89Zr-Radiolabeled Trastuzumab Imaging in Orthotopic and Metastatic Breast Tumors
Abstract
:1. Introduction
2. Experimental Section
2.1. Flow Cytometry
2.2. 89Zr Production and Antibody Labeling
2.3. Immunoreactive Fraction
2.4. Animal Studies
2.4.1. Biodistribution Studies
2.4.2. MicroPET/CT Studies
2.5. Immunohistochemistry
2.6. Statistical Analysis
3. Results and Discussion
3.1. Trastuzumab Conjugation, Radiolabeling, and Stability Testing
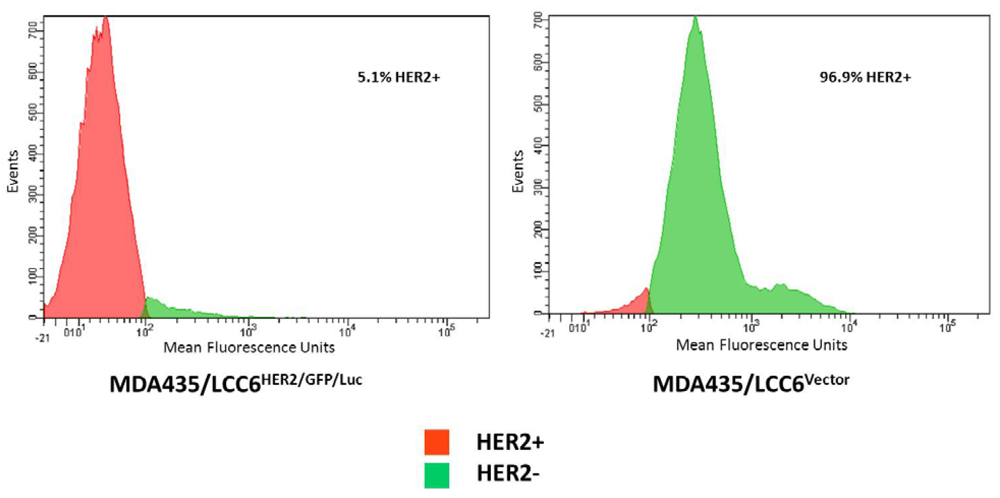
3.2. Flow Cytometry and Binding Studies

3.3. Biodistribution Studies

3.4. Imaging Studies


3.5. Immunohistochemistry
3.6. Discussion

4. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Citri, A.; Yarden, Y. EGF-ERBB signalling: Towards the systems level. Nat. Rev. Mol. Cell Biol. 2006, 7, 505–516. [Google Scholar] [CrossRef]
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987, 235, 177–182. [Google Scholar]
- Slamon, D.J.; Godolphin, W.; Jones, L.A.; Holt, J.A.; Wong, S.G.; Keith, D.E.; Levin, W.J.; Stuart, S.G.; Udove, J.; Ullrich, A.; et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989, 244, 707–712. [Google Scholar]
- Ignatiadis, M.; Desmedt, C.; Sotiriou, C.; de Azambuja, E.; Piccart, M. HER-2 as a target for breast cancer therapy. Clin. Cancer Res. 2009, 15, 1848–1852. [Google Scholar] [CrossRef]
- Whenham, N.; D'Hondt, V.; Piccart, M.J. HER2-positive breast cancer: From trastuzumab to innovatory anti-HER2 strategies. Clin. Breast Cancer 2008, 8, 38–49. [Google Scholar] [CrossRef]
- Banerjee, S.; Smith, I.E. Management of small HER2-positive breast cancers. Lancet Oncol. 2010, 11, 1193–1199. [Google Scholar] [CrossRef]
- Goel, S.; Chirgwin, J.; Francis, P.; Stuart-Harris, R.; Dewar, J.; Mileshkin, L.; Snyder, R.; Michael, M.; Koczwara, B. Rational use of trastuzumab in metastatic and locally advanced breast cancer: Implications of recent research. Breast 2011, 20, 101–110. [Google Scholar] [CrossRef]
- Guarneri, V.; Barbieri, E.; Dieci, M.V.; Piacentini, F.; Conte, P. Anti-HER2 neoadjuvant and adjuvant therapies in HER2 positive breast cancer. Cancer Treat. Rev. 2010, 36, S62–S66. [Google Scholar] [CrossRef]
- Cobleigh, M.A.; Vogel, C.L.; Tripathy, D.; Robert, N.J.; Scholl, S.; Fehrenbacher, L.; Wolter, J.M.; Paton, V.; Shak, S.; Lieberman, G.; et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J. Clin. Oncol. 1999, 17, 2639–2648. [Google Scholar]
- Dawood, S.; Broglio, K.; Buzdar, A.U.; Hortobagyi, G.N.; Giordano, S.H. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: An institutional-based review. J. Clin. Oncol. 2010, 28, 92–98. [Google Scholar]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef]
- Esteva, F.J.; Yu, D.; Hung, M.C.; Hortobagyi, G.N. Molecular predictors of response to trastuzumab and lapatinib in breast cancer. Nat. Rev. Clin. Oncol. 2010, 7, 98–107. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.; Schwartz, J.N.; Hagerty, K.L.; Allred, D.C.; Cote, R.J.; Dowsett, M.; Fitzgibbons, P.L.; Hanna, W.M.; Langer, A.; et al. American Society of Clinical Oncology/Collegeof American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch. Pathol. Lab. Med. 2007, 131, 18–43. [Google Scholar]
- Moelans, C.B.; de Weger, R.A.; van der Wall, E.; van Diest, P.J. Current technologies for HER2 testing in breast cancer. Crit. Rev. Oncol. Hematol. 2011, 80, 380–392. [Google Scholar]
- Rasbridge, S.A.; Gillett, C.E.; Seymour, A.M.; Patel, K.; Richards, M.A.; Rubens, R.D.; Millis, R.R. The effects of chemotherapy on morphology, cellular proliferation, apoptosis and oncoprotein expression in primary breast carcinoma. Br. J. Cancer 1994, 70, 335–341. [Google Scholar] [CrossRef]
- van de Ven, S.; Smit, V.T.; Dekker, T.J.; Nortier, J.W.; Kroep, J.R. Discordances in ER, PR and HER2 receptors after neoadjuvant chemotherapy in breast cancer. Cancer Treat. Rev. 2011, 37, 422–430. [Google Scholar]
- Guarneri, V.; Giovannelli, S.; Ficarra, G.; Bettelli, S.; Maiorana, A.; Piacentini, F.; Barbieri, E.; Dieci, M.V.; D'Amico, R.; Jovic, G.; et al. Comparison of HER-2 and hormone receptor expression in primary breast cancers and asynchronous paired metastases: Impact on patient management. Oncologist 2008, 13, 838–844. [Google Scholar] [CrossRef]
- Solomayer, E.F.; Becker, S.; Pergola-Becker, G.; Bachmann, R.; Kramer, B.; Vogel, U.; Neubauer, H.; Wallwiener, D.; Huober, J.; Fehm, T.N. Comparison of HER2 status between primary tumor and disseminated tumor cells in primary breast cancer patients. Breast Cancer Res. Treat. 2006, 98, 179–184. [Google Scholar] [CrossRef]
- Zurrida, S.; Montagna, E.; Naninato, P.; Colleoni, M.; Goldhirsch, A. Receptor status (ER, PgR and HER2) discordance between primary tumor and locoregional recurrence in breast cancer. Ann. Oncol. 2011, 22, 479–480. [Google Scholar] [CrossRef]
- Sekido, Y.; Umemura, S.; Takekoshi, S.; Suzuki, Y.; Tokuda, Y.; Tajima, T.; Osamura, R.Y. Heterogeneous gene alterations in primary breast cancer contribute to discordance between primary and asynchronous metastatic/recurrent sites: HER2 gene amplification and p53 mutation. Int. J. Oncol. 2003, 22, 1225–1232. [Google Scholar]
- Lear-Kaul, K.C.; Yoon, H.R.; Kleinschmidt-DeMasters, B.K.; McGavran, L.; Singh, M. Her-2/neu status in breast cancer metastases to the central nervous system. Arch. Pathol. Lab. Med. 2003, 127, 1451–1457. [Google Scholar]
- Niu, G.; Li, Z.; Cao, Q.; Chen, X. Monitoring therapeutic response of human ovarian cancer to 17-DMAG by noninvasive PET imaging with (64)Cu-DOTA-trastuzumab. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1510–1519. [Google Scholar] [CrossRef]
- Milenic, D.E.; Wong, K.J.; Baidoo, K.E.; Nayak, T.K.; Regino, C.A.; Garmestani, K.; Brechbiel, M.W. Targeting HER2: A report on the in vitro and in vivo pre-clinical data supporting trastuzumab as a radioimmunoconjugate for clinical trials. MAbs 2010, 2, 550–564. [Google Scholar] [CrossRef]
- Capala, J.; Bouchelouche, K. Molecular imaging of HER2-positive breast cancer: A step toward an individualized ‘image and treat’ strategy. Curr. Opin. Oncol. 2010, 22, 559–566. [Google Scholar] [CrossRef]
- Oude Munnink, T.H.; Korte, M.A.; Nagengast, W.B.; Timmer-Bosscha, H.; Schroder, C.P.; Jong, J.R.; Dongen, G.A.; Jensen, M.R.; Quadt, C.; Hooge, M.N.; et al. 89Zr-trastuzumab PET visualises HER2 downregulation by the HSP90 inhibitor NVP-AUY922 in a human tumour xenograft. Eur. J. Cancer 2010, 46, 678–684. [Google Scholar]
- Wong, J.Y.; Raubitschek, A.; Yamauchi, D.; Williams, L.E.; Wu, A.M.; Yazaki, P.; Shively, J.E.; Colcher, D.; Somlo, G. A pretherapybiodistribution and dosimetry study of indium-111-radiolabeledtrastuzumab in patients with human epidermal growth factor receptor 2-overexpressing breast cancer. Cancer Biother. Radiopharm. 2010, 25, 387–394. [Google Scholar] [CrossRef]
- McCabe, K.E.; Wu, A.M. Positive progress in immunoPET—Not just a coincidence. Cancer Biother. Radiopharm. 2010, 25, 253–261. [Google Scholar] [CrossRef]
- Dijkers, E.C.; Oude Munnink, T.H.; Kosterink, J.G.; Brouwers, A.H.; Jager, P.L.; de Jong, J.R.; van Dongen, G.A.; Schroder, C.P.; Lub-de Hooge, M.N.; de Vries, E.G. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin. Pharmacol. Ther. 2010, 87, 586–592. [Google Scholar] [CrossRef]
- Hoeben, B.A.; Kaanders, J.H.; Franssen, G.M.; Troost, E.G.; Rijken, P.F.; Oosterwijk, E.; van Dongen, G.A.; Oyen, W.J.; Boerman, O.C.; Bussink, J. PET of hypoxia with 89Zr-labeled cG250-F(ab')2 in head and neck tumors. J. Nucl. Med. 2010, 51, 1076–1083. [Google Scholar] [CrossRef]
- Borjesson, P.K.; Jauw, Y.W.; de Bree, R.; Roos, J.C.; Castelijns, J.A.; Leemans, C.R.; van Dongen, G.A.; Boellaard, R. Radiation dosimetry of 89Zr-labeled chimeric monoclonal antibody U36 as used for immuno-PET in head and neck cancer patients. J. Nucl. Med. 2009, 50, 1828–1836. [Google Scholar]
- Aerts, H.J.; Dubois, L.; Perk, L.; Vermaelen, P.; van Dongen, G.A.; Wouters, B.G.; Lambin, P. Disparity between in vivo EGFR expression and 89Zr-labeled cetuximab uptake assessed with PET. J. Nucl. Med. 2009, 50, 123–131. [Google Scholar]
- Nagengast, W.B.; de Vries, E.G.; Hospers, G.A.; Mulder, N.H.; de Jong, J.R.; Hollema, H.; Brouwers, A.H.; van Dongen, G.A.; Perk, L.R.; Lub-de Hooge, M.N. In vivo VEGF imaging with radiolabeledbevacizumab in a human ovarian tumor xenograft. J. Nucl. Med. 2007, 48, 1313–1319. [Google Scholar] [CrossRef]
- Verel, I.; Visser, G.W.; Boellaard, R.; Stigter-van Walsum, M.; Snow, G.B.; van Dongen, G.A. 89Zr immuno-PET: Comprehensive procedures for the production of 89Zr-labeled monoclonal antibodies. J. Nucl. Med. 2003, 44, 1271–1281. [Google Scholar]
- Perk, L.R.; Vosjan, M.J.; Visser, G.W.; Budde, M.; Jurek, P.; Kiefer, G.E.; van Dongen, G.A. p-Isothiocyanatobenzyl-desferrioxamine: A new bifunctionalchelate for facile radiolabeling of monoclonal antibodies with zirconium-89 for immuno-PET imaging. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 250–259. [Google Scholar] [CrossRef]
- Vosjan, M.J.; Perk, L.R.; Visser, G.W.; Budde, M.; Jurek, P.; Kiefer, G.E.; van Dongen, G.A. Conjugation and radiolabeling of monoclonal antibodies with zirconium-89 for PET imaging using the bifunctional chelate p-isothiocyanatobenzyl-desferrioxamine. Nat. Protoc. 2010, 5, 739–743. [Google Scholar] [CrossRef]
- Holland, J.P.; Sheh, Y.; Lewis, J.S. Standardized methods for the production of high specific-activity zirconium-89. Nucl. Med. Biol. 2009, 36, 729–739. [Google Scholar]
- Lindmo, T.; Boven, E.; Cuttitta, F.; Fedorko, J.; Bunn, P.A., Jr. Determination of the immunoreactive fraction of radiolabeled monoclonal antibodies by linear extrapolation to binding at infinite antigen excess. J. Immunol. Methods 1984, 72, 77–89. [Google Scholar] [CrossRef]
- Chanda, D.; Isayeva, T.; Kumar, S.; Siegal, G.P.; Szafran, A.A.; Zinn, K.R.; Reddy, V.V.; Ponnazhagan, S. Systemic osteoprotegerin gene therapy restores tumor-induced bone loss in a therapeutic model of breast cancer bone metastasis. Mol. Ther. 2008, 16, 871–878. [Google Scholar] [CrossRef]
- Holland, J.P.; Caldas-Lopes, E.; Divilov, V.; Longo, V.A.; Taldone, T.; Zatorska, D.; Chiosis, G.; Lewis, J.S. Measuring the pharmacodynamic effects of a novel Hsp90 inhibitor on HER2/neu expression in mice using Zr-DFO-trastuzumab. PLoSOne 2010, 5, e8859. [Google Scholar]
- Dijkers, E.C.; Kosterink, J.G.; Rademaker, A.P.; Perk, L.R.; van Dongen, G.A.; Bart, J.; de Jong, J.R.; de Vries, E.G.; Lub-de Hooge, M.N. Development and characterization of clinical-grade 89Zr-trastuzumab for HER2/neuimmunoPET imaging. J. Nucl. Med. 2009, 50, 974–981. [Google Scholar]
- di Cosimo, S.; Baselga, J. Management of breast cancer with targeted agents: Importance of heterogeneity. [corrected]. Nat. Rev. Clin. Oncol. 2010, 7, 139–147. [Google Scholar] [CrossRef]
- Bartlett, A.I.; Starcyznski, J.; Robson, T.; Maclellan, A.; Campbell, F.M.; van de Velde, C.J.; Hasenburg, A.; Markopoulos, C.; Seynaeve, C.; Rea, D.; et al. Heterogeneous HER2 gene amplification: Impact on patient outcome and a clinically relevant definition. Am. J. Clin. Pathol. 2011, 136, 266–274. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chang, A.J.; DeSilva, R.; Jain, S.; Lears, K.; Rogers, B.; Lapi, S. 89Zr-Radiolabeled Trastuzumab Imaging in Orthotopic and Metastatic Breast Tumors. Pharmaceuticals 2012, 5, 79-93. https://doi.org/10.3390/ph5010079
Chang AJ, DeSilva R, Jain S, Lears K, Rogers B, Lapi S. 89Zr-Radiolabeled Trastuzumab Imaging in Orthotopic and Metastatic Breast Tumors. Pharmaceuticals. 2012; 5(1):79-93. https://doi.org/10.3390/ph5010079
Chicago/Turabian StyleChang, Albert J., Ravindra DeSilva, Sandeep Jain, Kimberley Lears, Buck Rogers, and Suzanne Lapi. 2012. "89Zr-Radiolabeled Trastuzumab Imaging in Orthotopic and Metastatic Breast Tumors" Pharmaceuticals 5, no. 1: 79-93. https://doi.org/10.3390/ph5010079



