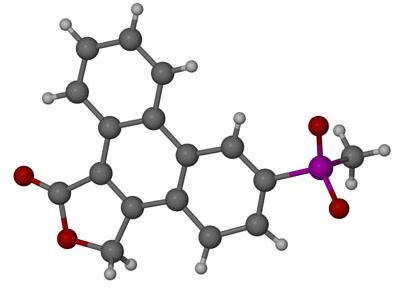
Structural Examination of 6-Methylsulphonylphenanthro- [9,10-C]-furan-1(3H)-one—A Rofecoxib Degradation Product
Abstract
:1. Introduction


2. Results and Discussion
2.1. Degradation Product Formation

2.2. Thermal Analysis

2.3. X-Ray Crystallographic Analysis
2.3.1. Unit Cell Determination
| I | Rofecoxib [9] | |
|---|---|---|
| Space group |  | P41212 |
| a | 8.4641 (1) Å | 11.374 (2) Å |
| b | 9.2247 (2) Å | 11.374 (2) Å |
| c | 9.4227 (2) Å | 22.939 (3) Å |
| α | 79.8987 (1) ° | 90 ° |
| β | 85.9977 (9) ° | 90° |
| γ | 67.5502 (2) ° | 90° |
| Volume | 669.41 (2) Å3 | 2967.6 (9) Å3 |
| Z | 2 | 8 |
2.3.2. Structure Determination and Refinement

2.3.3. Geometrical Analysis
| Donor --- H…Acceptor | Distance (Å) | Angle (°) |
|---|---|---|
| D - H H…A D…A | D - H…A | |
| C(1) --H(1A) …O(3B) i | 0.98 2.56 3.444(3) | 150 |
| C(15) --H(15) …O(12) ii | 0.95 2.54 3.168(2) | 124 |
| C(21) --H(21) …O(3B) ii | 0.95 2.50 2.904(2) | 106 |
| X--H…Cg (Pi-Ring) / Cg(A) … Cg(B) | Distance (Å) H…Cg X…Cg / Cg…Cg | Angle (°) X—H…Cg |
|---|---|---|
| C(9) --H(9B) …Cg(4) i | 2.681 3.447 | 134.4 |
| Cg(1) … Cg(3)ii | 3.554(1) | |
| Cg(1) … Cg(4)ii | 3.724(1) | |
| Cg(2) … Cg(2)iii | 3.812(2) | |
| Cg(2) … Cg(3)iii | 3.813(1) | |
| Cg(3) … Cg(3)ii | 3.596(1) | |
| Cg(4) … Cg(1)ii | 3.724(1) |

2.4. X-Ray Diffraction Powder Analysis

2.5. Mechanism

3. Experimental Section
3.1. General
3.2. Structure Determination and Crystal Data
4. Conclusions
Acknowledgements
References and Notes
- Lévesque, H.; Lafont, O. L'aspirine à travers les siècles : rappel historique. La revue de médecine interne 2000, 21, 8–17. [Google Scholar] [CrossRef]
- Celotti, F.; Laufer, S. Anti-inflammatory drugs: New multitarget compounds to face an old problem. The dual inhibition concept. Pharmacol. Res. 2001, 43, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Nicoll-Griffith, D.A.; Yergey, J.A.; Trimble, L.A.; Silva, J.M.; Li, C.; Chauret, N.; Gauthier, J.Y.; Grimm, E.; Leger, S.; Roy, P.; Therien, M.; Wang, Z.Y.; Prasit, P.; Zamboni, R.; Young, R.N.; Brideau, C.; Chan, C.C.; Mancini, J.; Riendeau, D. Synthesis, characterization, and activity of metabolites derived from the cyclooxygenase-2 inhibitor rofecoxib (MK-0966, Vioxx (TM)). Bioorg. Med. Chem. Lett. 2000, 10, 2683–2686. [Google Scholar] [PubMed]
- Rawat, S.; Jain, S.K. Rofecoxib-beta-cyclodextrin inclusion complex for solubility enhancement. Pharmazie 2003, 58, 639–641. [Google Scholar]
- Amini, M.; Hamedani, M.P.; Vosooghi, M.; Nabavi, M.; Shafiee, A. Pre-column derivatization of rofecoxib for determination in serum by HPLC. Anal. Bioanal. Chem. 2005, 382, 1265–1268. [Google Scholar]
- Nemutlu, E.; Oezaltin, N.; Altinoez, S. Determination of rofecoxib, in the presence of its photodegradation product, in pharmaceutical preparations by micellar electrokinetic capillary chromatography. Anal. Bioanal. Chem. 2004, 378, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Shehata, M.A.; Ashour, A.; Hassan, N.Y.; Fayed, A.S.; El-Zeany, B.A. Liquid chromatography and chemometric methods for determination of rofecoxib in presence of its photodegradate and alkaline degradation products. Anal. Chim. Acta 2004, 519, 23–30. [Google Scholar]
- Mao, B.; Abrahim, A.; Ge, Z.; Ellison, D.K.; Hartman, R.; Prabhu, S.V.; Reamer, R.A.; Wyvratt, J. Examination of rofecoxib solution decomposition under alkaline and photolytic stress conditions. J. Pharm. Biomed. Anal. 2002, 28, 1101–1113. [Google Scholar]
- Rekha, K.S.; Vyas, K.; Raju, C.M.H.; Chandrashekar, B.; Reddy, G.O. Viox, a COX-II inhibitor. Acta Crystallogr. C 2000, 56, E68–E68. [Google Scholar]
- Yvon, K.; Jeitschko, W.; Parthe, E. Lazy Pulverix, a Computer-Program, for Calculating X-Ray and Neutron-Diffraction Powder Patterns. J. Appl. Crystallogr. 1977, 10, 73–74. [Google Scholar] [CrossRef]
- Clayden, J.; Greeves, N.; Warren, S.; Wothers, P. Organic Chemistry; Oxford University Press: New York, NY, USA, 2001. [Google Scholar]
- Neckers, D.C.; Doyle, M.P. Organic Chemistry; John Wiley & Sons: New York, NY, USA, 1977. [Google Scholar]
- Hart, H. Organic Chemistry: A Short Course., 7th ed; Houghton Mifflin Company: Boston, MA, USA, 1987. [Google Scholar]
- Woolf, E.; Fu, I.; Matuszewski, B. Determination of rofecoxib, a cyclooxygenase-2 specific inhibitor, in human plasma using high-performance liquid chromatography with post-column photochemical derivatization and fluorescence detection. J. Chromatogr. B 1999, 730, 221–227. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dean, P.M. Structural Examination of 6-Methylsulphonylphenanthro- [9,10-C]-furan-1(3H)-one—A Rofecoxib Degradation Product. Pharmaceuticals 2010, 3, 369-378. https://doi.org/10.3390/ph3020369
Dean PM. Structural Examination of 6-Methylsulphonylphenanthro- [9,10-C]-furan-1(3H)-one—A Rofecoxib Degradation Product. Pharmaceuticals. 2010; 3(2):369-378. https://doi.org/10.3390/ph3020369
Chicago/Turabian StyleDean, Pamela M. 2010. "Structural Examination of 6-Methylsulphonylphenanthro- [9,10-C]-furan-1(3H)-one—A Rofecoxib Degradation Product" Pharmaceuticals 3, no. 2: 369-378. https://doi.org/10.3390/ph3020369
APA StyleDean, P. M. (2010). Structural Examination of 6-Methylsulphonylphenanthro- [9,10-C]-furan-1(3H)-one—A Rofecoxib Degradation Product. Pharmaceuticals, 3(2), 369-378. https://doi.org/10.3390/ph3020369