The Therapeutic Potential of Four Main Compounds of Zanthoxylum nitidum (Roxb.) DC: A Comprehensive Study on Biological Processes, Anti-Inflammatory Effects, and Myocardial Toxicity
Abstract
:1. Introduction
2. Results
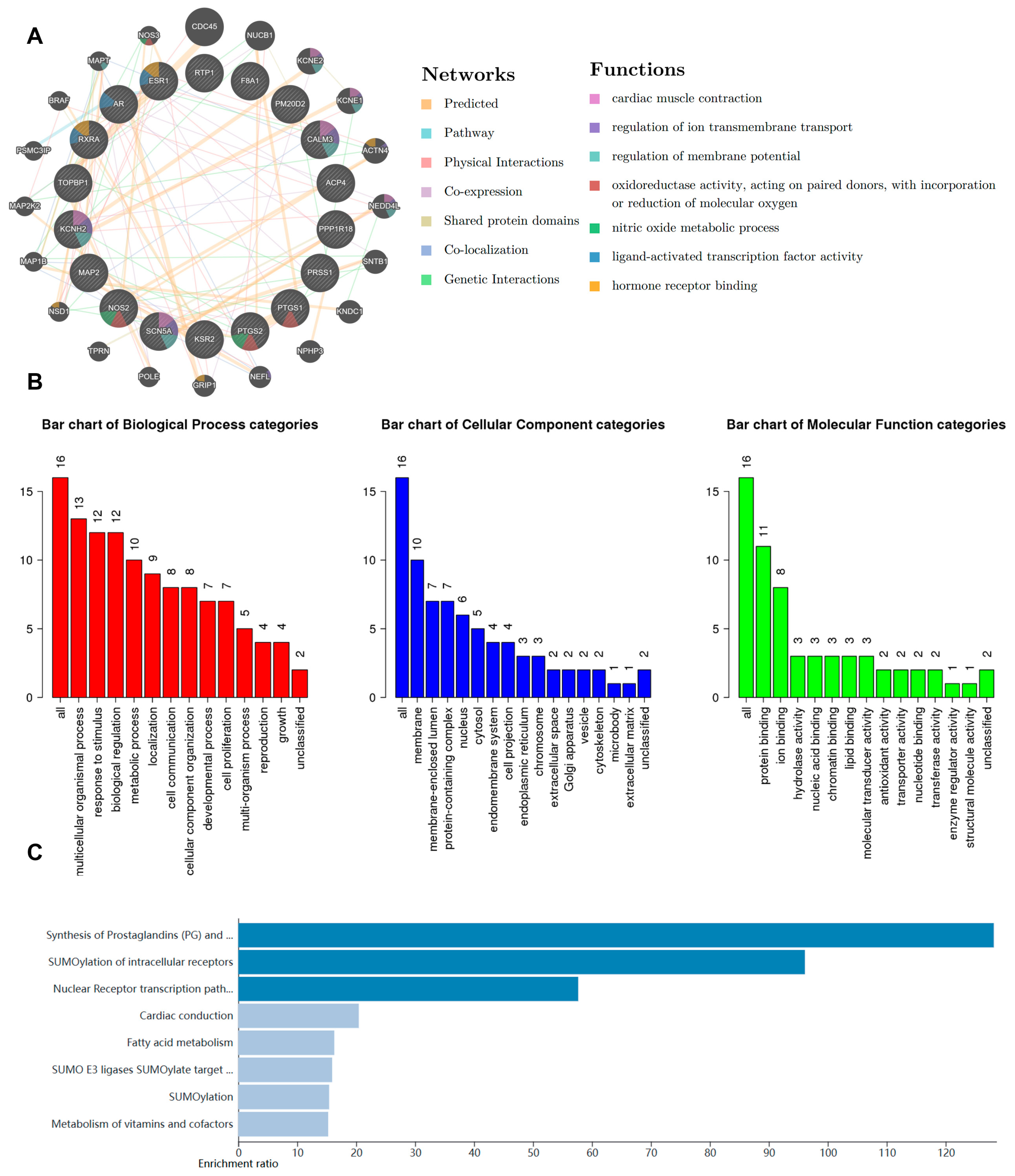
2.1. Bioinformatic Analysis of Z. nitidum
2.2. Determination of the Concentrations of NC, CHE, MAG, and HE in Z. nitidum
2.3. The Function of NC Was Predicted through Proteomics
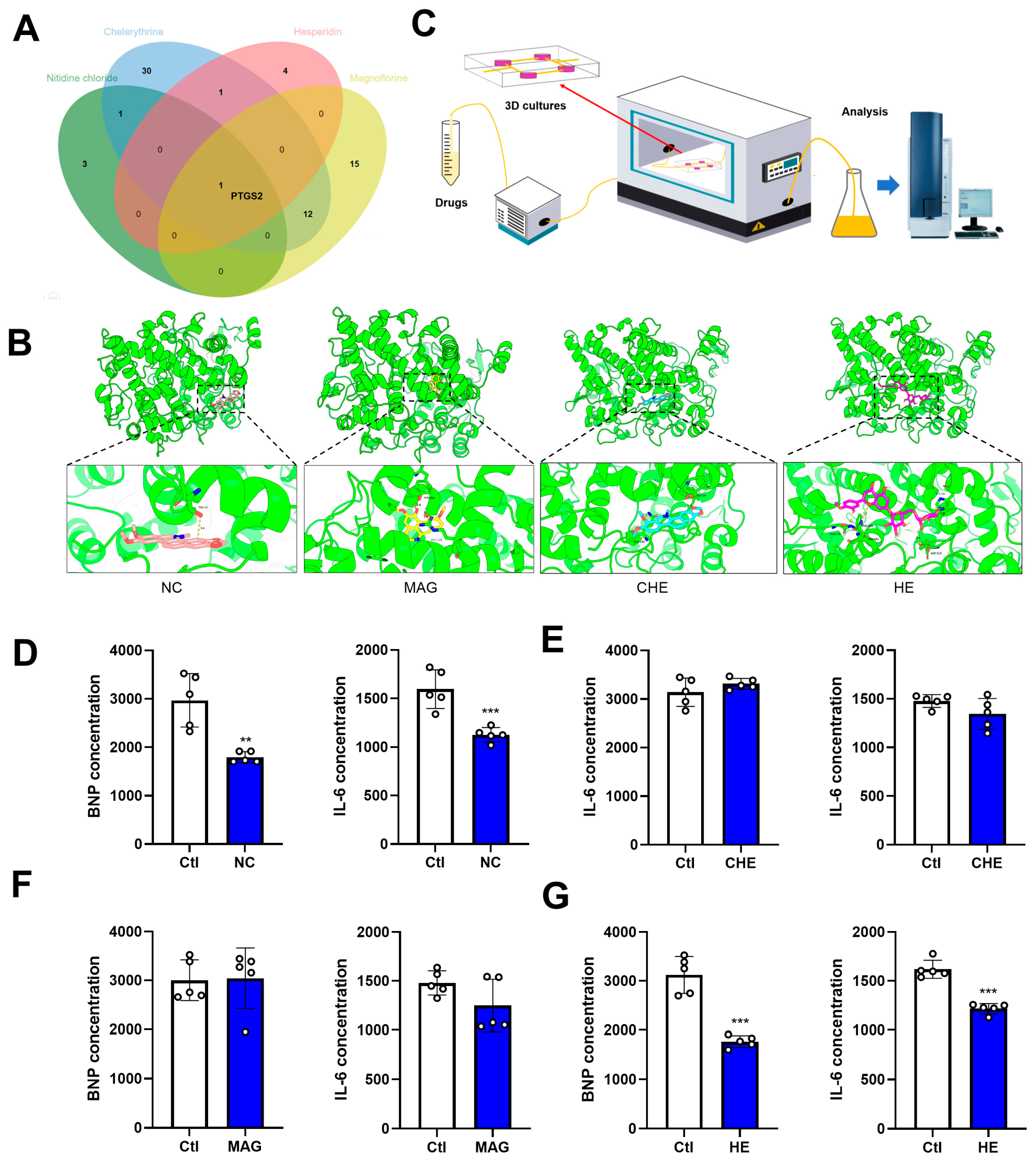
2.4. Prediction of Potential Targets and Pharmacological Characteristics of CHE, MAG, and HE Based on Bioinformatic Analysis
2.5. Effects of NC, CHE, MAG, and HE on Myocardial Cell Activity
2.6. Effects of NC, CHE, MAG, and HE on Myocardial IK1
2.7. Effects of NC, CHE, MAG, and HE on Myocardial ICa-L
2.8. Predictions of NC, CHE, MAG, and HE Targets and Their Anti-Inflammatory Effects on the Heart
3. Discussion
4. Materials and Methods
4.1. Reagent
4.2. Zanthoxylum nitidum (Roxb.) DC. Ethanol Extract Preparation
4.3. High-Performance Liquid Chromatography
4.4. Ultra-Performance Liquid Chromatography with Electrospray Ionization Quadrupole Time of Flight Mass (UPLC/ESI/qTOF-MS)
4.5. Organ-on-a-Chip Models
4.6. Prediction of Compound-Related Targets
4.7. Construction of a Network Model
4.8. Analysis of the Functional and Pathway Enrichment of the Main Compounds
4.9. Protein–Protein Interaction Networks
4.10. Analysis Using Metascape
4.11. Molecular Docking
4.12. Primary Culture of Neonatal Mouse Cardiomyocytes (NMCMs)
4.13. Electrode Control
4.14. Experimental Instruments
4.15. Patch Clamp Technology
4.16. CCK-8 Assay
4.17. Data Processing
4.18. Ethics Statement
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, Q.; Pu, H.; Guan, H.; Ma, C.; Zhang, Y.; Ding, W.; Cheng, X.; Ji, L.; Wang, Z.; Wang, C. Rapid Identification and Pharmacokinetic Studies of Multiple Active Alkaloids in Rat Plasma through Uplc-Q-Tof-Ms and Uplc-Ms/Ms after the Oral Administration of Zanthoxylum nitidum Extract. J. Pharm. Biomed. Anal. 2020, 186, 113232. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Li, C.; Wu, G. Insight into the Inhibitory Effects of Zanthoxylum nitidum against Helicobacter Pylori Urease and Jack Bean Urease: Kinetics and Mechanism. J. Ethnopharmacol. 2020, 249, 112419. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, Y.; Amoroso, V.; Acma, F.; Guiang, M.M.; Wu, H. Comparison of Production of Bioactive Components in Zanthoxylum nitidum Taproots from Different Regions in Southern China. Biomed. Chromatogr. 2023, 37, e5602. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Ma, R.; Yang, Y.; Mo, Z.; Pu, X.; Li, C. Zanthoxylum nitidum (Roxb.) Dc: Traditional Uses, Phytochemistry, Pharmacological Activities and Toxicology. J. Ethnopharmacol. 2020, 260, 112946. [Google Scholar] [CrossRef] [PubMed]
- Del Poeta, M.; Chen, S.F.; Von Hoff, D.; Dykstra, C.C.; Wani, M.C.; Manikumar, G.; Heitman, J.; Wall, M.E.; Perfect, J.R. Comparison of in Vitro Activities of Camptothecin and Nitidine Derivatives against Fungal and Cancer Cells. Antimicrob. Agents Chemother. 1999, 43, 2862–2868. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.T.; Pezzuto, J.M.; Kinghorn, A.D.; Hughes, S.H. Evaluation of Natural Products as Inhibitors of Human Immunodeficiency Virus Type 1 (Hiv-1) Reverse Transcriptase. J. Nat. Prod. 1991, 54, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Yue, R.; Ma, J.; Li, W.; Zhao, Z.; Li, H.; Shen, Y.; Hu, Z.; Lv, C.; Xu, X.; et al. Nitidine Chloride Exerts Anti-Inflammatory Action by Targeting Topoisomerase I and Enhancing Il-10 Production. Pharmacol. Res. 2019, 148, 104368. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Xiong, D.D.; He, R.Q.; Lai, Z.F.; Liu, L.M.; Huang, Z.G.; Yang, X.; Wu, H.Y.; Yang, L.H.; Ma, J.; et al. Identifying Tf-Mirna-Mrna Regulatory Modules in Nitidine Chloride Treated Hcc Xenograft of Nude Mice. Am. J. Transl. Res. 2019, 11, 7503–7522. [Google Scholar] [PubMed]
- Xu, H.; Cao, T.; Zhang, X.; Shi, Y.; Zhang, Q.; Chai, S.; Yu, L.; Jin, G.; Ma, J.; Wang, P.; et al. Nitidine Chloride Inhibits Sin1 Expression in Osteosarcoma Cells. Mol. Ther. Oncolytics 2019, 12, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Piperdi, S.; Gorlick, R. Activation of the Raf/Mitogen-Activated Protein/Extracellular Signal-Regulated Kinase Kinase/Extracellular Signal-Regulated Kinase Pathway Mediates Apoptosis Induced by Chelerythrine in Osteosarcoma. Clin. Cancer Res. 2008, 14, 6396–6404. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Sun, S.; Meng, Y.; Wang, L.; Liu, H.; Shi, W.; Zhang, Q.; Xu, W.; Sun, B.; Xu, J. Benzophenanthridine Alkaloid Chelerythrine Elicits Necroptosis of Gastric Cancer Cells Via Selective Conjugation at the Redox Hyperreactive C-Terminal Sec(498) Residue of Cytosolic Selenoprotein Thioredoxin Reductase. Molecules 2023, 28, 6842. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Singh, A.; Phogat, J.; Dahuja, A.; Dabur, R. Magnoflorine Prevent the Skeletal Muscle Atrophy Via Akt/Mtor/Foxo Signal Pathway and Increase Slow-Myhc Production in Streptozotocin-Induced Diabetic Rats. J. Ethnopharmacol. 2021, 267, 113510. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Han, L.; Cao, B.; Yang, X.; Zhu, X.; Yang, B.; Zhao, H.; Qiao, W. Use of Magnoflorine-Phospholipid Complex to Permeate Blood-Brain Barrier and Treat Depression in the Cums Animal Model. Drug Deliv. 2019, 26, 566–574. [Google Scholar] [CrossRef]
- Xiong, H.; Wang, J.; Ran, Q.; Lou, G.; Peng, C.; Gan, Q.; Hu, J.; Sun, J.; Yao, R.; Huang, Q. Hesperidin: A Therapeutic Agent for Obesity. Drug Des. Devel Ther. 2019, 13, 3855–3866. [Google Scholar] [CrossRef] [PubMed]
- Abd Elmaaboud, M.A.; Estfanous, R.S.; Atef, A.; Kabel, A.M.; Alnemari, K.A.; Naguib, T.M.; Alsufyani, S.E.; Darwish, H.W.; Arab, H.H. Dapagliflozin/Hesperidin Combination Mitigates Lipopolysaccharide-Induced Alzheimer’s Disease in Rats. Pharmaceuticals 2023, 16, 1370. [Google Scholar] [CrossRef] [PubMed]
- Heng, W.S.; Cheah, S.C. Chelerythrine Chloride Downregulates Β-Catenin and Inhibits Stem Cell Properties of Non-Small Cell Lung Carcinoma. Molecules 2020, 25, 224. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Kuang, T.; Du, H.; Li, Q.; Feng, T.; Zhang, Y.; Fan, G. Magnoflorine: A Review of Its Pharmacology, Pharmacokinetics and Toxicity. Pharmacol. Res. 2020, 152, 104632. [Google Scholar] [CrossRef] [PubMed]
- Roohbakhsh, A.; Parhiz, H.; Soltani, F.; Rezaee, R.; Iranshahi, M. Neuropharmacological Properties and Pharmacokinetics of the Citrus Flavonoids Hesperidin and Hesperetin—A Mini-Review. Life Sci. 2014, 113, 1–6. [Google Scholar] [CrossRef] [PubMed]
- de la Peña, J.B.; Lee, H.L.; Yoon, S.Y.; Kim, G.H.; Lee, Y.S.; Cheong, J.H. The Involvement of Magnoflorine in the Sedative and Anxiolytic Effects of Sinomeni Caulis Et Rhizoma in Mice. J. Nat. Med. 2013, 67, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, R.; Markandeya, Y.S.; Kamp, T.J.; Makielski, J.C.; January, C.T.; Eckhardt, L.L. Ik1-Enhanced Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes: An Improved Cardiomyocyte Model to Investigate Inherited Arrhythmia Syndromes. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H1611–H1621. [Google Scholar] [CrossRef] [PubMed]
- Miake, J.; Marbán, E.; Nuss, H.B. Biological Pacemaker Created by Gene Transfer. Nature 2002, 419, 132–133. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Qiao, X.; Zhang, L.; Wang, D.; Zhang, L.; Feng, Q.; Wu, B.; Cao, J.; Liu, Q. I(K1) Channel Agonist Zacopride Suppresses Ventricular Arrhythmias in Conscious Rats with Healing Myocardial Infarction. Life Sci. 2019, 239, 117075. [Google Scholar] [CrossRef] [PubMed]
- Girmatsion, Z.; Biliczki, P.; Bonauer, A.; Wimmer-Greinecker, G.; Scherer, M.; Moritz, A.; Bukowska, A.; Goette, A.; Nattel, S.; Hohnloser, S.H.; et al. Changes in Microrna-1 Expression and Ik1 up-Regulation in Human Atrial Fibrillation. Heart Rhythm. 2009, 6, 1802–1809. [Google Scholar] [CrossRef]
- Gómez, R.; Caballero, R.; Barana, A.; Amorós, I.; Calvo, E.; López, J.A.; Klein, H.; Vaquero, M.; Osuna, L.; Atienza, F.; et al. Nitric Oxide Increases Cardiac Ik1 by Nitrosylation of Cysteine 76 of Kir2.1 Channels. Circ. Res. 2009, 105, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Wible, B.A.; De Biasi, M.; Majumder, K.; Taglialatela, M.; Brown, A.M. Cloning and Functional Expression of an Inwardly Rectifying K+ Channel from Human Atrium. Circ. Res. 1995, 76, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.W.; Li, W.; Guo, K.; Chen, X.M.; Chen, Y.H.; Li, C.Y.; Zhao, B.C.; Zhao, J.; Wang, H.; Wang, Y.P.; et al. Antiarrhythmic Effects and Potential Mechanism of Wenxin Keli in Cardiac Purkinje Cells. Heart Rhythm. 2016, 13, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Ni, J.; Xia, X.; Jiang, M.; Bai, G. Searching for Synergistic Calcium Antagonists and Novel Therapeutic Regimens for Coronary Heart Disease Therapy from a Traditional Chinese Medicine, Suxiao Jiuxin Pill. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018, 1092, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Baumann, S.; Fas, S.C.; Giaisi, M.; Müller, W.W.; Merling, A.; Gülow, K.; Edler, L.; Krammer, P.H.; Li-Weber, M. Wogonin Preferentially Kills Malignant Lymphocytes and Suppresses T-Cell Tumor Growth by Inducing Plcgamma1- and Ca2+-Dependent Apoptosis. Blood 2008, 111, 2354–2363. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Lee, J.; Chung, J.J.; Jung, Y.; Kim, S.H. Integrating Organs-on-Chips: Multiplexing, Scaling, Vascularization, and Innervation. Trends Biotechnol. 2020, 38, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, K.S.; Müller, I.; Malcomber, S.; Carmichael, P.L.; Bouwmeester, H. Implementing Organ-on-Chip in a Next-Generation Risk Assessment of Chemicals: A Review. Arch. Toxicol. 2022, 96, 711–741. [Google Scholar] [CrossRef]
- Zhao, Y.; Kankala, R.K.; Wang, S.B.; Chen, A.Z. Multi-Organs-on-Chips: Towards Long-Term Biomedical Investigations. Molecules 2019, 24, 675. [Google Scholar] [CrossRef]
- Kim, H.; Kim, E.J.; Ngo, H.V.; Nguyen, H.D.; Park, C.; Choi, K.H.; Park, J.B.; Lee, B.J. Cellular Efficacy of Fattigated Nanoparticles and Real-Time Ros Occurrence Using Microfluidic Hepatocarcinoma Chip System: Effect of Anticancer Drug Solubility and Shear Stress. Pharmaceuticals 2023, 16, 1330. [Google Scholar] [CrossRef]
- Zhu, M.; Niu, J.; Jiang, J.; Dong, T.; Chen, Y.; Yang, X.; Liu, P. Chelerythrine Inhibits the Progression of Glioblastoma by Suppressing the Tgfb1-Erk1/2/Smad2/3-Snail/Zeb1 Signaling Pathway. Life Sci. 2022, 293, 120358. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, A.H.; Liu, S.B.; Qiu, S.; Li, X.N.; Zhang, T.L.; Liu, L.; Wang, X.J. Cell Metabolomics Identify Regulatory Pathways and Targets of Magnoline against Prostate Cancer. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018, 1102–1103, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Shah, J. Protective Effects of Hesperidin through Attenuation of Ki67 Expression against Dmba-Induced Breast Cancer in Female Rats. Life Sci. 2021, 285, 119957. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Jin, J.; Zou, G.; Sui, Y.; Han, Y.; Zhao, D.; Liu, L. Hesperidin Prevents Hyperglycemia in Diabetic Rats by Activating the Insulin Receptor Pathway. Exp. Ther. Med. 2021, 21, 53. [Google Scholar] [CrossRef] [PubMed]
- Amat-Ur-Rasool, H.; Ahmed, M.; Hasnain, S.; Ahmed, A.; Carter, W.G. In Silico Design of Dual-Binding Site Anti-Cholinesterase Phytochemical Heterodimers as Treatment Options for Alzheimer’s Disease. Curr. Issues Mol. Biol. 2021, 44, 152–175. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, R.; Duan, H.X.; Zhang, M.M.; He, L.; Ye, X.; Wei, D.N.; Wu, C.J. Research Progress Regarding Potential Effects of Traditional Chinese Medicine on Postoperative Intestinal Obstruction. J. Pharm. Pharmacol. 2021, 73, 1007–1022. [Google Scholar] [CrossRef] [PubMed]
- Dridi, H.; Kushnir, A.; Zalk, R.; Yuan, Q.; Melville, Z.; Marks, A.R. Intracellular Calcium Leak in Heart Failure and Atrial Fibrillation: A Unifying Mechanism and Therapeutic Target. Nat. Rev. Cardiol. 2020, 17, 732–747. [Google Scholar] [CrossRef]
- Li, W.; Luo, X.; Ulbricht, Y.; Wagner, M.; Piorkowski, C.; El-Armouche, A.; Guan, K. Establishment of an Automated Patch-Clamp Platform for Electrophysiological and Pharmacological Evaluation of Hipsc-Cms. Stem Cell Res. 2019, 41, 101662. [Google Scholar] [CrossRef] [PubMed]
- Little, H.J. L-Type Calcium Channel Blockers: A Potential Novel Therapeutic Approach to Drug Dependence. Pharmacol. Rev. 2021, 73, 127–154. [Google Scholar] [CrossRef]
- Xu, H.; Zhao, M.; Liang, S.; Huang, Q.; Xiao, Y.; Ye, L.; Wang, Q.; He, L.; Ma, L.; Zhang, H.; et al. The Effects of Puerarin on Rat Ventricular Myocytes and the Potential Mechanism. Sci. Rep. 2016, 6, 35475. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xie, W.; Yu, J.; Ellory, C.; Wilkins, R.; Zhu, Y.; Ma, Y.L. Ion Channel Targeted Mechanisms of Anti-Arrhythmic Chinese Herbal Medicine Xin Su Ning. Front. Pharmacol. 2019, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- Isaev, D.; Yang, K.S.; Shabbir, W.; Howarth, F.C.; Oz, M. Capsaicin Inhibits Multiple Voltage-Gated Ion Channels in Rabbit Ventricular Cardiomyocytes in Trpv1-Independent Manner. Pharmaceuticals 2022, 15, 1187. [Google Scholar] [CrossRef] [PubMed]
- Paloschi, V.; Sabater-Lleal, M.; Middelkamp, H.; Vivas, A.; Johansson, S.; van der Meer, A.; Tenje, M.; Maegdefessel, L. Organ-on-a-Chip Technology: A Novel Approach to Investigate Cardiovascular Diseases. Cardiovasc. Res. 2021, 117, 2742–2754. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.; Torisawa, Y.S.; Hamilton, G.A.; Kim, H.J.; Ingber, D.E. Microengineered Physiological Biomimicry: Organs-on-Chips. Lab Chip 2012, 12, 2156–2164. [Google Scholar] [CrossRef] [PubMed]
- Shamsol Azman, A.N.S.; Tan, J.J.; Abdullah, M.N.H.; Bahari, H.; Lim, V.; Yong, Y.K. Network Pharmacology and Molecular Docking Analysis of Active Compounds in Tualang Honey against Atherosclerosis. Foods 2023, 12, 1779. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Sakharkar, M.K.; Yang, J. Probing the Mechanism of Action (Moa) of Solanum Nigrum Linn against Breast Cancer Using Network Pharmacology and Molecular Docking. SN Appl. Sci. 2023, 5, 133. [Google Scholar] [CrossRef]
- Hong, Y.; Xu, W.Q.; Feng, J.; Lou, H.; Liu, H.; Wang, L.; Cui, H.; Jiang, L.T.; Xu, R.C.; Xu, H.H.; et al. Nitidine Chloride Induces Cardiac Hypertrophy in Mice by Targeting Autophagy-Related 4b Cysteine Peptidase. Acta Pharmacol. Sin. 2023, 44, 561–572. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wang, Q.; Liu, L.; Shi, Y.; Hong, Y.; Xu, W.; Xu, H.; Feng, J.; Xie, M.; Li, Y.; et al. The Therapeutic Potential of Four Main Compounds of Zanthoxylum nitidum (Roxb.) DC: A Comprehensive Study on Biological Processes, Anti-Inflammatory Effects, and Myocardial Toxicity. Pharmaceuticals 2024, 17, 524. https://doi.org/10.3390/ph17040524
Li X, Wang Q, Liu L, Shi Y, Hong Y, Xu W, Xu H, Feng J, Xie M, Li Y, et al. The Therapeutic Potential of Four Main Compounds of Zanthoxylum nitidum (Roxb.) DC: A Comprehensive Study on Biological Processes, Anti-Inflammatory Effects, and Myocardial Toxicity. Pharmaceuticals. 2024; 17(4):524. https://doi.org/10.3390/ph17040524
Chicago/Turabian StyleLi, Xiaohan, Qi Wang, Ling Liu, Yang Shi, Yang Hong, Wanqing Xu, Henghui Xu, Jing Feng, Minzhen Xie, Yang Li, and et al. 2024. "The Therapeutic Potential of Four Main Compounds of Zanthoxylum nitidum (Roxb.) DC: A Comprehensive Study on Biological Processes, Anti-Inflammatory Effects, and Myocardial Toxicity" Pharmaceuticals 17, no. 4: 524. https://doi.org/10.3390/ph17040524






