Unraveling the Mechanism of Xiaochaihu Granules in Alleviating Yeast-Induced Fever Based on Network Analysis and Experimental Validation
Abstract
:1. Introduction
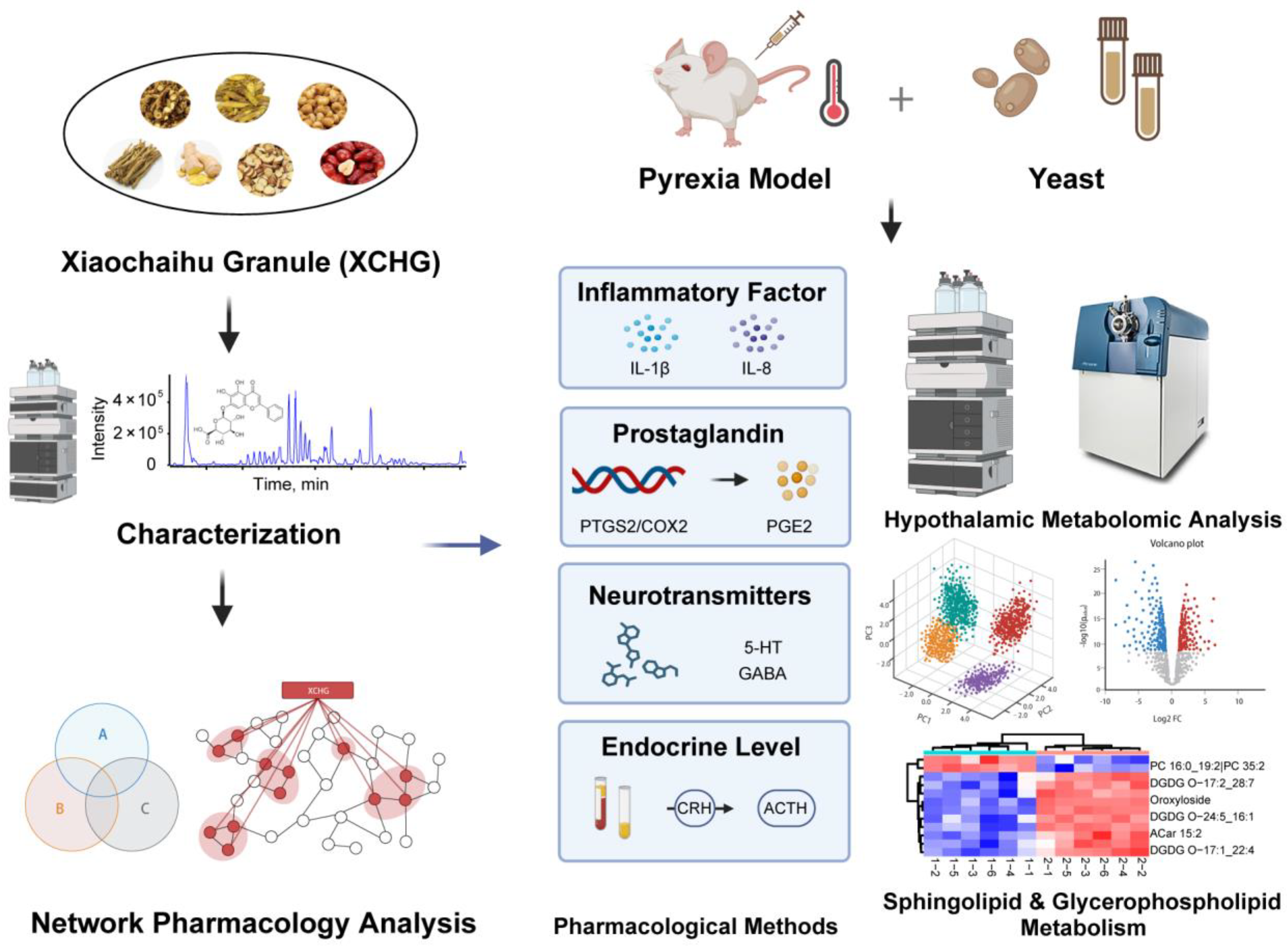
2. Results
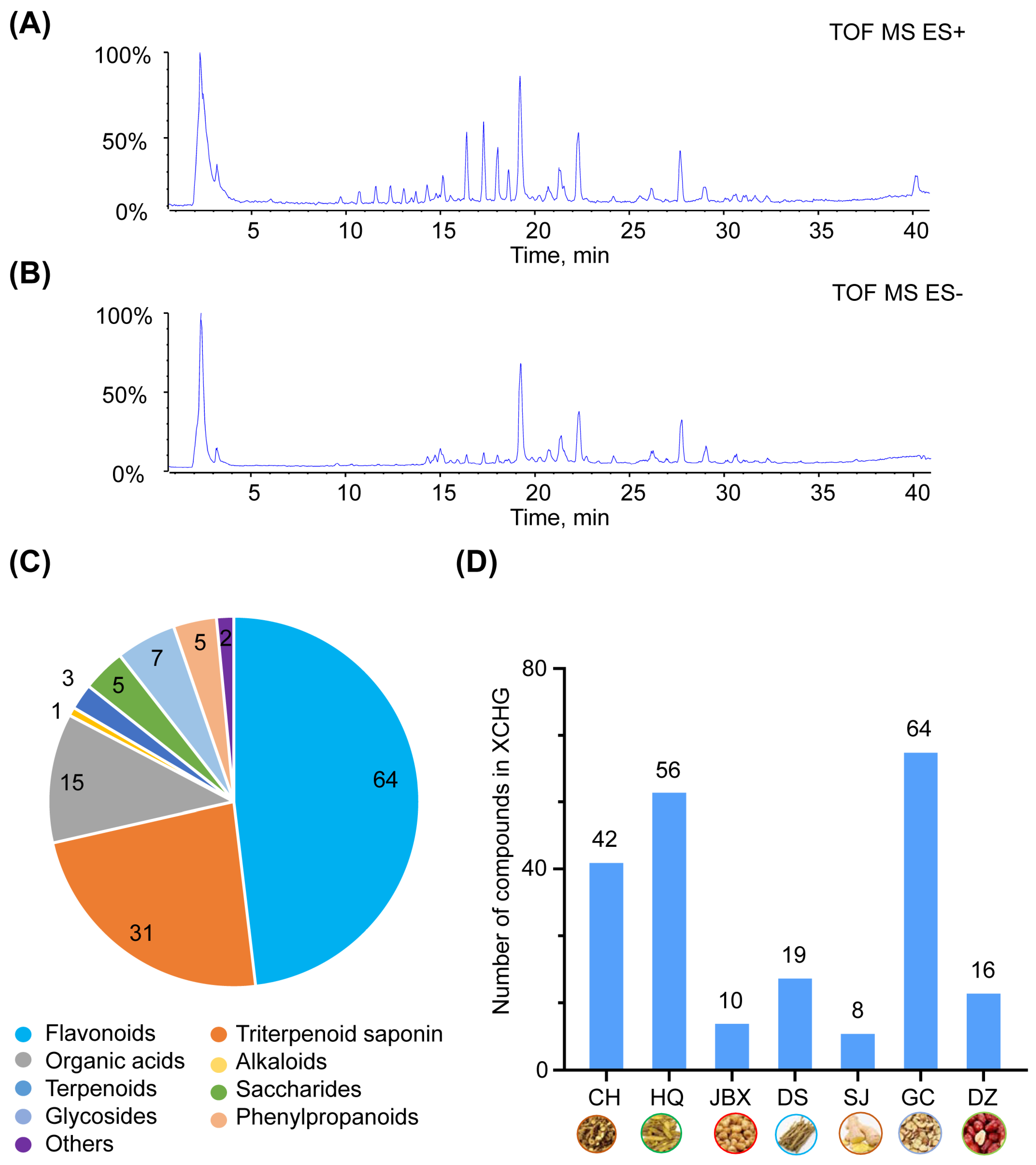
2.1. Chemical Composition of XCHG
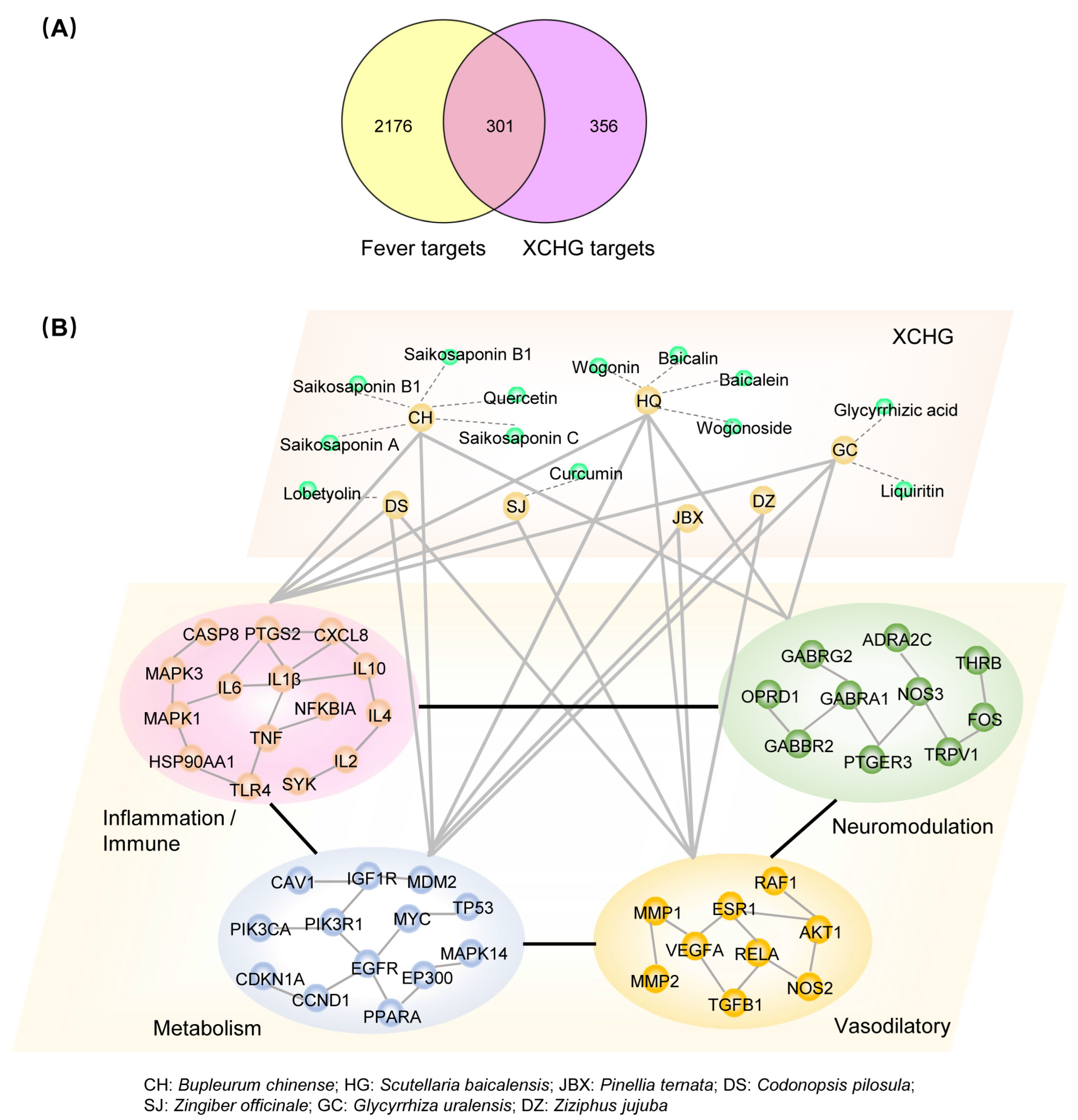
2.2. XCHG Affects the Biological Functional Modules Related to Fever
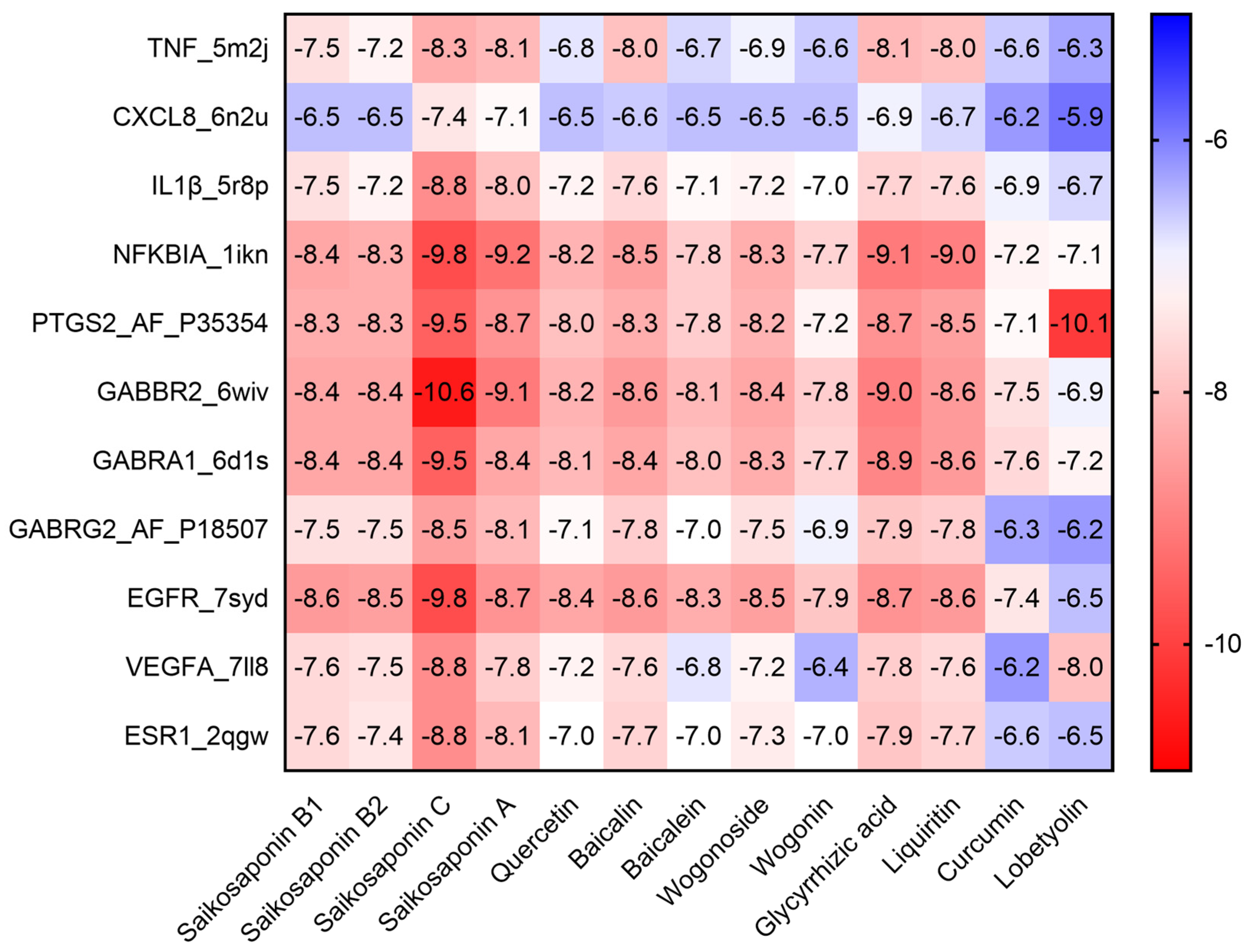
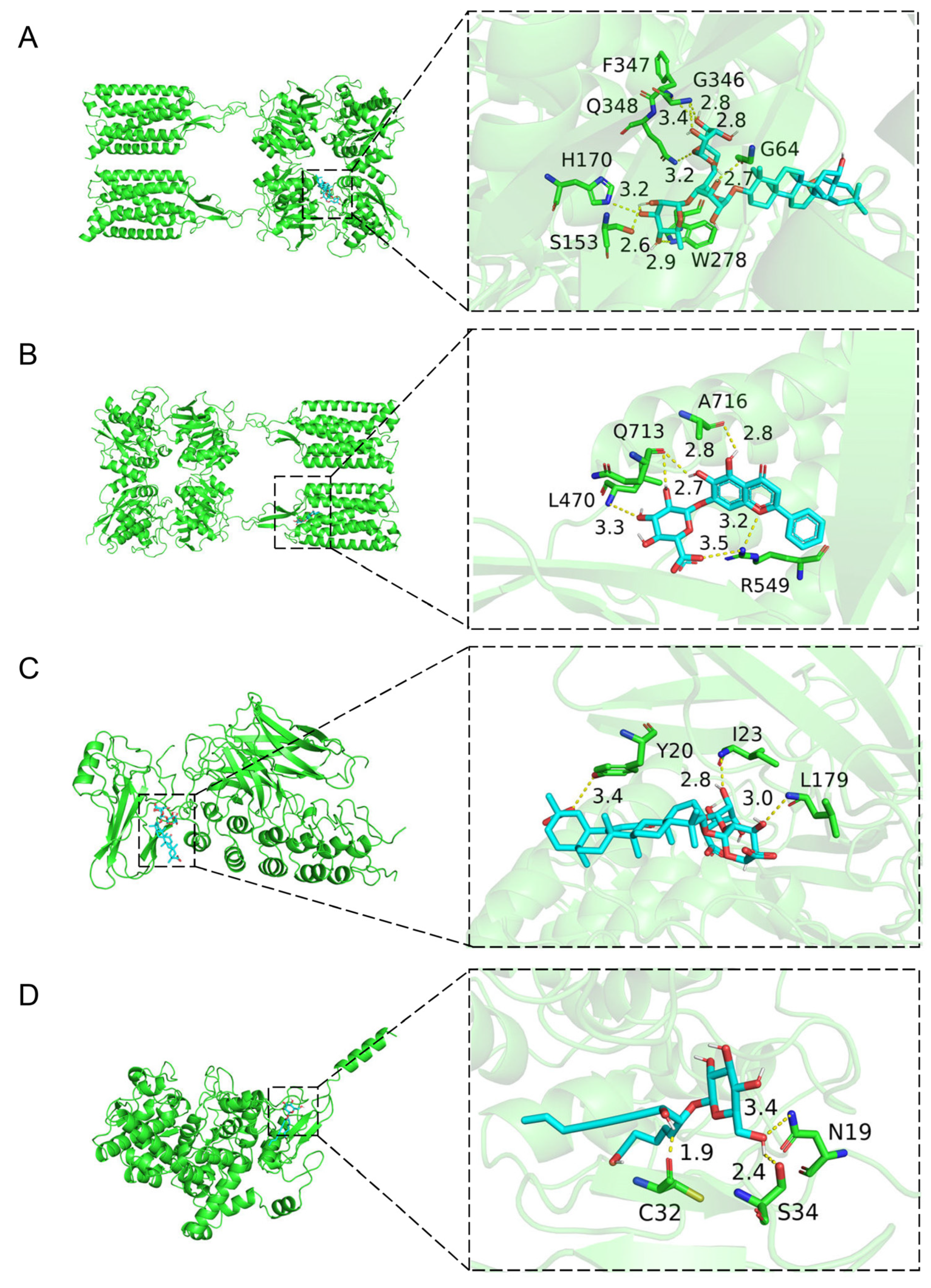
2.3. Molecular Docking of the Active Compounds and Core Targets
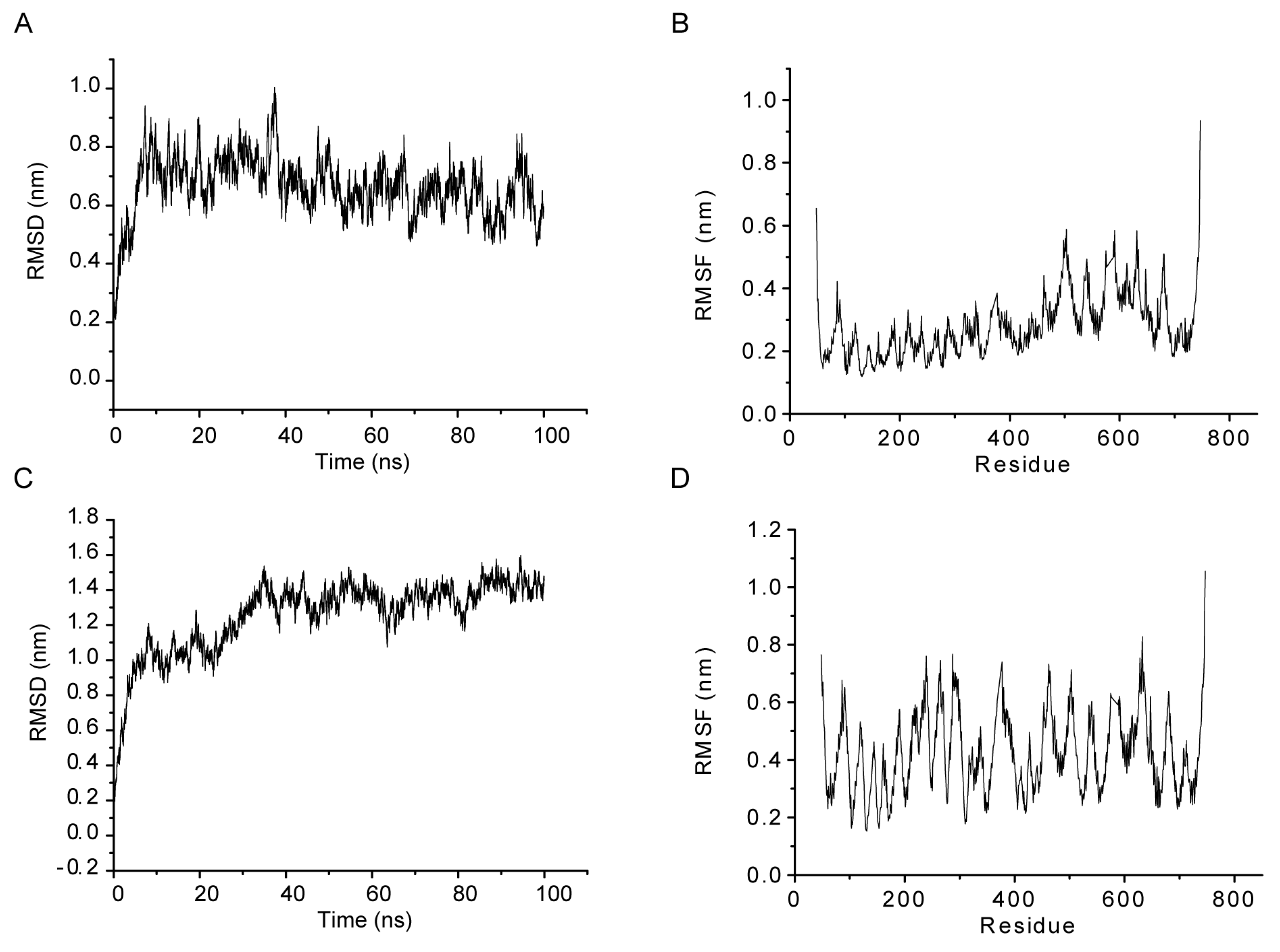
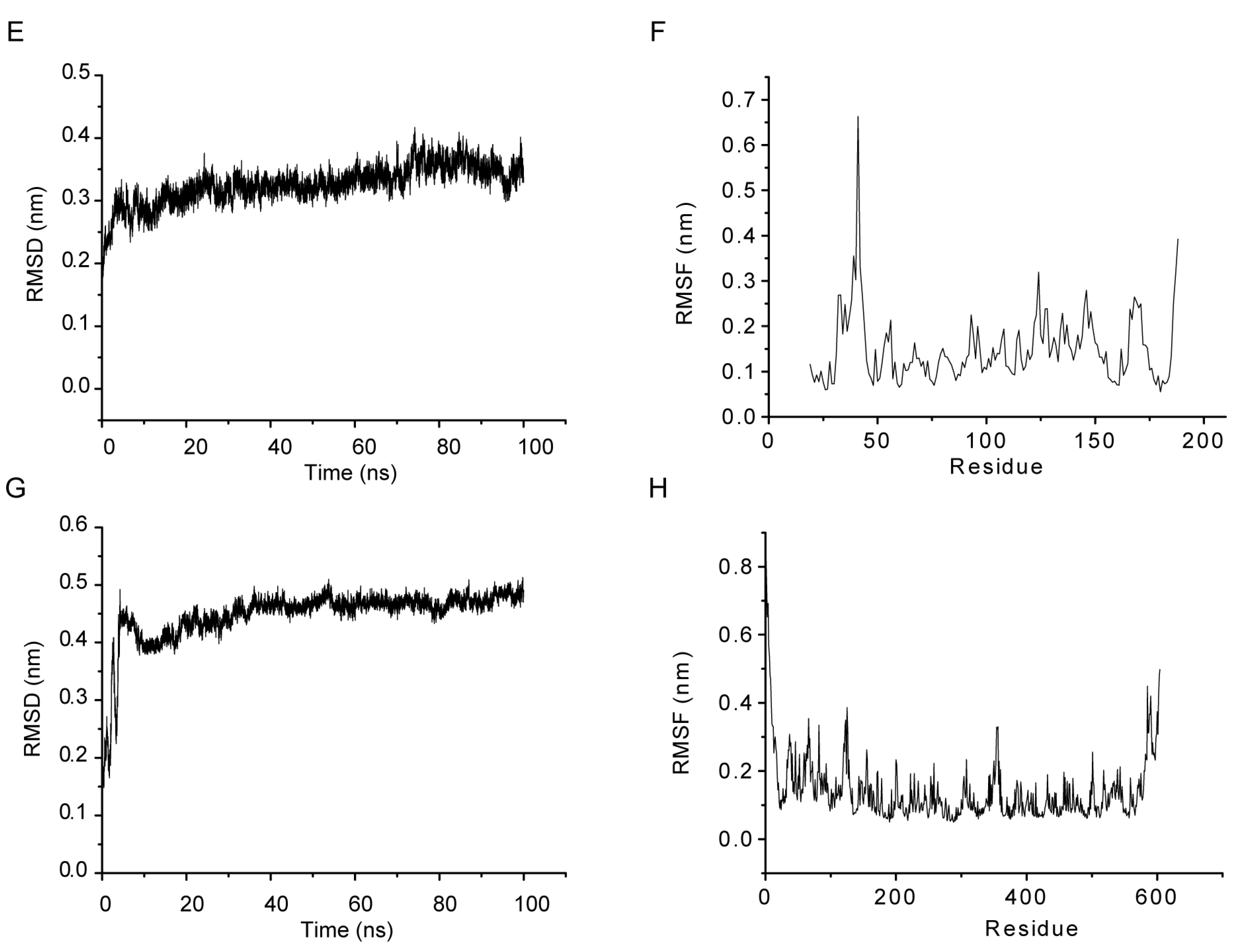
2.4. MD Simulations
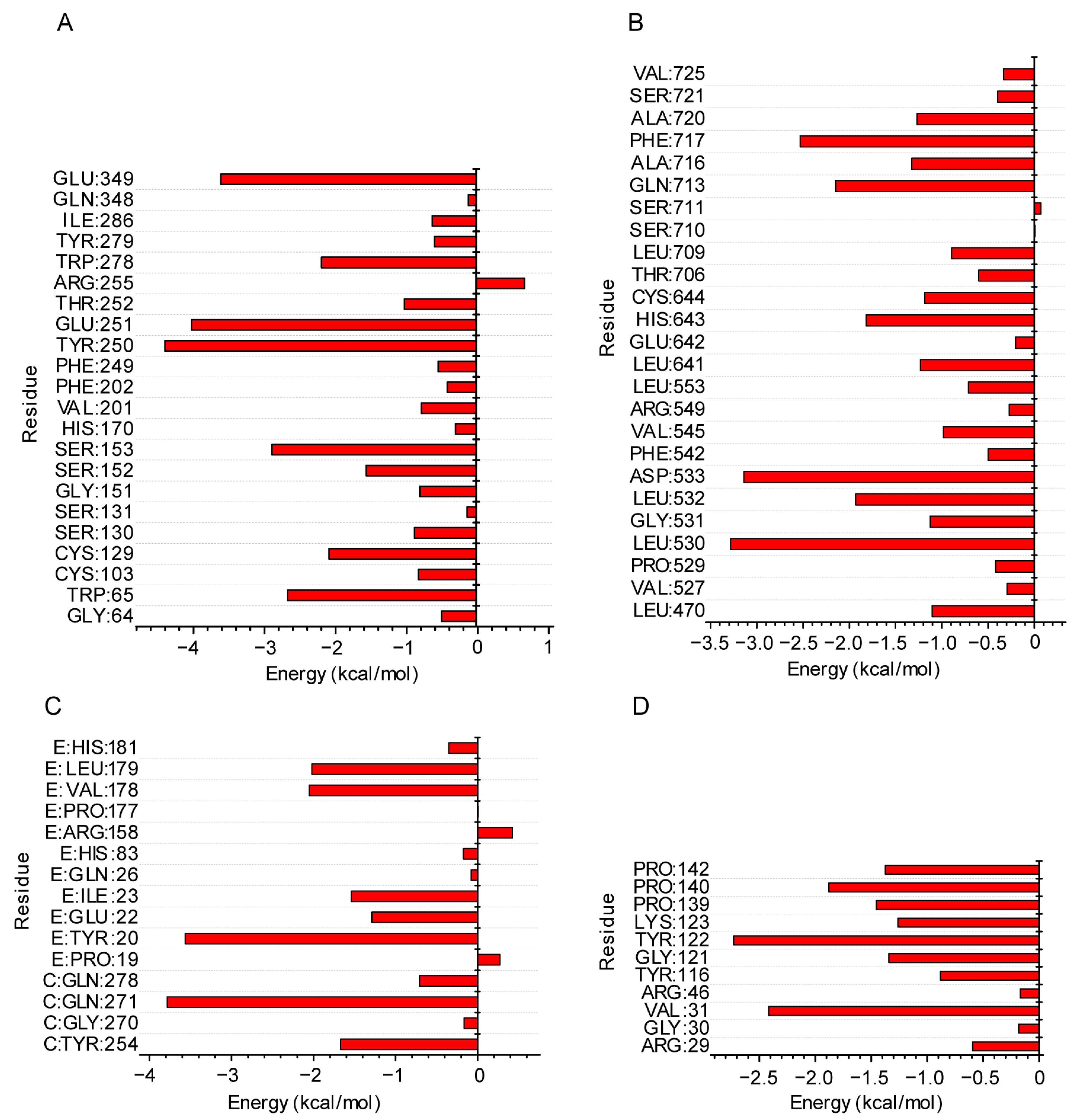
2.5. Molecular Mechanics of the Poisson–Boltzmann Surface Area (MMPBSA) Analysis from the MD Simulation Trajectory
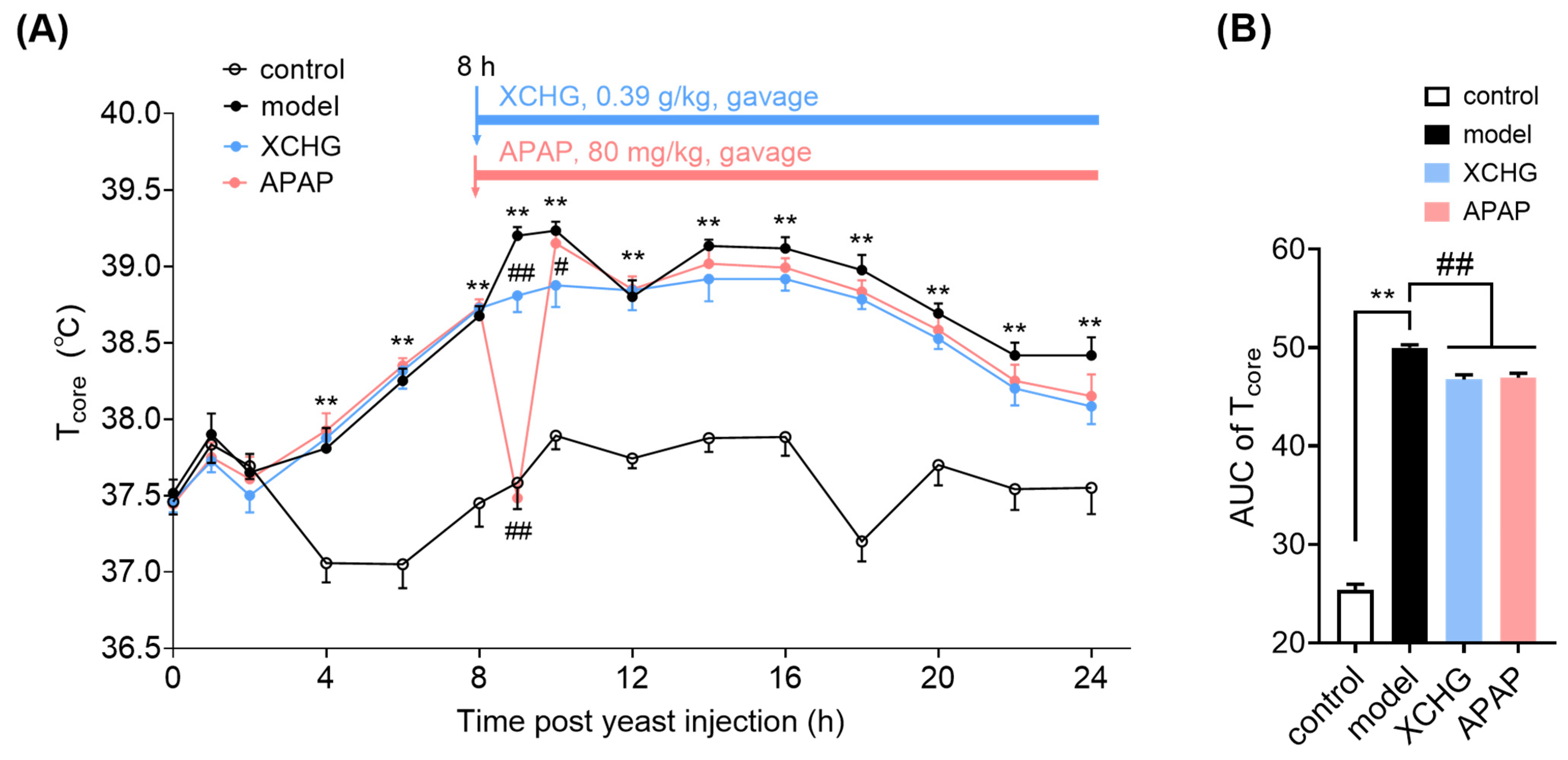
2.6. XCHG Alleviates Yeast-Induced Fever in Mice
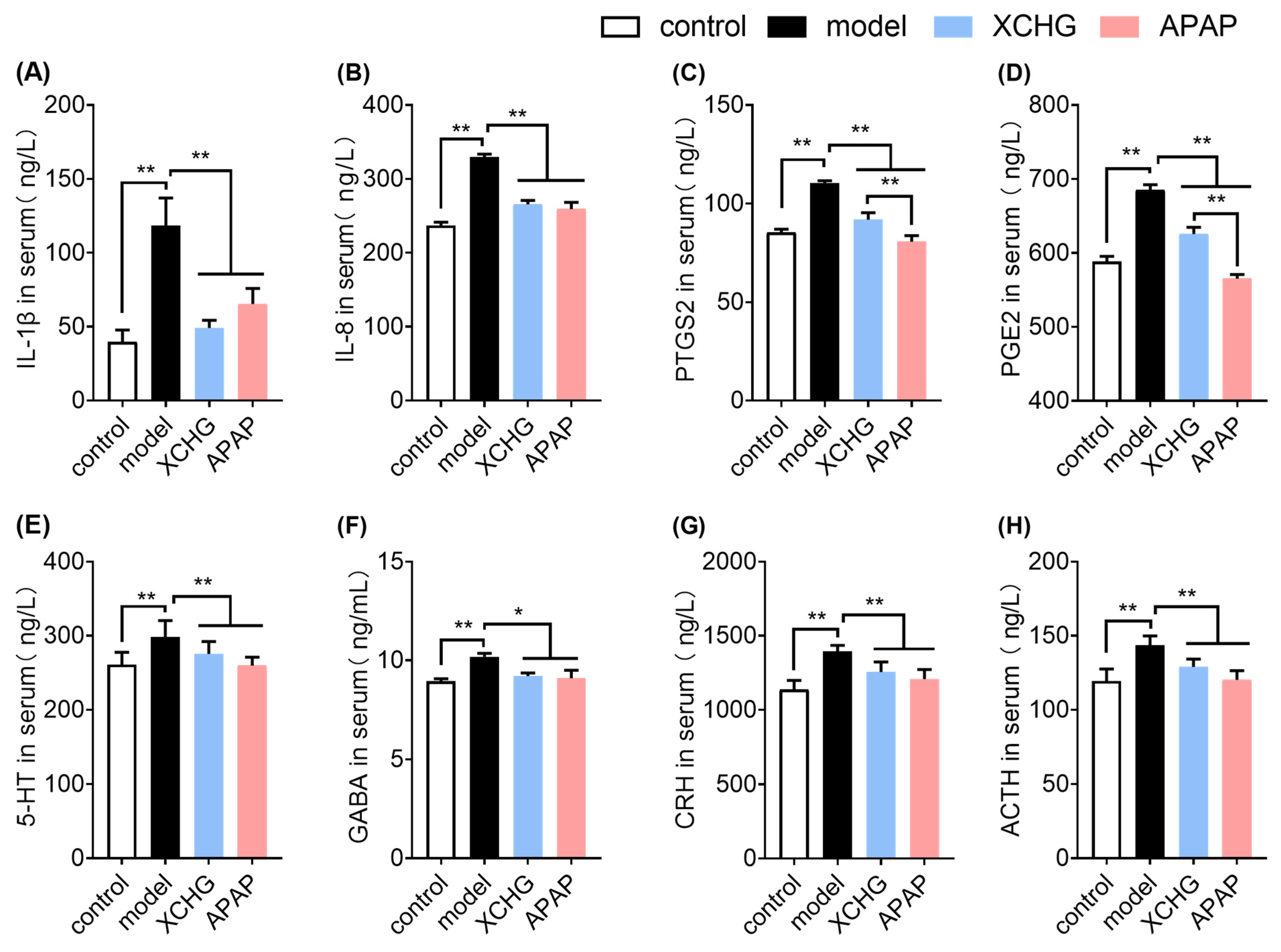
2.7. XCHG Reduces IL-1β and IL-8 Expression in Serum
2.8. XCHG Inhibits PTGS2 and PGE2 Levels in Serum
2.9. XCHG Lowers 5-HT and GABA Levels in Serum
2.10. XCHG Decreases CRH and ACTH Levels in Serum
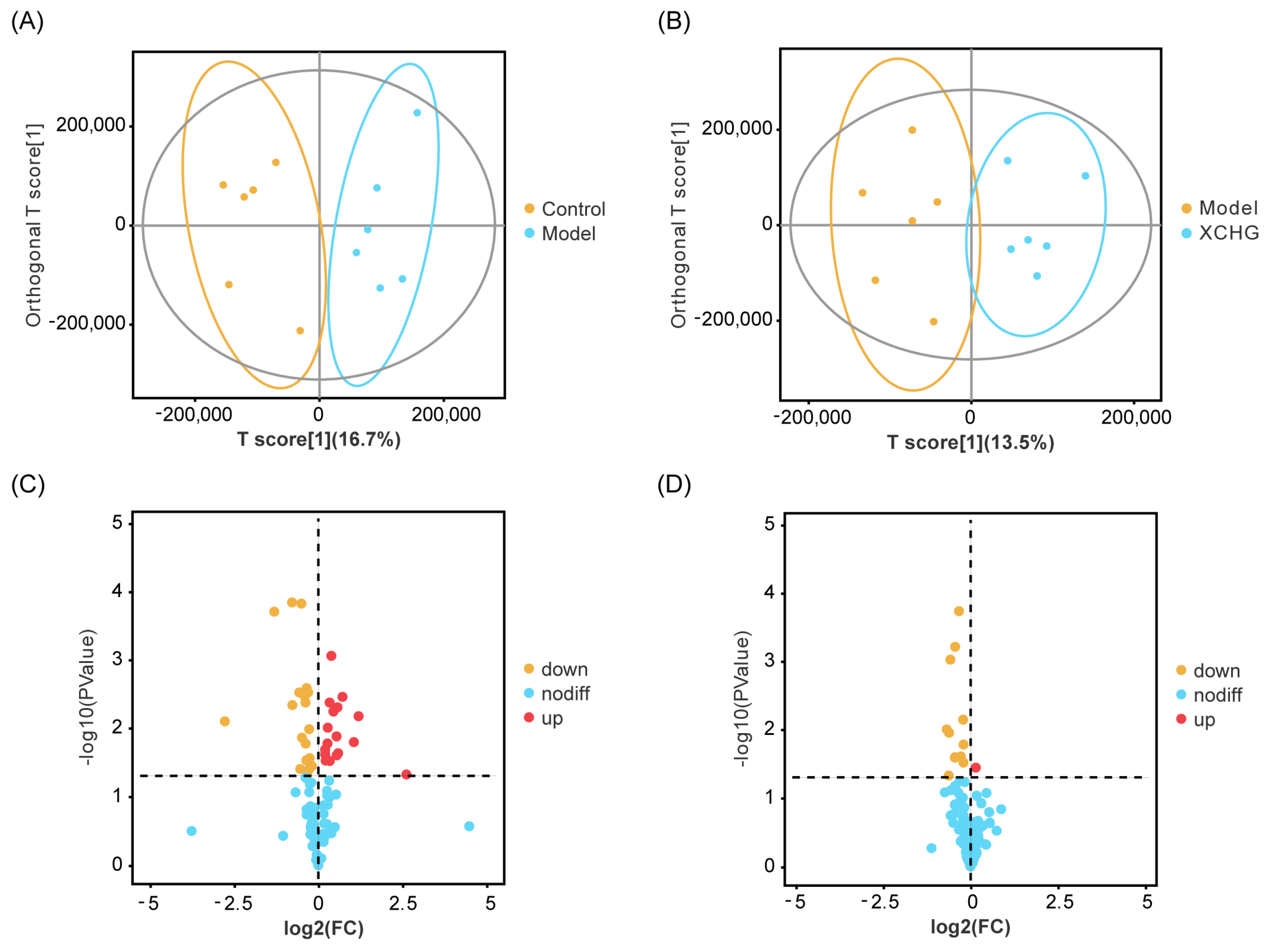
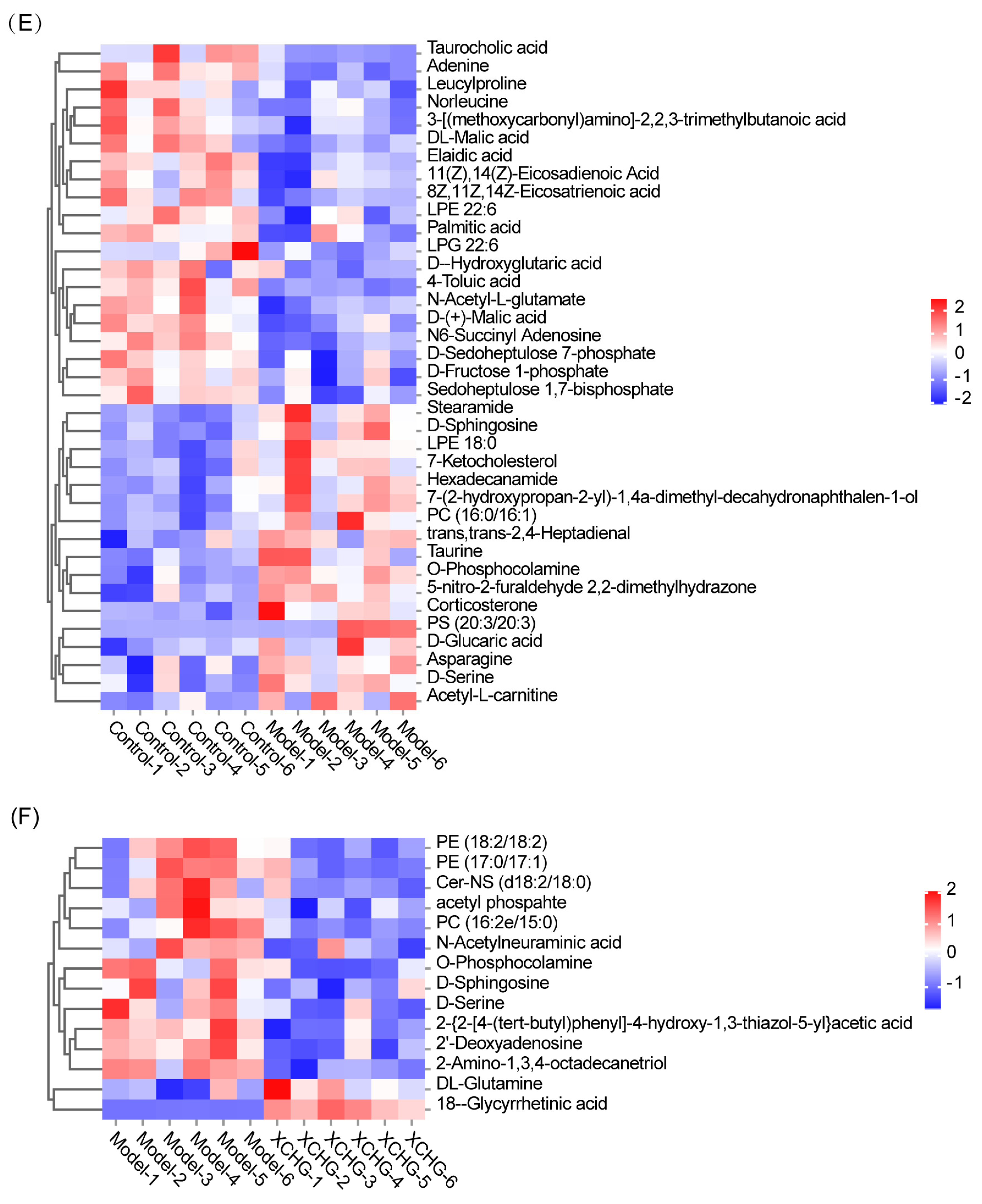
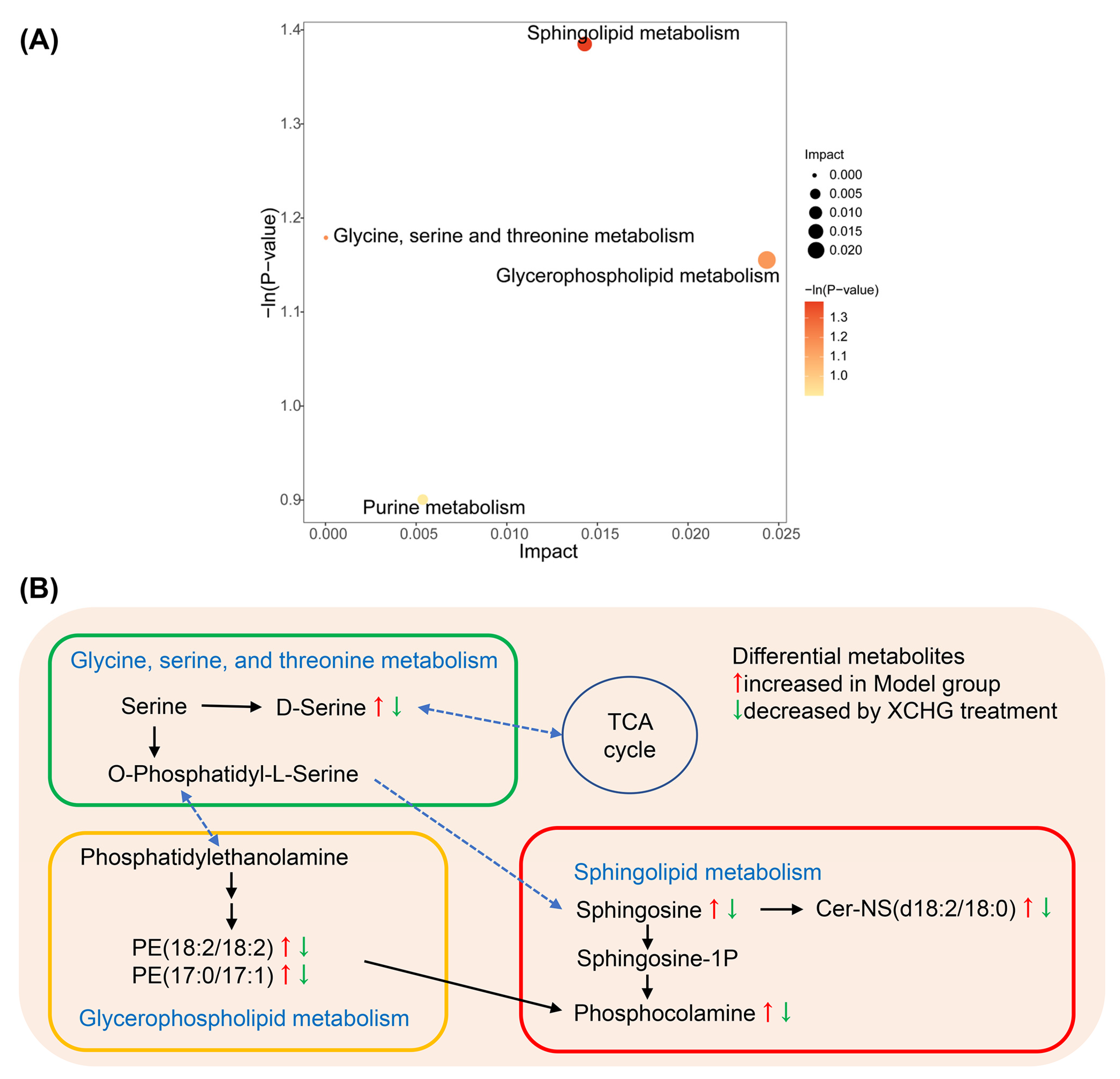
2.11. XCHG Regulates the Hypothalamic Metabolites and Metabolic Pathways
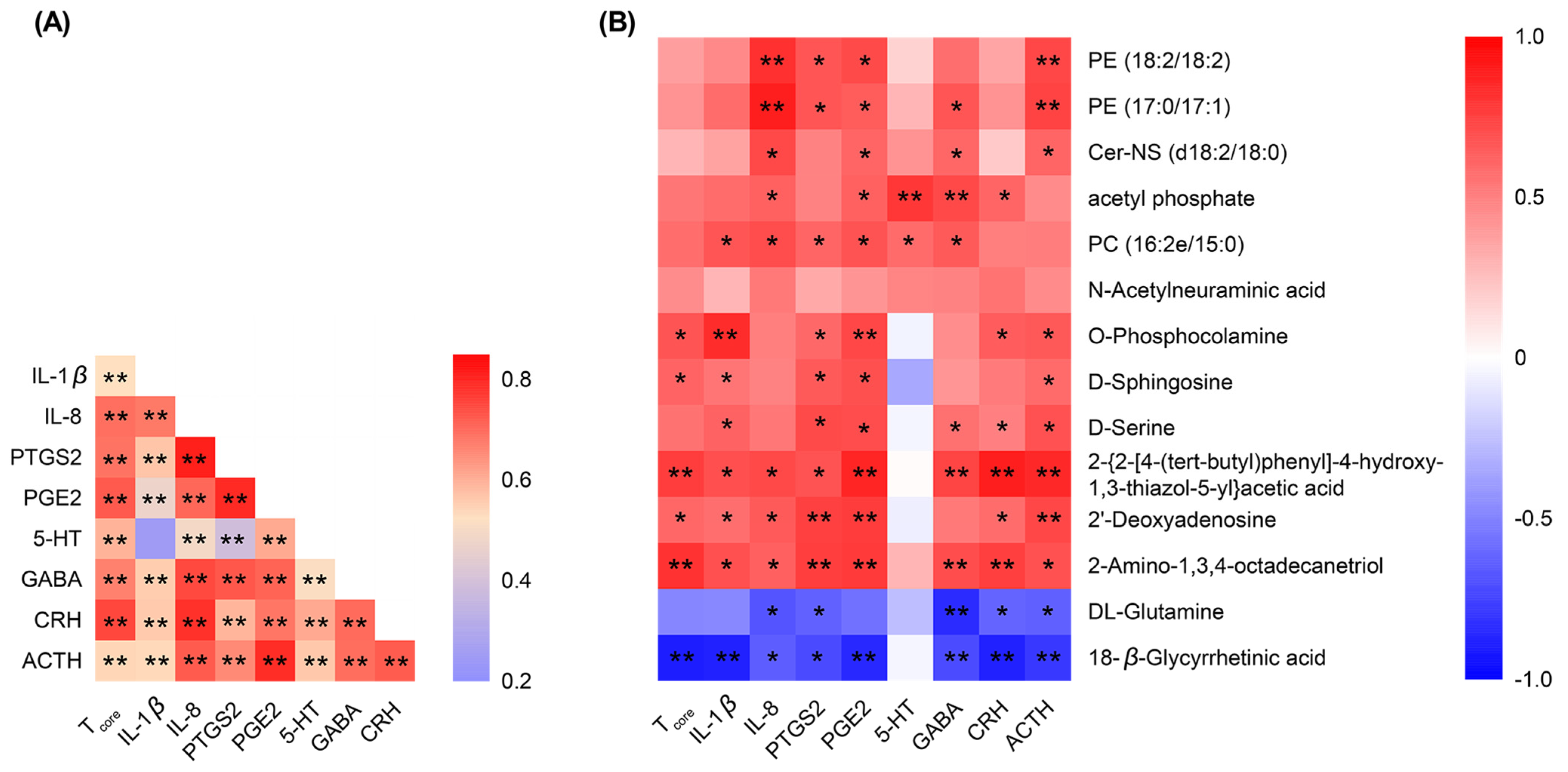
2.12. Spearman’s Correlation Analysis of the Detected Indicators
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Chemical Composition of XCHG
4.2.1. Sample Pretreatment
4.2.2. UFLC-Q-TOF-MS/MS Analysis
4.2.3. Quantification Analysis
4.3. Network Analysis
4.3.1. Target Prediction and Module Network Construction
4.3.2. Molecular Docking
4.3.3. MD Simulation
4.3.4. Molecular Mechanics Poisson–Boltzmann Surface Area (MMPBSA) Calculation
4.4. Experimental Verification
4.4.1. Animal Experimental Design
4.4.2. ELISA Analysis of Serum Biochemical Indicators
4.4.3. Untargeted Metabolomics Analysis of the Hypothalamus
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT | 5-hydroxytryptamine |
| ACTH | Adrenocorticotrophin |
| ADME | Absorption, distribution, metabolism, and excretion |
| APAP | Acetaminophen |
| AUC | Area under the curve |
| BPCs | Basic peak chromatograms |
| Cer | Ceramide |
| Cer-NS | Ceramide-N-(tetracosanoyl)-sphingosine |
| CH | Dried root of Bupleurum chinense DC. [Apiaceae] |
| COVID-19 | Coronavirus disease 2019 |
| COX | Cyclooxygenase |
| CRH | Corticotropin releasing hormone |
| CXCL8 | Interleukin-8 |
| DL | Drug-likeness |
| DS | Dried root of Codonopsis pilosula (Franch.) Nannf. [Campanulaceae] |
| DZ | Dried ripe fruit of Ziziphus jujuba Mill. [Rhamnaceae] |
| EGFR | Epidermal growth factor receptor |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| ESR1 | Estrogen receptor 1 |
| GABA | γ-aminobutyric acid |
| GABBR1 | γ-aminobutyric acid B receptor 1 |
| GABBR2 | γ-aminobutyric acid B receptor 2 |
| GABRG2 | GABA-A receptor |
| GC | Dried root of Glycyrrhiza uralensis Fisch. Ex DC. [Fabaceae] |
| GO | Gene ontology |
| Hbonds | Hydrogen bonds |
| HQ | Dried root of Scutellaria baicalensis Georgi [Lamiaceae] |
| IL | Interleukin |
| JBX | Ginger-processed Pinellia ternata (Thunb.) Makino [Araceae] |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| MD | Molecular dynamics |
| MMPBSA | Molecular mechanics Poisson–Boltzmann surface area |
| NFKBIA | NF-κB inhibitor IκBα |
| NF-κB | Nuclear factor kappa-B |
| OB | Oral bioavailability |
| OPLS-DA | Orthogonal partial least squares discriminant analysis |
| PC | Phosphatidylcholine |
| PE | Phosphatidylethanolamine |
| PGE2 | Prostaglandin E2 |
| PGs | Prostaglandins |
| PPI | Protein–protein interactions |
| PTGS | Prostaglandin endoperoxide synthases |
| Rg | Radius of gyration |
| RMSD | Root mean square deviation |
| RMSF | Root mean square fluctuation |
| S1P | Sphingosine-1-phosphate |
| SASA | Solvent accessible surface area |
| SJ | Fresh root of Zingiber officinale Roscoe [Zingiberaceae] |
| SphKs | Sphingosine kinase |
| SSs | Saikosaponins |
| STAT3 | Signal transducer and activator of transcription 3 |
| TCM | Traditional Chinese medicine |
| Tcore | Core body temperature |
| TNF | Tumor necrosis factor |
| UFLC-Q-TOF-MS/MS | Ultra-fast liquid chromatography/quadrupole-time-of-flight tandem mass spectrometry |
| UHPLC-MS | Ultra-high-performance liquid chromatography–tandem mass spectrometry |
| VEGFA | Vascular endothelial growth factor A |
References
- Bakalli, I.; Klironomi, D.; Kola, E.; Celaj, E. The management of fever in children. Minerva Pediatr. 2022, 74, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.L.; Liu, H.M.; Luo, C.H.; He, Y.N.; Wang, F.; Huang, H.Z.; Han, L.; Yang, M.; Xu, R.C.; Zhang, D.K. Fever and antipyretic supported by traditional Chinese medicine: A multi-pathway regulation. Front. Pharmacol. 2021, 12, 583279. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dou, W.; Tang, Y.; Goorha, S.; Ballou, L.R.; Blatteis, C.M. Acetaminophen: Antipyretic or hypothermic in mice? In either case, PGHS-1b (COX-3) is irrelevant. Prostaglandins Other Lipid Mediat. 2008, 85, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Jóźwiak-Bebenista, M.; Nowak, J.Z. Paracetamol: Mechanism of action, applications and safety concern. Acta Pol. Pharm. 2014, 71, 11–23. Available online: https://europepmc.org/article/MED/24779190 (accessed on 7 January 2023). [PubMed]
- Spence, J.D.; Grosser, T.; FitzGerald, G.A. Acetaminophen, Nonsteroidal Anti-Inflammatory Drugs, and Hypertension. Hypertension 2022, 79, 1922–1926. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, S.S.; Botting, R.M.; Goorha, S.; Colville-Nash, P.R.; Willoughby, D.A.; Ballou, L.R. Acetaminophen-induced hypothermia in mice is mediated by a prostaglandin endoperoxide synthase 1 gene-derived protein. Proc. Natl. Acad. Sci. USA 2004, 101, 11165–11169. [Google Scholar] [CrossRef]
- Aminoshariae, A.; Khan, A. Acetaminophen: Old drug, new issues. J. Endod. 2015, 41, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Gao, Y.; Yuan, Y.; Yang, K.; Shi, S.; Zhang, J.; Tian, J. Efficacy and safety of integrated traditional Chinese and western medicine for vorona virus disease 2019 (COVID-19): A systematic review and meta-analysis. Pharmacol. Res. 2020, 158, 104896. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Xu, N.; Zhou, Y.; Song, J.; Liu, J.; Zhu, H.; Jiang, J.; Xu, Y.; Li, R. Contribution of traditional Chinese medicine combined with conventional western medicine treatment for the novel coronavirus disease (COVID-19), current evidence with systematic review and meta-analysis. Phytother. Res. 2021, 35, 5992–6009. [Google Scholar] [CrossRef]
- Huang, P.; Li, Y.; Huang, B.; Zhao, S.; Chen, L.; Guan, H.; Chen, Y.; Feng, Y.; Huang, X.; Deng, Y.; et al. A five-dimensional network meta-analysis of Chinese herbal injections for treating acute tonsillitis combined with western medicine. Front. Pharmacol. 2022, 13, 888073. [Google Scholar] [CrossRef]
- Tang, G.; Li, S.; Zhang, C.; Chen, H.; Wang, N.; Feng, Y. Clinical efficacies, underlying mechanisms and molecular targets of Chinese medicines for diabetic nephropathy treatment and management. Acta Pharm. Sin. B 2021, 11, 2749–2767. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Cai, C.; Fang, J.; Wu, Q.; Zhao, J.; Wang, Z.; Guo, P.; Zheng, L.; Liu, A. Systems pharmacology dissection of pharmacological mechanisms of Xiaochaihu decoction against human coronavirus. BMC Complement. Med. Ther. 2023, 23, 252. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Liang, P.; Ma, Y.; Sun, Q.; Pu, Q.; Dong, L.; Luo, G.; Mazhar, M.; Liu, J.; Wang, R.; et al. Research progress of traditional Chinese medicine against COVID-19. Biomed. Pharmacother. 2021, 137, 111310. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Q.; Zou, S.H.; Yang, J.B.; Cai, J.; Zhang, Y.; Wang, Z.L. A survey and analysis of using traditional Chinese medicine during pregnancy. Int. J. Clin. Exp. Med. 2015, 8, 19496–19500. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4694499/ (accessed on 7 December 2023). [PubMed]
- Yang, L. Clinical effect of Xiaochaihu granule combined with oseltamivir in the treatment of childhood flu. Med. Inf. 2019, 32, 161–163. [Google Scholar]
- Enomoto, M.D.Y.; Nakamura, M.D.P.Y.; Enomoto, M.D.P.N.; Fujisawa, M.D.P.T.; Inui, M.D.P.N.; Suda, T. Japanese herbal medicine-induced pneumonitis: A review of 73 patients. Respir. Investig. 2017, 55, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; Pharmacopoeia Commission of the Ministry of Public Health of PRC: Beijing, China, 2020.
- Sun, M.; Xie, M.; Gao, l. Influences of integrated and separated “Xiao chaihu decoction”on fever due to different-dose LPS in rats. Shanghai J. Tradit. Chin. Med. 2003, 37, 44–47. [Google Scholar] [CrossRef]
- Bi, C.; Zheng, R.; Jiang, Z.; Zhang, J.; He, Y.; Wu, H.; Yao, H.; Wang, Y.; Liu, H. Progress in pharmacological study of Xiaochaihu Granules. J. Pharm. Res. 2020, 39, 590–596. [Google Scholar] [CrossRef]
- Chen, P.; Rao, H.; Wu, H.; Wang, Y.; Su, W.; Li, P. Discovery of the major bioactive compounds and action mechanisms responsible for the protective effects of Xiaochaihu Decoction on COVID-19 based on molecular docking and network pharmacology methods. Chin. J. Mod. Appl. Pharm. 2021, 38, 2665–2674. [Google Scholar] [CrossRef]
- Yu, X.; Miao, Z.; Zhang, L.; Zhu, L.; Sheng, H. Extraction, purification, structure characteristics, biological activities and pharmaceutical application of Bupleuri Radix Polysaccharide: A review. Int. J. Biol. Macromol. 2023, 237, 124146. [Google Scholar] [CrossRef]
- Jiang, P.; Ji, X.; Xia, J.; Xu, M.; Hao, F.; Tong, H.; Jiao, L. Structure and potential anti-fatigue mechanism of polysaccharides from Bupleurum chinense DC. Carbohydr. Polym. 2023, 306, 120608. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Wang, C.; Yang, X.; Ren, H. Phytochemical investigation on the roots of Bupleurum chinense DC. and their chemotaxonomic significance. Biochem. Syst. Ecol. 2023, 111, 104730. [Google Scholar] [CrossRef]
- Cao, T.Q.; Vu, N.K.; Woo, M.H.; Min, B.S. New polyacetylene and other compounds from Bupleurum chinense and their chemotaxonomic significance. Biochem. Syst. Ecol. 2020, 92, 104090. [Google Scholar] [CrossRef]
- Wang, Z.-L.; Wang, S.; Kuang, Y.; Hu, Z.-M.; Qiao, X.; Ye, M. A comprehensive review on phytochemistry, pharmacology, and flavonoid biosynthesis of Scutellaria baicalensis. Pharm. Biol. 2018, 56, 465–484. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-B.; Jiang, Y.; Chen, F. Separation methods used for Scutellaria baicalensis active components. J. Chromatogr. B 2004, 812, 277–290. [Google Scholar] [CrossRef]
- Zhao, T.; Tang, H.; Xie, L.; Zheng, Y.; Ma, Z.; Sun, Q.; Li, X. Scutellaria baicalensis Georgi. (Lamiaceae): A review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Pharm. Pharmacol. 2019, 71, 1353–1369. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Y.; Zhao, J.L.; Zhang, M.; Li, F.; Zhao, T.; Yang, L.Q. Sedative, hypnotic and anticonvulsant activities of the ethanol fraction from Rhizoma Pinelliae Praeparatum. J. Ethnopharmacol. 2011, 135, 325–329. [Google Scholar] [CrossRef]
- Zou, T.; Wang, J.; Wu, X.; Yang, K.; Zhang, Q.; Wang, C.; Wang, X.; Zhao, C. A review of the research progress on Pinellia ternata (Thunb.) Breit.: Botany, traditional uses, phytochemistry, pharmacology, toxicity and quality control. Heliyon 2023, 9, e22153. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Nie, B.; Yao, G.; Yang, H.; Ye, R.; Yuan, Z. Pinellia ternata (Thunb.) Makino Preparation promotes sleep by increasing REM sleep. Nat. Prod. Res. 2019, 33, 3326–3329. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.-J.; Wang, Z.-X.; Cui, F.; Zhang, Q.-N.; Song, P.-P.; Li, B.; Tang, Z.-S.; Hu, F.-D.; Shi, X.-F. Characterization of chemical composition variations in raw and processed Codonopsis Radix by integrating metabolomics and glycomics based on multiple chromatography-mass spectrometry technology. J. Sep. Sci. 2022, 45, 2375–2393. [Google Scholar] [CrossRef]
- Luan, F.; Ji, Y.; Peng, L.; Liu, Q.; Cao, H.; Yang, Y.; He, X.; Zeng, N. Extraction, purification, structural characteristics and biological properties of the polysaccharides from Codonopsis pilosula: A review. Carbohydr. Polym. 2021, 261, 117863. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.H.; Blunden, G.; Tanira, M.O.; Nemmar, A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food Chem. Toxicol. 2008, 46, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Li, Z.; Song, W.; Wang, Y.; Liang, W.; Li, K.; Tang, S.; Wang, Q.; Qiao, X.; Zhou, D.; et al. Bioactive Constituents of Glycyrrhiza uralensis (Licorice): Discovery of the Effective Components of a Traditional Herbal Medicine. J. Nat. Prod. 2016, 79, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Bao, T.; Mo, J.; Ni, J.; Chen, W. Research advances in bioactive components and health benefits of jujube (Ziziphus jujuba Mill.) fruit. J. Zhejiang Univ. Sci. B 2021, 22, 431–449. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, X.; Zeng, Y.; Zhou, Z.; Zhao, D.; Qin, F.; Zhang, B.; Yao, W.; Mao, Y.; Zhou, L.; et al. Network pharmacology combined with molecular docking and experimental verification to elucidate functional mechanism of Fufang Zhenzhu Tiaozhi against type 2 diabetes mellitus. J. Biomol. Struct. Dyn. 2023, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhang, Y.; Wang, D.; Yang, B.; Shen, Y.Q. Computer especially AI-assisted drug virtual screening and design in traditional Chinese medicine. Phytomedicine 2022, 107, 154481. [Google Scholar] [CrossRef] [PubMed]
- Joshi, T.; Joshi, T.; Sharma, P.; Chandra, S.; Pande, V. Molecular docking and molecular dynamics simulation approach to screen natural compounds for inhibition of Xanthomonas oryzae pv. Oryzae by targeting peptide deformylase. J. Biomol. Struct. Dyn. 2021, 39, 823–840. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lu, F.; Liu, H.; Zhao, H.; Liu, Y.; Fu, S.; Wang, M.; Xie, Z.; Yu, H.; Huang, Z.; et al. An integrated strategy by using target tissue metabolomics biomarkers as pharmacodynamic surrogate indices to screen antipyretic components of Qingkaikling injection. Sci. Rep. 2017, 7, 6310. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, X.; Huang, N.; Liu, R.; Sun, R. A comprehensive review and perspectives on pharmacology and toxicology of saikosaponins. Phytomedicine 2018, 50, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Yue, G.G.; Chan, B.C.; Kwok, H.F.; To, M.H.; Hon, K.L.; Fung, K.P.; Lau, C.B.; Leung, P.C. Screening for anti-inflammatory and bronchorelaxant activities of 12 commonly used Chinese herbal medicines. Phytother. Res. 2012, 26, 915–925. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Liu, J.G.; Li, H.; Yang, H.M. Pharmacological effects of active components of Chinese herbal medicine in the treatment of alzheimer’s disease: A review. Am. J. Chin. Med. 2016, 44, 1525–1541. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Wang, Y.; Zhang, Z.; Liu, T.; Zhang, J.; Cao, Y.; Zhang, B.; Feng, W.; Zheng, X.; Li, K. Exploring the efficacy mechanism and material basis of three processed Coptidis Rhizoma via metabolomics strategy. J. Pharm. Biomed. Anal. 2023, 232, 115450. [Google Scholar] [CrossRef] [PubMed]
- Chrubasik, S.; Pittler, M.H.; Roufogalis, B.D. Zingiberis rhizoma: A comprehensive review on the ginger effect and efficacy profiles. Phytomedicine 2005, 12, 684–701. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Chen, J.; Peng, F.; Sun, C.; Lei, Y.; Chen, G.; Li, G.; Yin, Y.; Lin, Z.; Wu, L.; et al. Pharmacological activities and pharmacokinetics of liquiritin: A review. J. Ethnopharmacol. 2022, 293, 115257. [Google Scholar] [CrossRef]
- Chen, J.; Kang, J.; Yuan, S.; O’Connell, P.; Zhang, Z.; Wang, L.; Liu, J.; Chen, R. Exploring the Mechanisms of Traditional Chinese Herbal Therapy in Gastric Cancer: A Comprehensive Network Pharmacology Study of the Tiao-Yuan-Tong-Wei decoction. Pharmaceuticals 2024, 17, 414. [Google Scholar] [CrossRef]
- Song, Y.; Slominski, R.M.; Qayyum, S.; Kim, T.-K.; Janjetovic, Z.; Raman, C.; Tuckey, R.C.; Song, Y.; Slominski, A.T. Molecular and structural basis of interactions of vitamin D3 hydroxyderivatives with aryl hydrocarbon receptor (AhR): An integrated experimental and computational study. Int. J. Biol. Macromol. 2022, 209, 1111–1123. [Google Scholar] [CrossRef]
- Wang, F.; Xu, Z.; Ren, L.; Tsang, S.Y.; Xue, H. GABAA receptor subtype selectivity underlying selective anxiolytic effect of baicalin. Neuropharmacology 2008, 55, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Maccioni, P.; Lorrai, I.; Carai, M.A.M.; Riva, A.; Morazzoni, P.; Mugnaini, C.; Corelli, F.; Gessa, G.L.; Colombo, G. Reducing effect of saikosaponin A, an active ingredient of Bupleurum falcatum, on alcohol self-administration in rats: Possible involvement of the GABAB receptor. Neurosci. Lett. 2016, 621, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.S.; Lee, Y.H.; Yeo, H.; Jung, E.; Lim, Y.; Shin, S.Y. Saikosaponin A and Saikosaponin C Reduce TNF-alpha-Induced TSLP Expression through Inhibition of MAPK-Mediated EGR1 Expression in HaCaT Keratinocytes. Int. J. Mol. Sci. 2022, 23, 4857. [Google Scholar] [CrossRef]
- Chen, S.; Wang, K.; Wang, H.; Gao, Y.; Nie, K.; Jiang, X.; Su, H.; Tang, Y.; Lu, F.; Dong, H.; et al. The therapeutic effects of saikosaponins on depression through the modulation of neuroplasticity: From molecular mechanisms to potential clinical applications. Pharmacol. Res. 2024, 201, 107090. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhou, X. Glycyrrhizin inhibits human neutrophil elastase-induced mucin 5AC overproduction in human bronchial epithelial cells. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2014, 39, 252–257. [Google Scholar] [CrossRef]
- Lai, M.-Y.; Hsiu, S.-L.; Tsai, S.-Y.; Hou, Y.-C.; Chao, P.-D.L. Comparison of metabolic pharmacokinetics of baicalin and baicalein in rats. J. Pharm. Pharmacol. 2010, 55, 205–209. [Google Scholar] [CrossRef]
- Nomura, Y.; Seki, H.; Suzuki, T.; Ohyama, K.; Mizutani, M.; Kaku, T.; Tamura, K.; Ono, E.; Horikawa, M.; Sudo, H.; et al. Functional specialization of UDP-glycosyltransferase 73P12 in licorice to produce a sweet triterpenoid saponin, glycyrrhizin. Plant J. 2019, 99, 1127–1143. [Google Scholar] [CrossRef]
- Bailly, C. Anticancer Properties of Lobetyolin, an Essential Component of Radix Codonopsis (Dangshen). Nat. Prod. Bioprospect. 2021, 11, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.; Jung, J.; Lee, Y.N.; Lee, Y.; Cho, H.; Na, E.; Hong, J.; Kim, E.; Lee, J.S.; Lee, J.S.; et al. GABBR2 mutations determine phenotype in rett syndrome and epileptic encephalopathy. Ann. Neurol. 2017, 82, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Fu, Z.; Frangaj, A.; Liu, J.; Mosyak, L.; Shen, T.; Slavkovich, V.N.; Ray, K.M.; Taura, J.; Cao, B.; et al. Structure of human GABA(B) receptor in an inactive state. Nature 2020, 584, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Lobanov, M.; Bogatyreva, N.S.; Galzitskaia, O.V. Radius of gyration is indicator of compactness of protein structure. Mol. Biol. 2008, 42, 701–706. Available online: https://europepmc.org/article/MED/18856071 (accessed on 7 December 2023). [CrossRef]
- Tran, T.-T.-N.; Tran, Q.-H.; Duong, C.Q.; Nguyen, Q.-T.; Tran, V.-T.; Le, M.-T.; Tran, V.-H.; Thai, K.-M. In silico approach to identify novel allosteric intracellular antagonist for blocking the interleukin-8/CXCR2 receptor signaling pathway. J. Biomol. Struct. Dyn. 2023, 41, 13154–13167. [Google Scholar] [CrossRef]
- Majewski, M.; Ruiz-Carmona, S.; Barril, X. An investigation of structural stability in protein-ligand complexes reveals the balance between order and disorder. Commun. Chem. 2019, 2, 110. [Google Scholar] [CrossRef]
- Malfatti, M.A.; Kuhn, E.A.; Murugesh, D.K.; Mendez, M.E.; Hum, N.; Thissen, J.B.; Jaing, C.J.; Loots, G.G. Manipulation of the Gut Microbiome Alters Acetaminophen Biodisposition in Mice. Sci. Rep. 2020, 10, 4571. [Google Scholar] [CrossRef]
- Tan, E.E.; Hopkins, R.A.; Lim, C.K.; Jamuar, S.S.; Ong, C.; Thoon, K.C.; Koh, M.J.; Shin, E.M.; Lian, D.W.; Weerasooriya, M.; et al. Dominant-negative NFKBIA mutation promotes IL-1β production causing hepatic disease with severe immunodeficiency. J. Clin. Investig. 2020, 130, 5817–5832. [Google Scholar] [CrossRef]
- Kaplanski, G.; Teysseire, N.; Farnarier, C.; Kaplanski, S.; Lissitzky, J.C.; Durand, J.M.; Soubeyrand, J.; Dinarello, C.A.; Bongrand, P. IL-6 and IL-8 production from cultured human endothelial cells stimulated by infection with Rickettsia conorii via a cell-associated IL-1 alpha-dependent pathway. J. Clin. Investig. 1995, 96, 2839–2844. [Google Scholar] [CrossRef]
- Huang, Y.H.; Lei, H.Y.; Liu, H.S.; Lin, Y.S.; Liu, C.C.; Yeh, T.M. Dengue virus infects human endothelial cells and induces IL-6 and IL-8 production. Am. J. Trop. Med. Hyg. 2000, 63, 71–75. [Google Scholar] [CrossRef]
- Soker, M.; Colpan, L.; Ece, A.; Devecioğlu, C.; Haspolat, K. Serum levels of IL-1 beta, sIL-2R, IL-6, IL-8, and TNF-alpha in febrile children with cancer and neutropenia. Med. Oncol. 2001, 18, 51–57. [Google Scholar] [CrossRef]
- Martín-Vázquez, E.; Cobo-Vuilleumier, N.; López-Noriega, L.; Lorenzo, P.I.; Gauthier, B.R. The PTGS2/COX2-PGE(2) signaling cascade in inflammation: Pro or anti? A case study with type 1 diabetes mellitus. Int. J. Biol. Sci. 2023, 19, 4157–4165. [Google Scholar] [CrossRef]
- Robb, C.T.; Goepp, M.; Rossi, A.G.; Yao, C. Non-steroidal anti-inflammatory drugs, prostaglandins, and COVID-19. Br. J. Pharmacol. 2020, 177, 4899–4920. [Google Scholar] [CrossRef]
- Li, Z.; Perlik, V.; Feleder, C.; Tang, Y.; Blatteis, C.M. Kupffer cell-generated PGE2 triggers the febrile response of guinea pigs to intravenously injected LPS. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R1262–R1270. [Google Scholar] [CrossRef]
- Voronova, I.P. 5-HT Receptors and Temperature Homeostasis. Biomolecules 2021, 11, 1914. [Google Scholar] [CrossRef]
- Bächtold, H.; Pletscher, A. Einfluss von Isonikotinsäurehydraziden auf den Verlauf der Körpertemperatur nach Reserpin, Monoaminen und Chlorpromazin. Experientia 1957, 13, 163–165. [Google Scholar] [CrossRef] [PubMed]
- Maillard, P.Y.; Baer, S.; Schaefer, É.; Desnous, B.; Villeneuve, N.; Lépine, A.; Fabre, A.; Lacoste, C.; El Chehadeh, S.; Piton, A.; et al. Molecular and clinical descriptions of patients with GABA(A) receptor gene variants (GABRA1, GABRB2, GABRB3, GABRG2): A cohort study, review of literature, and genotype-phenotype correlation. Epilepsia 2022, 63, 2519–2533. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Han, Z.; Wu, Z.; Xia, Y.; Yang, G.; Yin, Y.; Ren, W. GABA regulates IL-1β production in macrophages. Cell Rep. 2022, 41, 111770. [Google Scholar] [CrossRef]
- Wu, C.; Qin, X.; Du, H.; Li, N.; Ren, W.; Peng, Y. The immunological function of GABAergic system. Front. Biosci. 2017, 22, 1162–1172. [Google Scholar] [CrossRef]
- Lim, J.S.; Kim, C.R.; Shin, K.S.; Lee, S.J.; Yoon, T.J.; Park, H.J. Synergistic effect of Korean red ginseng extract and GABA mixture on the IgE production in mice via Th1/Th2 cell balance. Food Sci. Biotechnol. 2021, 30, 1571–1580. [Google Scholar] [CrossRef]
- Zhu, Z.; Shi, Z.; Xie, C.; Gong, W.; Hu, Z.; Peng, Y. A novel mechanism of Gamma-aminobutyric acid (GABA) protecting human umbilical vein endothelial cells (HUVECs) against H2O2-induced oxidative injury. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 217, 68–75. [Google Scholar] [CrossRef]
- Kim, J.K.; Kim, Y.S.; Lee, H.-M.; Jin, H.S.; Neupane, C.; Kim, S.; Lee, S.-H.; Min, J.-J.; Sasai, M.; Jeong, J.-H.; et al. GABAergic signaling linked to autophagy enhances host protection against intracellular bacterial infections. Nat. Commun. 2018, 9, 4184. [Google Scholar] [CrossRef]
- Zhou, B.; Xu, Q.; Guo, J.; Chen, Q.; Lv, Q.; Xiao, K.; Zhu, H.; Zhao, J.; Liu, Y. Necroptosis Contributes to LPS-Induced Activation of the Hypothalamic-Pituitary-Adrenal Axis in a Piglet Model. Int. J. Mol. Sci. 2022, 23, 11218. [Google Scholar] [CrossRef]
- Xie, F.; Xu, L.; Zhu, H.; Chen, Y.; Li, Y.; Nong, L.; Zeng, Y.; Cen, S. The potential antipyretic mechanism of Ellagic Acid with brain metabolomics using rats with yeast-induced fever. Molecules 2022, 27, 2465. [Google Scholar] [CrossRef]
- Huang, Y.; Yao, P.; Leung, K.W.; Wang, H.; Kong, X.P.; Dong, T.T.X.; Chen, Y.; Qin, Q.W.; Tsim, K.W.K. The Chinese medicinal herbs of spleen-meridian property regulate body temperature in yeast-induced fever rats. Phytomedicine 2020, 74, 152815. [Google Scholar] [CrossRef]
- Li, L.; Yan, X.; Chen, F.; Zheng, L.; Hu, Y.; He, F.; Ni, H.; Chen, F.; Li, Q. A comprehensive review of the metabolism of citrus flavonoids and their binding to bitter taste receptors. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1763–1793. [Google Scholar] [CrossRef]
- Han, J.; Ye, M.; Xu, M.; Sun, J.; Wang, B.; Guo, D. Characterization of flavonoids in the traditional Chinese herbal medicine-Huangqin by liquid chromatography coupled with electrospray ionization mass spectrometry. J. Chromatogr. B 2007, 848, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Bureau, G.; Longpré, F.; Martinoli, M.G. Resveratrol and quercetin, two natural polyphenols, reduce apoptotic neuronal cell death induced by neuroinflammation. J. Neurosci. Res. 2008, 86, 403–410. [Google Scholar] [CrossRef]
- Bao, Y.; Li, C.; Shen, H.; Nan, F. Determination of saikosaponin derivatives in Radix bupleuri and in pharmaceuticals of the chinese multiherb remedy xiaochaihu-tang using liquid chromatographic tandem mass spectrometry. Anal. Chem. 2004, 76, 4208–4216. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.S.; Im, H.T.; Lee, K.T. Saikosaponin B2 suppresses inflammatory responses through IKK/IkappaBalpha/NF-kappaB signaling inactivation in LPS-induced RAW 264.7 macrophages. Inflammation 2019, 42, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Kao, T.C.; Shyu, M.H.; Yen, G.C. Glycyrrhizic acid and 18beta-glycyrrhetinic acid inhibit inflammation via PI3K/Akt/GSK3beta signaling and glucocorticoid receptor activation. J. Agric. Food Chem. 2010, 58, 8623–8629. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Jin, Y.; Shin, J.H.; Kim, I.D.; Lee, H.K.; Park, S.; Han, P.L.; Lee, J.K. Glycyrrhizic acid affords robust neuroprotection in the postischemic brain via anti-inflammatory effect by inhibiting HMGB1 phosphorylation and secretion. Neurobiol. Dis. 2012, 46, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.T.; Niu, K.C.; Chang, C.K.; Lin, M.T.; Chang, C.P. Curcumin inhibits the increase of glutamate, hydroxyl radicals and PGE2 in the hypothalamus and reduces fever during LPS-induced systemic inflammation in rabbits. Eur. J. Pharmacol. 2008, 593, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, T.; Liu, S.; Liu, J.; Fang, S.; Tan, S.; Zeng, Y.; Zhang, B.; Li, W. Dissecting the molecular mechanism of cepharanthine against COVID-19, based on a network pharmacology strategy combined with RNA-sequencing analysis, molecular docking, and molecular dynamics simulation. Comput. Biol. Med. 2022, 151, 106298. [Google Scholar] [CrossRef]
- Subhaswaraj, P.; Siddhardha, B. Molecular docking and molecular dynamic simulation approaches for drug development and repurposing of drugs for severe acute respiratory syndrome-Coronavirus-2. In Computational Approaches for Novel Therapeutic and Diagnostic Designing to Mitigate SARS-CoV-2 Infection; Parihar, A., Ed.; Academic Press: San Diego, CA, USA, 2022; pp. 207–246. [Google Scholar]
- Sun, Z.; Wang, Y.; Pang, X.; Wang, X.; Zeng, H. Mechanisms of polydatin against spinal cord ischemia-reperfusion injury based on network pharmacology, molecular docking and molecular dynamics simulation. Bioorg. Chem. 2023, 140, 106840. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ren, L.; Li, W.; Zhang, Y.; Zhang, S.; Ge, B.; Yang, H.; Du, G.; Tang, B.; Wang, H.; et al. GABAergic signaling as a potential therapeutic target in cancers. Biomed. Pharmacother. 2023, 161, 114410. [Google Scholar] [CrossRef]
- Ni, S.-H.; OuYang, X.-L.; Liu, X.; Lin, J.-H.; Li, Y.; Sun, S.-N.; Deng, J.-P.; Han, X.-W.; Zhang, X.-J.; Li, H.; et al. A molecular phenotypic screen reveals that lobetyolin alleviates cardiac dysfunction in 5/6 nephrectomized mice by inhibiting osteopontin. Phytomedicine 2022, 107, 154412. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, Y.; Tian, S.; Xu, L.; Hou, T. Assessing the performance of MM/PBSA and MM/GBSA methods. 4. Accuracies of MM/PBSA and MM/GBSA methodologies evaluated by various simulation protocols using PDBbind data set. Phys. Chem. Chem. Phys. 2014, 16, 16719–16729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, D.; Li, X.; Li, Z.; Chao, J.; Qin, X. Metabolomic study of the fever model induced by baker’s yeast and the antipyretic effects of aspirin in rats using nuclear magnetic resonance and gas chromatography–mass spectrometry. J. Pharm. Biomed. Anal. 2013, 81–82, 168–177. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Li, S.; Dai, Y.; Li, X.; Wang, Q.; Wang, G.; Ma, Y.; Gu, X.; Zhang, C. The potential antipyretic mechanism of Gardeniae Fructus and its Heat-processed products with plasma metabolomics using rats with yeast-induced fever. Front. Pharmacol. 2019, 10, 491. [Google Scholar] [CrossRef] [PubMed]
- Aslam, B.; Hussain, A.; Bari, M.U.; Faisal, M.N.; Sindhu, Z.U.D.; Alonaizan, R.; Al-Akeel, R.K.; Naz, S.; Khan, R.U. Anti-pyretic, analgesic, and anti-inflammatory activities of meloxicam and curcumin co-encapsulated PLGA nanoparticles in acute experimental models. Metabolites 2023, 13, 935. [Google Scholar] [CrossRef]
- Nakamura, K. Central circuitries for body temperature regulation and fever. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R1207–R1228. [Google Scholar] [CrossRef] [PubMed]
- Brito, H.O.; Barbosa, F.L.; Reis, R.C.D.; Fraga, D.; Borges, B.S.; Franco, C.R.C.; Zampronio, A.R. Evidence of substance P autocrine circuitry that involves TNF-alpha, IL-6, and PGE2 in endogenous pyrogen-induced fever. J. Neuroimmunol. 2016, 293, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Brunton, L.; Chabner, B.; Knollman, B. Goodman & Gillman’s the Pharmacological Basis of Therapeutics, 12th ed.; Brunton, L.L., Ed.; The McGraw-Hill Professional: New York, NY, USA, 2010; pp. 951–952. [Google Scholar]
- Spaulding, S.C.; Bollag, W.B. The role of lipid second messengers in aldosterone synthesis and secretion. J. Lipid Res. 2022, 63, 100191. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.H.; Yang, D.Y.; Lin, C.Y.; Chen, T.M.; Tang, C.H.; Huang, Y.L. Sphingosine-1-phosphate suppresses chondrosarcoma metastasis by upregulation of tissue inhibitor of metalloproteinase 3 through suppressing miR-101 expression. Mol. Oncol. 2017, 11, 1380–1398. [Google Scholar] [CrossRef]
- Lucki, N.C.; Li, D.; Sewer, M.B. Sphingosine-1-phosphate rapidly increases cortisol biosynthesis and the expression of genes involved in cholesterol uptake and transport in H295R adrenocortical cells. Mol. Cell. Endocrinol. 2012, 348, 165–175. [Google Scholar] [CrossRef]
- Standoli, S.; Rapino, C.; Di Meo, C.; Rudowski, A.; Kämpfer-Kolb, N.; Volk, L.M.; Thomas, D.; Trautmann, S.; Schreiber, Y.; Meyer Zu Heringdorf, D.; et al. Sphingosine kinases at the intersection of pro-inflammatory LPS and anti-inflammatory endocannabinoid signaling in BV2 mouse microglia cells. Int. J. Mol. Sci. 2023, 24, 8508. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Dan, W.; Liu, J.; Ha, P.; Zhou, T.; Guo, X.; Hou, W. The use of traditional chinese medicine in relieving EGFR-TKI-associated diarrhea based on network pharmacology and data mining. Evid. Based Complement. Altern. Med. 2021, 2021, 5530898. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Lai, X.; Wang, X.; Yao, X.; Wang, W.; Li, S. Network pharmacology to explore the anti-inflammatory mechanism of Xuebijing in the treatment of sepsis. Phytomedicine 2021, 85, 153543. [Google Scholar] [CrossRef] [PubMed]
- Husain, S.; Mohamed, R.; Abd Halim, K.B.; Mohd Mutalip, S.S. Homology modeling of human BAP1 and analysis of its binding properties through molecular docking and molecular dynamics simulation. J. Biomol. Struct. Dyn. 2022, 41, 7158–7173. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Huang, Y.-J.; Tao, Z.-Y.; Huang, H.-Y.; Luo, L.-H.; Zhang, Y.-Q. Discussion on the mechanism of Lingguizhugan Decoction in treating hypertension based on network pharmacology and molecular simulation technology. J. Biomol. Struct. Dyn. 2023, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.R., III; McGee, T.D., Jr.; Swails, J.M.; Homeyer, N.; Gohlke, H.; Roitberg, A.E. MMPBSA.py: An Efficient Program for End-State Free Energy Calculations. J. Chem. Theory Comput. 2012, 8, 3314–3321. [Google Scholar] [CrossRef] [PubMed]
- Valdés-Tresanco, M.S.; Valdés-Tresanco, M.E.; Valiente, P.A.; Moreno, E. gmx_MMPBSA: A New Tool to Perform End-State Free Energy Calculations with GROMACS. J. Chem. Theory Comput. 2021, 17, 6281–6291. [Google Scholar] [CrossRef]
- Liu, C.; Su, H.; Wan, H.; Qin, Q.; Wu, X.; Kong, X.; Lin, N. Forsythoside A exerts antipyretic effect on yeast-induced pyrexia mice via inhibiting transient receptor potential vanilloid 1 function. Int. J. Biol. Sci. 2017, 13, 65–75. [Google Scholar] [CrossRef]
- Bhat, A.S.; Tandan, S.K.; Kumar, D.; Krishna, V.; Prakash, V.R. Interaction between inhibitors of inducible nitric oxide synthase and cyclooxygenase in Brewer’s yeast induced pyrexia in mice: An isobolographic study. Eur. J. Pharmacol. 2005, 511, 137–142. [Google Scholar] [CrossRef]
- Ochi, R.; Suemaru, K.; Kawasaki, H.; Araki, H. Effect of Brewer’s yeast-induced pyrexia on aminophylline-elicited convulsions in mice. Acta Med. Okayama 2009, 63, 273–280. [Google Scholar] [CrossRef]
- Wu, Y.; Peng, Y.; Song, C.; Li, L.; Ma, H.; Li, D.; Wang, F.; Yang, J.; Song, S.; Wu, C. Separation and identification of multiple constituents in Xiao Chai Hu Decoction (Sho-saiko-to) by bioactivity-guided fractionation combined with LC-ESI-QTOFMS/MS. Biomed. Chromatogr. 2015, 29, 1146–1166. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Duan, J.A.; Qian, D.; Tang, Y.; Qian, Y.; Wu, D.; Su, S.; Shang, E. Rapid determination of amino acids in fruits of Ziziphus jujuba by hydrophilic interaction ultra-high-performance liquid chromatography coupled with triple-quadrupole mass spectrometry. J. Agric. Food Chem. 2013, 61, 2709–2719. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Liu, J.; Wang, M.; Liu, Y.; Meng, X.; Zhang, T.; Qi, Y.; Zhang, B.; Liu, H.; Sun, X.; et al. Exploring on the bioactive markers of Codonopsis Radix by correlation analysis between chemical constituents and pharmacological effects. J. Ethnopharmacol. 2019, 236, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.Q.; Leung, A.K.; Chan, C.L.; Su, T.; Li, W.D.; Li, S.M.; Fong, D.W.; Yu, Z.L. UHPLC UHD Q-TOF MS/MS analysis of the impact of sulfur fumigation on the chemical profile of Codonopsis Radix (Dangshen). Analyst 2014, 139, 505–516. [Google Scholar] [CrossRef]
- Chen, T.; Wang, X.; Chen, P.; Zheng, Y.; He, Y.; Zeng, X.; Peng, W.; Su, W. Chemical components analysis and in vivo metabolite profiling of Jian’er Xiaoshi oral liquid by UHPLC-Q-TOF-MS/MS. J. Pharm. Biomed. Anal. 2022, 211, 114629. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Zhu, Z.; Zhang, H.; Zhao, L.; Liu, Y.; Dong, X.; Lou, Z.; Zhang, G.; Chai, Y. Analysis of phenolic and triterpenoid compounds in licorice and rat plasma by high-performance liquid chromatography diode-array detection, time-of-flight mass spectrometry and quadrupole ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Porzel, A.; Wessjohann, L.A. Comparative metabolite profiling and fingerprinting of medicinal licorice roots using a multiplex approach of GC–MS, LC–MS and 1D NMR techniques. Phytochemistry 2012, 76, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.C.; Lin, Y.-H.; Chang, T.-M.; Huang, W.-Y. Identification of two licorice species, Glycyrrhiza uralensis and Glycyrrhiza glabra, based on separation and identification of their bioactive components. Food Chem. 2012, 132, 2188–2193. [Google Scholar] [CrossRef]
- Zhang, Q.; Ye, M. Chemical analysis of the Chinese herbal medicine Gan-Cao (licorice). J. Chromatogr. A 2009, 1216, 1954–1969. [Google Scholar] [CrossRef]
- Yin, Q.; Wang, P.; Zhang, A.; Sun, H.; Wu, X.; Wang, X. Ultra-performance LC-ESI/quadrupole-TOF MS for rapid analysis of chemical constituents of Shaoyao-Gancao decoction. J. Sep. Sci. 2013, 36, 1238–1246. [Google Scholar] [CrossRef]
- Shang, Z.; Liu, C.; Qiao, X.; Ye, M. Chemical analysis of the Chinese herbal medicine licorice (Gan-Cao): An update review. J. Ethnopharmacol. 2022, 299, 115686. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Zeng, M.; Chen, L.; Cao, Z.; Cai, H.; Yang, G. Chemical and Absorption Signatures of Xiao Chai Hu Tang. Rapid Commun. Mass Spectrom. 2018, 32, 1107–1125. [Google Scholar] [CrossRef]
- Seo, O.N.; Kim, G.-S.; Kim, Y.-H.; Park, S.; Jeong, S.W.; Lee, S.J.; Jin, J.S.; Shin, S.C. Determination of polyphenol components of Korean Scutellaria baicalensis Georgi using liquid chromatography–tandem mass spectrometry: Contribution to overall antioxidant activity. J. Funct. Foods 2013, 5, 1741–1750. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Z.; Li, M.; Yuan, Y.; Cui, S.; Chen, J.; Li, R. An integrated strategy for profiling the chemical components of Scutellariae Radix and their exogenous substances in rats by ultra-high-performance liquid chromatography/quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2020, 34, e8823. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Li, R.; Song, W.; Miao, W.-J.; Liu, J.; Chen, H.-B.; Guo, D.-A.; Ye, M. A targeted strategy to analyze untargeted mass spectral data: Rapid chemical profiling of Scutellaria baicalensis using ultra-high performance liquid chromatography coupled with hybrid quadrupole orbitrap mass spectrometry and key ion filtering. J. Chromatogr. A 2016, 1441, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Montero, L.; Ibáñez, E.; Russo, M.; di Sanzo, R.; Rastrelli, L.; Piccinelli, A.L.; Celano, R.; Cifuentes, A.; Herrero, M. Metabolite profiling of licorice (Glycyrrhiza glabra) from different locations using comprehensive two-dimensional liquid chromatography coupled to diode array and tandem mass spectrometry detection. Anal. Chim. Acta 2016, 913, 145–159. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, M.-K.; Liao, X.; Zhu, X.-M.; Peng, S.-L.; Ding, L.-S. Rapid identification of compounds in Glycyrrhiza uralensis by liquid chromatography/tandem mass spectrometry. Chin. J. Anal. Chem. 2004, 32, 174–178. Available online: https://api.semanticscholar.org/CorpusID:100157464 (accessed on 6 April 2022).
- Morimoto, S.; Harioka, T.; Shoyama, Y. Purification and characterization of flavone-specific β-glucuronidase from callus cultures of Scutellaria baicalensis Georgi. Planta 1995, 195, 535–540. [Google Scholar] [CrossRef]
- Lei, T.; Chen, S.; Wang, K.; Zhang, D.; Dong, L.; Lv, C.; Wang, J.; Lu, J. Characterization and discrimination of raw and vinegar-baked Bupleuri radix based on UHPLC-Q-TOF-MS coupled with multivariate statistical analysis. Biomed. Chromatogr. 2018, 32, e4044. [Google Scholar] [CrossRef] [PubMed]
- Xuan, C.; Zhao, X.; Feng, F.; Qiao, B. The study of chemical components of Glycyrrhiza in Shanxi. Nat. Prod. Res. Dev. 2000, 12, 18–22. Available online: https://europepmc.org/article/CBA/333984 (accessed on 6 April 2022).
- Miyaichi, Y.; Imoto, Y.; Tomimori, T.; Namba, T. Studies on the Nepalese crude drugs. IX. On the flavonoid constituents of the root of Scutellaria scandens Buch.-Ham. ex D. Don. Chem. Pharm. Bull. 1988, 36, 2371. [Google Scholar] [CrossRef]
- Liu, G.; Rajesh, N.; Wang, X.; Zhang, M.; Wu, Q.; Li, S.; Chen, B.; Yao, S. Identification of flavonoids in the stems and leaves of Scutellaria baicalensis Georgi. J. Chromatogr. B 2011, 879, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Ma, J.; Chen, Y.; Tian, Q.; Shen, Y.; Wang, X.; Chen, B.; Yao, S. Investigation of flavonoid profile of Scutellaria bacalensis Georgi by high performance liquid chromatography with diode array detection and electrospray ion trap mass spectrometry. J. Chromatogr. A 2009, 1216, 4809–4814. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Yang, N.; Zhao, Y.; Sheng, X.; Yang, S.; Li, Y. A rapid classification and identification method applied to the analysis of glycosides in Bupleuri radix and liquorice by ultra high performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. J. Sep. Sci. 2018, 41, 3791–3805. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Zheng, C.-J.; Wu, L.-J.; Wu, S.-Y.; Cai, Y.; Chen, G.-Y.; Han, C.-R.; Song, X.-P. Bioactive flavonoid derivatives from Scutellaria luzonica. Chem. Nat. Compd. 2018, 54, 350–353. [Google Scholar] [CrossRef]
- Ren, Q.; Xia, T.; Quan, X.-G.; Ding, L.; Wang, H.-Y. Antileukemic activity of the chemical constituents from Scutellaria barbata D. Don. Acta Chromatogr. 2017, 29, 399–413. [Google Scholar] [CrossRef]
- Lee, S.M.; Min, B.S.; Lee, C.G.; Kim, K.S.; Kho, Y.H. Cytotoxic triterpenoids from the fruits of Zizyphus jujuba. Planta Med. 2003, 69, 1051–1054. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Park, J.G.; Lee, Y.H.; Lee, C.G.; Min, B.S.; Kim, J.H.; Lee, H.K. Anti-complementary activity of triterpenoides from fruits of Zizyphus jujuba. Biol. Pharm. Bull. 2004, 27, 1883–1886. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, R.; Chen, J.; Liu, M.; Liu, M.; Liu, B.; Yi, L.; Liu, S. Application of multifold characteristic ion filtering combined with statistical analysis for comprehensive profiling of chemical constituents in anti-renal interstitial fibrosis I decoction by ultra-high performance liquid chromatography coupled with hybrid quadrupole-orbitrap high resolution mass spectrometry. J. Chromatogr. A 2019, 1600, 197–208. [Google Scholar] [CrossRef]
| Plant Name | Pharmacognostic Potential | Chemical Composition |
|---|---|---|
| Bupleurum chinense DC. | Antioxidant, antipyretic, immunoregulation, antitumor, hepatoprotection, anti-inflammatory, antiarrhythmic, antifatigue [21,22] | Flavonoids (quercetin, luteolin, apigenin-8-C-β-D-glucoside), triterpenoid saponins (saikosaponins A, C, and D), phenylpropanoids (ferulic acid), coumarins [23,24] |
| Scutellaria baicalensis Georgi | Antiviral, anti-allergic, antitumor, anti-bacterial, antioxidant, anti-inflammatory, hepatoprotective, and neuroprotective activities [25,26] | Flavonoids (baicalin, baicalein, wogonin, wogonoside), phenylethanoid glycosides (martynoside) [25,27] |
| Pinellia ternata (Thunb.) Makino | Sedative, hypnotic, and anticonvulsant activities [28], antitumor, antitussive, antiasthmatic, anti-gastric ulcer, and antidiarrheal effects [29] | Alkaloids (L-Tyrosine, Guanosine, Adenosine), flavonoids (baicalin, Daidzein), phenylpropanoids (Coniferin), others (gingerol) [29,30] |
| Codonopsis pilosula (Franch.) Nannf. | Neuroprotective, immunomodulatory, antitumor, anti-inflammatory, antioxidant, hepatoprotective, anti-hypoxia, antifatigue, and prebiotic activities [31] | Saccharides (rhamnose, arabinose, oligosaccharides, polysaccharides), alkaloids (codonopyrrolidiums A, B), glycosides (lobetyolin), amino acids, [31,32] |
| Zingiber officinale Roscoe | Immunomodulatory, antitumorigenic, anti-inflammatory, anti-apoptotic, anti-hyperglycemic, anti-lipidemic and anti-emetic actions [33] | Gingerols {[4]-, [6]-, [7]-, [8]-, and [10]-gingerol, 6-gingerol, 6-shogaol, [4]-, [6]-, [8]-, [10]- and [12]-shogaol}, volatile oil (curcumene, terpineol, borneol) [33] |
| Glycyrrhiza uralensis Fisch. ex DC. | Antioxidant, anti-inflammatory, antiviral, antidiabetic, skin-whitening, and cholinergic activities [34] | Phenolic (kaempferol, Gancaonin I, Liquiritigenin, Formononetin), flavonoids (Schaftoside, liquiritin, Isoliquiritin), and triterpenoid saponins (glycyrrhizin, glycyrrhetinic acid) [34] |
| Ziziphus jujuba Mill. | Anticancer, antioxidant, anti-inflammatory, anti-hyperlipidemic, anti-hyperglycemic, immunoregulatory, neuroprotective, sedative, and antiviral functions [35] | Polyphenols (Gallic acid, Caffeic acid, Chlorogenic acid, Rutin), polysaccharides, amino acids, nucleotides, fatty acids, dietary fiber, alkaloids [35] |
| Complexes | Average Binding Energy (kcal/mol) | ||||
|---|---|---|---|---|---|
| ΔVDWAALS | ΔEEL | ΔEGB | ΔESURF | ΔTOTAL | |
| GABBR2_6wiv-saikosaponinc | −56.62 ± 4.36 | −102.76 ± 7.25 | 96.44 ± 4.61 | −7.96 ± 0.3 | −70.90 ± 4.46 |
| GABBR2_6wiv-baicalin | −55.51 ± 3.58 | −55.55 ± 9.36 | 63.14 ± 5.26 | −7.33 ± 0.22 | −55.25 ± 4.40 |
| NFKBIA_1ikn-glycyrrhizicacid | −53.99 ± 2.57 | −44.82 ± 8.35 | 64.53 ± 5.85 | −6.61 ± 0.25 | −40.89 ± 4.64 |
| PTGS2_AF_P35354-lobetyolin | −34.62 ± 3.97 | −29.21 ± 11.19 | 41.89 ± 8.05 | −5.27 ± 0.55 | −27.22 ± 4.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Wu, H.; Li, P.; Peng, W.; Wang, Y.; Zhang, X.; Zhang, A.; Li, J.; Meng, F.; Wang, W.; et al. Unraveling the Mechanism of Xiaochaihu Granules in Alleviating Yeast-Induced Fever Based on Network Analysis and Experimental Validation. Pharmaceuticals 2024, 17, 475. https://doi.org/10.3390/ph17040475
Chen X, Wu H, Li P, Peng W, Wang Y, Zhang X, Zhang A, Li J, Meng F, Wang W, et al. Unraveling the Mechanism of Xiaochaihu Granules in Alleviating Yeast-Induced Fever Based on Network Analysis and Experimental Validation. Pharmaceuticals. 2024; 17(4):475. https://doi.org/10.3390/ph17040475
Chicago/Turabian StyleChen, Xiuli, Hao Wu, Peibo Li, Wei Peng, Yonggang Wang, Xiaoli Zhang, Ao Zhang, Jinliang Li, Fenzhao Meng, Weiyue Wang, and et al. 2024. "Unraveling the Mechanism of Xiaochaihu Granules in Alleviating Yeast-Induced Fever Based on Network Analysis and Experimental Validation" Pharmaceuticals 17, no. 4: 475. https://doi.org/10.3390/ph17040475






