In Vivo Evaluation of Miconazole-Nitrate-Loaded Transethosomal Gel Using a Rat Model Infected with Candida albicans
Abstract
:1. Introduction
2. Results and Discussion
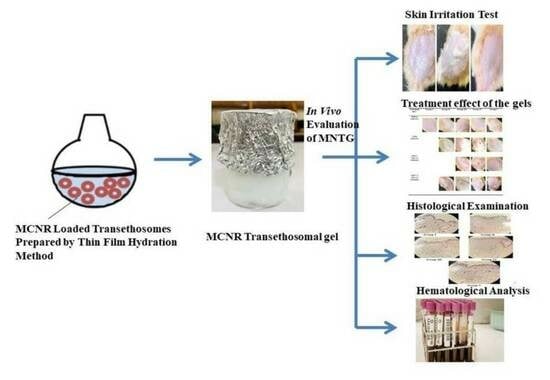
2.1. Patch Test (Skin Irritancy Test)
2.2. Treatment Effect of the Gels
2.3. Histological Examination
2.4. Hematological Analysis of Total and Differential WBC Count
3. Materials and Methods
3.1. Materials
3.2. Preparation of Miconazole Nitrate (MCNR) Transethosomal Formulation
3.3. Preparation of Transethosomal Gel and MCNR Gel
3.4. In Vivo Evaluation of MCNR Transethosomal Gel
3.4.1. Animals
3.4.2. Experimental Design
3.4.3. Patch Test (Skin Irritancy Test)
3.4.4. Immunosuppressed Animal Preparation
3.4.5. Fungal Strain Preparation
3.4.6. Development of Fungal Infection
3.4.7. Clinical Examinations
3.4.8. Histopathological Analysis
3.4.9. Assessment of Blood Parameters
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qushawy, M.; Nasr, A.; Abd-Alhaseeb, M.; Swidan, S. Design, optimization and characterization of a transfersomal gel using miconazole nitrate for the treatment of candida skin infections. Pharmaceutics 2018, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Coronado-Castellote, L.; Jiménez-Soriano, Y. Clinical and microbiological diagnosis of oral candidiasis. J. Clin. Exp. Dent. 2013, 5, e279–e286. [Google Scholar] [CrossRef] [PubMed]
- Sardi, J.; Scorzoni, L.; Bernardi, T.; Fusco-Almeida, A.; Mendes Giannini, M.J.S. Candida species: Current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J. Med. Microbiol. 2013, 62, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Slavin, M.; Van Hal, S.; Sorrell, T.C.; Lee, A.; Marriott, D.; Daveson, K.; Kennedy, K.; Hajkowicz, K.; Halliday, C.; Athan, E.; et al. Invasive infections due to filamentous fungi other than Aspergillus: Epidemiology and determinants of mortality. Clin. Microbiol. Infect. 2015, 21, 490.e1–490.e10. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Denning, D.W.; Gow, N.A.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv113. [Google Scholar] [CrossRef] [PubMed]
- Seyedmousavi, S.; Samerpitak, K.; Rijs, A.; Melchers, W.; Mouton, J.; Verweij, P.; de Hoog, G. Antifungal susceptibility patterns of opportunistic fungi in the genera Verruconis and Ochroconis. Antimicrob. Agents Chemother. 2014, 58, 3285–3292. [Google Scholar] [CrossRef] [PubMed]
- Lass-Flörl, C. Current challenges in the diagnosis of fungal infections. In Human Fungal Pathogen Identification; Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 3–15. [Google Scholar]
- Mendes, A.; Silva, A.C.; Catita, J.A.M.; Cerqueira, F.; Gabriel, C.; Lopes, C.M. Miconazole-loaded nanostructured lipid carriers (NLC) for local delivery to the oral mucosa: Improving antifungal activity. Coll. Surf. B Biointerfaces 2013, 111, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Mulani, H.; Bhise, K.S. QbD Approach in the formulation and evaluation of Miconazole Nitrate loaded ethosomal cream-o-gel. Int. Res. J. Pharm. Sci. 2017, 8, 1–13. [Google Scholar]
- Aljaeid, B.M.; Hosny, K.M. Miconazole-loaded solid lipid nanoparticles: Formulation and evaluation of a novel formula with high bioavailability and antifungal activity. Int. J. Nanomed. 2016, 11, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Mir-Palomo, S.; Nácher, A.; Díez-Sales, O.; Busó, M.O.V.; Caddeo, C.; Manca, M.L.; Manconi, M.; Fadda, A.M.; Saurí, A.R. Inhibition of skin inflammation by baicalin ultradeformable vesicles. Int. J. Pharm. 2016, 511, 23–29. [Google Scholar] [CrossRef]
- Faisal, W.; Soliman, G.M.; Hamdan, A.M. Enhanced skin deposition and delivery of voriconazole using ethosomal preparations. J. Liposome Res. 2018, 28, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Zahid, S.R.; Upmanyu, N.; Dangi, S.; Ray, S.K.; Jain, P.; Parkhe, G. Ethosome: A novel vesicular carrier for transdermal drug delivery. J. Drug Deliv. Ther. 2018, 8, 318–326. [Google Scholar] [CrossRef]
- Sharma, S.; Kaur, V.; Mahajan, K.; Singh, S.; Arora, P.; Arora, V.; Yadav, G. Antifungal Gel of Miconazole Nitrate: A Comparative Effect Study with Accumulation of Antioxidants and Surfactants. Acta Sci. Pharm. Sci. 2018, 2, 51–55. [Google Scholar]
- Ahmed, T.A.; Alzahrani, M.M.; Sirwi, A.; Alhakamy, N.A. Study the antifungal and ocular permeation of ketoconazole from ophthalmic formulations containing trans-ethosomes nanoparticles. Pharmaceutics 2021, 13, 151. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, K.J.; Parab, B.S.; Shidhaye, S.S. Nano-transethosomes: A novel tool for drug delivery through skin. Indian J. Pharm. Educ. Res. 2021, 55, s1–s10. [Google Scholar] [CrossRef]
- Abdulbaqi, I.M.; Darwis, Y.; Khan, N.A.K.; Assi, R.A.; Khan, A.A. Ethosomal nanocarriers: The impact of constituents and formulation techniques on ethosomal properties, in vivo studies, and clinical trials. Int. J. Nanomed. 2016, 11, 2279–2304. [Google Scholar] [CrossRef] [PubMed]
- Jondhalekar, T.; Aher, S.; Saudagar, R. Transethosome: Novel vesicular carrier for enhanced transdermal drug delivery system. Res. J. Pharm. Technol. 2017, 10, 1816–1819. [Google Scholar] [CrossRef]
- Chen, Z.; Li, B.; Liu, T.; Wang, X.; Zhu, Y.; Wang, L.; Wang, X.; Niu, X.; Xiao, Y.; Sun, Q. Evaluation of paeonol-loaded transethosomes as transdermal delivery carriers. Eur. J. Pharm. Sci. 2017, 99, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Garg, V.; Singh, H.; Bhatia, A.; Raza, K.; Singh, S.K.; Singh, B.; Beg, S. Systematic development of transethosomal gel system of piroxicam: Formulation optimization, in vitro evaluation, and ex vivo assessment. AAPS Pharmscitech 2017, 18, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Asghar, Z.; Jamshaid, T.; Sajid-ur-Rehman, M.; Jamshaid, U.; Gad, H.A. Novel Transethosomal Gel Containing Miconazole Nitrate; Development, Characterization, and Enhanced Antifungal Activity. Pharmaceutics 2023, 15, 2537. [Google Scholar] [CrossRef] [PubMed]
- Nisbet, S.J. Absence of human skin irritation and allergenic potential after repeated patch applications of a lamellar moisturizer. J. Cosmet. Dermatol. 2019, 18, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Barel, A.; Lambrecht, R.; Clarys, P.; Morrison Jr, B.; Paye, M. A comparative study of the effects on the skin of a classical bar soap and a syndet cleansing bar in normal use conditions and in the soap chamber test. Skin Res. Technol. 2001, 7, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Farage, M.A.; Maibach, H.I.; Andersen, K.E.; Lachapelle, J.M.; Kern, P.; Ryan, C.; Ely, J.; Kanti, A. Historical perspective on the use of visual grading scales in evaluating skin irritation and sensitization. Contact Dermat. 2011, 65, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Wang, J.; Ma, M.; Tan, F.; Li, N. Skin targeted lipid vesicles as novel nano-carrier of ketoconazole: Characterization, in vitro and in vivo evaluation. J. Mater. Sci. Mater. Med. 2015, 26, 175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Guo, M.; Zhu, T.; Xiong, H.; Zhu, L.-M. A careob-like nanofibers with a sustained drug release profile for promoting skin wound repair and inhibiting hypertrophic scar. Compos. Part B Eng. 2022, 236, 109790. [Google Scholar] [CrossRef]
- Wahedi, H.M.; Chae, J.K.; Subedi, L.; Kang, M.C.; Cho, H.; Kim, S.; Kim, S.Y. NED416, a novel synthetic Sirt1 activator, promotes cutaneous wound healing via the MAPK/Rho pathway. Int. J. Mol. Med. 2020, 46, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Farghaly Aly, U.; Abou-Taleb, H.A.; Abdellatif, A.A.; Sameh Tolba, N. Formulation and evaluation of simvastatin polymeric nanoparticles loaded in hydrogel for optimum wound healing purpose. Drug Des. Dev. Ther. 2019, 13, 1567–1580. [Google Scholar] [CrossRef] [PubMed]
- Yokota, J.; Kyotani, S. Influence of nanoparticle size on the skin penetration, skin retention and anti-inflammatory activity of non-steroidal anti-inflammatory drugs. J. Chin. Med. Assoc. 2018, 81, 511–519. [Google Scholar] [CrossRef]
- Ramadon, D.; McCrudden, M.T.; Courtenay, A.J.; Donnelly, R.F. Enhancement strategies for transdermal drug delivery systems: Current trends and applications. Drug Deliv. Transl. Res. 2022, 12, 758–791. [Google Scholar] [CrossRef] [PubMed]
- Dhopavkar, S.; Kadu, P. Transfersomes-a Boon for Transdermal Delivery. Indo Am. J. Pharm. Sci. 2017, 4, 2908–2919. [Google Scholar]
- Albash, R.; Abdelbary, A.A.; Refai, H.; El-Nabarawi, M.A. Use of transethosomes for enhancing the transdermal delivery of olmesartan medoxomil: In vitro, ex vivo, and in vivo evaluation. Int. J. Nanomed. 2019, 14, 1953–1968. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, M.M.; Khalil, I.A.; Khalil, M.A. Sertaconazole nitrate loaded nanovesicular systems for targeting skin fungal infection: In-vitro, ex-vivo and in-vivo evaluation. Int. J. Pharm. 2017, 527, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Narang, J. Topical nanoemulgel: A novel pathway for investigating alopecia. J. Nanomed. Nanotechnol. 2017, 8, 1000472. [Google Scholar] [CrossRef]
- Kaur, A.; Gupta, S.; Tyagi, A.; Sharma, R.K.; Ali, J.; Gabrani, R.; Dang, S. Development of nanoemulsion based gel loaded with phytoconstituents for the treatment of urinary tract infection and in vivo biodistribution studies. Adv. Pharm. Bull. 2017, 7, 611. [Google Scholar] [CrossRef] [PubMed]
- Castro, N.R.; Cristal dos Santos, C.P.; de Campos, V.E.B.; Cardoso, V.; Vermelho, A.B.; dos Santos, E.P.; Mansur, C.R.E. Development of hybrid vesicular nanosystems composed of lipids and chitosan for octyl methoxycinnamate encapsulation. Coll. Surf. A Physicochem. Eng. Asp. 2021, 608, 125476. [Google Scholar] [CrossRef]
- Ijaz, M.; Akhtar, N. Fatty acids based α-Tocopherol loaded nanostructured lipid carrier gel: In vitro and in vivo evaluation for moisturizing and anti-aging effects. J. Cosmet. Dermatol. 2020, 19, 3067–3076. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, S.; Ahmad, S.; Ali, B.; Usman, F.; Jabeen, Q.; Sajid-ur-Rehman, M.; Ahmed, M.; Zubair, H.M.; Alkazmi, L.; Batiha, G.E.-S. Chemical profiling and evaluation of toxicological, antioxidant, anti-inflammatory, anti-nociceptive and tyrosinase inhibitory potential of Portulacaria afra using in-vitro, in-vivo and in-silico studies. Arab. J. Chem. 2023, 16, 104784. [Google Scholar] [CrossRef]
- James, O.; Sunday, A.B. Evaluation of acute dermal irritation and wound contraction by Gymnema sylvestre and Datura metel extracts in rats. Am. J. Biomed. Life Sci. 2014, 2, 83–88. [Google Scholar] [CrossRef]
- Adegbenro, O.O.; Opeyemi, O.T. Development and estimation of anti-inflammatory activity of topical etoricoxib emulgel by carrageenan induced paw oedema method. Univers. J. Pharm. Res. 2019, 4, 23–28. [Google Scholar] [CrossRef]
- Zuang, V.; Alonso, M.-A.; Botham, P.A.; Eskes, C.; Fentem, J.; Liebsch, M.; van de Sandt, J.J.M. Skin Irritation and Corrosion. Altern. Lab. Anim. 2005, 33, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.R.; Muralidharan, S.; Parasuraman, S. In Vitro and in Vivo evaluation of microspheres loaded topical gel delivery system of ketoconazole in male rats against Candida Glabrata. J. Pharm. Sci. Res. 2014, 6, 376. [Google Scholar]
- Shadomy, S. Laboratory studies with antifungal agents: Susceptibility tests and bioassays. In Manual of Clinical Microbiology; American Society for Microbiology: Washington, DC, USA, 1985; pp. 991–999. [Google Scholar]
- Hamad, A.M.; Ahmed, H.G. Association of some carbohydrates with estrogen expression in breast lesions among Sudanese females. J. Histotechnol. 2018, 41, 2–9. [Google Scholar] [CrossRef]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques; Elsevier Health Sciences: Philadelphia, PA, USA, 2018. [Google Scholar]
- Sadaf, F.; Saleem, R.; Ahmed, M.; Ahmad, S.I. Healing potential of cream containing extract of Sphaeranthus indicus on dermal wounds in Guinea pigs. J. Ethnopharmacol. 2006, 107, 161–163. [Google Scholar] [CrossRef] [PubMed]
- SM, S.; KL, B.; PY, P. Assessment of Anti-Dermatophytic Activity of Zincoderm Gm Cream in Experimental Tinea Pedis in Wistar Rats. Int. J. Pharm. Chem. Biol. Sci. 2015, 5, 683. [Google Scholar]
- Suresh Kumar, P. Anti-fungal activity of Leptadenia reticulata in rat animal model in vivo. J. Basic Appl. Biol. 2008, 2, 9–13. [Google Scholar]
- Khiljee, T.; Akhtar, N. Investigation of antiaging and skin rejuvenation potential of phytoconstituents from Pyrus communis loaded topical emulgel. Pak. J. Pharm. Sci. 2019, 32, 293–300. [Google Scholar] [PubMed]
- Sridhar, K.; Charles, A.L. In vitro antioxidant activity of Kyoho grape extracts in DPPH and ABTS assays: Estimation methods for EC50 using advanced statistical programs. Food Chem. 2019, 275, 41–49. [Google Scholar] [CrossRef] [PubMed]




| Time (h) | MNG | Marketed Cream | MNTG | |||
|---|---|---|---|---|---|---|
| Erythema | Edema | Erythema | Edema | Erythema | Edema | |
| 0 h | 0 | 0 | 0 | 0 | 0 | 0 |
| 24 h | 1 | 1 | 1 | 0 | 1 | 1 |
| 48 h | 3 | 4 | 0 | 2 | 0 | 0 |
| Mean ± SD | 1.3 ± 1.57 | 1.6 ± 1.20 | 0.3 ± 0.21 | 0.6 ± 0.18 | 0.3 ± 0.04 | 0.3 ± 0.05 |
| Primary cutaneous irritation index | 2.9 | 0.9 | 0.6 | |||
| Subject | White Blood Cells/mcL |
|---|---|
| Group I (normal) | 9800 ± 145 |
| Group II (infected + untreated) | 4300 ± 265 |
| Group III (infected + treated with MNG) | 6700 ± 318 |
| Group IV (infected + treated with marketed cream) | 8800 ± 256 |
| Group V (infected + treated with MNTG) | 9500 ± 240 |
| Subject | Lymphocytes (%) | Eosinophils (%) |
|---|---|---|
| Group I (normal) | 43 | 1 |
| Group II (infected + untreated) | 15 | 6 |
| Group III (infected + treated with MNG) | 30 | 3 |
| Group IV (infected + treated with marketed cream) | 37 | 2 |
| Group V (infected + treated with MNTG) | 41 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asghar, Z.; Jamshaid, T.; Jamshaid, U.; Madni, A.; Akhtar, N.; Lashkar, M.O.; Gad, H.A. In Vivo Evaluation of Miconazole-Nitrate-Loaded Transethosomal Gel Using a Rat Model Infected with Candida albicans. Pharmaceuticals 2024, 17, 546. https://doi.org/10.3390/ph17050546
Asghar Z, Jamshaid T, Jamshaid U, Madni A, Akhtar N, Lashkar MO, Gad HA. In Vivo Evaluation of Miconazole-Nitrate-Loaded Transethosomal Gel Using a Rat Model Infected with Candida albicans. Pharmaceuticals. 2024; 17(5):546. https://doi.org/10.3390/ph17050546
Chicago/Turabian StyleAsghar, Zara, Talha Jamshaid, Usama Jamshaid, Asadullah Madni, Naheed Akhtar, Manar O. Lashkar, and Heba A. Gad. 2024. "In Vivo Evaluation of Miconazole-Nitrate-Loaded Transethosomal Gel Using a Rat Model Infected with Candida albicans" Pharmaceuticals 17, no. 5: 546. https://doi.org/10.3390/ph17050546