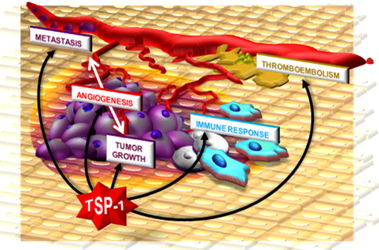
Thrombospondin-1 as a Paradigm for the Development of Antiangiogenic Agents Endowed with Multiple Mechanisms of Action
Abstract
:1. Neovascularization

2. Antiangiogenic Compounds
| Molecule | AGF bound | Reference |
|---|---|---|
| TSP-1 | FGF2, VEGF, HGF, HIV-1 Tat, TGF-β1 | [5,6,7,8], [9,10], [11], [12], [13] |
| α2-macroglobulin | FGF2, VEGF, TGF-β, IL8, TNF | [14], [15], [16], [17], [18] |
| heparin | FGF2, VEGF, HIV-1 Tat, HGF | [19], [20], [21], [22] |
| pentraxin-3 (PTX3) | FGF2, FGF8 | [23] |
| factor VII-activating protease | FGF2, PDGF | [24], [25] |
| platelet factor 4 (PF-4) | FGF2, VEGF | [26], [27] |
| SPARC | VEGF, PDGF | [28], [29] |
| CXCL13 | FGF2 | [30] |
| gangliosides | FGF2 | [31] |
| fibstatin (fibronectin fragment) | FGF2 | [32] |
| vitronectin | FGF2 | [33] |
| soluble VEGF receptor (VEGFR)-1 | VEGF | [34] |
| ADAMTS1 | VEGF | [35] |
| heparin affin regulatory peptide (HARP) | VEGF | [36] |
| connective tissue growth factor (CTGF) | VEGF | [37] |
| soluble endoglin | TGF-β1 | [38] |
| decorin | TGF-β1 | [39] |
| secretory component | IL8 | [40] |

3. Structure and Biological Activity of TSP
| MOLECULE | MECHANISM OF ACTION |
|---|---|
| inhibition of AGF expression/release by producing cells | |
| homocysteine | lowering FGF2 levels [43] |
| interleukin (IL)-12 | lowering FGF2 levels [44] |
| TSP-1 | lowering FGF2 levels [45] |
| inhibition/interference with AGF receptors on ECs | |
| IL-1, IFN-γ | TK- FGF receptors (TK-FGFR) down-regulation [46] |
| anosmin-1 | TK-FGFR occupancy [47] |
| thromboxane | inhibition of TK-FGFR1 internalization [48] |
| soluble form of TKR | formation of heterodimers with TK-FGFR1 [49] |
| antithrombin | HSPG down-regulation [50] |
| PF4 | HSPG occupancy [51], unknown [52] |
| MOLECULE | MECHANISM OF ACTION |
| endostatin | HSPG occupancy [53] |
| kallistatin | HSPG occupancy [54] |
| histidine-rich glycoprotein | HSPG occupancy [51] |
| endosulfatases | HSPG desulfation [55,56] |
| heparinase | HSPG degradation [57] |
| TSP-1 | HSPG occupancy [9], integrin occupancy [48] |
| inhibition/interference with AGF-activated second messengers in ECs | |
| heat-shock proteins 70 and 90 | down-regulation of pAkt, c-Raf-1 and ERK1/2 [58] |
| sprouty proteins | inhibition of TK-FGFR signalling [59] |
| homeobox gene GAX | inhibition of NF-kB signalling [60] |
| semaphorin-3F | inhibition of ERK1/2 signalling [61] |
| angiostatin [a plasminogen (Plg) fragment] | inhibition of ERK1/2 signalling [62] |
| ghrelin | inhibition of TKR/MAPK signalling [63] |
| lysophosphatidylcholine | inhibition of ras/ERK1/2 signalling [64] |
| pigment epithelium-derived factor | inhibition of Fyn signalling [65] |
| TSP-1 | inhibition of VEGF-mediated Akt signalling [66] |
| modification of EC apoptosis, phenotype, responsiveness to AGFs | |
| cleaved HMW kininogen | tropomyosin engagement [67] |
| IL-4 | alteration of cell cycle [68] |
| kininostatin (kininogen fragment) | inhibition of cyclin D1 expression [69] |
| vitamin D3-binding protein | CD36 engagement [70] |
| endostatin | Shb activation [71] |
| histidine-rich glycoprotein | tropomyosin engagement [72] |
| endostatin | cytoskeleton organization [73], Shb activation [71] |
| TSP-1 | TNF-α over-expression [74], CD36 engagement [66,75], apoptosis [45,66,74], ECM modification [76] |
| inhibition/interference with angiogenesis effectors | |
| IL-12 | inhibition of FGF-induced proteases [44] |
| tissue inhibitor metalloproteinase (TIMP)-2, 4 | inhibition of FGF-induced proteases [77] |
| kallistatin | inhibition of FGF-induced proteases [54] |
| TSP-1 | inhibition of FGF-induced proteases [78], binding to matrix metalloproteinase-2 (MMP-2) [79] |
| unknown mechanism of action | |
| collagen I [80], alphastatin (fibrinogen fragment) [81], CXCL14 [82], IL-12 [83], IP-10 [84], vasostatin [85], vasculostatin (fragment of brain angiogenesis inhibitor-1) [86], TGF-β1 [87], TNFs [88], somatostatin [89], retinoids [90], apolipoprotein(a) [91], prolactin (16 kDa fragment) [92] | |


3.1. Direct effects of TSP-1
3.2. Indirect effects of TSP-1
| Ligand | Binding domain in TSP-1 | Reference | ||
|---|---|---|---|---|
| free molecules (body fluids) | AGFs | FGF2 | • type III repeats | [7] |
| VEGF | • type I repeats | [37] | ||
| HGF | • 3D conformation | [11] | ||
| HIV-1 Tat | N.D. | [12] | ||
| TGF-β | • 2nd type I repeats (RFK sequence) | [132,133,134] | ||
| • type I repeats (WSXW sequence) | [133,134] | |||
| PDGF-BB | • 3D conformation | [135] | ||
| proteases and regulators | MMP-2 | • type I repeats | [136] | |
| Plg/plasmin | N.D. | [137,138,139] | ||
| tissue Plg activator | N.D. | [140] | ||
| urokinase Plg activator | N.D. | [141] | ||
| neutrophil elastase | • type III repeats | [142] | ||
| cathepsin G | • type III repeats | [142,143] | ||
| tissue factor inhibitor | N.D. | [144] | ||
| others | heparin | • N-ter domain [motifs Hep I (aa 17-35) & Hep II (aa 78-94)] | [103,145] | |
| • type I repeats | [146,147] | |||
| • signature domain | [148] | |||
| histidine-rich glycoprotein | N.D. | [149] | ||
| factor V | N.D. | [150] | ||
| angiocidin | • 2nd and 3rd type I repeats (CSVTCG sequence) | [151] | ||
| calumenin | • N-ter domain (aa 21- 228) | [152] | ||
| endostatin | N.D. | [153] | ||
| cell surface receptors | CD36 | • type I repeats | [112] | |
| CD47 | • C-ter domain | [118,122,154] | ||
| HSPGs | • N-ter domain [motifs Hep I (aa 17-35) & Hep II (aa 78-94)] | [103,145] | ||
| • signature domain | [148] | |||
| sulfated glycolipids | • N-ter domain | [155] | ||
| • 3D conformation | [155] | |||
| LRP | • N-ter domain | [126,155] | ||
| VLDL receptor | • N-ter domain | [156,157] | ||
| calreticulin | • N-ter domain (aa 17-35) | [126,158] | ||
| integrins | • N-ter domain | [107,129,159,160,161] | ||
| • type I repeats | [128,130,162] | |||
| • type III repeats (RGD sequence) | [118,163] | |||
| ECM | collagen I | N.D. | [164] | |
| collagen V | • procollagen domain + type I & II repeats | [165,166] | ||
| fibronectin | • N-ter domain + type I & II repeats | [167,168] | ||
| laminin | N.D. | [165] | ||
| fibrinogen/fibrin | • N-ter domain | [157] | ||
| • procollagen domain | [146] | |||
| • type I repeats | [169,170] | |||
| von Willebrand factor | • signature domain | [171] | ||
| dermatan sulfate | • N-ter domain (KKTR sequence) | [172] | ||
| chondroitin sulfate | • N-ter domain | [155] | ||
| IGF-binding protein-5 | • N.D. | [173] | ||

4. Therapeutic Exploitation of TSP-1 as an Antiangiogenic Agent
4.1. TSP-1 upregulation
| Molecule | References |
|---|---|
| glucose | [179] |
| peroxisome proliferator-activated receptor agonist fenofibrate | [180] |
| trichostatin-A | [181] |
| retinoic acid | [182,183] |
| somatostatin receptor subtype 2 | [10] |
| cyclic adenosine 5'-monophosphate-activated guanine nucleotide exchange factor for Rap1 | [184] |
| angiostatin | [185] |
| PHA -665752 (a small molecule, ATP-competitive inhibitor of c-Met receptor) | [186] |
| delta4-tibolone | [187] |
| phorbol 12-myristate 13-acetate | [183] |
| fibulin-5 | [188] |
| angiotensin II and its agonist CGP42112A | [189,190] |
| endostatin | [191] |
| estradiol | [192] |
| progesterone and raloxifene | [193] |
| IL-6 | [183] |
| IL-18 | [194] |
| erythropoietin | [195] |
| epidermal growth factor | [196] |
| TFG-β1, FGF2 | [197] |
| thrombin | [198] |
| inhibitors of DNA methyltransferases and histone deacetylases | [199] |
| CD26-processed chemokines CXCL12 and CCL5 | [200] |
4.2. Gene therapy

4.3. TSP-1-based peptides and peptidomimetics

4.3.1. Characterization of TSP-1 active domains and sequences
| Pro-angiogenic peptides | Mechanism | Reference |
| peptides from the N-ter domain |
| [78,103,129] |
| antiangiogenic peptides | ||
| integrin–binding sequence of the N-ter domain |
| [129] |
| sequences in the pro-collagen domain |
| [227] |
| various peptides from the second and third type I repeats |
| [228,234,235] |
| peptide from the type III repeats |
| [8] |
| peptide 4N1 in the C-ter domain |
| [122] |
4.3.2. Modifications of TSP-1-derived peptides and generation of peptidomimetics
5. Conclusions

Acknowledgements
References
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar]
- Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995, 1, 27–31. [Google Scholar] [CrossRef]
- Rusnati, M.; Presta, M. Extracellular angiogenic growth factor interactions: an angiogenesis interactome survey. Endothelium 2006, 13, 93–111. [Google Scholar]
- Presta, M.; Dell'Era, P.; Mitola, S.; Moroni, E.; Ronca, R.; Rusnati, M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005, 16, 159–178. [Google Scholar] [Green Version]
- Taraboletti, G.; Belotti, D.; Borsotti, P.; Vergani, V.; Rusnati, M.; Presta, M.; Giavazzi, R. The 140-kilodalton antiangiogenic fragment of thrombospondin-1 binds to basic fibroblast growth factor. Cell Growth Differ. 1997, 8, 471–479. [Google Scholar]
- Margosio, B.; Marchetti, D.; Vergani, V.; Giavazzi, R.; Rusnati, M.; Presta, M.; Taraboletti, G. Thrombospondin 1 as a scavenger for matrix-associated fibroblast growth factor 2. Blood 2003, 102, 4399–4406. [Google Scholar]
- Margosio, B.; Rusnati, M.; Bonezzi, K.; Cordes, B.L.; Annis, D.S.; Urbinati, C.; Giavazzi, R.; Presta, M.; Ribatti, D.; Mosher, D.F.; Taraboletti, G. Fibroblast growth factor-2 binding to the thrombospondin-1 type III repeats, a novel antiangiogenic domain. Int. J. Biochem. Cell Biol. 2008, 40, 700–709. [Google Scholar]
- Colombo, G.; Margosio, B.; Ragona, L.; Neves, M.; Bonifacio, S.; Annis, D.S.; Stravalaci, M.; Tomaselli, S.; Giavazzi, R.; Rusnati, M.; Presta, M.; Zetta, L.; Mosher, D.F.; Ribatti, D.; Gobbi, M.; Taraboletti, G. Non-peptidic thrombospondin-1-mimics as fibroblast growth factor-2 inhibitors: an integrated strategy for the development of new antiangiogenic compounds. J. Biol. Chem. 2010, in press. [Google Scholar]
- Gupta, K.; Gupta, P.; Wild, R.; Ramakrishnan, S.; Hebbel, R.P. Binding and displacement of vascular endothelial growth factor (VEGF) by thrombospondin: effect on human microvascular endothelial cell proliferation and angiogenesis. Angiogenesis 1999, 3, 147–158. [Google Scholar]
- Laklai, H.; Laval, S.; Dumartin, L.; Rochaix, P.; Hagedorn, M.; Bikfalvi, A.; Le Guellec, S.; Delisle, M.B.; Schally, A.V.; Susini, C.; Pyronnet, S.; Bousquet, C. Thrombospondin-1 is a critical effector of oncosuppressive activity of sst2 somatostatin receptor on pancreatic cancer. Proc. Natl. Acad. Sci. USA 2009, 106, 17769–17774. [Google Scholar]
- Lamszus, K.; Joseph, A.; Jin, L.; Yao, Y.; Chowdhury, S.; Fuchs, A.; Polverini, P.J.; Goldberg, I.D.; Rosen, E.M. Scatter factor binds to thrombospondin and other extracellular matrix components. Am. J. Pathol. 1996, 149, 805–819. [Google Scholar]
- Rusnati, M.; Taraboletti, G.; Urbinati, C.; Tulipano, G.; Giuliani, R.; Molinari-Tosatti, M.P.; Sennino, B.; Giacca, M.; Tyagi, M.; Albini, A.; Noonan, D.; Giavazzi, R.; Presta, M. Thrombospondin-1/HIV-1 tat protein interaction: modulation of the biological activity of extracellular Tat. FASEB J. 2000, 14, 1917–1930. [Google Scholar]
- Murphy-Ullrich, J.E.; Schultz-Cherry, S.; Hook, M. Transforming growth factor-beta complexes with thrombospondin. Mol. Biol. Cell 1992, 3, 181–188. [Google Scholar]
- Asplin, I.R.; Wu, S.M.; Mathew, S.; Bhattacharjee, G.; Pizzo, S.V. Differential regulation of the fibroblast growth factor (FGF) family by alpha(2)-macroglobulin: evidence for selective modulation of FGF-2-induced angiogenesis. Blood 2001, 97, 3450–3457. [Google Scholar]
- Bhattacharjee, G.; Asplin, I.R.; Wu, S.M.; Gawdi, G.; Pizzo, S.V. The conformation-dependent interaction of alpha 2-macroglobulin with vascular endothelial growth factor. A novel mechanism of alpha 2-macroglobulin/growth factor binding. J. Biol. Chem. 2000, 275, 26806–26811. [Google Scholar]
- Feige, J.J.; Negoescu, A.; Keramidas, M.; Souchelnitskiy, S.; Chambaz, E.M. Alpha 2-macroglobulin: a binding protein for transforming growth factor-beta and various cytokines. Horm. Res. 1996, 45, 227–232. [Google Scholar]
- Kurdowska, A.; Alden, S.M.; Noble, J.M.; Stevens, M.D.; Carr, F.K. Involvement of alpha-2-macroglobulin receptor in clearance of interleukin 8-alpha-2-macroglobulin complexes by human alveolar macrophages. Cytokine 2000, 12, 1046–1053. [Google Scholar]
- LaMarre, J.; Wollenberg, G.K.; Gonias, S.L.; Hayes, M.A. Cytokine binding and clearance properties of proteinase-activated alpha 2-macroglobulins. Lab. Invest. 1991, 65, 3–14. [Google Scholar]
- Rusnati, M.; Presta, M. Interaction of angiogenic basic fibroblast growth factor with endothelial cell heparan sulfate proteoglycans. Biological implications in neovascularization. Int. J. Clin. Lab. Res. 1996, 26, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Norrby, K. 2.5 kDa and 5.0 kDa heparin fragments specifically inhibit microvessel sprouting and network formation in VEGF165-mediated mammalian angiogenesis. Int. J. Exp. Pathol. 2000, 81, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Rusnati, M.; Coltrini, D.; Oreste, P.; Zoppetti, G.; Albini, A.; Noonan, D.; d'Adda di Fagagna, F.; Giacca, M.; Presta, M. Interaction of HIV-1 Tat protein with heparin. Role of the backbone structure, sulfation, and size. J. Biol. Chem. 1997, 272, 11313–11320. [Google Scholar] [PubMed]
- Lietha, D.; Chirgadze, D.Y.; Mulloy, B.; Blundell, T.L.; Gherardi, E. Crystal structures of NK1-heparin complexes reveal the basis for NK1 activity and enable engineering of potent agonists of the MET receptor. Embo. J. 2001, 20, 5543–5555. [Google Scholar]
- Rusnati, M.; Camozzi, M.; Moroni, E.; Bottazzi, B.; Peri, G.; Indraccolo, S.; Amadori, A.; Mantovani, A.; Presta, M. Selective recognition of fibroblast growth factor-2 by the long pentraxin PTX3 inhibits angiogenesis. Blood 2004, 104, 92–99. [Google Scholar]
- Shibamiya, A.; Muhl, L.; Tannert-Otto, S.; Preissner, K.T.; Kanse, S.M. Nucleic acids potentiate Factor VII-activating protease (FSAP)-mediated cleavage of platelet-derived growth factor-BB and inhibition of vascular smooth muscle cell proliferation. Biochem.J. 2007, 404, 45–50. [Google Scholar]
- Etscheid, M.; Beer, N.; Kress, J.A.; Seitz, R.; Dodt, J. Inhibition of bFGF/EGF-dependent endothelial cell proliferation by the hyaluronan-binding protease from human plasma. Eur. J. Cell Biol. 2004, 82, 597–604. [Google Scholar]
- Lozano, R.M.; Redondo-Horcajo, M.; Jimenez, M.A.; Zilberberg, L.; Cuevas, P.; Bikfalvi, A.; Rico, M.; Gimenez-Gallego, G. Solution structure and interaction with basic and acidic fibroblast growth factor of a 3-kDa human platelet factor-4 fragment with antiangiogenic activity. J. Biol. Chem. 2001, 276, 35723–35734. [Google Scholar]
- Bikfalvi, A. Platelet factor 4: an inhibitor of angiogenesis. Semin. Thromb. Hemost. 2004, 30, 379–385. [Google Scholar]
- Kupprion, C.; Motamed, K.; Sage, E.H. SPARC (BM-40, osteonectin) inhibits the mitogenic effect of vascular endothelial growth factor on microvascular endothelial cells. J. Biol. Chem. 1998, 273, 29635–29640. [Google Scholar]
- Raines, E.W.; Lane, T.F.; Iruela-Arispe, M.L.; Ross, R.; Sage, E.H. The extracellular glycoprotein SPARC interacts with platelet-derived growth factor (PDGF)-AB and -BB and inhibits the binding of PDGF to its receptors. Proc. Natl. Acad. Sci. USA 1992, 89, 1281–1285. [Google Scholar]
- Spinetti, G.; Camarda, G.; Bernardini, G.; Romano Di Peppe, S.; Capogrossi, M.C.; Napolitano, M. The chemokine CXCL13 (BCA-1) inhibits FGF-2 effects on endothelial cells. Biochem. Biophys. Res. Commun. 2001, 289, 19–24. [Google Scholar]
- Rusnati, M.; Tanghetti, E.; Urbinati, C.; Tulipano, G.; Marchesini, S.; Ziche, M.; Presta, M. Interaction of fibroblast growth factor-2 (FGF-2) with free gangliosides: biochemical characterization and biological consequences in endothelial cell cultures. Mol. Biol. Cell 1999, 10, 313–327. [Google Scholar]
- Bossard, C.; Van den Berghe, L.; Laurell, H.; Castano, C.; Cerutti, M.; Prats, A.C.; Prats, H. Antiangiogenic properties of fibstatin, an extracellular FGF-2-binding polypeptide. Cancer Res. 2004, 64, 7507–7512. [Google Scholar]
- Hollier, B.; Harkin, D.G.; Leavesley, D.; Upton, Z. Responses of keratinocytes to substrate-bound vitronectin: growth factor complexes. Exp. Cell Res. 2005, 305, 221–232. [Google Scholar]
- Shibuya, M. Vascular endothelial growth factor receptor-1 (VEGFR-1/Flt-1): a dual regulator for angiogenesis. Angiogenesis 2006, 9, 225–230; discussion 231. [Google Scholar] [CrossRef] [PubMed]
- Luque, A.; Carpizo, D.R.; Iruela-Arispe, M.L. ADAMTS1/METH1 inhibits endothelial cell proliferation by direct binding and sequestration of VEGF165. J. Biol. Chem. 2003, 278, 23656–23665. [Google Scholar]
- Heroult, M.; Bernard-Pierrot, I.; Delbe, J.; Hamma-Kourbali, Y.; Katsoris, P.; Barritault, D.; Papadimitriou, E.; Plouet, J.; Courty, J. Heparin affin regulatory peptide binds to vascular endothelial growth factor (VEGF) and inhibits VEGF-induced angiogenesis. Oncogene 2004, 23, 1745–1753. [Google Scholar]
- Inoki, I.; Shiomi, T.; Hashimoto, G.; Enomoto, H.; Nakamura, H.; Makino, K.; Ikeda, E.; Takata, S.; Kobayashi, K.; Okada, Y. Connective tissue growth factor binds vascular endothelial growth factor (VEGF) and inhibits VEGF-induced angiogenesis. Faseb J. 2002, 16, 219–221. [Google Scholar]
- Venkatesha, S.; Toporsian, M.; Lam, C.; Hanai, J.; Mammoto, T.; Kim, Y.M.; Bdolah, Y.; Lim, K.H.; Yuan, H.T.; Libermann, T.A.; Stillman, I.E.; Roberts, D.; D'Amore, P.A.; Epstein, F.H.; Sellke, F.W.; Romero, R.; Sukhatme, V.P.; Letarte, M.; Karumanchi, S.A. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat. Med. 2006, 12, 642–649. [Google Scholar]
- O'Connor-McCourt, M.D.; Wakefield, L.M. Latent transforming growth factor-beta in serum. A specific complex with alpha 2-macroglobulin. J. Biol. Chem. 1987, 262, 14090–14099. [Google Scholar] [PubMed]
- Kemeny, L.; Szolnoky, G.; Kenderessy, A.S.; Gyulai, R.; Kiss, M.; Michel, G.; Nagy, K.; Ruzicka, T.; Dobozy, A. Identification of a soluble interleukin-8 inhibitor in the supernatant of polymorphonuclear leukocytes. Immunol. Lett. 1998, 64, 23–29. [Google Scholar]
- Eggert, A.; Ikegaki, N.; Kwiatkowski, J.; Zhao, H.; Brodeur, G.M.; Himelstein, B.P. High-level expression of angiogenic factors is associated with advanced tumor stage in human neuroblastomas. Clin. Cancer Res. 2000, 6, 1900–1908. [Google Scholar]
- Barthlen, W.; Flaadt, D.; Girgert, R.; Conzelmann, J.; Schweizer, P.; Zugmaier, G.; Buck, M.; Knabbe, C. Significance of heparin-binding growth factor expression on cells of solid pediatric tumors. J. Pediatr. Surg. 2003, 38, 1296–1304. [Google Scholar]
- Chang, P.Y.; Lu, S.C.; Lee, C.M.; Chen, Y.J.; Dugan, T.A.; Huang, W.H.; Chang, S.F.; Liao, W.S.; Chen, C.H.; Lee, Y.T. Homocysteine inhibits arterial endothelial cell growth through transcriptional downregulation of fibroblast growth factor-2 involving G protein and DNA methylation. Circ. Res. 2008, 102, 933–941. [Google Scholar]
- Meeran, S.M.; Katiyar, S.; Elmets, C.A.; Katiyar, S.K. Interleukin-12 deficiency is permissive for angiogenesis in UV radiation-induced skin tumors. Cancer Res. 2007, 67, 3785–3793. [Google Scholar]
- Zak, S.; Treven, J.; Nash, N.; Gutierrez, L.S. Lack of thrombospondin-1 increases angiogenesis in a model of chronic inflammatory bowel disease. Int. J. Colorectal Dis. 2008, 23, 297–304. [Google Scholar]
- Norioka, K.; Mitaka, T.; Mochizuki, Y.; Hara, M.; Kawagoe, M.; Nakamura, H. Interaction of interleukin-1 and interferon-gamma on fibroblast growth factor-induced angiogenesis. Jpn. J. Cancer Res. 1994, 85, 522–529. [Google Scholar]
- Hu, Y.; Guimond, S.E.; Travers, P.; Cadman, S.; Hohenester, E.; Turnbull, J.E.; Kim, S.H.; Bouloux, P.M. Novel mechanisms of fibroblast growth factor receptor 1 regulation by extracellular matrix protein anosmin-1. J. Biol. Chem. 2009, 284, 29905–29920. [Google Scholar]
- Ashton, A.W.; Cheng, Y.; Helisch, A.; Ware, J.A. Thromboxane A2 receptor agonists antagonize the proangiogenic effects of fibroblast growth factor-2: role of receptor internalization, thrombospondin-1, and alpha(v)beta3. Circ. Res. 2004, 94, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Gunn, M.; Dell, K.; Tseng, A., Jr.; Williams, L. A truncated form of fibroblast growth factor receptor 1 inhibits signal transduction by multiple types of fibroblast growth factor receptor. J. Biol. Chem. 1992, 267, 1470–1476. [Google Scholar]
- Zhang, W.; Chuang, Y.J.; Swanson, R.; Li, J.; Seo, K.; Leung, L.; Lau, L.F.; Olson, S.T. Antiangiogenic antithrombin down-regulates the expression of the proangiogenic heparan sulfate proteoglycan, perlecan, in endothelial cells. Blood 2004, 103, 1185–1191. [Google Scholar] [PubMed]
- Brown, K.J.; Parish, C.R. Histidine-rich glycoprotein and platelet factor 4 mask heparan sulfate proteoglycans recognized by acidic and basic fibroblast growth factor. Biochemistry 1994, 33, 13918–13927. [Google Scholar]
- Sulpice, E.; Bryckaert, M.; Lacour, J.; Contreres, J.O.; Tobelem, G. Platelet factor 4 inhibits FGF2-induced endothelial cell proliferation via the extracellular signal-regulated kinase pathway but not by the phosphatidylinositol 3-kinase pathway. Blood 2002, 100, 3087–3094. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, P.; Xie, L.; Kalluri, R. Endogenous inhibitors of angiogenesis. Cancer Res. 2005, 65, 3967–3979. [Google Scholar]
- Miao, R.Q.; Agata, J.; Chao, L.; Chao, J. Kallistatin is a new inhibitor of angiogenesis and tumor growth. Blood 2002, 100, 3245–3252. [Google Scholar]
- Wang, S.; Ai, X.; Freeman, S.D.; Pownall, M.E.; Lu, Q.; Kessler, D.S.; Emerson, C.P., Jr. QSulf1, a heparan sulfate 6-O-endosulfatase, inhibits fibroblast growth factor signaling in mesoderm induction and angiogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 4833–4838. [Google Scholar]
- Lai, J.P.; Sandhu, D.S.; Shire, A.M.; Roberts, L.R. The tumor suppressor function of human sulfatase 1 (SULF1) in carcinogenesis. J. Gastrointest. Cancer 2008, 39, 149–158. [Google Scholar]
- Chua, C.C.; Rahimi, N.; Forsten-Williams, K.; Nugent, M.A. Heparan sulfate proteoglycans function as receptors for fibroblast growth factor-2 activation of extracellular signal-regulated kinases 1 and 2. Circ. Res. 2004, 94, 316–323. [Google Scholar]
- Kaur, G.; Belotti, D.; Burger, A.M.; Fisher-Nielson, K.; Borsotti, P.; Riccardi, E.; Thillainathan, J.; Hollingshead, M.; Sausville, E.A.; Giavazzi, R. Antiangiogenic properties of 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin: an orally bioavailable heat shock protein 90 modulator. Clin. Cancer Res. 2004, 10, 4813–4821. [Google Scholar]
- Hanafusa, H.; Torii, S.; Yasunaga, T.; Nishida, E. Sprouty1 and Sprouty2 provide a control mechanism for the Ras/MAPK signalling pathway. Nat. Cell Biol. 2002, 4, 850–858. [Google Scholar]
- Patel, S.; Leal, A.D.; Gorski, D.H. The homeobox gene Gax inhibits angiogenesis through inhibition of nuclear factor-kappaB-dependent endothelial cell gene expression. Cancer Res. 2005, 65, 1414–1424. [Google Scholar]
- Kessler, O.; Shraga-Heled, N.; Lange, T.; Gutmann-Raviv, N.; Sabo, E.; Baruch, L.; Machluf, M.; Neufeld, G. Semaphorin-3F is an inhibitor of tumor angiogenesis. Cancer Res. 2004, 64, 1008–1015. [Google Scholar]
- Redlitz, A.; Daum, G.; Sage, E.H. Angiostatin diminishes activation of the mitogen-activated protein kinases ERK-1 and ERK-2 in human dermal microvascular endothelial cells. J. Vasc. Res. 1999, 36, 28–34. [Google Scholar]
- Baiguera, S.; Conconi, M.T.; Guidolin, D.; Mazzocchi, G.; Malendowicz, L.K.; Parnigotto, P.P.; Spinazzi, R.; Nussdorfer, G.G. Ghrelin inhibits in vitro angiogenic activity of rat brain microvascular endothelial cells. Int. J. Mol. Med. 2004, 14, 849–854. [Google Scholar] [PubMed]
- Rikitake, Y.; Kawashima, S.; Yamashita, T.; Ueyama, T.; Ishido, S.; Hotta, H.; Hirata, K.; Yokoyama, M. Lysophosphatidylcholine inhibits endothelial cell migration and proliferation via inhibition of the extracellular signal-regulated kinase pathway. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Kanda, S.; Mochizuki, Y.; Nakamura, T.; Miyata, Y.; Matsuyama, T.; Kanetake, H. Pigment epithelium-derived factor inhibits fibroblast-growth-factor-2-induced capillary morphogenesis of endothelial cells through Fyn. J. Cell Sci. 2005, 118, 961–970. [Google Scholar]
- Sun, J.; Hopkins, B.D.; Tsujikawa, K.; Perruzzi, C.; Adini, I.; Swerlick, R.; Bornstein, P.; Lawler, J.; Benjamin, L.E. Thrombospondin-1 modulates VEGF-A-mediated Akt signaling and capillary survival in the developing retina. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1344–H1351. [Google Scholar]
- Zhang, J.C.; Donate, F.; Qi, X.; Ziats, N.P.; Juarez, J.C.; Mazar, A.P.; Pang, Y.P.; McCrae, K.R. The antiangiogenic activity of cleaved high molecular weight kininogen is mediated through binding to endothelial cell tropomyosin. Proc. Natl. Acad. Sci. USA 2002, 99, 12224–12229. [Google Scholar]
- Kim, J.; Cheon, I.S.; Won, Y.J.; Na, H.J.; Kim, Y.M.; Choe, J. IL-4 inhibits cell cycle progression of human umbilical vein endothelial cells by affecting p53, p21(Waf1), cyclin D1, and cyclin E expression. Mol. Cells 2003, 16, 92–96. [Google Scholar] [PubMed]
- Guo, Y.L.; Wang, S.; Colman, R.W. Kininostatin, an angiogenic inhibitor, inhibits proliferation and induces apoptosis of human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Kanda, S.; Mochizuki, Y.; Miyata, Y.; Kanetake, H.; Yamamoto, N. Effects of vitamin D(3)-binding protein-derived macrophage activating factor (GcMAF) on angiogenesis. J. Natl. Cancer Inst. 2002, 94, 1311–1319. [Google Scholar]
- Dixelius, J.; Larsson, H.; Sasaki, T.; Holmqvist, K.; Lu, L.; Engstrom, A.; Timpl, R.; Welsh, M.; Claesson-Welsh, L. Endostatin-induced tyrosine kinase signaling through the Shb adaptor protein regulates endothelial cell apoptosis. Blood 2000, 95, 3403–3411. [Google Scholar]
- Guan, X.; Juarez, J.C.; Qi, X.; Shipulina, N.V.; Shaw, D.E.; Morgan, W.T.; McCrae, K.R.; Mazar, A.P.; Donate, F. Histidine-proline rich glycoprotein (HPRG) binds and transduces anti-angiogenic signals through cell surface tropomyosin on endothelial cells. Thromb. Haemost. 2004, 92, 403–412. [Google Scholar]
- Dixelius, J.; Cross, M.; Matsumoto, T.; Sasaki, T.; Timpl, R.; Claesson-Welsh, L. Endostatin regulates endothelial cell adhesion and cytoskeletal organization. Cancer Res. 2002, 62, 1944–1947. [Google Scholar]
- Rege, T.A.; Stewart, J., Jr.; Dranka, B.; Benveniste, E.N.; Silverstein, R.L.; Gladson, C.L. Thrombospondin-1-induced apoptosis of brain microvascular endothelial cells can be mediated by TNF-R1. J. Cell Physiol. 2009, 218, 94–103. [Google Scholar]
- Dawson, D.W.; Volpert, O.V.; Pearce, S.F.; Schneider, A.J.; Silverstein, R.L.; Henkin, J.; Bouck, N.P. Three distinct D-amino acid substitutions confer potent antiangiogenic activity on an inactive peptide derived from a thrombospondin-1 type 1 repeat. Mol. Pharmacol. 1999, 55, 332–338. [Google Scholar]
- Zhou, L.; Isenberg, J.S.; Cao, Z.; Roberts, D.D. Type I collagen is a molecular target for inhibition of angiogenesis by endogenous thrombospondin-1. Oncogene 2006, 25, 536–545. [Google Scholar]
- Lafleur, M.A.; Handsley, M.M.; Knauper, V.; Murphy, G.; Edwards, D.R. Endothelial tubulogenesis within fibrin gels specifically requires the activity of membrane-type-matrix metalloproteinases (MT-MMPs). J. Cell Sci. 2002, 115, 3427–3438. [Google Scholar]
- Donnini, S.; Morbidelli, L.; Taraboletti, G.; Ziche, M. ERK1-2 and p38 MAPK regulate MMP/TIMP balance and function in response to thrombospondin-1 fragments in the microvascular endothelium. Life Sci. 2004, 74, 2975–2985. [Google Scholar]
- Fears, C.Y.; Grammer, J.R.; Stewart, J.E., Jr.; Annis, D.S.; Mosher, D.F.; Bornstein, P.; Gladson, C.L. Low-density lipoprotein receptor-related protein contributes to the antiangiogenic activity of thrombospondin-2 in a murine glioma model. Cancer Res. 2005, 65, 9338–9346. [Google Scholar]
- Kroon, M.E.; van Schie, M.L.; van der Vecht, B.; van Hinsbergh, V.W.; Koolwijk, P. Collagen type 1 retards tube formation by human microvascular endothelial cells in a fibrin matrix. Angiogenesis 2002, 5, 257–265. [Google Scholar]
- Staton, C.A.; Brown, N.J.; Rodgers, G.R.; Corke, K.P.; Tazzyman, S.; Underwood, J.C.; Lewis, C.E. Alphastatin, a 24-amino acid fragment of human fibrinogen, is a potent new inhibitor of activated endothelial cells in vitro and in vivo. Blood 2004, 103, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Shellenberger, T.D.; Wang, M.; Gujrati, M.; Jayakumar, A.; Strieter, R.M.; Burdick, M.D.; Ioannides, C.G.; Efferson, C.L.; El-Naggar, A.K.; Roberts, D.; Clayman, G.L.; Frederick, M.J. BRAK/CXCL14 is a potent inhibitor of angiogenesis and a chemotactic factor for immature dendritic cells. Cancer Res. 2004, 64, 8262–8270. [Google Scholar]
- Sgadari, C.; Angiolillo, A.L.; Tosato, G. Inhibition of angiogenesis by interleukin-12 is mediated by the interferon-inducible protein 10. Blood 1996, 87, 3877–3882. [Google Scholar]
- Angiolillo, A.L.; Sgadari, C.; Taub, D.D.; Liao, F.; Farber, J.M.; Maheshwari, S.; Kleinman, H.K.; Reaman, G.H.; Tosato, G. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J. Exp. Med. 1995, 182, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Pike, S.E.; Yao, L.; Jones, K.D.; Cherney, B.; Appella, E.; Sakaguchi, K.; Nakhasi, H.; Teruya-Feldstein, J.; Wirth, P.; Gupta, G.; Tosato, G. Vasostatin, a calreticulin fragment, inhibits angiogenesis and suppresses tumor growth. J. Exp. Med. 1998, 188, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Brat, D.J.; Devi, N.S.; Van Meir, E.G. Vasculostatin, a proteolytic fragment of Brain Angiogenesis Inhibitor 1, is an antiangiogenic and antitumorigenic factor. Oncogene 2005.
- Pepper, M.S.; Belin, D.; Montesano, R.; Orci, L.; Vassalli, J.D. Transforming growth factor-beta 1 modulates basic fibroblast growth factor-induced proteolytic and angiogenic properties of endothelial cells in vitro. J. Cell Biol. 1990, 111, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Nariuchi, H.; Tsuruoka, N.; Nishihara, T.; Beitz, J.G.; Calabresi, P.; Frackelton, A.R., Jr. Actions of TNF and IFN-gamma on angiogenesis in vitro. J. Invest. Dermatol. 1990, 95, 85S–89S. [Google Scholar] [PubMed]
- Grant, M.B.; Caballero, S.; Millard, W.J. Inhibition of IGF-I and b-FGF stimulated growth of human retinal endothelial cells by the somatostatin analogue, octreotide: a potential treatment for ocular neovascularization. Regul. Pept. 1993, 48, 267–278. [Google Scholar]
- Ribatti, D.; Alessandri, G.; Baronio, M.; Raffaghello, L.; Cosimo, E.; Marimpietri, D.; Montaldo, P.G.; De Falco, G.; Caruso, A.; Vacca, A.; Ponzoni, M. Inhibition of neuroblastoma-induced angiogenesis by fenretinide. Int. J. Cancer. 2001, 94, 314–321. [Google Scholar]
- Schulter, V.; Koolwijk, P.; Peters, E.; Frank, S.; Hrzenjak, A.; Graier, W.F.; van Hinsbergh, V.W.; Kostner, G.M. Impact of apolipoprotein(a) on in vitro angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Duenas, Z.; Torner, L.; Corbacho, A.M.; Ochoa, A.; Gutierrez-Ospina, G.; Lopez-Barrera, F.; Barrios, F.A.; Berger, P.; Martinez de la Escalera, G.; Clapp, C. Inhibition of rat corneal angiogenesis by 16-kDa prolactin and by endogenous prolactin-like molecules. Invest. Ophthalmol. Vis. Sci. 1999, 40, 2498–2505. [Google Scholar]
- Russo, K.; Ragone, R.; Facchiano, A.M.; Capogrossi, M.C.; Facchiano, A. Platelet-derived growth factor-BB and basic fibroblast growth factor directly interact in vitro with high affinity. J. Biol. Chem. 2002, 277, 1284–1291. [Google Scholar] [PubMed]
- Tucker, R.P. The thrombospondin type 1 repeat superfamily. Int. J. Biochem. Cell Biol. 2004, 36, 969–974. [Google Scholar]
- Karagiannis, E.D.; Popel, A.S. A systematic methodology for proteome-wide identification of peptides inhibiting the proliferation and migration of endothelial cells. Proc. Natl. Acad. Sci. USA 2008, 105, 13775–13780. [Google Scholar]
- Adams, J.C. Functions of the conserved thrombospondin carboxy-terminal cassette in cell-extracellular matrix interactions and signaling. Int. J. Biochem. Cell Biol. 2004, 36, 1102–1114. [Google Scholar]
- Kvansakul, M.; Adams, J.C.; Hohenester, E. Structure of a thrombospondin C-terminal fragment reveals a novel calcium core in the type 3 repeats. Embo. J. 2004, 23, 1223–1233. [Google Scholar]
- Hogg, P.J. Thrombospondin 1 as an enzyme inhibitor. Thromb. Haemost. 1994, 72, 787–792. [Google Scholar]
- Carlson, C.B.; Bernstein, D.A.; Annis, D.S.; Misenheimer, T.M.; Hannah, B.L.; Mosher, D.F.; Keck, J.L. Structure of the calcium-rich signature domain of human thrombospondin-2. Nat. Struct. Mol Biol. 2005, 12, 910–914. [Google Scholar]
- Iruela-Arispe, M.L.; Lombardo, M.; Krutzsch, H.C.; Lawler, J.; Roberts, D.D. Inhibition of angiogenesis by thrombospondin-1 is mediated by 2 independent regions within the type 1 repeats. Circulation 1999, 100, 1423–1431. [Google Scholar]
- Camozzi, M.; Rusnati, M.; Bugatti, A.; Bottazzi, B.; Mantovani, A.; Bastone, A.; Inforzato, A.; Vincenti, S.; Bracci, L.; Mastroianni, D.; Presta, M. Identification of an antiangiogenic FGF2-binding site in the N terminus of the soluble pattern recognition receptor PTX3. J. Biol. Chem. 2006, 281, 22605–22613. [Google Scholar]
- Motegi, K.; Harada, K.; Ohe, G.; Jones, S.J.; Ellis, I.R.; Crouch, D.H.; Schor, S.L.; Schor, A.M. Differential involvement of TGF-beta1 in mediating the motogenic effects of TSP-1 on endothelial cells, fibroblasts and oral tumour cells. Exp. Cell Res. 2008, 314, 2323–2333. [Google Scholar]
- Taraboletti, G.; Morbidelli, L.; Donnini, S.; Parenti, A.; Granger, H.J.; Giavazzi, R.; Ziche, M. The heparin binding 25 kDa fragment of thrombospondin-1 promotes angiogenesis and modulates gelatinase and TIMP-2 production in endothelial cells. Faseb J. 2000, 14, 1674–1676. [Google Scholar]
- Ferrari do Outeiro-Bernstein, M.A.; Nunes, S.S.; Andrade, A.C.; Alves, T.R.; Legrand, C.; Morandi, V. A recombinant NH(2)-terminal heparin-binding domain of the adhesive glycoprotein, thrombospondin-1, promotes endothelial tube formation and cell survival: a possible role for syndecan-4 proteoglycan. Matrix Biol. 2002, 21, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Murphy-Ullrich, J.E.; Gurusiddappa, S.; Frazier, W.A.; Hook, M. Heparin-binding peptides from thrombospondins 1 and 2 contain focal adhesion-labilizing activity. J. Biol. Chem. 1993, 268, 26784–26789. [Google Scholar]
- Adams, J.C.; Bentley, A.A.; Kvansakul, M.; Hatherley, D.; Hohenester, E. Extracellular matrix retention of thrombospondin 1 is controlled by its conserved C-terminal region. J. Cell Sci. 2008, 121, 784–795. [Google Scholar]
- Calzada, M.J.; Sipes, J.M.; Krutzsch, H.C.; Yurchenco, P.D.; Annis, D.S.; Mosher, D.F.; Roberts, D.D. Recognition of the N-terminal modules of thrombospondin-1 and thrombospondin-2 by alpha6beta1 integrin. J. Biol. Chem. 2003, 278, 40679–40687. [Google Scholar]
- Vogel, T.; Guo, N.H.; Krutzsch, H.C.; Blake, D.A.; Hartman, J.; Mendelovitz, S.; Panet, A.; Roberts, D.D. Modulation of endothelial cell proliferation, adhesion, and motility by recombinant heparin-binding domain and synthetic peptides from the type I repeats of thrombospondin. J. Cell Biochem. 1993, 53, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, P. Thrombospondins function as regulators of angiogenesis. J. Cell Commun. Signal 2009, 189–200. [Google Scholar]
- Asch, A.S.; Barnwell, J.; Silverstein, R.L.; Nachman, R.L. Isolation of the thrombospondin membrane receptor. J. Clin. Invest. 1987, 79, 1054–1061. [Google Scholar]
- Swerlick, R.A.; Lee, K.H.; Wick, T.M.; Lawley, T.J. Human dermal microvascular endothelial but not human umbilical vein endothelial cells express CD36 in vivo and in vitro. J. Immunol. 1992, 148, 78–83. [Google Scholar] [PubMed]
- Dawson, D.W.; Pearce, S.F.; Zhong, R.; Silverstein, R.L.; Frazier, W.A.; Bouck, N.P. CD36 mediates the In vitro inhibitory effects of thrombospondin-1 on endothelial cells. J. Cell Biol. 1997, 138, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Primo, L.; Ferrandi, C.; Roca, C.; Marchio, S.; di Blasio, L.; Alessio, M.; Bussolino, F. Identification of CD36 molecular features required for its in vitro angiostatic activity. FASEB J. 2005, 19, 1713–1715. [Google Scholar] [PubMed]
- Zhang, X.; Kazerounian, S.; Duquette, M.; Perruzzi, C.; Nagy, J.A.; Dvorak, H.F.; Parangi, S.; Lawler, J. Thrombospondin-1 modulates vascular endothelial growth factor activity at the receptor level. FASEB J. 2009, 23, 3368–3376. [Google Scholar]
- Isenberg, J.S.; Martin-Manso, G.; Maxhimer, J.B.; Roberts, D.D. Regulation of nitric oxide signalling by thrombospondin 1: implications for anti-angiogenic therapies. Nat. Rev. Cancer 2009, 9, 182–194. [Google Scholar]
- Jimenez, B.; Volpert, O.V.; Crawford, S.E.; Febbraio, M.; Silverstein, R.L.; Bouck, N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat. Med. 2000, 6, 41–48. [Google Scholar]
- Li, K.; Yang, M.; Yuen, P.M.; Chik, K.W.; Li, C.K.; Shing, M.M.; Lam, H.K.; Fok, T.F. Thrombospondin-1 induces apoptosis in primary leukemia and cell lines mediated by CD36 and Caspase-3. Int. J. Mol. Med. 2003, 12, 995–1001. [Google Scholar]
- Saumet, A.; Slimane, M.B.; Lanotte, M.; Lawler, J.; Dubernard, V. Type 3 repeat/C-terminal domain of thrombospondin-1 triggers caspase-independent cell death through CD47/alphavbeta3 in promyelocytic leukemia NB4 cells. Blood 2005, 106, 658–667. [Google Scholar]
- Gao, A.G.; Lindberg, F.P.; Dimitry, J.M.; Brown, E.J.; Frazier, W.A. Thrombospondin modulates alpha v beta 3 function through integrin-associated protein. J. Cell Biol. 1996, 135, 533–544. [Google Scholar]
- Gao, A.G.; Lindberg, F.P.; Finn, M.B.; Blystone, S.D.; Brown, E.J.; Frazier, W.A. Integrin-associated protein is a receptor for the C-terminal domain of thrombospondin. J. Biol. Chem. 1996, 271, 21–24. [Google Scholar]
- Brown, E.J.; Frazier, W.A. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 2001, 11, 130–135. [Google Scholar]
- Kanda, S.; Shono, T.; Tomasini-Johansson, B.; Klint, P.; Saito, Y. Role of thrombospondin-1-derived peptide, 4N1K, in FGF-2-induced angiogenesis. Exp. Cell Res. 1999, 252, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.S.; Outeiro-Bernstein, M.A.; Juliano, L.; Vardiero, F.; Nader, H.B.; Woods, A.; Legrand, C.; Morandi, V. Syndecan-4 contributes to endothelial tubulogenesis through interactions with two motifs inside the pro-angiogenic N-terminal domain of thrombospondin-1. J Cell Physiol. 2008, 214, 828–837. [Google Scholar]
- Oganesian, A.; Armstrong, L.C.; Migliorini, M.M.; Strickland, D.K.; Bornstein, P. Thrombospondins use the VLDL receptor and a nonapoptotic pathway to inhibit cell division in microvascular endothelial cells. Mol. Biol. Cell 2008, 19, 563–571. [Google Scholar]
- Orr, A.W.; Pedraza, C.E.; Pallero, M.A.; Elzie, C.A.; Goicoechea, S.; Strickland, D.K.; Murphy-Ullrich, J.E. Low density lipoprotein receptor-related protein is a calreticulin coreceptor that signals focal adhesion disassembly. J. Cell Biol. 2003, 161, 1179–1189. [Google Scholar]
- Orr, A.W.; Elzie, C.A.; Kucik, D.F.; Murphy-Ullrich, J.E. Thrombospondin signaling through the calreticulin/LDL receptor-related protein co-complex stimulates random and directed cell migration. J. Cell Sci. 2003, 116, 2917–2927. [Google Scholar]
- Staniszewska, I.; Zaveri, S.; Del Valle, L.; Oliva, I.; Rothman, V.L.; Croul, S.E.; Roberts, D.D.; Mosher, D.F.; Tuszynski, G.P.; Marcinkiewicz, C. Interaction of alpha9beta1 integrin with thrombospondin-1 promotes angiogenesis. Circ. Res. 2007, 100, 1308–1316. [Google Scholar]
- Short, S.M.; Derrien, A.; Narsimhan, R.P.; Lawler, J.; Ingber, D.E.; Zetter, B.R. Inhibition of endothelial cell migration by thrombospondin-1 type-1 repeats is mediated by beta1 integrins. J. Cell Biol. 2005, 168, 643–653. [Google Scholar]
- Chandrasekaran, L.; He, C.Z.; Al-Barazi, H.; Krutzsch, H.C.; Iruela-Arispe, M.L.; Roberts, D.D. Cell contact-dependent activation of alpha3beta1 integrin modulates endothelial cell responses to thrombospondin-1. Mol. Biol. Cell 2000, 11, 2885–2900. [Google Scholar]
- Calzada, M.J.; Zhou, L.; Sipes, J.M.; Zhang, J.; Krutzsch, H.C.; Iruela-Arispe, M.L.; Annis, D.S.; Mosher, D.F.; Roberts, D.D. Alpha4beta1 integrin mediates selective endothelial cell responses to thrombospondins 1 and 2 in vitro and modulates angiogenesis in vivo. Circ. Res. 2004, 94, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Greenaway, J.; Lawler, J.; Moorehead, R.; Bornstein, P.; Lamarre, J.; Petrik, J. Thrombospondin-1 inhibits VEGF levels in the ovary directly by binding and internalization via the low density lipoprotein receptor-related protein-1 (LRP-1). J. Cell Physiol. 2007, 210, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Schultz-Cherry, S.; Chen, H.; Mosher, D.F.; Misenheimer, T.M.; Krutzsch, H.C.; Roberts, D.D.; Murphy-Ullrich, J.E. Regulation of transforming growth factor-beta activation by discrete sequences of thrombospondin 1. J. Biol. Chem. 1995, 270, 7304–7310. [Google Scholar]
- Young, G.D.; Murphy-Ullrich, J.E. The tryptophan-rich motifs of the thrombospondin type 1 repeats bind VLAL motifs in the latent transforming growth factor-beta complex. J. Biol. Chem. 2004, 279, 47633–47642. [Google Scholar]
- Miao, W.M.; Seng, W.L.; Duquette, M.; Lawler, P.; Laus, C.; Lawler, J. Thrombospondin-1 type 1 repeat recombinant proteins inhibit tumor growth through transforming growth factor-beta-dependent and -independent mechanisms. Cancer Res. 2001, 61, 7830–7839. [Google Scholar]
- Hogg, P.J.; Hotchkiss, K.A.; Jimenez, B.M.; Stathakis, P.; Chesterman, C.N. Interaction of platelet-derived growth factor with thrombospondin 1. Biochem. J. 1997, 326, (Pt 3). 709–716. [Google Scholar] [PubMed]
- Bein, K.; Simons, M. Thrombospondin type 1 repeats interact with matrix metalloproteinase 2. Regulation of metalloproteinase activity. J. Biol. Chem. 2000, 275, 32167–32173. [Google Scholar] [PubMed]
- Rabhi-Sabile, S.; de Romeuf, C.; Pidard, D. On the mechanism of plasmin-induced aggregation of human platelets: implication of secreted von Willebrand factor. Thromb. Haemost. 1998, 79, 1191–1198. [Google Scholar]
- Anonick, P.K.; Yoo, J.K.; Webb, D.J.; Gonias, S.L. Characterization of the antiplasmin activity of human thrombospondin-1 in solution. Biochem. J. 1993, 289, (Pt 3). 903–909. [Google Scholar] [PubMed]
- Silverstein, R.L.; Leung, L.L.; Harpel, P.C.; Nachman, R.L. Complex formation of platelet thrombospondin with plasminogen. Modulation of activation by tissue activator. J. Clin. Invest. 1984, 74, 1625–1633. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R.L.; Nachman, R.L.; Leung, L.L.; Harpel, P.C. Activation of immobilized plasminogen by tissue activator. Multimolecular complex formation. J. Biol. Chem. 1985, 260, 10346–10352. [Google Scholar] [PubMed]
- Silverstein, R.L.; Nachman, R.L.; Pannell, R.; Gurewich, V.; Harpel, P.C. Thrombospondin forms complexes with single-chain and two-chain forms of urokinase. J. Biol. Chem. 1990, 265, 11289–11294. [Google Scholar]
- Hogg, P.J.; Jimenez, B.M.; Chesterman, C.N. Identification of possible inhibitory reactive centers in thrombospondin 1 that may bind cathepsin G and neutrophil elastase. Biochemistry 1994, 33, 6531–6537. [Google Scholar]
- Hogg, P.J.; Owensby, D.A.; Chesterman, C.N. Thrombospondin 1 is a tight-binding competitive inhibitor of neutrophil cathepsin G. Determination of the kinetic mechanism of inhibition and localization of cathepsin G binding to the thrombospondin 1 type 3 repeats. J. Biol. Chem. 1993, 268, 21811–21818. [Google Scholar] [PubMed]
- Mast, A.E.; Stadanlick, J.E.; Lockett, J.M.; Dietzen, D.J.; Hasty, K.A.; Hall, C.L. Tissue factor pathway inhibitor binds to platelet thrombospondin-1. J. Biol. Chem. 2000, 275, 31715–31721. [Google Scholar]
- Feitsma, K.; Hausser, H.; Robenek, H.; Kresse, H.; Vischer, P. Interaction of thrombospondin-1 and heparan sulfate from endothelial cells. Structural requirements of heparan sulfate. J. Biol. Chem. 2000, 275, 9396–9402. [Google Scholar] [PubMed]
- Panetti, T.S.; Kudryk, B.J.; Mosher, D.F. Interaction of recombinant procollagen and properdin modules of thrombospondin-1 with heparin and fibrinogen/fibrin. J. Biol. Chem. 1999, 274, 430–437. [Google Scholar]
- Yu, H.; Tyrrell, D.; Cashel, J.; Guo, N.H.; Vogel, T.; Sipes, J.M.; Lam, L.; Fillit, H.M.; Hartman, J.; Mendelovitz, S.; Panel, A.; Roberts, D.D. Specificities of heparin-binding sites from the amino-terminus and type 1 repeats of thrombospondin-1. Arch. Biochem. Biophys. 2000, 374, 13–23. [Google Scholar]
- Lawler, J.; Ferro, P.; Duquette, M. Expression and mutagenesis of thrombospondin. Biochemistry 1992, 31, 1173–1180. [Google Scholar]
- Silverstein, R.L.; Leung, L.L.; Harpel, P.C.; Nachman, R.L. Platelet thrombospondin forms a trimolecular complex with plasminogen and histidine-rich glycoprotein. J. Clin. Invest. 1985, 75, 2065–2073. [Google Scholar]
- Isordia-Salas, I.; Manns, J.M.; Sainz, I.; Parekh, H.; DeLa Cadena, R.A. Thromsbospondin-1 binds to the heavy chain of elastase activated coagulation factor V (FVaHNE) and enhances thrombin generation on the surface of a promyelocytic cell line. Thromb. Res. 2005, 116, 533–543. [Google Scholar]
- Zhou, J.; Rothman, V.L.; Sargiannidou, I.; Dimitrov, S.; Qiu, C.; Smith, E.; Sheffield, J.; Sharma, M.; Tuszynski, G.P. Cloning and characterization of angiocidin, a tumor cell binding protein for thrombospondin-1. J. Cell Biochem. 2004, 92, 125–146. [Google Scholar]
- Hansen, G.A.; Vorum, H.; Jacobsen, C.; Honore, B. Calumenin but not reticulocalbin forms a Ca2+-dependent complex with thrombospondin-1. A potential role in haemostasis and thrombosis. Mol. Cell Biochem. 2009, 320, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Faye, C.; Moreau, C.; Chautard, E.; Jetne, R.; Fukai, N.; Ruggiero, F.; Humphries, M.J.; Olsen, B.R.; Ricard-Blum, S. Molecular interplay between endostatin, integrins, and heparan sulfate. J. Biol. Chem. 2009, 284, 22029–22040. [Google Scholar] [PubMed]
- Floquet, N.; Dedieu, S.; Martiny, L.; Dauchez, M.; Perahia, D. Human thrombospondin's (TSP-1) C-terminal domain opens to interact with the CD-47 receptor: a molecular modeling study. Arch. Biochem. Biophys. 2008, 478, 103–109. [Google Scholar]
- Elzie, C.A.; Murphy-Ullrich, J.E. The N-terminus of thrombospondin: the domain stands apart. Int. J. Biochem. Cell Biol. 2004, 36, 1090–1101. [Google Scholar]
- Mikhailenko, I.; Krylov, D.; Argraves, K.M.; Roberts, D.D.; Liau, G.; Strickland, D.K. Cellular internalization and degradation of thrombospondin-1 is mediated by the amino-terminal heparin binding domain (HBD). High affinity interaction of dimeric HBD with the low density lipoprotein receptor-related protein. J. Biol. Chem. 1997, 272, 6784–6791. [Google Scholar] [PubMed]
- Tan, K.; Duquette, M.; Liu, J.H.; Zhang, R.; Joachimiak, A.; Wang, J.H.; Lawler, J. The structures of the thrombospondin-1 N-terminal domain and its complex with a synthetic pentameric heparin. Structure 2006, 14, 33–42. [Google Scholar]
- Michalak, M.; Corbett, E.F.; Mesaeli, N.; Nakamura, K.; Opas, M. Calreticulin: one protein, one gene, many functions. Biochem. J. 1999, 344, (Pt 2). 281–292. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Calzada, M.J.; Sipes, J.M.; Cashel, J.A.; Krutzsch, H.C.; Annis, D.S.; Mosher, D.F.; Roberts, D.D. Interactions of thrombospondins with alpha4beta1 integrin and CD47 differentially modulate T cell behavior. J. Cell Biol. 2002, 157, 509–519. [Google Scholar]
- Calzada, M.J.; Roberts, D.D. Novel integrin antagonists derived from thrombospondins. Curr. Pharm. Des. 2005, 11, 849–866. [Google Scholar]
- Furrer, J.; Luy, B.; Basrur, V.; Roberts, D.D.; Barchi, J.J., Jr. Conformational analysis of an alpha3beta1 integrin-binding peptide from thrombospondin-1: implications for antiangiogenic drug design. J. Med. Chem. 2006, 49, 6324–6333. [Google Scholar]
- Krutzsch, H.C.; Choe, B.J.; Sipes, J.M.; Guo, N.; Roberts, D.D. Identification of an alpha(3)beta(1) integrin recognition sequence in thrombospondin-1. J. Biol. Chem. 1999, 274, 24080–24086. [Google Scholar]
- Calzada, M.J.; Annis, D.S.; Zeng, B.; Marcinkiewicz, C.; Banas, B.; Lawler, J.; Mosher, D.F.; Roberts, D.D. Identification of novel beta1 integrin binding sites in the type 1 and type 2 repeats of thrombospondin-1. J. Biol. Chem. 2004, 279, 41734–41743. [Google Scholar]
- Lahav, J.; Schwartz, M.A.; Hynes, R.O. Analysis of platelet adhesion with a radioactive chemical crosslinking reagent: interaction of thrombospondin with fibronectin and collagen. Cell 1982, 31, 253–262. [Google Scholar]
- Mumby, S.M.; Raugi, G.J.; Bornstein, P. Interactions of thrombospondin with extracellular matrix proteins: selective binding to type V collagen. J. Cell Biol. 1984, 98, 646–652. [Google Scholar]
- Galvin, N.J.; Vance, P.M.; Dixit, V.M.; Fink, B.; Frazier, W.A. Interaction of human thrombospondin with types I-V collagen: direct binding and electron microscopy. J. Cell Biol. 1987, 104, 1413–1422. [Google Scholar]
- Sottile, J.; Hocking, D.C. Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions. Mol. Biol. Cell 2002, 13, 3546–3559. [Google Scholar]
- Lahav, J.; Lawler, J.; Gimbrone, M.A. Thrombospondin interactions with fibronectin and fibrinogen. Mutual inhibition in binding. Eur. J. Biochem. 1984, 145, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Bale, M.D.; Mosher, D.F. Thrombospondin is a substrate for blood coagulation factor XIIIa. Biochemistry 1986, 25, 5667–5673. [Google Scholar]
- Bale, M.D.; Mosher, D.F. Effects of thrombospondin on fibrin polymerization and structure. J. Biol. Chem. 1986, 261, 862–868. [Google Scholar]
- Pimanda, J.E.; Annis, D.S.; Raftery, M.; Mosher, D.F.; Chesterman, C.N.; Hogg, P.J. The von Willebrand factor-reducing activity of thrombospondin-1 is located in the calcium-binding/C-terminal sequence and requires a free thiol at position 974. Blood 2002, 100, 2832–2838. [Google Scholar]
- Merle, B.; Malaval, L.; Lawler, J.; Delmas, P.; Clezardin, P. Decorin inhibits cell attachment to thrombospondin-1 by binding to a KKTR-dependent cell adhesive site present within the N-terminal domain of thrombospondin-1. J. Cell Biochem. 1997, 67, 75–83. [Google Scholar]
- Moralez, A.M.; Maile, L.A.; Clarke, J.; Busby, W.H., Jr.; Clemmons, D.R. Insulin-like growth factor binding protein-5 (IGFBP-5) interacts with thrombospondin-1 to induce negative regulatory effects on IGF-I actions. J. Cell Physiol. 2005, 203, 328–334. [Google Scholar]
- Taraboletti, G.; Benelli, R.; Borsotti, P.; Rusnati, M.; Presta, M.; Giavazzi, R.; Ruco, L.; Albini, A. Thrombospondin-1 inhibits Kaposi's sarcoma (KS) cell and HIV-1 Tat-induced angiogenesis and is poorly expressed in KS lesions. J. Pathol. 1999, 188, 76–81. [Google Scholar]
- Yang, Z.; Strickland, D.K.; Bornstein, P. Extracellular matrix metalloproteinase 2 levels are regulated by the low density lipoprotein-related scavenger receptor and thrombospondin 2. J. Biol. Chem. 2001, 276, 8403–8408. [Google Scholar]
- Iruela-Arispe, M.L.; Luque, A.; Lee, N. Thrombospondin modules and angiogenesis. Int. J. Biochem. Cell Biol. 2004, 36, 1070–1078. [Google Scholar]
- Rodriguez-Manzaneque, J.C.; Lane, T.F.; Ortega, M.A.; Hynes, R.O.; Lawler, J.; Iruela-Arispe, M.L. Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor. Proc. Natl. Acad. Sci. USA 2001, 98, 12485–12490. [Google Scholar]
- Qian, X.; Wang, T.N.; Rothman, V.L.; Nicosia, R.F.; Tuszynski, G.P. Thrombospondin-1 modulates angiogenesis in vitro by up-regulation of matrix metalloproteinase-9 in endothelial cells. Exp. Cell Res. 1997, 235, 403–412. [Google Scholar]
- Wang, S.; Skorczewski, J.; Feng, X.; Mei, L.; Murphy-Ullrich, J.E. Glucose up-regulates thrombospondin 1 gene transcription and transforming growth factor-beta activity through antagonism of cGMP-dependent protein kinase repression via upstream stimulatory factor 2. J. Biol. Chem. 2004, 279, 34311–34322. [Google Scholar] [PubMed]
- Panigrahy, D.; Kaipainen, A.; Huang, S.; Butterfield, C.E.; Barnes, C.M.; Fannon, M.; Laforme, A.M.; Chaponis, D.M.; Folkman, J.; Kieran, M.W. PPARalpha agonist fenofibrate suppresses tumor growth through direct and indirect angiogenesis inhibition. Proc. Natl. Acad. Sci. USA 2008, 105, 985–990. [Google Scholar]
- Kang, J.H.; Kim, S.A.; Chang, S.Y.; Hong, S.; Hong, K.J. Inhibition of trichostatin A-induced antiangiogenesis by small-interfering RNA for thrombospondin-1. Exp. Mol. Med. 2007, 39, 402–411. [Google Scholar]
- Castle, V.P.; Ou, X.; O'Shea, S.; Dixit, V.M. Induction of thrombospondin 1 by retinoic acid is important during differentiation of neuroblastoma cells. J. Clin. Invest. 1992, 90, 1857–1863. [Google Scholar]
- Kim, S.A.; Kang, J.H.; Cho, I.; Bae, S.W.; Hong, K.J. Cell-type specific regulation of thrombospondin-1 expression and its promoter activity by regulatory agents. Exp. Mol. Med. 2001, 33, 117–123. [Google Scholar]
- Doebele, R.C.; Schulze-Hoepfner, F.T.; Hong, J.; Chlenski, A.; Zeitlin, B.D.; Goel, K.; Gomes, S.; Liu, Y.; Abe, M.K.; Nor, J.E.; Lingen, M.W.; Rosner, M.R. A novel interplay between Epac/Rap1 and mitogen-activated protein kinase kinase 5/extracellular signal-regulated kinase 5 (MEK5/ERK5) regulates thrombospondin to control angiogenesis. Blood 2009, 114, 4592–4600. [Google Scholar]
- Lee, T.Y.; Muschal, S.; Pravda, E.A.; Folkman, J.; Abdollahi, A.; Javaherian, K. Angiostatin regulates the expression of antiangiogenic and proapoptotic pathways via targeted inhibition of mitochondrial proteins. Blood 2009, 114, 1987–1998. [Google Scholar] [CrossRef] [PubMed]
- Puri, N.; Khramtsov, A.; Ahmed, S.; Nallasura, V.; Hetzel, J.T.; Jagadeeswaran, R.; Karczmar, G.; Salgia, R. A selective small molecule inhibitor of c-Met, PHA665752, inhibits tumorigenicity and angiogenesis in mouse lung cancer xenografts. Cancer Res. 2007, 67, 3529–3534. [Google Scholar] [PubMed]
- Mirkin, S.; Mahony, M.C.; Archer, D.F. Effect of tibolone and its metabolites on vascular endothelial growth factor isoforms 121 and 165 and thrombospondin-1 mRNA in Ishikawa cells. Menopause 2004, 11, 82–88. [Google Scholar]
- Albig, A.R.; Schiemann, W.P. Fibulin-5 antagonizes vascular endothelial growth factor (VEGF) signaling and angiogenic sprouting by endothelial cells. DNA Cell Biol. 2004, 23, 367–379. [Google Scholar]
- Naito, T.; Masaki, T.; Nikolic-Paterson, D.J.; Tanji, C.; Yorioka, N.; Kohno, N. Angiotensin II induces thrombospondin-1 production in human mesangial cells via p38 MAPK and JNK: a mechanism for activation of latent TGF-beta1. Am. J. Physiol. Renal Physiol. 2004, 286, F278–F287. [Google Scholar]
- Fischer, J.W.; Stoll, M.; Hahn, A.W.; Unger, T. Differential regulation of thrombospondin-1 and fibronectin by angiotensin II receptor subtypes in cultured endothelial cells. Cardiovasc. Res. 2001, 51, 784–791. [Google Scholar]
- Ding, I.; Sun, J.Z.; Fenton, B.; Liu, W.M.; Kimsely, P.; Okunieff, P.; Min, W. Intratumoral administration of endostatin plasmid inhibits vascular growth and perfusion in MCa-4 murine mammary carcinomas. Cancer Res. 2001, 61, 526–531. [Google Scholar]
- Hyder, S.M.; Liang, Y.; Wu, J. Estrogen regulation of thrombospondin-1 in human breast cancer cells. Int. J. Cancer. 2009, 125, 1045–1053. [Google Scholar]
- Navarro, F.J.; Mirkin, S.; Archer, D.F. Effect of raloxifene, 17beta-estradiol, and progesterone on mRNA for vascular endothelial growth factor isoforms 121 and 165 and thrombospondin-1 in Ishikawa cells. Fertil. Steril. 2003, 79, 1409–1415. [Google Scholar]
- Kim, J.; Kim, C.; Kim, T.S.; Bang, S.I.; Yang, Y.; Park, H.; Cho, D. IL-18 enhances thrombospondin-1 production in human gastric cancer via JNK pathway. Biochem. Biophys. Res. Commun. 2006, 344, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Congote, L.F.; DiFalco, M.R.; Gibbs, B.F. Thrombospondin 1, produced by endothelial cells under the action of erythropoietin, stimulates thymidine incorporation into erythroid cells and counteracts the inhibitory action of insulin-like growth factor binding protein 3. Cytokine 2005, 30, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Soula-Rothhut, M.; Coissard, C.; Sartelet, H.; Boudot, C.; Bellon, G.; Martiny, L.; Rothhut, B. The tumor suppressor PTEN inhibits EGF-induced TSP-1 and TIMP-1 expression in FTC-133 thyroid carcinoma cells. Exp. Cell Res. 2005, 304, 187–201. [Google Scholar]
- Horiguchi, H.; Jin, L.; Ruebel, K.H.; Scheithauer, B.W.; Lloyd, R.V. Regulation of VEGF-A, VEGFR-I, thrombospondin-1, -2, and -3 expression in a human pituitary cell line (HP75) by TGFbeta1, bFGF, and EGF. Endocrine 2004, 24, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Sales, V.; Vila, V.; Ferrando, M.; Reganon, E. Atorvastatin neutralizes the up-regulation of thrombospondin-1 induced by thrombin in human umbilical vein endothelial cells. Endothelium 2007, 14, 233–238. [Google Scholar]
- Hellebrekers, D.M.; Jair, K.W.; Vire, E.; Eguchi, S.; Hoebers, N.T.; Fraga, M.F.; Esteller, M.; Fuks, F.; Baylin, S.B.; van Engeland, M.; Griffioen, A.W. Angiostatic activity of DNA methyltransferase inhibitors. Mol. Cancer Ther. 2006, 5, 467–475. [Google Scholar]
- Liu, Z.; Christensson, M.; Forslow, A.; De Meester, I.; Sundqvist, K.G. A CD26-controlled cell surface cascade for regulation of T cell motility and chemokine signals. J. Immunol. 2009, 183, 3616–3624. [Google Scholar]
- Bocci, G.; Francia, G.; Man, S.; Lawler, J.; Kerbel, R.S. Thrombospondin 1, a mediator of the antiangiogenic effects of low-dose metronomic chemotherapy. Proc. Natl. Acad. Sci. USA 2003, 100, 12917–12922. [Google Scholar]
- Hamano, Y.; Sugimoto, H.; Soubasakos, M.A.; Kieran, M.; Olsen, B.R.; Lawler, J.; Sudhakar, A.; Kalluri, R. Thrombospondin-1 associated with tumor microenvironment contributes to low-dose cyclophosphamide-mediated endothelial cell apoptosis and tumor growth suppression. Cancer Res. 2004, 64, 1570–1574. [Google Scholar]
- Zhao, H.Y.; Ooyama, A.; Yamamoto, M.; Ikeda, R.; Haraguchi, M.; Tabata, S.; Furukawa, T.; Che, X.F.; Iwashita, K.; Oka, T.; Fukushima, M.; Nakagawa, M.; Ono, M.; Kuwano, M.; Akiyama, S. Down regulation of c-Myc and induction of an angiogenesis inhibitor, thrombospondin-1, by 5-FU in human colon cancer KM12C cells. Cancer Lett. 2008, 270, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Damber, J.E.; Vallbo, C.; Albertsson, P.; Lennernas, B.; Norrby, K. The anti-tumour effect of low-dose continuous chemotherapy may partly be mediated by thrombospondin. Cancer Chemother. Pharmacol. 2006, 58, 354–360. [Google Scholar]
- Yoo, G.H.; Piechocki, M.P.; Ensley, J.F.; Nguyen, T.; Oliver, J.; Meng, H.; Kewson, D.; Shibuya, T.Y.; Lonardo, F.; Tainsky, M.A. Docetaxel induced gene expression patterns in head and neck squamous cell carcinoma using cDNA microarray and PowerBlot. Clin. Cancer Res. 2002, 8, 3910–3921. [Google Scholar]
- Cinatl, J., Jr.; Kotchetkov, R.; Blaheta, R.; Driever, P.H.; Vogel, J.U.; Cinatl, J. Induction of differentiation and suppression of malignant phenotype of human neuroblastoma BE(2)-C cells by valproic acid: enhancement by combination with interferon-alpha. Int. J. Oncol. 2002, 20, 97–106. [Google Scholar]
- Bocci, G.; Falcone, A.; Fioravanti, A.; Orlandi, P.; Di Paolo, A.; Fanelli, G.; Viacava, P.; Naccarato, A.G.; Kerbel, R.S.; Danesi, R.; Del Tacca, M.; Allegrini, G. Antiangiogenic and anticolorectal cancer effects of metronomic irinotecan chemotherapy alone and in combination with semaxinib. Br. J. Cancer. 2008, 98, 1619–1629. [Google Scholar]
- Allegrini, G.; Falcone, A.; Fioravanti, A.; Barletta, M.T.; Orlandi, P.; Loupakis, F.; Cerri, E.; Masi, G.; Di Paolo, A.; Kerbel, R.S.; Danesi, R.; Del Tacca, M.; Bocci, G. A pharmacokinetic and pharmacodynamic study on metronomic irinotecan in metastatic colorectal cancer patients. Br. J. Cancer. 2008, 98, 1312–1319. [Google Scholar]
- Castle, V.P.; Dixit, V.M.; Polverini, P.J. Thrombospondin-1 suppresses tumorigenesis and angiogenesis in serum- and anchorage-independent NIH 3T3 cells. Lab. Invest. 1997, 77, 51–61. [Google Scholar]
- Zhang, X.; Xu, J.; Lawler, J.; Terwilliger, E.; Parangi, S. Adeno-associated virus-mediated antiangiogenic gene therapy with thrombospondin-1 type 1 repeats and endostatin. Clin. Cancer Res. 2007, 13, 3968–3976. [Google Scholar]
- Liu, P.; Wang, Y.; Li, Y.H.; Yang, C.; Zhou, Y.L.; Li, B.; Lu, S.H.; Yang, R.C.; Cai, Y.L.; Tobelem, G.; Caen, J.; Han, Z.C. Adenovirus-mediated gene therapy with an antiangiogenic fragment of thrombospondin-1 inhibits human leukemia xenograft growth in nude mice. Leuk. Res. 2003, 27, 701–708. [Google Scholar]
- Yee, K.O.; Streit, M.; Hawighorst, T.; Detmar, M.; Lawler, J. Expression of the type-1 repeats of thrombospondin-1 inhibits tumor growth through activation of transforming growth factor-beta. Am. J. Pathol. 2004, 165, 541–552. [Google Scholar]
- Miyata, Y.; Koga, S.; Takehara, K.; Kanetake, H.; Kanda, S. Expression of thrombospondin-derived 4N1K peptide-containing proteins in renal cell carcinoma tissues is associated with a decrease in tumor growth and angiogenesis. Clin. Cancer Res. 2003, 9, 1734–1740. [Google Scholar]
- Amagasaki, K.; Sasaki, A.; Kato, G.; Maeda, S.; Nukui, H.; Naganuma, H. Antisense-mediated reduction in thrombospondin-1 expression reduces cell motility in malignant glioma cells. Int. J. Cancer. 2001, 94, 508–512. [Google Scholar]
- Dameron, K.M.; Volpert, O.V.; Tainsky, M.A.; Bouck, N. Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin-1. Science 1994, 265, 1582–1584. [Google Scholar]
- Dameron, K.M.; Volpert, O.V.; Tainsky, M.A.; Bouck, N. The p53 tumor suppressor gene inhibits angiogenesis by stimulating the production of thrombospondin. Cold Spring Harb. Symp. Quant. Biol. 1994, 59, 483–489. [Google Scholar]
- Gautam, A.; Densmore, C.L.; Melton, S.; Golunski, E.; Waldrep, J.C. Aerosol delivery of PEI-p53 complexes inhibits B16-F10 lung metastases through regulation of angiogenesis. Cancer Gene Ther. 2002, 9, 28–36. [Google Scholar]
- Suarez, Y.; Fernandez-Hernando, C.; Yu, J.; Gerber, S.A.; Harrison, K.D.; Pober, J.S.; Iruela-Arispe, M.L.; Merkenschlager, M.; Sessa, W.C. Dicer-dependent endothelial microRNAs are necessary for postnatal angiogenesis. Proc. Natl. Acad. Sci. USA 2008, 105, 14082–14087. [Google Scholar]
- Dews, M.; Homayouni, A.; Yu, D.; Murphy, D.; Sevignani, C.; Wentzel, E.; Furth, E.E.; Lee, W.M.; Enders, G.H.; Mendell, J.T.; Thomas-Tikhonenko, A. Augmentation of tumor angiogenesis by a Myc-activated microRNA cluster. Nat. Genet. 2006, 38, 1060–1065. [Google Scholar]
- Qin, H.; Shao, Q.; Thomas, T.; Kalra, J.; Alaoui-Jamali, M.A.; Laird, D.W. Connexin26 regulates the expression of angiogenesis-related genes in human breast tumor cells by both GJIC-dependent and -independent mechanisms. Cell Commun. Adhes. 2003, 10, 387–393. [Google Scholar]
- Yang, G.; Cai, K.Q.; Thompson-Lanza, J.A.; Bast, R.C., Jr.; Liu, J. Inhibition of breast and ovarian tumor growth through multiple signaling pathways by using retrovirus-mediated small interfering RNA against Her-2/neu gene expression. J. Biol. Chem. 2004, 279, 4339–4345. [Google Scholar]
- de Fraipont, F.; Keramidas, M.; El Atifi, M.; Chambaz, E.M.; Berger, F.; Feige, J.J. Expression of the thrombospondin 1 fragment 167-569 in C6 glioma cells stimulates tumorigenicity despite reduced neovascularization. Oncogene 2004, 23, 3642–3649. [Google Scholar]
- Wells, J.A.; McClendon, C.L. Reaching for high-hanging fruit in drug discovery at protein-protein interfaces. Nature 2007, 450, 1001–1009. [Google Scholar]
- Robinson, J.A.; Demarco, S.; Gombert, F.; Moehle, K.; Obrecht, D. The design, structures and therapeutic potential of protein epitope mimetics. Drug Discov. Today 2008, 13, 944–951. [Google Scholar]
- Murray, J.K.; Gellman, S.H. Targeting protein-protein interactions: lessons from p53/MDM2. Biopolymers 2007, 88, 657–686. [Google Scholar]
- Sulochana, K.N.; Ge, R. Developing antiangiogenic peptide drugs for angiogenesis-related diseases. Curr. Pharm. Des. 2007, 13, 2074–2086. [Google Scholar]
- Tolsma, S.S.; Volpert, O.V.; Good, D.J.; Frazier, W.A.; Polverini, P.J.; Bouck, N. Peptides derived from two separate domains of the matrix protein thrombospondin-1 have anti-angiogenic activity. J. Cell Biol. 1993, 122, 497–511. [Google Scholar]
- Lee, N.V.; Sato, M.; Annis, D.S.; Loo, J.A.; Wu, L.; Mosher, D.F.; Iruela-Arispe, M.L. ADAMTS1 mediates the release of antiangiogenic polypeptides from TSP1 and 2. Embo. J. 2006, 25, 5270–5283. [Google Scholar]
- Bruel, A.; Touhami-Carrier, M.; Thomaidis, A.; Legrand, C. Thrombospondin-1 (TSP-1) and TSP-1-derived heparin-binding peptides induce promyelocytic leukemia cell differentiation and apoptosis. Anticancer Res. 2005, 25, 757–764. [Google Scholar]
- Zhang, X.; Galardi, E.; Duquette, M.; Delic, M.; Lawler, J.; Parangi, S. Antiangiogenic treatment with the three thrombospondin-1 type 1 repeats recombinant protein in an orthotopic human pancreatic cancer model. Clin. Cancer Res. 2005, 11, 2337–2344. [Google Scholar]
- Zhang, X.; Connolly, C.; Duquette, M.; Lawler, J.; Parangi, S. Continuous administration of the three thrombospondin-1 type 1 repeats recombinant protein improves the potency of therapy in an orthotopic human pancreatic cancer model. Cancer Lett. 2007, 247, 143–149. [Google Scholar]
- Yee, K.O.; Connolly, C.M.; Duquette, M.; Kazerounian, S.; Washington, R.; Lawler, J. The effect of thrombospondin-1 on breast cancer metastasis. Breast Cancer Res. Treat. 2009, 114, 85–96. [Google Scholar]
- Guo, N.H.; Krutzsch, H.C.; Inman, J.K.; Shannon, C.S.; Roberts, D.D. Antiproliferative and antitumor activities of D-reverse peptides derived from the second type-1 repeat of thrombospondin-1. J. Pept. Res. 1997, 50, 210–221. [Google Scholar]
- Jimenez, B.; Volpert, O.V.; Reiher, F.; Chang, L.; Munoz, A.; Karin, M.; Bouck, N. c-Jun N-terminal kinase activation is required for the inhibition of neovascularization by thrombospondin-1. Oncogene 2001, 20, 3443–3448. [Google Scholar]
- Guo, N.; Krutzsch, H.C.; Inman, J.K.; Roberts, D.D. Thrombospondin 1 and type I repeat peptides of thrombospondin 1 specifically induce apoptosis of endothelial cells. Cancer Res. 1997, 57, 1735–1742. [Google Scholar]
- Manna, P.P.; Frazier, W.A. CD47 mediates killing of breast tumor cells via Gi-dependent inhibition of protein kinase A. Cancer Res. 2004, 64, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Lih, C.J.; Wei, W.; Cohen, S.N. Txr1: a transcriptional regulator of thrombospondin-1 that modulates cellular sensitivity to taxanes. Genes Dev. 2006, 20, 2082–2095. [Google Scholar]
- Rath, G.M.; Schneider, C.; Dedieu, S.; Sartelet, H.; Morjani, H.; Martiny, L.; El Btaouri, H. Thrombospondin-1 C-terminal-derived peptide protects thyroid cells from ceramide-induced apoptosis through the adenylyl cyclase pathway. Int. J. Biochem. Cell Biol. 2006, 38, 2219–2228. [Google Scholar]
- Goldfarb, D.S.; Gariepy, J.; Schoolnik, G.; Kornberg, R.D. Synthetic peptides as nuclear localization signals. Nature 1986, 322, 641–644. [Google Scholar]
- Nestor, J.J., Jr. The medicinal chemistry of peptides. Curr Med Chem. 2009, 16, 4399–4418. [Google Scholar]
- Bogdanov, A., Jr.; Marecos, E.; Cheng, H.C.; Chandrasekaran, L.; Krutzsch, H.C.; Roberts, D.D.; Weissleder, R. Treatment of experimental brain tumors with trombospondin-1 derived peptides: an in vivo imaging study. Neoplasia 1999, 1, 438–445. [Google Scholar] [PubMed]
- Coronella, J.; Li, L.; Johnson, K.; Pirie-Shepherd, S.; Roxas, G.; Levin, N. Selective activity against proliferating tumor endothelial cells by CVX-22, a thrombospondin-1 mimetic CovX-Body. Anticancer Res. 2009, 29, 2243–2252. [Google Scholar]
- Molckovsky, A.; Siu, L.L. First-in-class, first-in-human phase I results of targeted agents: Highlights of the 2008 American Society of Clinical Oncology meeting. J. Hematol. Oncol. 2008, 1, 20. [Google Scholar]
- Reiher, F.K.; Volpert, O.V.; Jimenez, B.; Crawford, S.E.; Dinney, C.P.; Henkin, J.; Haviv, F.; Bouck, N.P.; Campbell, S.C. Inhibition of tumor growth by systemic treatment with thrombospondin-1 peptide mimetics. Int. J. Cancer. 2002, 98, 682–689. [Google Scholar]
- Haviv, F.; Bradley, M.F.; Kalvin, D.M.; Schneider, A.J.; Davidson, D.J.; Majest, S.M.; McKay, L.M.; Haskell, C.J.; Bell, R.L.; Nguyen, B.; Marsh, K.C.; Surber, B.W.; Uchic, J.T.; Ferrero, J.; Wang, Y.C.; Leal, J.; Record, R.D.; Hodde, J.; Badylak, S.F.; Lesniewski, R.R.; Henkin, J. Thrombospondin-1 mimetic peptide inhibitors of angiogenesis and tumor growth: design, synthesis, and optimization of pharmacokinetics and biological activities. J. Med. Chem. 2005, 48, 2838–2846. [Google Scholar] [PubMed]
- Greenaway, J.; Henkin, J.; Lawler, J.; Moorehead, R.; Petrik, J. ABT-510 induces tumor cell apoptosis and inhibits ovarian tumor growth in an orthotopic, syngeneic model of epithelial ovarian cancer. Mol. Cancer Ther. 2009, 8, 64–74. [Google Scholar]
- Anderson, J.C.; Grammer, J.R.; Wang, W.; Nabors, L.B.; Henkin, J.; Stewart, J.E., Jr.; Gladson, C.L. ABT-510, a modified type 1 repeat peptide of thrombospondin, inhibits malignant glioma growth in vivo by inhibiting angiogenesis. Cancer Biol. Ther. 2007, 6, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Quesada, A.J.; Nelius, T.; Yap, R.; Zaichuk, T.A.; Alfranca, A.; Filleur, S.; Volpert, O.V.; Redondo, J.M. In vivo upregulation of CD95 and CD95L causes synergistic inhibition of angiogenesis by TSP1 peptide and metronomic doxorubicin treatment. Cell Death Differ. 2005, 12, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Viloria-Petit, A.; Miquerol, L.; Yu, J.L.; Gertsenstein, M.; Sheehan, C.; May, L.; Henkin, J.; Lobe, C.; Nagy, A.; Kerbel, R.S.; Rak, J. Contrasting effects of VEGF gene disruption in embryonic stem cell-derived versus oncogene-induced tumors. EMBO. J. 2003, 22, 4091–4102. [Google Scholar] [CrossRef] [PubMed]
- Rusk, A.; Cozzi, E.; Stebbins, M.; Vail, D.; Graham, J.; Valli, V.; Henkin, J.; Sharpee, R.; Khanna, C. Cooperative activity of cytotoxic chemotherapy with antiangiogenic thrombospondin-I peptides, ABT-526 in pet dogs with relapsed lymphoma. Clin. Cancer Res. 2006, 12, 7456–7464. [Google Scholar]
- Hoekstra, R.; de Vos, F.Y.; Eskens, F.A.; Gietema, J.A.; van der Gaast, A.; Groen, H.J.; Knight, R.A.; Carr, R.A.; Humerickhouse, R.A.; Verweij, J.; de Vries, E.G. Phase I safety, pharmacokinetic, and pharmacodynamic study of the thrombospondin-1-mimetic angiogenesis inhibitor ABT-510 in patients with advanced cancer. J. Clin. Oncol. 2005, 23, 5188–5197. [Google Scholar] [PubMed]
- Baker, L.H.; Rowinsky, E.K.; Mendelson, D.; Humerickhouse, R.A.; Knight, R.A.; Qian, J.; Carr, R.A.; Gordon, G.B.; Demetri, G.D. Randomized, phase II study of the thrombospondin-1-mimetic angiogenesis inhibitor ABT-510 in patients with advanced soft tissue sarcoma. J. Clin. Oncol. 2008, 26, 5583–5588. [Google Scholar]
- Gordon, M.S.; Mendelson, D.; Carr, R.; Knight, R.A.; Humerickhouse, R.A.; Iannone, M.; Stopeck, A.T. A phase 1 trial of 2 dose schedules of ABT-510, an antiangiogenic, thrombospondin-1-mimetic peptide, in patients with advanced cancer. Cancer 2008, 113, 3420–3429. [Google Scholar] [PubMed]
- Ebbinghaus, S.; Hussain, M.; Tannir, N.; Gordon, M.; Desai, A.A.; Knight, R.A.; Humerickhouse, R.A.; Qian, J.; Gordon, G.B.; Figlin, R. Phase 2 study of ABT-510 in patients with previously untreated advanced renal cell carcinoma. Clin. Cancer Res. 2007, 13, 6689–6695. [Google Scholar]
- Markovic, S.N.; Suman, V.J.; Rao, R.A.; Ingle, J.N.; Kaur, J.S.; Erickson, L.A.; Pitot, H.C.; Croghan, G.A.; McWilliams, R.R.; Merchan, J.; Kottschade, L.A.; Nevala, W.K.; Uhl, C.B.; Allred, J.; Creagan, E.T. A phase II study of ABT-510 (thrombospondin-1 analog) for the treatment of metastatic melanoma. Am. J. Clin. Oncol. 2007, 30, 303–309. [Google Scholar]
- Yang, Q.; Tian, Y.; Liu, S.; Zeine, R.; Chlenski, A.; Salwen, H.R.; Henkin, J.; Cohn, S.L. Thrombospondin-1 peptide ABT-510 combined with valproic acid is an effective antiangiogenesis strategy in neuroblastoma. Cancer Res. 2007, 67, 1716–1724. [Google Scholar]
- Gietema, J.A.; Hoekstra, R.; de Vos, F.Y.; Uges, D.R.; van der Gaast, A.; Groen, H.J.; Loos, W.J.; Knight, R.A.; Carr, R.A.; Humerickhouse, R.A.; Eskens, F.A. A phase I study assessing the safety and pharmacokinetics of the thrombospondin-1-mimetic angiogenesis inhibitor ABT-510 with gemcitabine and cisplatin in patients with solid tumors. Ann. Oncol. 2006, 17, 1320–1327. [Google Scholar]
- Hoekstra, R.; de Vos, F.Y.; Eskens, F.A.; de Vries, E.G.; Uges, D.R.; Knight, R.; Carr, R.A.; Humerickhouse, R.; Verweij, J.; Gietema, J.A. Phase I study of the thrombospondin-1-mimetic angiogenesis inhibitor ABT-510 with 5-fluorouracil and leucovorin: a safe combination. Eur. J. Cancer 2006, 42, 467–472. [Google Scholar]
- Clackson, T.; Wells, J.A. A hot spot of binding energy in a hormone-receptor interface. Science 1995, 267, 383–386. [Google Scholar]
- Thanos, C.D.; DeLano, W.L.; Wells, J.A. Hot-spot mimicry of a cytokine receptor by a small molecule. Proc. Natl. Acad. Sci. USA 2006, 103, 15422–15427. [Google Scholar]
- Martin-Manso, G.; Galli, S.; Ridnour, L.A.; Tsokos, M.; Wink, D.A.; Roberts, D.D. Thrombospondin 1 promotes tumor macrophage recruitment and enhances tumor cell cytotoxicity of differentiated U937 cells. Cancer Res. 2008, 68, 7090–7099. [Google Scholar]
- Yang, M.; Li, K.; Ng, M.H.; Yuen, P.M.; Fok, T.F.; Li, C.K.; Hogg, P.J.; Chong, B.H. Thrombospondin-1 inhibits in vitro megakaryocytopoiesis via CD36. Thromb. Res. 2003, 109, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Castelli, R.; Porro, F.; Tarsia, P. The heparins and cancer: review of clinical trials and biological properties. Vasc. Med. 2004, 9, 205–213. [Google Scholar]
- John, A.S.; Hu, X.; Rothman, V.L.; Tuszynski, G.P. Thrombospondin-1 (TSP-1) up-regulates tissue inhibitor of metalloproteinase-1 (TIMP-1) production in human tumor cells: exploring the functional significance in tumor cell invasion. Exp. Mol. Pathol. 2009, 87, 184–188. [Google Scholar]
- Kang, S.Y.; Halvorsen, O.J.; Gravdal, K.; Bhattacharya, N.; Lee, J.M.; Liu, N.W.; Johnston, B.T.; Johnston, A.B.; Haukaas, S.A.; Aamodt, K.; Yoo, S.; Akslen, L.A.; Watnick, R.S. Prosaposin inhibits tumor metastasis via paracrine and endocrine stimulation of stromal p53 and Tsp-1. Proc. Natl. Acad. Sci. USA 2009, 106, 12115–12120. [Google Scholar]
- Koskimaki, J.E.; Karagiannis, E.D.; Rosca, E.V.; Vesuna, F.; Winnard, P.T., Jr.; Raman, V.; Bhujwalla, Z.M.; Popel, A.S. Peptides derived from type IV collagen, CXC chemokines, and thrombospondin-1 domain-containing proteins inhibit neovascularization and suppress tumor growth in MDA-MB-231 breast cancer xenografts. Neoplasia 2009, 11, 1285–1291. [Google Scholar] [PubMed]
- Li, Z.; He, L.; Wilson, K.; Roberts, D. Thrombospondin-1 inhibits TCR-mediated T lymphocyte early activation. J. Immunol. 2001, 166, 2427–2436. [Google Scholar]
- Johansson, U.; Higginbottom, K.; Londei, M. CD47 ligation induces a rapid caspase-independent apoptosis-like cell death in human monocytes and dendritic cells. Scand. J. Immunol. 2004, 59, 40–49. [Google Scholar]
- Turpie, B.; Yoshimura, T.; Gulati, A.; Rios, J.D.; Dartt, D.A.; Masli, S. Sjogren's syndrome-like ocular surface disease in thrombospondin-1 deficient mice. Am. J. Pathol. 2009, 175, 1136–1147. [Google Scholar]
- Yang, K.; Vega, J.L.; Hadzipasic, M.; Schatzmann Peron, J.P.; Zhu, B.; Carrier, Y.; Masli, S.; Rizzo, L.V.; Weiner, H.L. Deficiency of thrombospondin-1 reduces Th17 differentiation and attenuates experimental autoimmune encephalomyelitis. J. Autoimmun. 2009, 32, 94–103. [Google Scholar]
- Tabib, A.; Krispin, A.; Trahtemberg, U.; Verbovetski, I.; Lebendiker, M.; Danieli, T.; Mevorach, D. Thrombospondin-1-N-terminal domain induces a phagocytic state and thrombospondin-1-C-terminal domain induces a tolerizing phenotype in dendritic cells. PLoS One 2009, 4, e6840. [Google Scholar]
- Yamauchi, Y.; Kuroki, M.; Imakiire, T.; Uno, K.; Abe, H.; Beppu, R.; Yamashita, Y.; Shirakusa, T. Opposite effects of thrombospondin-1 via CD36 and CD47 on homotypic aggregation of monocytic cells. Matrix Biol. 2002, 21, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Bonnefoy, A.; Daenens, K.; Feys, H.B.; De Vos, R.; Vandervoort, P.; Vermylen, J.; Lawler, J.; Hoylaerts, M.F. Thrombospondin-1 controls vascular platelet recruitment and thrombus adherence in mice by protecting (sub)endothelial VWF from cleavage by ADAMTS13. Blood 2006, 107, 955–964. [Google Scholar]
- Voit, S.; Udelhoven, M.; Lill, G.; Aktas, B.; Nieswandt, B.; Schror, K.; Weber, A.A. The C-terminal peptide of thrombospondin-1 stimulates distinct signaling pathways but induces an activation-independent agglutination of platelets and other cells. FEBS Lett. 2003, 544, 240–245. [Google Scholar]
- Farrow, B.; Berger, D.H.; Rowley, D. Tumor-derived pancreatic stellate cells promote pancreatic cancer cell invasion through release of thrombospondin-2. J. Surg. Res. 2009, 156, 155–160. [Google Scholar]
- Ghoneim, C.; Soula-Rothhut, M.; Rothhut, B. Thrombospondin-1 in differentiated thyroid cancer: Dr. Jekyll and Mr. Hyde. Connect. Tissue Res. 2008, 49, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Sid, B.; Langlois, B.; Sartelet, H.; Bellon, G.; Dedieu, S.; Martiny, L. Thrombospondin-1 enhances human thyroid carcinoma cell invasion through urokinase activity. Int. J. Biochem. Cell Biol. 2008, 40, 1890–1900. [Google Scholar]
- Albo, D.; Berger, D.H.; Rothman, V.L.; Tuszynski, G.P. Role of urokinase plasminogen activator receptor in thrombospondin 1-mediated tumor cell invasion. J. Surg. Res. 1999, 82, 331–338. [Google Scholar]
- Robinet, A.; Emonard, H.; Banyai, L.; Laronze, J.Y.; Patthy, L.; Hornebeck, W.; Bellon, G. Collagen-binding domains of gelatinase A and thrombospondin-derived peptides impede endocytic clearance of active gelatinase A and promote HT1080 fibrosarcoma cell invasion. Life Sci. 2008, 82, 376–382. [Google Scholar]
- Rath, G.M.; Schneider, C.; Dedieu, S.; Rothhut, B.; Soula-Rothhut, M.; Ghoneim, C.; Sid, B.; Morjani, H.; El Btaouri, H.; Martiny, L. The C-terminal CD47/IAP-binding domain of thrombospondin-1 prevents camptothecin- and doxorubicin-induced apoptosis in human thyroid carcinoma cells. Biochim. Biophys. Acta 2006, 1763, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rusnati, M.; Urbinati, C.; Bonifacio, S.; Presta, M.; Taraboletti, G. Thrombospondin-1 as a Paradigm for the Development of Antiangiogenic Agents Endowed with Multiple Mechanisms of Action. Pharmaceuticals 2010, 3, 1241-1278. https://doi.org/10.3390/ph3041241
Rusnati M, Urbinati C, Bonifacio S, Presta M, Taraboletti G. Thrombospondin-1 as a Paradigm for the Development of Antiangiogenic Agents Endowed with Multiple Mechanisms of Action. Pharmaceuticals. 2010; 3(4):1241-1278. https://doi.org/10.3390/ph3041241
Chicago/Turabian StyleRusnati, Marco, Chiara Urbinati, Silvia Bonifacio, Marco Presta, and Giulia Taraboletti. 2010. "Thrombospondin-1 as a Paradigm for the Development of Antiangiogenic Agents Endowed with Multiple Mechanisms of Action" Pharmaceuticals 3, no. 4: 1241-1278. https://doi.org/10.3390/ph3041241
APA StyleRusnati, M., Urbinati, C., Bonifacio, S., Presta, M., & Taraboletti, G. (2010). Thrombospondin-1 as a Paradigm for the Development of Antiangiogenic Agents Endowed with Multiple Mechanisms of Action. Pharmaceuticals, 3(4), 1241-1278. https://doi.org/10.3390/ph3041241