Google Scholar] [CrossRef] [PubMed]
- Oura, K.; Morishita, A.; Tani, J.; Masaki, T. Antitumor Effects and Mechanisms of Metabolic Syndrome Medications on Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2022, 9, 1279–1298. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Jin, Y.X.; Shen, W.; Neng, J.; Wu, T.; Li, Y.J.; Fu, Z.W. Low dose streptozotocin (STZ) combined with high energy intake can effectively induce type 2 diabetes through altering the related gene expression. Asia Pac. J. Clin. Nutr. 2007, 16 (Suppl. S1), 412–417. [Google Scholar] [PubMed]
- Zhang, S.; Xu, H.; Yu, X.; Wu, Y.; Sui, D. Metformin ameliorates diabetic nephropathy in a rat model of low-dose streptozotocin-induced diabetes. Exp. Ther. Med. 2017, 14, 383–390. [Google Scholar] [CrossRef]
- Farrag, E.A.E.; Hammad, M.O.; Safwat, S.M.; Hamed, S.; Hellal, D. Artemisinin attenuates type 2 diabetic cardiomyopathy in rats through modulation of AGE-RAGE/HMGB-1 signaling pathway. Sci. Rep. 2023, 13, 11043. [Google Scholar] [CrossRef]
- Abdelrazik Soliman, N.G.; Abdel-Hamid, A.A.M.; El-Hawwary, A.A.; Ellakkany, A. Impact of liraglutide on microcirculation in experimental diabetic cardiomyopathy. Acta Histochem. 2020, 122, 151533. [Google Scholar] [CrossRef]
- Moron, M.S.; Depierre, J.W.; Mannervik, B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim. Biophys. Acta 1979, 582, 67–78. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Delides, A.; Spooner, R.J.; Goldberg, D.M.; Neal, F.E. An optimized semi-automatic rate method for serum glutathione reductase activity and its application to patients with malignant disease. J. Clin. Pathol. 1976, 29, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Su, Q.; Wang, Y.; Dai, R.; Lu, Y.; Su, B.; Zhao, Y. Effect of atorvastatin (Lipitor) on myocardial apoptosis and caspase-8 activation following coronary microembolization. Cell Biochem. Biophys. 2011, 61, 399–406. [Google Scholar] [CrossRef]







| Groups | Parameters | ||
|---|---|---|---|
| Body Weight (g) | HW/BW Ratio (mg/g) | Glucose (mg/dL) | |
| Nondiabetic control | 389.2 ± 17.76 | 2.619 ± 0.27 | 39.37 ± 6.22 |
| Diabetic control | 252.0 ± 13.16 ### | 4.751 ± 0.49 ### | 323.2 ± 27.64 ### |
| Liraglutide-treated nondiabetic rats | 340.8 ± 10.20 | 2.774 ± 0.1 | 37.83 ± 5.82 |
| Liraglutide-treated diabetic rats | 220.0 ± 13.20 | 2.798 ± 0.13 *** | 62.38 ± 3.83 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alobaid, S.M.; Alshahrani, R.M.; Alonazi, A.S.; Alrasheed, N.M.; Alamin, M.A.; Alshammari, T.K.; Bin Dayel, A.F.; Elnagar, D.M.; Alotaibi, R.R.; Almuthnabi, L.A.; et al. Liraglutide Attenuates Diabetic Cardiomyopathy via the ILK/PI3K/AKT/PTEN Signaling Pathway in Rats with Streptozotocin-Induced Type 2 Diabetes Mellitus. Pharmaceuticals 2024, 17, 374. https://doi.org/10.3390/ph17030374
Alobaid SM, Alshahrani RM, Alonazi AS, Alrasheed NM, Alamin MA, Alshammari TK, Bin Dayel AF, Elnagar DM, Alotaibi RR, Almuthnabi LA, et al. Liraglutide Attenuates Diabetic Cardiomyopathy via the ILK/PI3K/AKT/PTEN Signaling Pathway in Rats with Streptozotocin-Induced Type 2 Diabetes Mellitus. Pharmaceuticals. 2024; 17(3):374. https://doi.org/10.3390/ph17030374
Chicago/Turabian StyleAlobaid, Shatha M., Rahaf M. Alshahrani, Asma S. Alonazi, Nawal M. Alrasheed, Maha A. Alamin, Tahani K. Alshammari, Anfal F. Bin Dayel, Doaa M. Elnagar, Rana R. Alotaibi, Lama A. Almuthnabi, and et al. 2024. "Liraglutide Attenuates Diabetic Cardiomyopathy via the ILK/PI3K/AKT/PTEN Signaling Pathway in Rats with Streptozotocin-Induced Type 2 Diabetes Mellitus" Pharmaceuticals 17, no. 3: 374. https://doi.org/10.3390/ph17030374