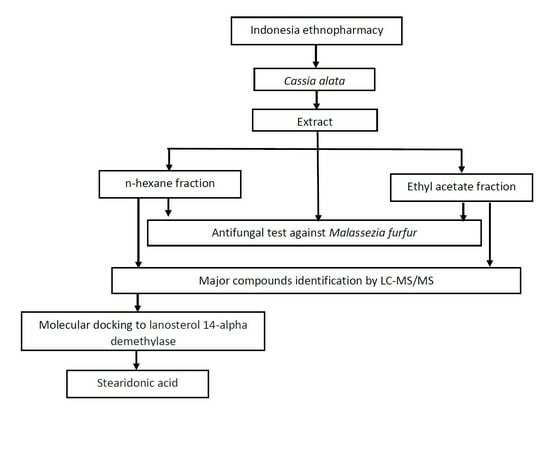
Cassia alata L.: A Study of Antifungal Activity against Malassezia furfur, Identification of Major Compounds, and Molecular Docking to Lanosterol 14-Alpha Demethylase
Abstract
:1. Introduction
2. Results
2.1. Preparation of C. alata Leaf Extract and Fractions
2.2. In Vitro Antifungal Activity
2.3. Identification of Major Compounds in Fractions
2.4. In Silico Study
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of C. alata Leaf Extract and Fractions
4.3. In Vitro Antifungal Activity Test
4.4. Identification of Major Compounds in the Fractions
4.5. Molecular Docking
4.5.1. Preparation of Enzyme
4.5.2. Preparation of Compounds
4.5.3. Active Site Prediction
4.5.4. Docking Simulation
4.5.5. Interaction Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ingle, K.P.; Deshmukh, A.G.; Padole, D.A.; Dudhare, M.S.; Moharil, M.P.; Khelurkar, V.C. Phytochemicals: Extraction methods, identification, and detection of bioactive compounds from plant extracts. J. Pharmacogn. Phytochem. 2017, 6, 32–36. [Google Scholar]
- Pandey, A.; Tripathi, S. Concept of standardization, extraction, and pre-phytochemical screening strategies for herbal drug. J. Pharmacogn. Phytochem. 2014, 2, 115–1199. [Google Scholar]
- Fatmawati, S.; Yuliana; Purnomo, A.S.; Bakar, M.F.A. Chemical constituents, usage and pharmacological activity of Cassia alata. Heliyon 2020, 6, e04396. [Google Scholar] [CrossRef]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Frontier Pharmacol. 2013, 4, 1–10. [Google Scholar] [CrossRef]
- Verpoorte, R. Forward. In Evidence-Based Validation of Herbal Medicine; Mukherjee, P.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 13–14. [Google Scholar]
- Hennebelle, T.; Weniger, B.; Joseph, H.; Sahpaz, S.; Bailleul, F. Senna alata. Fitoterapia 2009, 80, 385–393. [Google Scholar] [CrossRef]
- Senna Species, Candle Bush, Candelabra Bush, Empress Candle Plant, Golden Candlestick. Available online: https://davesgarden.com/guides/pf/go/371 (accessed on 4 March 2024).
- Hassan, S.W.; Umar, R.A.; Ladan, M.J.; Nyemike, P.; Wasagu, R.S.U.; Lawal, M.; Eboh, A.A. Nutritive value, phytochemical and antifungal properties of Pergularia tomentosa L. (Asclepiadaceae). Int. J. Pharmacol. 2007, 3, 334–340. [Google Scholar] [CrossRef]
- Edegbo, E.; Okolo, M.L.O.; Adegoke, A.S.; Omatola, C.A.; Idache, B.M.; Abraham, J.O.; Akor, M.E.; Zakari, D.A.; Alaba, A.Z.; Omale, S.; et al. Phytochemical screening and antifungal activity of Cassia alata (Linn.) crude leaf extracts. Afri. J. Microbiol. Res. 2023, 17, 176–183. [Google Scholar] [CrossRef]
- Sujatha, J.; Rajesh, K.S.; Lakshmi, T. Antidermatophytic, the anticancer and antioxidant activity of Cassia alata ethanolic extract and its phytochemical analysis. Int. J. Res. Pharm. Sci. 2019, 10, 838–845. [Google Scholar] [CrossRef]
- Selvi, V.; Isaivani, I.; Karpagam, S. Studies on antimicrobial activities from flower extracts of Cassia alata Linn. Int. J. Curr. Sci. 2012, 4, 299–303. [Google Scholar]
- Sule, W.F.; Okonko, I.O.; Omo-Ogun, S.; Ojezele, M.O.; Ojezele, O.J.; Alli, J.A.; Soyemi, E.T.; Olaonipekun, T.O. Phytochemical properties and in-vitro antifungal activity of Senna alata Linn. crude stem bark extract. J. Med. Plants Res. 2011, 5, 176–183. [Google Scholar] [CrossRef]
- Phongpaichit, S.; Pujenjob, N.; Rukachaisirikul, V.; Ongsakul, M. Antifungal activity from leaf extracts of Cassia alata L., Cassia fistula L. and Cassia tora L. Songklanakarin J. Sci. Technol. 2004, 26, 741–748. [Google Scholar]
- Akinmoladun, A.C.; Obuotor, E.M.; Farombi, E.O. Evaluation of antioxidant and free radical scavenging capacities of some Nigerian indigenous medicinal plants. J. Med. Food 2010, 13, 444–451. [Google Scholar] [CrossRef]
- Adiana, M.A.; Mazura, M.P. Study on Senna alata and its different extracts by Fourier Transform Infrared Spectroscopy and two-dimensional correlation infrared spectroscopy. J. Mol. Struct. 2011, 991, 84–91. [Google Scholar] [CrossRef]
- Rahman, M.S.; Ali, M.Y.; Ali, M.U. In vitro screening of two flavonoid compounds isolated from cassia alata L. leaves for fungicidal activities. J. Biol. Sci. 2008, 16, 142–193. [Google Scholar] [CrossRef]
- Saito, S.; Silva, G.; Santos, R.X.; Gosmann, G.; Pungartnik, C.; Brendel, M. Astragalin from Cassia alata induces DNA adducts in vitro and repairable DNA damage in the yeast Saccharomyces cerevisiae. Int. J. Mol. Sci. 2012, 13, 2846–2862. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Xu, L.; Zou, Z.; Yang, S. Studies on chemical constituents from leaves of Cassia alata. Zhongguo Zhong Yao Za Zhi 2009, 34, 861–863. [Google Scholar]
- Khare, C.P. Indian Medicinal Plants: An Illustrated dictionary; Springer Press: London, UK, 2007; pp. 167–168. [Google Scholar]
- Faergemann, J. Management of seborrheic dermatitis and pityriasis versicolor. Am. J. Clin. Dermatol. 2000, 1, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Gaitanis, G.; Magiatis, P.; Hantschke, M.; Bassukas, I.D.; Velegraki, A. The Malassezia genus in skin and systemic diseases. Clin. Microbiol. Rev. 2012, 25, 106–141. [Google Scholar] [CrossRef]
- Gao, Z.; Perez-Perez, G.I.; Chen, Y.; Blaser, M.J. Quantitation of major human cutaneous bacterial and fungal populations. J. Clin. Microbiol. 2010, 48, 3575–3581. [Google Scholar] [CrossRef]
- Gupta, A.K.; Bluhm, R.; Barlow, J.O.; Fleischer, A.B., Jr.; Feldman, S.R. Prescribing practices for seborrheic dermatitis vary with the physician’s specialty: Implications for clinical practice. J. Dermatolog. Treat. 2004, 15, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Dessinioti, C.; Katsambas, A. Seborrheic dermatitis: Etiology, risk factors, and treatments: Facts and controversies. Clin. Dermatol. 2013, 31, 343–351. [Google Scholar] [CrossRef]
- Wikramanayake, T.C.; Borda, L.J.; Miteva, M.; Paus, R. Seborrheic dermatitis-Looking beyond Malassezia. Exp. Dermatol. 2019, 28, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.R.; Messenger, A.G.; Tosti, A.; Hordinsky, M.; Hay, R.J.; Wang, X.; Zachariae, C.; Kerr, K.M.; Henry, J.P.; Rust, R.C.; et al. A comprehensive pathophysiology of dandruff and seborrheic dermatitis-Towards a more precise definition of scalp health. Acta Derm-Venereol. 2013, 93, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Mustarichie, R.; Wicaksono, I.A.; Hayati, C. Anti-alopecia characteristics of ethanol extract, n-hexane, ethyl acetate and water fractions of Malvaviscus arboreus Cav. Res. J. Pharm. Technol. 2018, 11, 5066–5072. [Google Scholar] [CrossRef]
- Becher, R.; Wirsel, S.G.R. Fungal cytochrome P450 sterol 14α-demethylase (CYP51) and azole resistance in plant and human pathogens. Appl. Microbiol. Biotechnol. 2012, 95, 825–840. [Google Scholar] [CrossRef]
- Baysal, V.; Yildirim, M.; Ozcanli, C.; Ceyhan, A.M. Itraconazole in the treatment of seborrheic dermatitis: A new treatment modality. Int. J. Dermatol. 2004, 43, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Mustarichie, R.; Rostinawati, T.; Pitaloka, D.A.E.; Saptarini, N.M.; Iskandar, Y. Herbal Therapy for the Treatment of Seborrhea Dermatitis. Clin. Cosmet. Investig. Dermatol. 2022, 2022, 2391–2405. [Google Scholar] [CrossRef]
- Dewick, P.M. Medicinal Natural Products. In A Biosynthetic Approach; Wiley: New York, NY, USA, 2002; pp. 1–507. [Google Scholar]
- Seigler, D.S. Plant Secondary Metabolism; Springer Science: New York, NY, USA, 1998; pp. 1–759. [Google Scholar]
- Wink, M. Modes of Action of Herbal Medicines and Plant Secondary Metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef]
- Svahn, S. Analysis of secondary metabolites from Aspergillus fumigatus and Penicillium nalgiovense. In Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Pharmacy; Uppsala University: Uppsala, Sweden, 2015. [Google Scholar]
- Balunas, M.J.; Kinghorn, A.D. Drug discovery from medicinal plants. Life Sci. 2005, 78, 431–441. [Google Scholar] [CrossRef]
- Saptarini, N.M.; Mustarichie, R.; Herawati, I.E.; Hadisoebroto, G. Isolation, identification, and quantification of major flavonoid in leaves of Pereskia Bleo (Kunth) DC. Int. J. Appl. Pharm. 2022, 14, 106–110. [Google Scholar] [CrossRef]
- Lapornik, B.; Prosek, M.; Wondra, A.G. Comparison of extracts prepared from plant by-products using different solvents and extraction time. J. Food Eng. 2005, 71, 214–222. [Google Scholar] [CrossRef]
- Wang, G.X.; Zhou, Z.; Jiang, D.X.; Han, J.; Wang, J.F.; Zhao, L.W.; Li, J. In vivo anthelmintic activity of five alkaloids from Macleaya microcarpa (Maxim) Fedde against Dactylogyrus intermedius in Carassius auratus. Vet. Parasitol. 2010, 171, 305–313. [Google Scholar] [CrossRef]
- Tiwari, P.; Kumar, B.; Kaur, M.; Kaur, G.; Kaur, H. Phytochemical screening and extraction: A review. Int Pharm Sci. 2011, 1, 98–106. [Google Scholar]
- Ketoconazole (Topical) (Monograph). Available online: https://www.drugs.com/monograph/ketoconazole-topical.html (accessed on 4 March 2024).
- Sulistyo, M.H.; Pani, S.; Kumaji, S. Pengaruh ekstrak daun ketepeng cina (Cassia alata L.) terhadap pertumbuhan jamur Malassezia Furfur penyebab ketombe. J. Health Techn. Sci. 2018, 1, 11–17. [Google Scholar] [CrossRef]
- Triana, O.; Prasetya, F.; Kuncoro, H.; Rijai, L. Aktivitas Antijamur Ekstrak Daun Ketepeng Cina (Cassia alata L.). J. Sains Dan Kesehat. 2016, 1, 311–315. [Google Scholar] [CrossRef]
- Cushnie, T.P.; Cushnie, B.; Echeverria, J.; Fowsantear, W.; Thammawat, S.; Dodgson, J.L.; Law, S.; Clow, S.M. Bioprospecting for antibacterial drugs: A multidisciplinary perspective on natural product source material, bioassay selection and avoidable pitfalls. Pharm. Res. 2020, 37, 125. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, C.; Song, L.; Sommerfeld, M.; Hu, Q. A high throughput nile red method for quantitative measurement of neutral lipids in microalgae. J. Microbiol. Methods 2009, 77, 41–47. [Google Scholar] [CrossRef]
- Fatahillah, R.; Fitriyani, D.; Wijayanti, F. In vitro anti-inflammatory activity of extract and fraction seed coat kebiul (Caesalpinia bonduc L.). J. Chem. 2022, 10, 42–50. [Google Scholar] [CrossRef]
- Promgool, T.; Pancharoen, O.; Deachathai, S. Antibacterial and antioxidative compounds from Cassia alata Linn. Songklanakarin J. Sci. Technol. 2014, 36, 459–463. [Google Scholar]
- Ghannoum, M.A.; Rice, L.B. Antifungal Agents: Mode of Action, Mechanisms of Resistance, and Correlation of These Mechanisms with Bacterial Resistance. Clin. Microbiol. Rev. 1999, 12, 501–517. [Google Scholar] [CrossRef]
- Walker, G.M.; White, N.A. Introduction to Fungal Physiology. In Fungi; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005; pp. 1–34. [Google Scholar] [CrossRef]
- Pinto, E.; Vale-Silva, L.; Cavaleiro, C.; Salgueiro, L. Antifungal activity of the clove essential oil from Syzygium aromaticum on Candida, Aspergillus and dermatophyte species. J. Med. Microbiol. 2009, 58, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.D.; Dizdaroglu, M.; Cooke, M.S. Oxidative DNA damage and disease: Induction, repair and significance. Mutat. Res. Rev. Mutat. Res. 2004, 567, 1–61. [Google Scholar] [CrossRef]
- Rautenbach, M.; Troskie, A.M.; Vosloo, J.A. Antifungal peptides: To be or not to be membrane active. Biochimie 2016, 130, 132–145. [Google Scholar] [CrossRef]
- Lagrouh, F.; Dakka, N.; Bakri, Y. The antifungal activity of Moroccan plants and the mechanism of action of secondary metabolites from plants. J. Mycol. Med. 2017, 27, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, L.P.; Zhang, J.D.; Li, Q.; Shen, H.; Chen, S.M.; He, L.J.; Yan, L.; Xu, G.T.; An, M.M.; et al. Synergistic Antifungal Effect of Glabridin and Fluconazole. PLoS ONE 2014, 9, e103442. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Fong, W.-P.; Tsang, P.W.-K. Novel antifungal activity of purpurin against Candida species in vitro. Med. Mycol. 2010, 48, 904–911. [Google Scholar] [CrossRef]
- Bitencourt, T.A.; Komoto, T.T.; Massaroto, B.G.; Miranda, C.E.S.; Beleboni, R.O.; Marins, M.; Fachin, A.L. Trans-chalcone and quercetin down-regulate fatty acid synthase gene expression and reduce ergosterol content in the human pathogenic dermatophyte Trichophyton rubrum. BMC Compl. Altern. Med. 2013, 13, 229. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.; Mikhova, B.; Naidenski, H.; Tsvetkova, I.; Kostova, I. Chemical composition and antimicrobial activity of wild garlic Allium ursinum of Bulgarian origin. Nat. Prod. Commun. 2009, 4, 1059–1062. [Google Scholar] [CrossRef]
- Timothy, S.Y.; Wazis, C.H.; Adati, R.G.; Maspalma, I.D. Antifungal activity of aqueous and ethanolic leaf extracts of Cassia alata Linn. J. Appl. Pharm. Sci. 2012, 2, 182–185. [Google Scholar] [CrossRef]
- Yun, D.G.; Lee, D.G. Silymarin exerts antifungal effects via membrane-targeted mode of action by increasing permeability and inducing oxidative stress. Biochim. Biophys. Acta Biomembr. 2017, 1859, 467–474. [Google Scholar] [CrossRef]
- Rocha, M.F.G.; Sales, J.A.; Rocha, M.G.; Galdino, L.M.; Angular, L.; Pireira-Neto, W.A.; Cordeiro, R.A.; Castelo-Branco, D.S.C.M.; Sidrim, J.J.C.; Brilhante, R.S.N. Antifungal effects of the flavonoids kaempferol and quercetin: A possible alternative for the control of fungal biofilms. Biofouling 2019, 35, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Proma, R.Z.; Khan, N. Phytochemical screening of plant extracts and GC-MS analysis of n-hexane extract of the leaves of Cassia alata Linn. J. Phytopharmacol. 2020, 9, 342–347. [Google Scholar] [CrossRef]
- Badr, A.N.; Ali, H.S.; Abdel-Razek, A.G.; Shaheta, M.G.; Albaridi, N.A. Bioactive components of pomegranate oil and their influence on mycotoxin secretion. Toxins 2020, 12, 748. [Google Scholar] [CrossRef]
- Altieri, C.; Cardillo, D.; Bevilacqua, A.; Sinigaglia, M. Inhibition of Aspergillus spp. and Penicillium spp. by fatty acids and their monoglycerides. J. Food Prot. 2007, 70, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Avis, T.J.; Bélanger, R.R. Specificity and mode of action of the antifungal fatty acid cis-9-heptadecenoic acid produced by Pseudozyma flocculosa. Appl. Environ. Microbiol. 2001, 67, 956–960. [Google Scholar] [CrossRef]
- Thibane, V.S.; Ells, R.; Hugo, A.; Albertyn, J.; Van Rensburg, W.J.J.; Van Wyk, P.W.; Kock, J.L.; Pohl, C.H. Polyunsaturated fatty acids cause apoptosis in C. albicans and C. dubliniensis biofilms. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 1463–1468. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Shono, F.; Kai, H.; Uno, T.; Uyeda, M. Inhibition of topoisomerases by fatty acids. J. Enzym. Inhib. 2000, 15, 357–366. [Google Scholar] [CrossRef]
- Yonezawa, Y.; Hada, T.; Uryu, K.; Tsuzuki, T.; Eitsuka, T.; Miyazawa, T.; Murakami-Nakai, C.; Yoshida, H.; Mizushina, Y. Inhibitory effect of conjugated eicosapentaenoic acid on mammalian DNA polymerase and topoisomerase activities and human cancer cell proliferation. Biochem. Pharmacol. 2005, 70, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y. Topoisomerase I inhibitors: Camptothecins and beyond. Nat. Rev. Cancer 2006, 6, 789–802. [Google Scholar] [CrossRef]
- Wood, R.; Lee, T. Metabolism of 2-hexadecynoate and inhibition of fatty acid elongation. J. Biol. Chem. 1981, 256, 12379–12386. [Google Scholar] [CrossRef]
- Parang, K.; Knaus, E.E.; Wiebe, L.I.; Sardari, S.; Daneshtalab, M.; Csizmadia, F. Synthesis and antifungal activities of myristic acid analogs. Arch. Pharm. 1996, 329, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.N.; Nosanchuk, J.D. Lipid droplet formation protects against gluco/lipotoxicity in Candida parapsilosis: An essential role of fatty acid desaturase Ole1. Cell Cycle 2011, 10, 3159–3167. [Google Scholar] [CrossRef] [PubMed]
- McGaw, L.J.; Jäger, A.K.; Van Staden, J. Isolation of antibacterial fatty acids from Schotia brachypetala. Fitoter 2002, 73, 431–433. [Google Scholar] [CrossRef] [PubMed]
- Astiti, N.P.A.; Ramona, Y. GC-MS analysis of active and applicable compounds in methanol extract of sweet star fruit (Averrhoa carambola L.) leaves. HAYATI J. Biosci. 2021, 28, 12–22. [Google Scholar] [CrossRef]
- Thibane, V.S.; Kock, J.L.; Ells, R.; van Wyk, P.W.; Pohl, C.H. Effect of marine polyunsaturated fatty acids on biofilm formation of Candida albicans and Candida dubliniensis. Mar. Drugs. 2010, 8, 2597–2604. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; He, B.; Ma, L.; Sun, Y.; Niu, Y.; Zeng, B. Recent Advances in Ergosterol Biosynthesis and Regulation Mechanisms in Saccharomyces cerevisiae. Indian J. Microbiol. 2017, 57, 270–277. [Google Scholar] [CrossRef]
- Pineda, R.; Vizcaino, S.; Garcia, C.M.; Gil, J.H.; Durango, D. Antifungal activity of extracts, essential oil and constituents from Petroselinum crispum against Colletotrichum acutatum. Rev. Fac. Nac. De Agron. Medellin 2018, 71, 8563–8572. [Google Scholar] [CrossRef]
- Mutasa, T.; Mangoyi, R.; Mukanganyama, S. The effects of Combretum zeyheri leaf extract on ergosterol synthesis in Candida albicans. J. Herbs Spices Med. Plants 2015, 21, 211–217. [Google Scholar] [CrossRef]
- Beena, P.; Rajesh, K.J.; Arul, B. Preliminary phytochemical screening of Cicer arietinum in folklore medicine for hepatoprotection. J. Innov. Pharm. Biol. Sci. 2016, 3, 153–159. [Google Scholar]
- Saptarini, N.M.; Mustarichie, R.; Aulifa, D.L.; Hendriani, R.; Herawati, I.E. Analysis of antioxidant and antibacterial activity of leaves of fig (Ficus carica L.) from Ciwidey Distric, West Java, Indonesia. Rasayan J. Chem. 2022, Special Issue, 172–179. [Google Scholar] [CrossRef]
- Martini, N.; Eloff, J.N. The preliminary isolation of several antibacterial compounds from Combretum erythrophyllum (Combretaceae). J. Ethnopharmacol. 1998, 62, 2555–2563. [Google Scholar] [CrossRef] [PubMed]
- Hasanuddin, S.; Erisman; Ramadhan, D.S.F.; Gozali, D.; Arba, M.; Mustarichie, R. Inhibitory activity, metabolite contents determination, and in silico prediction of parsley leaves fraction (Petrocelinum crispum MILL) as antifungal agent of Malassezia furfur. Rasayan J. Chem. 2022, 15, 1539–1546. [Google Scholar] [CrossRef]
- Apweiler, R.; Bairoch, S.; Wu, C.H.; Barker, W.C.; Boeckmann, B.; Ferro, S.; Gasteiger, E.; Huang, H.; Lopez, R.; Magrane, M.; et al. UniProt: The universal protein knowledgebase. Nucleic Acids Res. 2004, 32, D115–D119. [Google Scholar] [CrossRef] [PubMed]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Cassarino, T.G.; Bertoni, M.; Bordoli, L.; et al. SWISS-MODEL: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014, 42, W252–W258. [Google Scholar] [CrossRef] [PubMed]
- McGinnis, S.; Madden, T.L. BLAST: At the core of a powerful and diverse set of sequence analysis tools. Nucleic Acids Res. 2004, 32, W20–W25. [Google Scholar] [CrossRef]
- Schwede, T.; Kopp, J.; Guex, N.; Peitsch, M.C. SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res. 2003, 31, 3381–3385. [Google Scholar] [CrossRef]
- Hollingsworth, S.A.; Karplus, P.A. A fresh look at the Ramachandran plot and the occurrence of standard structures in proteins. Biomol. Concepts 2010, 1, 271–283. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 1–14. [Google Scholar] [CrossRef]






| Compound | Concentration (mg/mL) | Inhibition Zone (mm) |
|---|---|---|
| Extract | 50 | 29.89 ± 1.21 * |
| 100 | 30.33 ± 1.92 * | |
| 150 | 32.11 ± 0.91 * | |
| 200 | 32.67 ± 0.61 * | |
| 300 | 33.78 ± 0.36 * | |
| 400 | 39.11 ± 0.34 * | |
| Ketoconazole | 20 | 19.44 ± 1.27 * |
| DMSO | 10 | 0 |
| Compound | Concentration (mg/mL) | Inhibition Zone (mm) |
|---|---|---|
| n-Hexane fraction | 250 | 6.54 ± 0.41 * |
| 300 | 7.89 ± 0.17 * | |
| 350 | 8.14 ± 0.15 * | |
| 400 | 15.76 ± 0.09 * | |
| Ethyl acetate fraction | 250 | 4.99 ± 0.32 * |
| 300 | 9.04 ± 0.02 * | |
| 350 | 9.32 ± 0.01 * | |
| 400 | 9.68 ± 0.01 * | |
| Ketoconazole | 20 | 19.34 ± 1.27 * |
| DMSO | 10 | 0 |
| Compound Name | Formula | Observed m/z | Neutral Mass (Da) | Observed RT (min) |
|---|---|---|---|---|
| 5,7,2′,5′-Tetrahydroxy-flavone | C15H10O6 | 287.0541 | 286.04774 | 5.09 |
| Kaempferol-3,7-diglucoside | C27H30O16 | 611.1624 | 610.15338 | 3.49 |
| Quercetin | C15H10O7 | 303.0494 | 302.04265 | 4.59 |
| Candidate mass C35H36N4O5 | C35H36N4O5 | 593.2758 | 592.26857 | 10.35 |
| Candidate mass C36H38N4O7 | C36H38N4O7 | 639.2825 | 638.27405 | 10.78 |
| Compound Name | Formula | Observed m/z | Neutral Mass (Da) | Observed RT (min) |
|---|---|---|---|---|
| 9-Ene-methyl palmitate | C17H32O2 | 291.2306 | 268.24023 | 8.84 |
| Stearidonic acid | C18H28O2 | 277.2154 | 276.20893 | 7.65 |
| Trichosanic acid | C18H30O2 | 279.2311 | 278.22458 | 8.07 |
| Candidate mass C36H38N4O7 | C36H38N4O7 | 639.2819 | 638.27405 | 10.77 |
| Candidate mass C34H34N4O5 | C34H34N4O5 | 579.2615 | 578.25292 | 11.37 |
| Compound | ∆G (kcal/mol) | Amino Acid Residue Hydrogen Bond | Other Amino Acid Residues Involved |
|---|---|---|---|
| Ketoconazole (positive control) | −7.9 | TYR90, HIS415, PRO409 | ALA261, PRO326, ILE327, PHE410, CYS417 |
| 9-Ene-methyl palmitate | −6.5 | LEU46 | PHE458, LEU457 |
| Stearidonic acid | −7.2 | SER456 | ILE331, LEU45 |
| Trichosanic acid | −7.2 | SER329 | ILE330, ILE327, ILE331, LEU457, TYR76, TYR22, TYR90, THR80, PHE458, PHE183, SER456, LEU79, VAL75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saptarini, N.M.; Mustarichie, R.; Hasanuddin, S.; Corpuz, M.J.-A.T. Cassia alata L.: A Study of Antifungal Activity against Malassezia furfur, Identification of Major Compounds, and Molecular Docking to Lanosterol 14-Alpha Demethylase. Pharmaceuticals 2024, 17, 380. https://doi.org/10.3390/ph17030380
Saptarini NM, Mustarichie R, Hasanuddin S, Corpuz MJ-AT. Cassia alata L.: A Study of Antifungal Activity against Malassezia furfur, Identification of Major Compounds, and Molecular Docking to Lanosterol 14-Alpha Demethylase. Pharmaceuticals. 2024; 17(3):380. https://doi.org/10.3390/ph17030380
Chicago/Turabian StyleSaptarini, Nyi Mekar, Resmi Mustarichie, Silviana Hasanuddin, and Mary Jho-Anne Tolentino Corpuz. 2024. "Cassia alata L.: A Study of Antifungal Activity against Malassezia furfur, Identification of Major Compounds, and Molecular Docking to Lanosterol 14-Alpha Demethylase" Pharmaceuticals 17, no. 3: 380. https://doi.org/10.3390/ph17030380