Abstract
At the time of birth, humans experience an induced pro-inflammatory beneficial event. The mediators of this encouraged activity, is a fleet of bacteria that assault all mucosal surfaces as well as the skin. Thus initiating effects that eventually provide the infant with immune tissue maturation. These effects occur beneath an emergent immune system surveillance and antigenic tolerance capability radar. Over time, continuous and regulated interactions with environmental as well as commensal microbial, viral, and other antigens lead to an adapted and maintained symbiotic state of tolerance, especially in the gastrointestinal tract (GIT) the organ site of the largest microbial biomass. However, the perplexing and much debated surprise has been that all microbes need not be targeted for destruction. The advent of sophisticated genomic techniques has led to microbiome studies that have begun to clarify the critical and important biochemical activities that commensal bacteria provide to ensure continued GIT homeostasis. Until recently, the GIT and its associated micro-biometabolome was a neglected factor in chronic disease development and end organ function. A systematic underestimation has been to undervalue the contribution of a persistent GIT dysbiotic (a gut barrier associated abnormality) state. Dysbiosis provides a plausible clue as to the origin of systemic metabolic disorders encountered in clinical practice that may explain the epidemic of chronic diseases. Here we further build a hypothesis that posits the role that subtle adverse responses by the GIT microbiome may have in chronic diseases. Environmentally/nutritionally/and gut derived triggers can maintain microbiome perturbations that drive an abnormal overload of dysbiosis. Live probiotic cultures with specific metabolic properties may assist the GIT microbiota and reduce the local metabolic dysfunctions. As such the effect may translate to a useful clinical treatment approach for patients diagnosed with a metabolic disease for end organs such as the kidney and liver. A profile emerges that shows that bacteria are diverse, abundant, and ubiquitous and have significantly influenced the evolution of the eukaryotic cell.
1. Introduction
The microbial communities that colonize the human GIT have been collectively referred to as the gut microbiota. The resident commensal cohort adapts to the local environmental/milieu conditions of the human host and establishes a complex ecosystem in which host–microbe, milieu–microbe and microbe–microbe interactions oversee the composition and dynamics of the GIT microbial and host cell community.
A recent analysis of gut microbial communities illustrates how the commensal community in the GIT, alter their make-up according to the milieu composition that is derived from different nutritional practices. The proposal has documented that there may be three predominant GIT microbial family types that predominate in the gut and have been designated as enterotypes. These include the Bacteroides, Prevotella, and Ruminococcus, families of bacteria [1]. A subsequent study that also investigated the association of dietary and environmental variables with the gut microbiota reported that the GIT microbiome was an entity with functional plasticity. In effect this flexibility is subject to environmental/nutritional signal(s) adaptation as evidenced by changed patterns of enterotype governance [2]. Wu and colleagues also reported, that the faecal communities clustered into enterotypes, and were distinguished primarily by the levels of Bacteroides and Prevotella that were present [1]. They concluded that the enterotypes were strongly associated with long-term diets, mainly protein and animal fat (Bacteroides) against carbohydrates (Prevotella). Furthermore, a controlled-feeding study of 10 subjects showed that microbiome compositions changed detectably within 24 h of initiating a high-fat/low-fiber or low-fat/high-fiber diet, whilst enterotype identity remained stable during the 10-day study. This data indicated that alternative GIT enterotype conditions could be dependent on long-term dietary patterns. Hence what is the biological significance of these studies, as yet, remains inconclusive [2]. However, enterpotype designation may not be as clear cut as envisaged, given that these human-associated bacterial diversity studies have categorized individuals into enterotypes/clusters based on the abundances of key bacterial genera in the gut microbiota. A recent meta-analysis of microbial community structures in humans recommends that multiple approaches may be required when testing and comparing for enterotypes [3].
As recent studies begin to report variations in gut metabolites our understanding of the host microbiome variations in health and disease progresses. For example it has been reported that in individuals with enriched gut microbe types (e.g., increased proportions of Prevotella in the gut exhibit a significantly higher plasma concentration of trimethylamine-N-oxide a pro-atherogenic metabolite) than individuals with a Bacteroides enterotype [4]. This very much indicating that enterotypes and their variations affect the host. Moreover Roager and colleagues [5] have recently shown that the ratio, of Prevotella spp to Bacteroides spp provides an additional stratification step that further fine tunes the profile of gut enterotypes. This may further enhance assessment of gut directed interventions in health and disease states.
Over the past several decades though, research has seen a refocusing of thinking and effort directed towards elucidating the critical inter-relationships that exist between the GIT microbiome and its host. This research has redefined the interactions between gut microbes and vertebrates, now recognising that the microbial active cohort and its mammalian host have shared co-evolutionary metabolic interactions that span millennia. Microbial interactions in the GIT provide the necessary cues for the development of regulated pro- and anti-inflammatory signals that promotes immunological tolerance, metabolic regulation and other factors which may then control local and extra-intestinal inflammation.
Furthermore, it is also becoming apparent that the GIT with its commensal cohort is a central regulator for the activities of end organs such as the kidneys, brain, adipose tissue, muscle and liver and as such may provide local prompts that are transmitted extra-intestinally to end organ sites. A scientific insight therefore has emerged that plausibly links the GIT with the physiology of end organ function that may influence health maintenance or trigger and support a disease state. Maintaining a healthy GIT milieu and epithelium with the administration of probiotics may constitute a novel therapeutic strategy for health.
2. Methods
A systematic search of the literature covering the years 2000–2014 was conducted using PubMed, the Cochrane Library, Science Direct, Scopus, EMBASE, MEDLINE and CINAHL.
2.1. Search Terms
Articles were identified using the search terms, “Probiotics” OR “Prebiotics” OR “Commensal Bacteria” OR symbiotics AND “Gastrointestinal Tract Diseases” and “Brain” AND “Kidney Disease” AND “Adipose Tissue” AND “Joint Diseases” AND “Liver Diseases” AND “Lung Diseases” AND “Immune Deficits”. The Inclusion criteria for this review were: (1) An RCT and/or cross-over clinical trial that used either a placebo comparator or other as a control; (2) Human participants (children, adolescents or adults); (3) The clinical study was published in English. A flow diagram of studies included and excluded is presented in Figure 1.
3. Clinical Studies
Probiotics are live bacterial cultures that are added to foods (e.g., yoghurts) and employed as dietary supplements, that when orally administered can improve the health of the host beyond their fundamental basic nutritional content [6]. Probiotic bacteria encompass those from different genera (as for example Lactobacilli, Bifidobacterium, Escherichia, Saccharomyces (a yeast), Streptococcus) giving rise to a variety of different species of each genera (i.e., Lactobacillus acidophilus; Lactobacillus bulgaricus, Lactobacillus rhamnosus); that lead to different strains within a species (i.e., Lactobacillus acidophilus La-1, Lactobacillus acidophilus NCFM). This taxonomic differentiation, importantly emphasizing that different strains from the same bacterial species may exhibit variable activity and as such may elaborate different physiological functions within the GIT [7] whilst exhibiting overlapping or specific therapeutic actions to different organ systems [8].
Figure 1.
Flow diagram of studies for limited review.
3.1. Probiotics and the GIT
Perhaps the most studied site for investigating probiotic efficacy is the GIT. It has been reported [9] that probiotic bacteria may operate on three levels of host functionality that enhances GIT and extra intestinal functions (Figure 2) namely, (i) interfering with the growth of pathogenic bacteria in the lumen of the GIT; (ii) strengthening the epithelial gut lining’s barrier function and mucosal immunity as well as mucus production; and (iii) beyond the gut, have an effect on the systemic immune system, as well as other cell and organ systems such as the liver. Numerous studies have reported the efficacious use of probiotics (Table 1) [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44]. Irritable bowel syndrome causes abdominal pain, bloating and alternating constipation and diarrhea. Clinical studies with probiotics overall have demonstrated efficacy for reducing abdominal pain [10,11,12,13,14,15,16,17,18]. In clinical trials [19,20,21] investigating functional abdominal pain and associated symptomatology two studies [19,20] that employed.
L. reuteri demonstrated efficacy. Several clinical studies have investigated the administration of probiotics in reducing antibiotic associated diarrhea [22,23,24,25,26,27,28,29]. Most studies demonstrated efficacy in reducing the development of antibiotic associated diarrhea and or duration of antibiotic associated diarrhea [23,24,26,27,28,29]. A number of clinical investigations have tested the efficacy of probiotics to reduce helicobacter pylori infection [30,31,32,33,34,35]. All studies included in this review bar one [30] reported an efficacious outcome with probiotic administration. Furthermore, several studies have also clinically investigated the efficacy of probiotics for functional gastrointestinal symptoms (e.g., pouchitis among others) [36,37,38,39,40,41]. All studies except two [39,41] reported an efficacious outcome. We reviewed three studies with very low weight or pretern birth infants and the efficacy of probiotics and the outcomes were contentious [42,43,44].
3.2. Probiotics and the Liver
A recent report has advanced the hypothesis that there exits a gut–liver axis that suggests the GIT microbiota may significantly affect liver physiology and act as a co–factor in the etiology of chronic liver disease [45]. This hypothesis has stemmed largely from the longstanding practice of using lactulose in the treatment of hepatic encephalopathy [46]. This then, suggesting gut microbiota involvement in the management of chronic liver disease. A GIT microbiota that sustains a persistent low level pro–inflammatory pathogenic profile could modulate liver damage caused by ethanol and other toxic compounds such as acetaldehyde, phenols and endotoxins.
Table 2 summarizes numerous studies that have employed probiotics in the treatment of chronic liver diseases reporting significant improvements [47,48,49,50,51,52,53,54,55,56,57]. Clinical studies that demonstrated efficacy were related to improving endotoxemia that in turn improved liver functionality [47,48,49,50]. It would seem that the probiotic actions most relevant to chronic liver diseases are modification of intestinal barrier function and the prevention of bacterial/toxin translocations. Increased GIT overloads with Gram-negative bacteria, increased permeability and impaired immunity may all contribute to increased bacterial/toxin translocations. Furthermore, a strong correlation between the rate of bacterial/toxin overload and the severity of cirrhosis was demonstrated [51,52,53,54,55]. Hence, multi-strain probiotics may alter gut flora and rescue the GIT microbiome towards a protective commensal bacteria profile with a concomitant increase in GIT epithelial barrier function.
3.3. Probiotics and Obesity
In vitro screening-experiments with bacteria from the genus Lactobacillus and bifidobacteria isolated from the human GIT have demonstrated significant cholesterol-lowering actions [58]. Recent findings suggest that a high-fat diet and the GIT bacterial cohort interact to promote early inflammatory changes in the gut that contribute to the development of obesity and insulin resistance [59].
Table 3 presents clinical studies that have investigated probiotic preparations in obesity [60,61,62,63,64]. The overall trend is that probiotic preparations could positively influence weight reduction. Specifically, in a study with healthy infants [60] it was demonstrated that probiotic administration significantly lowered levels of palmitoleic acid and significantly increased levels of putrescine. The data suggest that palmitoleic acid a major monounsaturated fatty acid (MUFA) that is strongly linked to visceral obesity was reduced with probiotic supplementation. While putrescine a polyamine with importance for gut integrity was beneficially increased. Probiotic supplementation in adulthood [61] and during the childhood (from birth to 10 years) [62] demonstrated that probiotics at least in part assisted with the control of abdominal visceral and subcutaneous fat. In an additional study administration of a multi-strain probiotic supplement provided a synergistic effect on overweight and obese individuals when provided with a weight loss diet [63]. In a further study with overweight children a multi-strain probiotic formulation significantly demonstrated decreased blood lipid profiles [64].
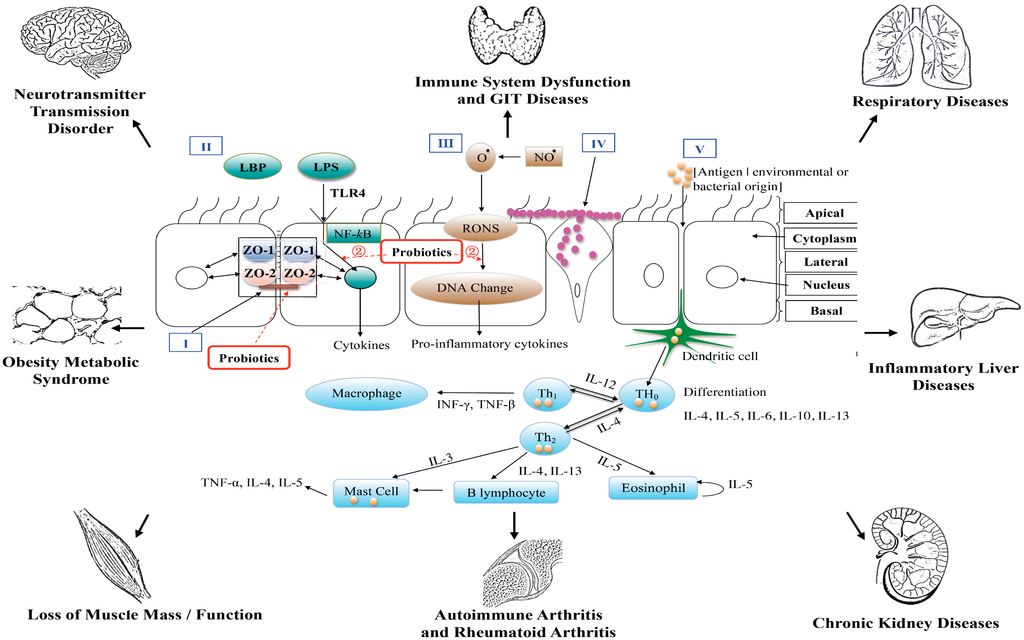
Figure 2.
A diagrammatic representation of the epithelial barrier of the gastrointestinal tract and end–organ associations.

Table 1.
Clinical studies of probiotics (with and without prebiotics) and gastrointestinal tract diseases.
| Participant Type | Study Type (N° Patients) | Treatment | Duration | Results | Ref. |
|---|---|---|---|---|---|
| Irritable Bowel Syndrome | |||||
| Irritable bowel syndrome –constipation predominant | DBPCT (34) | 1 × 108 CFU/g B. lactis DN-173 010 125g/b.i.d. | 4 weeks | ↑Abdominal girth and gastrointestinal transit ↓Symptoms scores of IBS | [10] |
| Irritable bowel syndrome | DBPCT (55) | 1 × 1010 CFU/cap L. rhamnosus GG/b.i.d. | 6 weeks | ↓Abdominal pain | [11] |
| Irritable bowel syndrome | RCT (77) | 1 × 1010 CFU B. infantis 35624/o.i.d. | 8 weeks | ↓Abdominal pain Normalization of Th1/Th2 balance | [12] |
| Irritable bowel syndrome | DBPCT (40) | 2 × 109 CFU/mL L. acidophilus-SDC 2012/o.i.d. | 4 weeks | ↓Abdominal pain or discomfort | [13] |
| Irritable bowel syndrome | DBPCT (52) | 2.5 × 1010 CFU/cap L. acidophilus CUL60 and CUL21 B. lactis CUL34 and B. bifidum CUL20/o.i.d. | 8 weeks | ↓Symptoms scores of IBS ↑Scores for quality of life, days without pain and satisfaction with bowel habit | [14] |
| Irritable bowel syndrome | DBPCT (52) | 5 × 107 CFU/mL L. paracasei ssp paracasei F19 5 × 107 CFU/mL L. acidophilus La5 5 × 107 CFU/mL B. lactis/Bb12 200 mL/b.i.d. | 8 weeks | No clear positive effect on IBS symptoms | [15] |
| Irritable bowel syndrome—diarrhea predominant | DBPCT (30) | 1 × 108 CFU/mL S. thermophiles/1 × 107 CFU/mL L. bulgaricus/ 1 × 107 CFU/mL L. acidophilus/1 × 107 CFU/mL B. Longum 200 mL/b.i.d. | 4 weeks | ↑IBS scores ↓Intestinal permeability | [16] |
| Irritable bowel syndrome | DBPCT(122) | 1 × 109 CFU/cap B. bifidum MIMBb75/o.i.d. | 4 weeks | ↑IBS scores | [17] |
| Irritable bowel syndrome –diarrhea predominant | DBPCT (297) | Inactivated L. acidophilus LB [dose administered not provided] | 6 weeks | ↓Number of stools | [18] |
| Functional Abdominal Pain/Associated Symptoms | |||||
| Functional gastroesphageal reflux | DBPCT(44) | 1 × 108 CFU/cap L. reuteri DSM 17938/o.i.d. | 4 weeks | ↓Median fasting antral area ↑Delta in gastric emptying rate ↓Median episodes per day of regurgitation | [19] |
| Functional abdominal pain | DBPCT (60) | 2 × 108 CFU/cap L. reuteri DSM 17938/b.i.d. | 4 weeks | ↓Abdominal pain | [20] |
| Functional gastrointestinal symptoms | DBPCT(17) | 1-5 × 1010 CFU L. rhamnosus GG/b.i.d | 2 weeks | No evidence of efficacy | [21] |
| Antibiotic Associated Diarrhea | |||||
| Antibiotic-associated diarrhea | DBPCT (2941) | 6 × 1010 CFU/cap L.acidophilus CUL60, and CUL21, B. bifidum CUL20, and B lactis CUL34/o.i.d | 8 weeks | No evidence of efficacy | [22] |
| Antibiotic-associated diarrhea | DBPCT (89) | 5 × 108 CFU/g L. acidophilus Cl1285 and L. casei 98 g/o.i.d. | During antibiotic treatment | Prevention of antibiotic-associated diarrhea in hospitalized patients | [23] |
| Antibiotic-associated diarrhea | DBPCT (255) | 50 or 100 × 109 CFU/cap L. acidophilus CL1285 and L. casei LBC80R/o.i.d. or b.i.d. during antibiotic treatment | 2 weeks | ↓Risk of antibiotic-associated diarrhea | [24] |
| Antibiotic-associated diarrhea | DBPCT (275) | 5 × 109 CFU/cap S. boulardii/b.i.d | during treatment + 1 week | No preventing effect on the development of antibiotic-associated diarrhea | [25] |
| Antibiotic-associated diarrhea | DBPCT (437) | 5 × 108 CFU/g L. acidophilus CL1285®/L. casei 98 g/o.i.d. during antibiotic treatment | 5 weeks | ↓Duration of diarrhea | [26] |
| Antibiotic-associated diarrhea | DBPCT (113) | 1 × 108 CFU/mL L. casei/L. bulgaricus/S. thermophilus 97mL/b.i.d. during antibiotic treatment | 2 weeks | ↓Risk of antibiotic-associated diarrhea | [27] |
| Antibiotic-associated diarrhea | DBPCT (229) | 4.5 × 1011 CFU/sachet B. breve, B. longum, B. infantis, L. acidophilus, L. plantarum, L. paracasei, L.delbrueckii subsp. bulgaricus, S. thermophilus/b.i.d | during antibiotic course+1 week | ↓Risk of antibiotic-associated diarrhea | [28] |
| Acute rotavirus diarrhea | DBPCT (64) | 4 × 1010 CFU/dose S. boulardii or 6.625 × 107 CFU/dose, L.acidophilus, 8.75 × 106 CFU/dose L. rhamnosus, B. longum and 1.375 × 107CFU/dose S. Boulardii/b.i.d | 5 days | ↓Median duration of diarrhea and fever in children who received the single species product ↓Vomiting in children who received the mixed species product | [29] |
| Helicobacter pylori eradication | |||||
| H. pylori therapy | DBPCT (107) | 1.25 × 109 CFU L. acidophilus, 1.25 × 109 CFU L.rhamnosus, 1.25 × 109 CFU B.bifidum and S. faecium (b.i.d) | 1 week | No evidence of increased efficacy | [30] |
| H. pylori therapy | Open label | 30 × 108 CFU B. infantis/b.i.d | 2 weeks | ↑ Cure rates | [31] |
| H. pylori therapy | DBPCT (88) | 1 × 106 CFU/g L. acidophilus LA-5/ 1 × 106 CFU/g B. lactis BB-12 125 g/b.i.d. during H. pylori eradication | 5 weeks | ↓Duration of antibiotics-associated diarrhea ↓Gastrointestinal complaints | [32] |
| H. pylori therapy | Open label (228) | 3 × 107 CFU L. acidophilus/o.i.d | 2 weeks | ↑ Cure rates | [33] |
| H. pylori therapy | Open label (90) | 1×108 CFU L.reuteri/o.i.d | 1 week | ↑ Cure rates ↓Frequency and the intensity of antibiotic-associated side-effects | [34] |
| H. pylori positive subjects | DBPCT (22) | 5 × 109 CFU/tablet dead L. reuteris DSMZ17648/4 tablets/b.i.d | 2 weeks | ↓ H. pylori | [35] |
| Functional gastrointestinal symptoms | |||||
| Chronic pouchitis | DBPCT (20) | (0.5–1) × 1010 CFU/capsule L. rhamnosus GG/2 caps, b.i.d | 12 weeks | ↑Ratio of total faecal lactobacilli to total faecal anaerobes ↑Frequency of lactobacilli-positive cultures in the pouch and afferent limb mucosal biopsy | [36] |
| Functional gastrointestinal symptoms | TBPCT (87) | 1.8 × 109 or 17.2 × 109 CFU/cap B. lactis HN019/o.i.d. | 2 weeks | ↓Whole gut transit time ↓Functional gastrointestinal symptoms | [37] |
| Healthy, postprandial intestinal gas-related symptom | DBPCT (61) | 2 × 109 CFU/cap B. coagulans/o.i.d. | 4 weeks | ↓Abdominal pain ↓Distension scoreNo significant differences in flatus, bloating and gas scores | [38] |
| Elderly patients receiving enteral feeding | DBPCT (123) | 2.5 × 1010 CFU/sachet B. longum BB536 or 5 × 1010CFU/sachet B. longum BB536/b.i.d | 16 weeks | ↑Bowel movements in patients with a low frequency of defecation ↓Bowel movements of patients with a high frequency of defecation | [39] |
| Elderly patients receiving enteral feeding | DBPCT (83) | 5 × 1010 CFU/sachet B. longum BB536/o.i.d | 16 weeks | No significant changes in the frequency of defecation | [39] |
| Women with mild digestive symptoms | DBPCT (197) | 1 × 108 CFU/g B. lactis DN-173 010 125g/b.i.d. | 4 weeks | ↑Gastrointestinal well-being ↓Digestive symptoms | [40] |
| Women with minor digestive symptoms | DBPCT (324) | 1. 107 CFU/g B. lactis and 9.26 × 106 CFU/g S. thermophilus and L. bulgaricus/125 g/b.i.d | 4 weeks | No improvement in GI well-being | [41] |
| Very low weight infants/preterm infants | |||||
| Very low-birth weight infants | DBPCT (221) | 3.5 × 1018 CFU/mL L. sporogenes | from first feed until discharge | No significant difference in the incidence of death or necrotizing enterocolitis ↓Feeding intolerance | [42] |
| Preterm infants | BRCT (81) | 2 × 107 CFU/g of milk powder B. lactis (daily milk volume increasing during treatment) | 4 weeks | ↓Intestinal permeability ↑Head growth | [43] |
| Prophylatic use in newborn infants | DBRCT (589) | L reuteri DSM 17938 Dose of 1 × 108 CFU/day | 12 weeks | ↓the onset of functional gastrointestinal disorders | [44] |
L. = Lactobacillus; B. = Bifidobacteria; P. = Propionibacterium; S. = Saccharomyces (boulardii); S. = Streptococcus (thermophilus); CFU: colony-forming unit; RCT: Randomized Controlled Trial; DBPCT: double bind placebo controlled trial; TBPCT: triple blind placebo controlled trial; o.i.d: once daily; b.i.d: twice daily; t.i.d: three times daily.

Table 2.
Clinical studies of probiotics and liver disease.
| Participant Type | Study Type (N°. Patients) | Treatment | Duration | Results | Ref. |
|---|---|---|---|---|---|
| Alcoholic liver disease | Open-label (66) | 0.9 × 108CFU/cap B. bifidum/0.9 × 109CFU/cap L. plantarum/o.i.d. | 5 days | Restoration of the bowel flora Improvement in alcohol-induced liver injury | [47] |
| Alcoholic liver disease | DBPCT (49) | 2.5–25 × 109CFU/cap E. coli Nissle twice the amount after 5 days/o.i.d. | 6 weeks | Improvement of intestinal colonization in the E. coli, ↓Endotoxemia Improvement of liver functions | [48] |
| Alcoholic liver disease | Open-label (20) | 6.5 × 109CFU/cap L. casei Shirota/t.i.d. | 4 weeks | Restore neutrophil function, ex vivo endotoxin- stimulated levels of sTNFR1, sTNFR2 and IL10 normalized TLR4 expression | [49] |
| Alcoholic liver disease, Nonalcoholic Fatty Liver Disease | Open-label (78) | 4.5 × 1011CFU/cap S. thermophilus/B. breve/B. longum/B. infantis/L. acidophilus/L. plantarum/L. casei/L. bulgaricus/o.i.d. | 12 weeks | Improvement of plasma level of MDA and 4-HE, whereas cytokines (TNF-alpha, IL-6, and IL-10) improved only in ALD patients | [50] |
| Cirrhosis | RCT (39) | E. Nissle/o.i.d. [dose administered not provided] | 12 weeks | Improvement in intestinal colonisation Improvement in liver function assessed with the Child-Pugh classification. | [51] |
| Cirrhosis | RCT (81) | 109CFU/capsule B. bifidus/L. acidophilus/L.bulgaricus S. thermophilus/t.i.d. | 2 weeks | ↓Escherichia coli count ↓Intestinal flora imbalance Improvement in debilitation, food intake, appetite, abdominal distension, and ascitic fluid | [52] |
| Cirrhosis | DBPCT (36) | 2 × 1010 CFU/cap L. acidophilus/L. bulgaricus/B. lactis/S. thermophilus/o.i.d. | 24 weeks | ↓Ammonia levels starting after 1 month of treatment in patients with baseline ammonia levels > 50 mmol/LNo effect on liver enzyme | [53] |
| Cirrhosis | RCT (8) | 1.8 × 1012CFU/cap S. thermophiles/B. breve/B. longum/B. infantis/L. acidophilus/L. plantarum/L. casei/L. bulgaricus/b.i.d. | 8 weeks | ↑Serum TNF-α ↓Plasma aldosterone. | [54] |
| Cirrhosis | RCT (50) | 2.1 × 107CFU/cap Bifidobacterium/L. acidophilus/Enterococcus/t.i.d. or 9 × 108CFU/cap B. subtilis/1 × 108 CFU/cap E. faecium/t.i.d. | 2 weeks | ↑ Bifidobacterium count ↓Fecal pH, fecal and blood ammonia. ↓Endotoxin in cirrhotic patients with endotoxemia (probiotics containing Bacillus subtilis and Enterococcus faecium) | [55] |
| Hepathic encephalopathy | DBPCT (55) | 1010 CFU/cap P. pentoseceus 5–33:3/L. mesenteroides 32–77:1/L. paracasei subspecies paracasei 19/L. plantarum 2592/o.i.d. | 4 weeks | ↑Fecal content of non-urease-producing
Lactobacillus species at the expense of these other bacterial species ↓Blood ammonia levels and reversal of HE ↓Endotoxemia. | [56] |
| Hepathic encephalopathy | DBPCT (60) | B. longum/o.i.d. And FOS [dose administered not provided] | 12 weeks | Improving neuropsychological testing, serum ammonia levels | [57] |
B. = Bacillus E. = Enterococcus; E. = Escherichia coli; P. = Pediacoccus.
Tien et al. [65] have reported that the anti-inflammatory effects of Lactobacillus casei are negatively associated with NF-κB activation. Figure 2 provides a diagrammatic view. Therefore, is has been hypothesised that health properties of probiotics could be related to peroxisome proliferator-activated receptor gamma (PPARg) activation, which then blocks the activity of NF-κB [66,67]. Hence it is interesting to note that over-consumption of food triggers GIT pro-inflammatory bacterial activity; this then may induce GIT metabolic dysfunction increasing the risk of metabolic diseases. Whereas a healthy diet with an optimally balanced GIT microbiota that promotes regulated/controlled PPARg activation could alleviate or suppress the risk of developing metabolic diseases such as T2DM.
3.4. Probiotics and the Brain
There is an increasing body of preclinical evidence that supports an important role that the gut microbiota may promote emotional behavior and may influence underlying brain mechanisms [68,69,70]. Studies with germ-free mice have demonstrated the important role of gut microbiota in brain development and resultant adult pain responses and emotional behaviors, as well as on adult hypothalamic-pituitary axis responsiveness.
Of the scant clinical trials that have investigated probiotics and brain behavior, the results have shown significant improvement in behavior with probiotic administration (Table 4) [71,72,73,74,75]. In one study assessing patients with traumatic brain injury, probiotic supplementation improved the anti-inflammatory clinical picture [74].
3.5. Probiotics and CKD
The dysfunction of the kidneys leads to disturbed renal metabolism and to impaired glomerular filtration and tubular secretion/reabsorption problems. This results in the retention of toxic solutes, which affect all organs of the body. It has been posited that toxins generated by gastrointestinal dysbiosis, and introduced into the body via the small and large bowel, may all contribute to CKD. They comprise advanced glycation end products, phenols and indoles [76]. Moreover, recent reports suggest that the bacterial load and the adverse products of the intestinal microbiota might influence chronic disease pathogenesis [1,2]. This is particularly relevant to the development of CKD, a disease of increasing prevalence in many Western societies. It has also been recently reported that the pharmacobiotic potential of the GIT micro–biometabolome may provide a plausible therapeutic role with the administration of live multi–strain probiotic cultures [77].
Although the current evidence as to the efficacy of probiotics to reduce uremic toxins is limited, the clinical evidence demonstrates that specific strains in a multiple–strain matrix configuration, in combination with prebiotics, may be most beneficial in reducing gut derived uremic toxins (Table 5) [78,79,80]. In addition, selecting probiotic species with known metabolic function, such as Streptococcus thermophilus, for metabolizing urea as a nitrogen growth source could contribute to reducing uremia.

Table 3.
Clinical studies of probiotics and obesity.
| Patients | Study Type (N° Patients) | Treatment | Duration | Results | Ref. |
|---|---|---|---|---|---|
| Healthy Infants | RCT (179) | 1 × 108 CFU/g L. paracasei ssp. paracasei F19/100 g b.i.d. | 28 weeks | ↓Palmitoleic acid ↑Putrescine | [60] |
| Adults with obese tendencies | DBPCT (87) | 5 × 108 CFU/g L. gasseri SBT2055 CFU/200 g daily | 12 weeks | ↓Abdominal visceral and subcutaneous fat areas ↓Body weight and other measures ↑High-molecular weight adiponectin in serum | [61] |
| Pregnant Women with obese tendencies | DBPCT (159) | 1 × 1010 CFU/cap L. rhamnosus GG/o.i.d. | 4 weeks | Moderation of the initial phase of excessive weight gain of the children, but not of the second phase of excessive weight gain | [62] |
| Obese Adults | DBPCT (75) | 1 × 108 CFU/mL L. acidophilus La5/ 1 × 108 CFU/mL B. BB12/ 1 × 108 CFU/mL L. casei DN001/o.i.d. | 8 weeks | ↓Expression of T-bet gene. | [63] |
| Overweight and obese children | TBPCT (70) | 2.0 × 108 CFU L. casei, L.s rhamnosus, S. thermophilus, B. breve, L. acidophilus, B. longum and L. bulgaricus with prebiotics (fructo oligosaccharides), Vitamin E, Vitamin A and Vitamin C/o.i.d | 8 weeks | ↓Serum triglycerides, total- and low density lipoprotein-cholesterol levels | [64] |
SBCT: single bind controlled trial.

Table 4.
Clinical studies of probiotics and brain disease.
| Participant Type | Study Type (N° Patients) | Treatment | Duration | Results | Ref. |
|---|---|---|---|---|---|
| Anxiety-depressive symptoms | DBPCT (132) | 108 CFU/capsule L. casei/65mL/i.o.d. | 3 weeks | Improvement in mood scores | [71] |
| Chronic fatigue syndrome | DBPCT (39) | 8 × 107 CFU/sachet L. casei strain Shirota/t.i.d. | 8 weeks | ↑Fecal total Bifidobacteria and Lactobacillus ↓Anxiety symptoms | [72] |
| Healthy adults | DBPCT (25) | 3 × 109CFU/sachet L. helveticus R0052/3 × 109CFU/cap B. longum R0175/i.o.d. | 2 weeks | ↓Behaviors indicative of anxiety | [73] |
| Traumatic brain injury | SBCT (52) | 0.5 × 108CFU/sachet B. longum/ 0.5 × 107CFU/sachet L. bulgaricus 0.5 ×107CFU/cap S. thermophilues/t.i.d. | 3 weeks | Adjustment of the Th1/Th2 imbalance ↓Infection rate ↓Use of antibiotics ↑Level of IL-12 | [74] |
| Healthy women with no gastrointestinal or psychiatric symptoms | DBPCT (36) | 1.2×109 CFU/cup S. Thermophilues L. bulgaricus/b.i.d. | 4 weeks | ↓Task-related response of a distributed functional network containing affective, viscerosensory, and somatosensory cortices | [75] |
3.6. Probiotics and Joint Disease
Patients diagnosed with joint diseases have been reported as predisposed to GIT disturbances [81].
There are a small number of human clinical trials (Table 6) [82,83,84,85,86] that have assessed the therapeutic efficacy of administering probiotics to patients with autoimmune arthritic diseases. However, there are no clinical studies that have investigated the role of probiotics in reducing the symptoms of osteoarthritis. A recent animal study though has provided plausible data that a probiotic strain investigated, namely, Lactobacillus casei could act as a potent nutraceutical modulator for the treatment of osteoarthritis. Pain was reduced, as were inflammatory responses, and articular cartilage degradation [87].
3.7. Probiotics and Respiratory Diseases
Respiratory allergies include allergic rhinitis, sinusitis and asthma. The advent of the hygiene hypothesis has proposed that the increase in allergic diseases reflects a decrease in infections during childhood [88]. Clinical trials have also suggested that the exposure to microbes through the GIT robustly shapes immune function [89].
Probiotics have been reported to exert a beneficial effect in the prevention as well as the treatment of allergic diseases through modification of immune system of host via the GIT ecosystem. This has prompted studies (Table 7) [90,91,92,93,94,95,96,97,98,99,100,101] of feeding probiotics in prevention as well as the treatment of respiratory allergies. The clinical data presents a contentious profile of probiotic efficacy. In a recent controlled study it was reported that long–term consumption of fermented milk containing Lactobacillus casei may improve the health status of children with allergic rhinitis, however no effect was found in asthmatic children [92].
3.8. Probiotics and Skin Conditions
Lactobacillus GG has been reported to reduce clinical symptoms, intestinal inflammation and mucosal barrier permeability in infants with allergic dermatitis [102].
Allergic conditions are caused by abnormal or exaggerated immune reactions of the skin. A range of symptoms can be expressed however the most common chronic allergic conditions of the skin are atopic dermatitis/eczema. Probiotics are reported to exert some benefit in such conditions, which is thought to be due to the immune modulating effects of the bacteria. Studies demonstrate that probiotics contribute to relief of symptoms and also prevention of atopic conditions in infants (Table 8) [103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119]. In one study a probiotic preparation induced the repair of ultra violet damaged skin [103].

Table 5.
Clinical studies of probiotics and chronic kidney disease.
| Patients | Study Type (N° Patients) | Treatment | Duration | Results | Ref. |
|---|---|---|---|---|---|
| Chronic kidney disease (stages 3 and 4) | DBPCT (13) | 1.5 × 109 CFU/cap L. acidophilus KB31/B. longum KB35/S. thermophilus KB27/2 capsules/t.i.d. | 24 weeks | Moderate changes in uric acid concentration No significant difference in serum creatinine concentration | [78] |
| Chronic kidney disease (stages 3 and 4) | DBPCT (246) | 1.5 × 109 CFU/cap L. acidophilus/B. longum/S. thermophilus/2 capsules/t.i.d. | 24 weeks | ↓Blood urea nitrogen. ↑Well-being with no serious adverse effects. | [79] |
| Chronic kidney disease | DBPCT (9) | 1 × 108 CFU L. casei strain Shirota/B. breve strain Yakult with 1.67 g galacto-oligosaccharides/t.i.d. | 4 weeks | ↑Quantity and normalization of the stools ↓Serum p-cresol level. | [80] |

Table 6.
Clinical studies of probiotics and joint diseases.
| Patients | Study Type (N° Patients) | Treatment | Duration | Results | Ref. |
|---|---|---|---|---|---|
| Rheumatoid arthritis Sulfasalazine treated patients | PCT (12) | 0.9 × 108 CFU/sachet L. acidophilus L10/B. lactis B94/S. salivarius K12/b.i.d | 12 weeks | No influence on the Sulfasalazine metabolism. | [83] |
| Rheumatoid arthritis | DBPCT (45) | 2 × 109 CFU/caplet B. coagulans GBI-30/b.i.d. | 8 weeks | ↓Pain scores. Improvement of global assessment and self-assessed disability | [84] |
| Rheumatoid arthritis | DBPCT (29) | 2 × 109 CFU/cap L.s reuteri RC-14/L. rhamnosus GR-1/b.i.d. | 12 weeks | No differences observed | [85] |
| Spondyloarthritis | DBPCT (63) | 1 × 108 CFU/g S. salivarius K12/4 × 108 CFU/g B. lactis LAFTI B94 1 × 108 CFU/g L. acidophilus LAFTI L100.8 g/b.i.d. | 3 weeks | No significant difference was noted between groups in any of the core domains | [86] |
S. = Streptococcus (salivarius); PCT: Placebo Clinical Trial

Table 7.
Clinical studies of probiotics and respiratory allergic diseases.
| Participant Type | Study Type (N°. Patients) | Treatment | Duration | Results | Ref. |
|---|---|---|---|---|---|
| Asthma and allergic rhinitis | DBPCT (101) | 2 × 109 CFU/cap L.gasseri/o.i.d. | 8 weeks | ↓Clinical symptom scores ↓TNF-α, IFN-γ, IL-12, and IL-13 production by the PBMCs | [90] |
| Grass pollen-dependent allergic rhino-conjunctivitis | DBPCT (30) | 2.5–25 × 109 CFU/cap E.coli Nissle 1917/2 caps/o.i.d | 24 weeks | No clinical evidence of efficacy | [91] |
| Allergic asthma and/or rhinitis | DBCT (187) | 1 × 1010 CFU/mL L. casei/100 mL/o.i.d. | 52 weeks | No difference | [92] |
| Perennial allergic rhinitis | DBPCT (49) | 3 × 108 CFU/mL L. acidophilus strain L-92/100 mL/o.i.d. | 8 weeks | No difference in IgE level or Th1/Th2 | [93] |
| Seasonal allergic rhinitis | DBPCT (20) | 1 × 105 CFU/mL L. casei Shirota/65mL/o.i.d. | 20 weeks | ↓Antigen-induced IL-5, IL-6 and IFN- γ ↑IgG ↓IgE | [94] |
| High-risk allergy children | DBPCT (105) | 5 × 109 CFU/capsule L.GG/2 cap, o.i.d 6-4 weeks before delivery and 6 months after birth | 30 weeks | No evidence of efficacy | [95] |
| High risk allergic disease infants | DBPCT (1223) | 5 × 109 CFU/cap L. rhamnosus GG/5 × 109 CFU/capsule L. reuteri LC705 2 × 108 CFU/cap B. breve Bb99/2 × 108 CFU/cap P. freudenreichii subspecies shermanii JS/b.i.d [4 weeks before delivery + 24 weeks] | 30 weeks | Protection from allergic disease only to cesarean-delivered children | [96] |
| Respiratory illness | DBPCT (523) | 2.5 × 106 CFU/mL L.rhamnosus GG (130 mL, t.i.c) | 28 weeks | ↓Occurence of respiratory illness | [97] |
| Japanese cedar pollinosis | DBPCT (44) | 5 × 1010 CFU B. longum BB536/b.i.d | 13 weeks | ↑ Bacteroides fragilis group | [98] |
| Infants | DBPCT (81) | 1 × 109 CFU/cap L. rhamnosus GG and 1 × 1010 CFU/cap B. lactis Bb-12/o.i.d | 40 weeks | ↓Risk of recurrent respiratory infections ↓ Acute otitis media ↓ Antibiotic use | [99] |
| Grass pollen-dependent allergic rhinitis | DBPCT (20) | 2 × 109 CFU/g B. lactis NCC2818/2g/o.i.d | 8 weeks | ↓Th-2 cytokines, secreted by stimulated blood lymphocytes ↓Total nasal symptom scores ↓Activated CD63 expressing basophils | [100] |
| Allergic rhinitis | DBPCT (31) | 5 × 109 CFU/mL L. Helveticus NCC1643 and × 107 CFU/mL L. paracasei ST11 / 80 mL. o.i.d | 4 weeks | ↓Nasal congestion and nasal pruritus ↓IL-5, IL-8 and IL-10 secretion by peripheral blood mononuclear cells and serum allergen-specific IgG4 | [101] |
PBMC peripheral blood mononuclear cells; P. freudenreichii: Propionibacterium freudenreichii.

Table 8.
Clinical studies of probiotics and skin conditions/diseases.
| Participant Type | Study Type (N°. Patients) | Treatment | Duration | Results | Ref. |
|---|---|---|---|---|---|
| UV induced skin damage | |||||
| Ultraviolet-induced skin damage | CT (139) | 5 × 108 CFU L. johnsoni/before UVR exposure | 3–6 weeks | Prevention the UV-induced decrease in Langerhans cell density ↑Factor XIIIa+ type I dermal dendrocytes ↓Dermal inflammatory cells ↑Minimal erythemal dose ↑ΔE* parameter | [103] |
| Pregnant women carrying high risk allergy babies | |||||
| High-risk allergy children | DBPCT (159) | 1 × 1010 CFU/cap L. rhamnosus strain GG/o.i.d. or b.i.d./3 weeks before delivery + 24 weeks | 4 weeks to mothers and 24 weeks to infants | ↓cumulative risk for developing eczema during the first 7 years of life | [104] |
| Pregnant women carrying high-risk allergy children | DBPCT (1223) mothers (925) infants | 5 × 109 CFU/cap L. rhamnosus GG 53103/5 × 109 CFU/cap/L. rhamnosus LC705 7061 CFU/cap/5 × 109 CFU/cap B. breve Bb99 13692 and 2 × 108 CFU/cap P. freudenreichii ssp. shermanii JS 7076/b.i.d.+ GOS daily | Mothers dosed with multi strain probiotics for 2 to 4 weeks before delivery then infants received probiotics +GOS for 24 weeks | Prevention of eczema at 2 years of age ↑Lactobacilli and Bifidobacteria in the gut. No effect on incidence of allergic diseases. ↑ CRP, IgA, IgE, IL-10 which were associated with ↓ risk of eczema. | [105,106] |
| Pre and post natal probiotic supplementation | DBPCT (61) | L. reuteri 1 × 108 CFU/day to mothers from week 36 of pregnancy and then to the infant for 24 months post delivery. | 52 weeks | ↓IgE-associated eczema and lowered allergen and mitogen responsiveness | [107] |
| Maternal probiotic supplementation during pregnancy | DBPCT (205) | L. rhamnosus LPR (CGMCC 1.3724) and B. longum BL999 (ATCC: BAA-999) or the combination ST11 and BL999 (ST11 BL999) consisting of L. paracasei ST11 (CNCM 1–2116) and B longum BL999. Dose 1 × 109 CFU/day provided in 1 sachet of 7 g/d (powder form) which was diluted in a glass of water. | 8 weeks | ↓risk of eczema in infants with allergic mothers positive for skin prick test. | [108] |
| Atopic dermatitis/eczema with/without cow’s milk/food allergies | |||||
| Atopic dermatitis | DBPCT (90) | 5 × 109 CFU/g L. acidophilus DDS-1/B. lactis UABLA-12/1g, b.i.d | 8 weeks | ↓SCORAD* score ↓CD4 and CD25 lymphocytes ↑CD8 Lymphocytes | [109] |
| High-risk atopic dermatitis children | PCT (15) | B. breve M-16V strain [dose administered not provided] | 4 weeks | ↑Proportion of Bifidobacteria in the fecal microflora ↓Proportion of aerobic bacteria ↓allergic symptoms | [110] |
| High-risk allergy children | DBPCT (132) | 0.5 × 106 CFU/cap LGG/2 capsules/o.i.d. | 28 weeks | Preventive effect on the incidence of eczema in high-risk children | [111] |
| High risk atopic eczema children | DBPCT (132) | 0.5 × 106 CFU/cap L. rhamnosus 53103 2 caps/o.i.d. | 28 weeks | Preventive effect on the incidence of eczema in high-risk children | [112] |
| Atopic dermatitis | DBPCT (58) | 1 × 106 CFU B. bifidum BGN4/1 × 106 CFU B. lactis AD011/1 × 106 CFU L. acidophilus AD031/o.i.d. | 32 weeks | ↓Cumulative incidence of eczema no difference in serum total IgE level or the sensitization against food allergens | [113] |
| High-risk atopic dermatitis children | DBPCT (102) | 1 × 109 CFU/sachet B. bifidum/1 × 109 CFU/sachet B. lactis/1 × 109 CFU/sachet L. lactis/o.i.d. 8 weeks before delivery + 58 weeks. | Prenatal administration to mothers and for 52 weeks to infants post birth | Preventive effect on the incidence of eczema in high-risk children | [114] |
| Atopic dermatitis | DBPCT (59) | 2 × 1010 CFU/g L. rhamnosus and B. Lactis [dose administered not provided] | 4 weeks | ↓SCORAD geometric mean score | [115] |
| Atopic eczema/dermatitis syndrome and food allergy | DBPCT (230) | 5 × 109 CFU/cap L. rhamnosus GG 53103 or 5 × 109 CFU/cap L. rhamnosus GG/5 × 109 CFU L. rhamnosus LC705/2 × 108 CFU/cap/B. breve Bbi99 and 2 × 109 CFU/capsule P. freudenreichii ssp. Shermanii JS/b.i.d. | 4 weeks | ↑Fecal IgA ↓Fecal alpha1-antitrypsin | [116] |
| Atopic dermatitis | DBPCT (66) | 1 × 109/sachet L. fermentum VRI-033 PCC (b.i.d) | 8 weeks | ↓SCORAD total scores | [117] |
| High-risk allergy children | DBPCT (425) | 6 × 109 CFU/day L. rhamnosus HN001 or 9 × 109 CFU/day B.animalis subsp lactis HN019/from 35 weeks gestation to 2 years after birth | 109 weeks | Protective effect of HN001 against eczema, when given for the first 2 years of life only, extended to at least 4 years of age. Protective effect against rhino-conjunctivitis | [118] |
| High-risk allergy children | DBPCT (474) | 6 × 109 CFU/cap L. rhamnosus/3 weeks before delivery + 2 years | 119 weeks | ↓Cumulative prevalence of eczema No effect on atopy | [119] |
* SCORAD = SCORing Atopic Dermatitis; UVR: Ultraviolet Radiation; UV-DL: Ultraviolet
4. Discussion
A diverse series of clinical trials implementing an assortment of probiotic preparations have frequently demonstrated efficacy, when investigating their administration effects on various end organ tissues (Table 1, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7 and Table 8). The central theme of this activity posits that the GIT can influence numerous end organ tissues beneficially. Further, the clinical studies indicate that the administration of probiotics may provide efficacy in restoring the GIT microbiome to a more balanced metabolic state. This possibly achieved by partly controlling the pathogenic bacterial cohort that in turn beneficially affects end organ physiology.
Hence in this review/commentary we have advanced the hypothesis that a dysbiotic GIT that is induced by a microbiome drift toward an over–growth of pathogenic bacteria may play a significant role in the induction of pro–inflammatory mediators that begin in the GIT and then may affect different end organs as shown in Figure 2. The disruption of the GIT epithelial barrier that can accompany chronic use of analgesic medications (e.g., NSAIDs) exacerbating local pro–inflammatory responses induced by the pathogenic commensal cohort is such an example. This activity can further disrupt GIT physiological and epithelial barrier function leading to disruption of controlled pro-inflammatory actions.
The gut mucosa is the largest and most dynamic immunological environment of the body. It's often the first point of pathogen/antigen exposure and many microbes use it as a base position entry into the rest of the body. The gut immune system therefore needs to be prepared to respond to pathogens while at the same time it is constantly exposed to innocuous environmental antigens, food particles and commensal pathogens and their respective metabolites, which need to be tolerated. Misdirected immune responses to harmless antigens are the underlying cause of food allergies and debilitating conditions such as inflammatory bowel diseases. GIT dysbiosis describes bacterial imbalances usually in the GIT. Such imbalances may increase the risk of developing GIT barrier dysfunction, via enterocyte hyper–permeability [leaky gut] to bacterial endotoxins or environmental antigens.
The published research data recommends that minimum doses required to elicit a therapeutic benefit is strain dependent. Shornikova and colleagues have reported that 107 bacteria of L. reuteri MM53 is sufficient to produce a beneficial effect [120]. However with other bacterial strains such as L. rhamnosus GG (lyophilised) 109 viable bacteria is a requisite dose [121].
Research studies have produced conflicting evidence, with some studies demonstrating a therapeutic benefit with doses of 107–108 CFU/dose [121]. Clinical research trials that have reported efficacious outcomes administered probiotic strains with ≥ 109 CFU/dose [120,122,123,124].
At present the best practice is to ensure that supplements contain strains with a concentration of 109 CFU/dose or higher unless research demonstrates conclusively that efficacy is achieved at lower doses. Also, it seems that multi–strain probiotics favor enhanced efficacy over single strains. In preparations with multiple strains a similar strain concentration should apply.
What is becoming increasingly clear is that the pharmacobiotic nature of probiotic strains in the form of nutritional and functional food additives to regulate the gut microbiome is an exciting growth area of therapeutics, developing alongside an increased scientific understanding of gut–microbiome symbiosis in health and disease.
Although, readdressing the broad definition accustomed to probiotics may be difficult given that different strains have been shown to ameliorate similar symptoms in different end organs, the published clinical studies show that probiotics may have drug like effects. Hence therefore as such there is a need to further define probiotics at the strain level according to specific activities demonstrated and the robustness of that effect. An effort that is both convoluted and intellectually challenging.
5. Future Prospective
A growing number of studies have shown a correlation between dysbiosis of the gastrointestinal microbiome and end-organ disease. With the transient modulatory effects that probiotics can induce on the gastrointestinal microbiotia, there emerges a significant potential to counterbalance gastrointestinal dysbiosis for health restoration. During this last decade, the efficacy of probiotic supplementation has been studied in number of human diseases, including numerous conditions as for example irritable bowel syndrome, inflammatory bowel diseases, obesity and numerous allergic diseases (Table 1, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7 and Table 8). Variations in probiotics species and strains used for clinical trials may be the primary reason for the variable effects that have been observed. This then serving to teach, that importantly standardized methods are required for the study of the gastrointestinal microbiome that, will allow valid comparisons from different groups to be made.
Modulation of the gut microbiota is one of the potential health-beneficial effects of probiotics. They have the capacity to modulate the intestinal microbiota by diverse mechanisms that include reduction of the luminal pH, competition for nutrients, secretion of anti microbial compounds or even prevention of pathogenic bacterial adhesion. Recent literature provides evidence that probiotics have immune-modulatory and anti-inflammatory effects. However, these effects can be strain-specific and species-dependant, thus knowing the physiological and the molecular mechanisms of each probiotic strain is an essential requisite for efficiently treating immune-mediated diseases. Thus contributing to the development of multi-strain probiotic formulas designed for specific interventions. This approach is paramount for testing probiotic efficacy otherwise the evidence will remain largely empirical, and clinical trial outcomes will vary and the potential of probiotics in disease treatments will remain obscure.
Furthermore, a common “hype” leveled at probiotics is that that they will cure all of disease. This posit is unequivocally disputed and not endorsed. An enhanced understanding of the functional GIT bacterial cohort that tolerates the host versus the pathogenic cohort that adversely affects the host will elucidate important relationships that exist between the indigenous microbiome and the human host. Probiotic preparations with specific metabolic properties (e.g., those strains that may increase mucus secretion) may provide clues for the future direction of clinical research into probiotics that confirm specific actions and doses to be administered for specific conditions. Hence the intention would then be to administer specific probiotic strains that beneficially modify the microbiome, albeit transiently, with specific beneficial actions directed at preventing or treating specific conditions (e.g., antibiotic associated diarrhea). Such research will lead to the further wide acceptance of live bacterial cultures as pharmacobiotic therapeutics.
Acknowledgments
Luis Vitetta has received National Institute of Complementary Medicine and National Health and Medical Research Council of Australia competitive funding and Industry support for research into probiotics.
Conflicts of interest
The authors have no further conflicts of interest relevant to the content of this review.
Author Contributions
LV conception and design of review. LV, RM, JYZ, AWL, SH and SC, read, amended and approved the final version of the manuscript.
References
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.; Raes, J.; Pelletier, E.; le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar]
- Koren, O.; Knights, D.; Gonzalez, A.; Waldron, L.; Segata, N.; Knight, R.; Huttenhower, C.; Ley, R.E. A guide to enterotypes across the human body: Meta-analysis of microbial community structures in human microbiome datasets. PLoS Comput. Biol. 2013, 9, e1002863. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Roager, H.M.; Licht, T.R.; Poulsen, S.K.; Larsen, T.M.; Bahl, M.I. Microbial enterotypes, inferred by the prevotella-to-bacteroides ratio, remained stable during a 6-month randomized controlled diet intervention with the new nordic diet. Appl. Environ. Microbiol. 2014, 80, 1142–1149. [Google Scholar] [CrossRef]
- Morelli, L.; Capurso, L. FAO/WHO Guidelines on Probiotics: 10 Years on. J. Clin. Gastroenterol. 2012, 46, S1–S2. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, H.M.; Koning, C.J.; Mulder, L.; Rombouts, F.M.; Beynen, A.C. Monostrain, multistrain and multispecies probiotics—A comparison of functionality and efficacy. Int. J. Food Microbiol. 2004, 96, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Vitetta, L.; Briskey, D.; Hayes, E.; Shing, C.; Peake, J. A review of the pharmacobiotic regulation of gastrointestinal inflammation by probiotics, commensal bacteria and prebiotics. Inflammopharmacology 2012, 20, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Rijkers, G.T.; Bengmark, S.; Enck, P.; Haller, D.; Herz, U.; Kalliomaki, M.; Kudo, S.; Lenoir-Wijnkoop, I.; Mercenier, A.; Myllyluoma, E.; et al. Guidance for Substantiating the Evidence for Beneficial Effects of Probiotics: Current Status and Recommendations for Future Research. J. Nutr. 2010, 140, 671S–676S. [Google Scholar]
- Agrawal, A.; Houghton, L.A.; Morris, J.; Reilly, B.; Guyonnet, D.; Goupil Feuillerat, N.; Schlumberger, A.; Jakob, S.; Whorwell, P.J. Clinical trial: The effects of a fermented milk product containing Bifidobacterium lactis DN-173 010 on abdominal distension and gastrointestinal transit in irritable bowel syndrome with constipation. Aliment. Pharmacol. Ther. 2009, 29, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Bausserman, M.; Michail, S. The use of Lactobacillus GG in irritable bowel syndrome in children: A double-blind randomized control trial. J. Pediatr. 2005, 147, 197–201. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, L.; McCarthy, J.; Kelly, P.; Hurley, G.; Luo, F.; Chen, K.; O’Sullivan, G.C.; Kiely, B.; Collins, J.K.; Shanahan, F.; Quigley, E.M. Lactobacillus and bifidobacterium in irritable bowel syndrome: Symptom responses and relationship to cytokine profiles. Gastroenterology 2005, 128, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Sinn, D.H.; Song, J.H.; Kim, H.J.; Lee, J.H.; Son, H.J.; Chang, D.K.; Kim, Y.H.; Kim, J.J.; Rhee, J.C.; Rhee, P.L. Therapeutic effect of Lactobacillus acidophilus-SDC 2012, 2013 in patients with irritable bowel syndrome. Dig. Dis. Sci. 2008, 53, 2714–2718. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.A.; Stimpson, J.; Wang, D.; Plummer, S.; Garaiova, I.; Barker, M.E.; Corfe, B.M. Clinical trial: A multistrain probiotic preparation significantly reduces symptoms of irritable bowel syndrome in a double-blind placebo-controlled study. Aliment. Pharmacol. Ther. 2009, 29, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Sondergaard, B.; Olsson, J.; Ohlson, K.; Svensson, U.; Bytzer, P.; Ekesbo, R. Effects of probiotic fermented milk on symptoms and intestinal flora in patients with irritable bowel syndrome: A randomized, placebo-controlled trial. Scand. J. Gastroenterol. 2011, 46, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Li, Y.Q.; Zuo, X.L.; Zhen, Y.B.; Yang, J.; Liu, C.H. Clinical trial: Effect of active lactic acid bacteria on mucosal barrier function in patients with diarrhoea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 2008, 28, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Guglielmetti, S.; Mora, D.; Gschwender, M.; Popp, K. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life—A double-blind, placebo-controlled study. Aliment. Pharmacol. Ther. 2011, 33, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Tarrerias, A.L.; Costil, V.; Vicari, F.; Letard, J.C.; Adenis-Lamarre, P.; Aisene, A.; Batistelli, D.; Bonnaud, G.; Carpentier, S.; Dalbies, P.; et al. The effect of inactivated Lactobacillus LB fermented culture medium on symptom severity: Observational investigation in 297 patients with diarrhea-predominant irritable bowel syndrome. Dig. Dis. 2011, 29, 588–591. [Google Scholar] [CrossRef] [PubMed]
- Indrio, F.; Riezzo, G.; Raimondi, F.; Bisceglia, M.; Filannino, A.; Cavallo, L.; Francavilla, R. Lactobacillus reuteri accelerates gastric emptying and improves regurgitation in infants. Eur. J. Clin. Invest. 2011, 41, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Romano, C.; Ferrau, V.; Cavataio, F.; Iacono, G.; Spina, M.; Lionetti, E.; Comisi, F.; Famiani, A.; Comito, D. Lactobacillus reuteri in children with functional abdominal pain (FAP). J. Paediatr. Child Health 2010. [Google Scholar] [CrossRef]
- Salminen, M.K.; Tynkkynen, S.; Rautelin, H.; Poussa, T.; Saxelin, M.; Ristola, M.; Valtonen, V.; Järvinen, A. The efficacy and safety of probiotic Lactobacillus rhamnosus GG on prolonged, noninfectious diarrhea in HIV Patients on antiretroviral therapy: A randomized, placebo-controlled, crossover study. HIV Clin. Trials 2004, 5, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.J.; Wareham, K.; Wang, D.; Bradley, C.; Hutchings, H.; Harris, W.; Dhar, A.; Brown, H.; Foden, A.; Gravenor, M.B. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2013, 382, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Beausoleil, M.; Fortier, N.; Guenette, S.; L’Ecuyer, A.; Savoie, M.; Franco, M.; Lachaine, J.; Weiss, K. Effect of a fermented milk combining Lactobacillus acidophilus Cl1285 and Lactobacillus casei in the prevention of antibiotic-associated diarrhea: A randomized, double-blind, placebo-controlled trial. Can. J. Gastroenterol. 2007, 21, 732–736. [Google Scholar] [PubMed]
- Gao, X.W.; Mubasher, M.; Fang, C.Y.; Reifer, C.; Miller, L.E. Dose-response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile-associated diarrhea prophylaxis in adult patients. Am. J. Gastroenterol. 2010, 105, 1636–1641. [Google Scholar] [CrossRef] [PubMed]
- Pozzoni, P.; Riva, A.; Bellatorre, A.G.; Amigoni, M.; Redaelli, E.; Ronchetti, A.; Stefani, M.; Tironi, R.; Molteni, E.E.; Conte, D.; et al. Saccharomyces boulardii for the prevention of antibiotic-associated diarrhea in adult hospitalized patients: A single-center, randomized, double-blind, placebo-controlled trial. Am. J. Gastroenterol. 2012, 107, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Sampalis, J.; Psaradellis, E.; Rampakakis, E. Efficacy of BIO K+ CL1285 in the reduction of antibiotic-associated diarrhea—A placebo controlled double-blind randomized, multi-center study. Arch. Med. Sci. 2010, 6, 56–64. [Google Scholar] [PubMed]
- Hickson, M.; D’Souza, A.L.; Muthu, N.; Rogers, T.R.; Want, S.; Rajkumar, C.; Bulpitt, C.J. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: Randomised double blind placebo controlled trial. BMJ 2007, 335, 80. [Google Scholar] [CrossRef] [PubMed]
- Selinger, C.P.; Bell, A.; Cairns, A.; Lockett, M.; Sebastian, S.; Haslam, N. Probiotic VSL#3 prevents antibiotic-associated diarrhoea in a double-blind, randomized, placebo-controlled clinical trial. J. Hosp Infect. 2013, 84, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Grandy, G.; Medina, M.; Soria, R.; Teran, C.G.; Araya, M. Probiotics in the treatment of acute rotavirus diarrhoea. A randomized, double-blind, controlled trial using two different probiotic preparations in Bolivian children. BMC Infect. Dis. 2010, 10, 253. [Google Scholar]
- Navarro-Rodriguez, T.; Silva, F.M.; Barbuti, R.C.; Mattar, R.; Moraes-Filho, J.P.; de Oliveira, M.N.; Bogsan, C.S.; Chinzon, D.; Eisig, J.N. Association of a probiotic to a Helicobacter pylori eradication regimen does not increase efficacy or decreases the adverse effects of the treatment: A prospective, randomized, double-blind, placebo-controlled study. BMC Gastroenterol. 2013, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Dajani, A.I.; Abu Hammour, A.M.; Yang, D.H.; Chung, P.C.; Nounou, M.A.; Yuan, K.Y.; Zakaria, M.A.; Schi, H.S. Do probiotics improve eradication response to Helicobacter pylori on standard triple or sequential therapy? Saudi J. Gastroenterol. 2013, 19, 113–120. [Google Scholar]
- De Vrese, M.; Kristen, H.; Rautenberg, P.; Laue, C.; Schrezenmeir, J. Probiotic lactobacilli and bifidobacteria in a fermented milk product with added fruit preparation reduce antibiotic associated diarrhea and Helicobacter pylori activity. J. Dairy Res. 2011, 78, 396–403. [Google Scholar]
- Du, Y.Q.; Su, T.; Fan, J.G.; Lu, Y.X.; Zheng, P.; Li, X.H.; Guo, C.Y.; Xu, P.; Gong, Y.F.; Li, Z.S. Adjuvant probiotics improve the eradication effect of triple therapy for Helicobacter pylori infection. World J. Gastroenterol. 2012, 18, 6302–6307. [Google Scholar] [CrossRef] [PubMed]
- Efrati, C.; Nicolini, G.; Cannaviello, C.; O’Sed, N.P.; Valabrega, S. Helicobacter pylori eradication: Sequential therapy and Lactobacillus reuteri supplementation. World J. Gastroenterol. 2012, 18, 6250–6254. [Google Scholar] [CrossRef] [PubMed]
- Mehling, H.; Busjahn, A. Non-viable Lactobacillus reuteri DSMZ 17648 (Pylopass) as a new approach to Helicobacter pylori control in humans. Nutrients 2013, 5, 3062–3073. [Google Scholar] [CrossRef] [PubMed]
- Kuisma, J.; Mentula, S.; Jarvinen, H.; Kahri, A.; Saxelin, M.; Farkkila, M. Effect of Lactobacillus rhamnosus GG on ileal pouch inflammation and microbial flora. Aliment. Pharmacol. Ther. 2003, 17, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Waller, P.A.; Gopal, P.K.; Leyer, G.J.; Ouwehand, A.C.; Reifer, C.; Stewart, M.E.; Miller, L.E. Dose-response effect of Bifidobacterium lactis HN019 on whole gut transit time and functional gastrointestinal symptoms in adults. Scand J. Gastroenterol. 2011, 46, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Kalman, D.S.; Schwartz, H.I.; Alvarez, P.; Feldman, S.; Pezzullo, J.C.; Krieger, D.R. A prospective, randomized, double-blind, placebo-controlled parallel-group dual site trial to evaluate the effects of a Bacillus coagulans-based product on functional intestinal gas symptoms. BMC Gastroenterol. 2009, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Kondo, J.; Xiao, J.Z.; Shirahata, A.; Baba, M.; Abe, A.; Ogawa, K.; Shimoda, T. Modulatory effects of Bifidobacterium longum BB536 on defecation in elderly patients receiving enteral feeding. World J. Gastroenterol. 2013, 19, 2162–2170. [Google Scholar] [CrossRef] [PubMed]
- Guyonnet, D.; Schlumberger, A.; Mhamdi, L.; Jakob, S.; Chassany, O. Fermented milk containing Bifidobacterium lactis DN-173 010 improves gastrointestinal well-being and digestive symptoms in women reporting minor digestive symptoms: A randomised, double-blind, parallel, controlled study. Br. J. Nutr. 2009, 102, 1654–1662. [Google Scholar] [CrossRef] [PubMed]
- Marteau, P.; Guyonnet, D.; Lafaye de Micheaux, P.; Gelu, S. A randomized, double-blind, controlled study and pooled analysis of two identical trials of fermented milk containing probiotic Bifidobacterium lactis CNCM I-2494 in healthy women reporting minor digestive symptoms. Neurogastroenterol. Motil. 2013, 25, 331–e252. [Google Scholar] [CrossRef] [PubMed]
- Sari, F.N.; Dizdar, E.A.; Oguz, S.; Erdeve, O.; Uras, N.; Dilmen, U. Oral probiotics: Lactobacillus sporogenes for prevention of necrotizing enterocolitis in very low-birth weight infants: A randomized, controlled trial. Eur. J. Clin. Nutr. 2011, 65, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Stratiki, Z.; Costalos, C.; Sevastiadou, S.; Kastanidou, O.; Skouroliakou, M.; Giakoumatou, A.; Petrohilou, V. The effect of a bifidobacter supplemented bovine milk on intestinal permeability of preterm infants. Early Hum. Dev. 2007, 83, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Indrio, F.; di Mauro, A.; Riezzo, G.; Civardi, E.; Intini, C.; Corvaglia, L.; Ballardini, E.; Bisceglia, M.; Cinquetti, M.; Brazzoduro, E.; et al. Prophylactic use of a probiotic in the prevention of colic, regurgitation, and functional constipation: A randomized clinical trial. JAMA Pediatr. 2014, 168, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Loguercio, C.; de Simone, T.; Federico, A.; Terracciano, F.; Tuccillo, C.; di Chicco, M.; Carteni, M. Gut-liver axis: A new point of attack to treat chronic liver damage? Am. J. Gastroenterol. 2002, 97, 2144–2146. [Google Scholar]
- Polson, J.; Lee, W.M. AASLD position paper: The management of acute liver failure. Hepatology 2005, 41, 1179–1197. [Google Scholar] [CrossRef] [PubMed]
- Kirpich, I.A.; Solovieva, N.V.; Leikhter, S.N.; Shidakova, N.A.; Lebedeva, O.V.; Sidorov, P.I.; Bazhukova, T.A.; Soloviev, A.G.; Barve, S.S.; McClain, C.J.; et al. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: A pilot study. Alcohol 2008, 42, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Stadlbauer, V.; Mookerjee, R.P.; Hodges, S.; Wright, G.A.; Davies, N.A.; Jalan, R. Effect of probiotic treatment on deranged neutrophil function and cytokine responses in patients with compensated alcoholic cirrhosis. J. Hepatol. 2008, 48, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Loguercio, C.; Federico, A.; Tuccillo, C.; Terracciano, F.; D’Auria, M.V.; de Simone, C.; del Vecchio Blanco, C. Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J. Clin. Gastroenterol. 2005, 39, 540–543. [Google Scholar] [CrossRef] [PubMed]
- Lata, J.; Jurankova, J.; Pribramska, V.; Fric, P.; Senkyrik, M.; Dite, P.; Kroupa, R. Effect of administration of Escherichia coli Nissle (Mutaflor) on intestinal colonisation, endo-toxemia, liver function and minimal hepatic encephalopathy in patients with liver cirrhosis. Vnitr. Lek. 2006, 52, 215–219. [Google Scholar] [PubMed]
- Lata, J.; Novotny, I.; Pribramska, V.; Jurankova, J.; Fric, P.; Kroupa, R.; Stiburek, O. The effect of probiotics on gut flora, level of endotoxin and Child-Pugh score in cirrhotic patients: Results of a double-blind randomized study. Eur. J. Gastroenterol. Hepatol. 2007, 19, 1111–1113. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.E.; Zhang, Y.; Zhang, J.; Dong, P.L.; Chen, M.; Duan, Z.P. Probiotic yogurt effects on intestinal flora of patients with chronic liver disease. Nurs. Res. 2010, 59, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Pereg, D.; Kotliroff, A.; Gadoth, N.; Hadary, R.; Lishner, M.; Kitay-Cohen, Y. Probiotics for patients with compensated liver cirrhosis: A double-blind placebo-controlled study. Nutrition 2011, 27, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.Y.; Wang, H.J.; Lu, Z.; Xu, S.Z. Intestinal microflora in patients with liver cirrhosis. Chin. J. Dig. Dis. 2004, 5, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Tandon, P.; Moncrief, K.; Madsen, K.; Arrieta, M.C.; Owen, R.J.; Bain, V.G.; Wong, W.W.; Ma, M.M. Effects of probiotic therapy on portal pressure in patients with cirrhosis: A pilot study. Liver Int. 2009, 29, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Duan, Z.P.; Ha, D.K.; Bengmark, S.; Kurtovic, J.; Riordan, S.M. Synbiotic modulation of gut flora: Effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology 2004, 39, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, M.; Greco, F.; Barone, G.; Gargante, M.P.; Malaguarnera, M.; Toscano, M.A. Bifidobacterium longum with fructo-oligosaccharide (FOS) treatment in minimal hepatic encephalopathy: A randomized, double-blind, placebo-controlled study. Dig. Dis. Sci. 2007, 52, 3259–3265. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.I.; Gibson, G.R. Cholesterol assimilation by lactic acid bacteria and bifidobacteria isolated from the human gut. Appl. Environ. Microbiol. 2002, 68, 4689–4693. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Lund, P.K. Role of intestinal inflammation as an early event in obesity and insulin resistance. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Chorell, E.; Karlsson Videhult, F.; Hernell, O.; Antti, H.; West, C.E. Impact of probiotic feeding during weaning on the serum lipid profile and plasma metabolome in infants. Br. J. Nutr. 2013, 110, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Kadooka, Y.; Sato, M.; Imaizumi, K.; Ogawa, A.; Ikuyama, K.; Akai, Y.; Okano, M.; Kagoshima, M.; Tsuchida, T. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur. J. Clin. Nutr. 2010, 64, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Luoto, R.; Kalliomaki, M.; Laitinen, K.; Isolauri, E. The impact of perinatal probiotic intervention on the development of overweight and obesity: Follow-up study from birth to 10 years. Int. J. Obes. (Lond) 2010, 34, 1531–1537. [Google Scholar] [CrossRef]
- Zarrati, M.; Shidfar, F.; Nourijelyani, K.; Mofid, V.; Hossein zadeh-Attar, M.J.; Bidad, K.; Najafi, F.; Gheflati, Z.; Chamari, M.; Salehi, E. Lactobacillus acidophilus La5, Bifidobacterium BB12, and Lactobacillus casei DN001 modulate gene expression of subset specific transcription factors and cytokines in peripheral blood mononuclear cells of obese and overweight people. Biofactors 2013, 39, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Safavi, M.; Farajian, S.; Kelishadi, R.; Mirlohi, M.; Hashemipour, M. The effects of synbiotic supplementation on some cardio-metabolic risk factors in overweight and obese children: A randomized triple-masked controlled trial. Int. J. Food Sci. Nutr. 2013, 64, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Tien, M.T.; Girardin, S.E.; Regnault, B.; le Bourhis, L.; Dillies, M.A.; Coppée, J.Y.; Bourdet-Sicard, R.; Sansonetti, P.J.; Pédron, T. Anti-inflammatory effect of Lactobacillus casei on Shigella-infected human intestinal epithelial cells. J. Immunol. 2006, 176, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.K.; Omaye, S.T. Metabolic diseases and pro- and prebiotics: Mechanistic insights. Nutr. Metab. (Lond.) 2012, 9, 60. [Google Scholar] [CrossRef]
- Amaral, F.A.; Sachs, D.; Costa, V.V.; Fagundes, C.T.; Cisalpino, D.; Cunha, T.M.; Ferreira, S.H.; Cunha, F.Q.; Silva, T.A.; Nicoli, J.R. Commensal microbiota is fundamental for the development of inflammatory pain. Proc. Natl. Acad. Sci. USA 2008, 105, 2193–2197. [Google Scholar] [CrossRef] [PubMed]
- Diaz Heijtz, R.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar]
- Neufeld, K.M.; Kang, N.; Bienenstock, J.; Foster, J.A. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 2011, 23, 255–264, e119. [Google Scholar]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Benton, D.; Williams, C.; Brown, A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur. J. Clin. Nutr. 2007, 61, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Bested, A.C.; Beaulne, T.M.; Katzman, M.A.; Iorio, C.; Berardi, J.M.; Logan, A.C. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog. 2009, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M.; et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011, 105, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Zhu, J.C.; Du, J.; Zhang, L.M.; Yin, H.H. Effects of probiotics on serum levels of Th1/Th2 cytokine and clinical outcomes in severe traumatic brain-injured patients: A prospective randomized pilot study. Crit. Care 2011, 15, R290. [Google Scholar] [CrossRef] [PubMed]
- Tillisch, K.; Labus, J.; Kilpatrick, L.; Jiang, Z.; Stains, J.; Ebrat, B.; Guyonnet, D.; Legrain-Raspaud, S.; Trotin, B.; Naliboff, B.; et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 2013, 144, 1394–1401. [Google Scholar]
- Vitetta, L.; Gobe, G. Uremia and chronic kidney disease: The role of the gut microflora and therapies with pro- and prebiotics. Mol. Nutr. Food Res. 2013, 57, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Vitetta, L.; Alford, H. The Pharmacobiotic Potential of the Gastrointestinal Tract Micro-Biometabolome–Probiotic Connect: A Brief Commentary. Drug Dev. Res. 2013. [Google Scholar] [CrossRef]
- Ranganathan, N.; Friedman, E.A.; Tam, P.; Rao, V.; Ranganathan, P.; Dheer, R. Probiotic dietary supplementation in patients with stage 3 and 4 chronic kidney disease: A 6-month pilot scale trial in Canada. Curr. Med. Res. Opin. 2009, 25, 1919–1930. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, N.; Ranganathan, P.; Friedman, E.A.; Joseph, A.; Delano, B.; Goldfarb, D.S.; Tam, P.; Rao, A.V.; Anteyi, E.; Musso, C.G. Pilot study of probiotic dietary supplementation for promoting healthy kidney function in patients with chronic kidney disease. Adv. Ther. 2010, 27, 634–647. [Google Scholar] [PubMed]
- Nakabayashi, I.; Nakamura, M.; Kawakami, K.; Ohta, T.; Kato, I.; Uchida, K.; Yoshida, M. Effects of synbiotic treatment on serum level of p-cresol in haemodialysis patients: A preliminary study. Nephrol. Dial. Transplant. 2011, 26, 1094–1098. [Google Scholar] [CrossRef] [PubMed]
- Chong, V.H.; Wang, C.L. Higher prevalence of gastrointestinal symptoms among patients with rheumatic disorders. Singap. Med. J. 2008, 49, 419–424. [Google Scholar]
- Hatakka, K.; Martio, J.; Korpela, M.; Herranen, M.; Poussa, T.; Laasanen, T.; Saxelin, M.; Vapaatalo, H.; Moilanen, E.; Korpela, R. Effects of probiotic therapy on the activity and activation of mild rheumatoid arthritis—A pilot study. Scand J. Rheumatol. 2003, 32, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Waller, R.D.; Stebbings, S.; Highton, J.; Orlovich, D.A.; Schmierer, D.; Fawcett, J.P. The effects of an orally administered probiotic on sulfasalazine metabolism in individuals with rheumatoid arthritis: A preliminary study. Int. J. Rheum. Dis. 2010, 13, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Mandel, D.R.; Eichas, K.; Holmes, J. Bacillus coagulans: A viable adjunct therapy for relieving symptoms of rheumatoid arthritis according to a randomized, controlled trial. BMC Complement. Altern. Med. 2010, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Pineda Mde, L.; Thompson, S.F.; Summers, K.; de Leon, F.; Pope, J.; Reid, G. A randomized, double-blinded, placebo-controlled pilot study of probiotics in active rheumatoid arthritis. Med. Sci. Monit. 2011, 17, CR347–CR354. [Google Scholar]
- Jenks, K.; Stebbings, S.; Burton, J.; Schultz, M.; Herbison, P.; Highton, J. Probiotic therapy for the treatment of spondyloarthritis: A randomized controlled trial. J. Rheumatol. 2010, 37, 2118–2125. [Google Scholar] [CrossRef] [PubMed]
- So, J.S.; Song, M.K.; Kwon, H.K.; Lee, C.G.; Chae, C.S.; Sahoo, A.; Jash, A.; Lee, S.H.; Park, Z.Y.; Im, S.H. Lactobacillus casei enhances type II collagen/glucosamine-mediated suppression of inflammatory responses in experimental osteoarthritis. Life Sci. 2011, 88, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Sironi, M.; Clerici, M. The hygiene hypothesis: An evolutionary perspective. Microbes Infect. 2010, 12, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Lin, Y.L.; Jan, R.L.; Chen, H.H.; Wang, J.Y. Randomized placebo-controlled trial of lactobacillus on asthmatic children with allergic rhinitis. Pediatr. Pulmonol. 2010, 45, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Dolle, S.; Berg, J.; Rasche, C.; Worm, M. Tolerability and clinical outcome of coseasonal treatment with Escherichia coli strain Nissle 1917 in grass pollen-allergic subjects. Int. Arch. Allergy Immunol. 2014, 163, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, M.; Agostoni, C.; Riva, E.; Salvini, F.; Ruscitto, A.; Zuccotti, G.V.; Radaelli, G.; Felicita Study Group. A randomized prospective double blind controlled trial on effects of long-term consumption of fermented milk containing Lactobacillus casei in pre-school children with allergic asthma and/or rhinitis. Pediatr. Res. 2007, 62, 215–220. [Google Scholar]
- Ishida, Y.; Nakamura, F.; Kanzato, H.; Sawada, D.; Hirata, H.; Nishimura, A.; Kajimoto, O.; Fujiwara, S. Clinical effects of Lactobacillus acidophilus strain L-92 on perennial allergic rhinitis: A double-blind, placebo-controlled study. J. Dairy Sci. 2005, 88, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Ivory, K.; Chambers, S.J.; Pin, C.; Prieto, E.; Arqués, J.L.; Nicoletti, C. Oral delivery of Lactobacillus casei Shirota modifies allergen-induced immune responses in allergic rhinitis. Clin. Exp. Allergy 2008, 38, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Kopp, M.V.; Hennemuth, I.; Heinzmann, A.; Urbanek, R. Randomized, double-blind, placebo-controlled trial of probiotics for primary prevention: No clinical effects of Lactobacillus GG supplementation. Pediatrics 2008, 121, e850–e856. [Google Scholar] [CrossRef] [PubMed]
- Kuitunen, M.; Kukkonen, K.; Juntunen-Backman, K.; Korpela, R.; Poussa, T.; Tuure, T.; Haahtela, T.; Savilahti, E. Probiotics prevent IgE-associated allergy until age 5 years in cesarean-delivered children but not in the total cohort. J Allergy Clin. Immunol. 2009, 123, 335–341. [Google Scholar]
- Kumpu, M.; Kekkonen, R.A.; Kautiainen, H.; Jarvenpaa, S.; Kristo, A.; Huovinen, P.; Pitkäranta, A.; Korpela, R.; Hatakka, K. Milk containing probiotic Lactobacillus rhamnosus GG and respiratory illness in children: A randomized, double-blind, placebo-controlled trial. Eur. J. Clin. Nutr. 2012, 66, 1020–1023. [Google Scholar]
- Odamaki, T.; Xiao, J.Z.; Iwabuchi, N.; Sakamoto, M.; Takahashi, N.; Kondo, S.; Miyaji, K.; Iwatsuki, K.; Togashi, H.; Enomoto, T.; et al. Influence of Bifidobacterium longum BB536 intake on faecal microbiota in individuals with Japanese cedar pollinosis during the pollen season. J. Med. Microbiol. 2007, 56, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Rautava, S.; Salminen, S.; Isolauri, E. Specific probiotics in reducing the risk of acute infections in infancy—A randomised, double-blind, placebo-controlled study. Br. J. Nutr. 2009, 101, 1722–1726. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Hacini-Rachinel, F.; Gosoniu, M.L.; Bourdeau, T.; Holvoet, S.; Doucet-Ladeveze, R.; Beaumont, M.; Mercenier, A.; Nutten, S. Immune-modulatory effect of probiotic Bifidobacterium lactis NCC2818 in individuals suffering from seasonal allergic rhinitis to grass pollen: An exploratory, randomized, placebo-controlled clinical trial. Eur. J. Clin. Nutr. 2013, 67, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Wassenberg, J.; Nutten, S.; Audran, R.; Barbier, N.; Aubert, V.; Moulin, J.; Mercenier, A.; Spertini, F. Effect of Lactobacillus paracasei ST11 on a nasal provocation test with grass pollen in allergic rhinitis. Clin. Exp. Allergy 2011, 41, 565–573. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, T.T.; di Sabatino, A. The exposure of infants to Lactobacillus rhamnosus GG in Finland. J. Pediatr. Gastroenterol. Nutr. 2006, 42, 476–478. [Google Scholar] [CrossRef] [PubMed]
- Bouilly-Gauthier, D.; Jeannes, C.; Maubert, Y.; Duteil, L.; Queille-Roussel, C.; Piccardi, N.; Montastier, C.; Manissier, P.; Piérard, G.; Ortonne, J.P. Clinical evidence of benefits of a dietary supplement containing probiotic and carotenoids on ultraviolet-induced skin damage. Br. J. Dermatol. 2010, 163, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Kalliomaki, M.; Salminen, S.; Poussa, T.; Isolauri, E. Probiotics during the first 7 years of life: A cumulative risk reduction of eczema in a randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2007, 119, 1019–1021. [Google Scholar] [CrossRef] [PubMed]
- Kukkonen, K.; Savilahti, E.; Haahtela, T.; Juntunen-Backman, K.; Korpela, R.; Poussa, T.; Tuure, T.; Kuitunen, M. Probiotics and prebiotic galacto-oligosaccharides in the prevention of allergic diseases: A randomized, double-blind, placebo-controlled trial. J. Allergy Clin. Immunol. 2007, 119, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Marschan, E.; Kuitunen, M.; Kukkonen, K.; Poussa, T.; Sarnesto, A.; Haahtela, T.; Korpela, R.; Savilahti, E.; Vaarala, O. Probiotics in infancy induce protective immune profiles that are characteristic for chronic low-grade inflammation. Clin. Exp. Allergy 2008, 38, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, A.; Abrahamsson, T.R.; Bjorksten, B.; Jenmalm, M.C. Pre- and post-natal Lactobacillus reuteri supplementation decreases allergen responsiveness in infancy. Clin. Exp. Allergy 2013, 43, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Rautava, S.; Kainonen, E.; Salminen, S.; Isolauri, E. Maternal probiotic supplementation during pregnancy and breast-feeding reduces the risk of eczema in the infant. J. Allergy Clin. Immunol. 2012, 130, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Gerasimov, S.V.; Vasjuta, V.V.; Myhovych, O.O.; Bondarchuk, L.I. Probiotic supplement reduces atopic dermatitis in preschool children: A randomized, double-blind, placebo-controlled, clinical trial. Am. J. Clin. Dermatol. 2010, 11, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Hattori, K.; Yamamoto, A.; Sasai, M.; Taniuchi, S.; Kojima, T.; Kobayashi, Y.; Iwamoto, H.; Namba, K.; Yaeshima, T. Effects of administration of bifidobacteria on fecal microflora and clinical symptoms in infants with atopic dermatitis. Arerugi 2003, 52, 20–30. [Google Scholar] [PubMed]
- Kalliomaki, M.; Salminen, S.; Arvilommi, H.; Kero, P.; Koskinen, P.; Isolauri, E. Probiotics in primary prevention of atopic disease: A randomised placebo-controlled trial. Lancet 2001, 357, 1076–1079. [Google Scholar] [CrossRef] [PubMed]
- Kalliomaki, M.; Salminen, S.; Poussa, T.; Arvilommi, H.; Isolauri, E. Probiotics and prevention of atopic disease: 4-Year follow-up of a randomised placebo-controlled trial. Lancet 2003, 361, 1869–1871. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kwon, J.H.; Ahn, S.H.; Lee, S.I.; Han, Y.S.; Choi, Y.O.; Lee, S.Y.; Ahn, K.M.; Ji, G.E. Effect of probiotic mix (Bifidobacterium bifidum, Bifidobacterium lactis, Lactobacillus acidophilus) in the primary prevention of eczema: A double-blind, randomized, placebo-controlled trial. Pediatr. Allergy Immunol. 2010, 21, e386–e393. [Google Scholar] [CrossRef] [PubMed]
- Niers, L.; Martin, R.; Rijkers, G.; Sengers, F.; Timmerman, H.; van Uden, N.; Smidt, H.; Kimpen, J.; Hoekstra, M. The effects of selected probiotic strains on the development of eczema (the PandA study). Allergy 2009, 64, 1349–1358. [Google Scholar]
- Sistek, D.; Kelly, R.; Wickens, K.; Stanley, T.; Fitzharris, P.; Crane, J. Is the effect of probiotics on atopic dermatitis confined to food sensitized children? Clin. Exp. Allergy 2006, 36, 629–633. [Google Scholar] [CrossRef]
- Viljanen, M.; Kuitunen, M.; Haahtela, T.; Juntunen-Backman, K.; Korpela, R.; Savilahti, E. Probiotic effects on faecal inflammatory markers and on faecal IgA in food allergic atopic eczema/dermatitis syndrome infants. Pediatr. Allergy Immunol. 2005, 16, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Weston, S.; Halbert, A.; Richmond, P.; Prescott, S.L. Effects of probiotics on atopic dermatitis: A randomised controlled trial. Arch. Dis. Child. 2005, 90, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Wickens, K.; Black, P.; Stanley, T.V.; Mitchell, E.; Barthow, C.; Fitzharris, P.; Purdie, G.; Crane, J. A protective effect of Lactobacillus rhamnosus HN001 against eczema in the first 2 years of life persists to age 4 years. Clin. Exp. Allergy 2012, 42, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Wickens, K.; Black, P.N.; Stanley, T.V.; Mitchell, E.; Fitzharris, P.; Tannock, G.W.; Purdie, G.; Crane, J.; Probiotic Study Group. A differential effect of 2 probiotics in the prevention of eczema and atopy: A double-blind, randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2008, 122, 788–794. [Google Scholar]
- Shornikova, A.V.; Casas, I.A.; Isolauri, E.; Mykkanen, H.; Vesikari, T. Lactobacillus reuteri as a therapeutic agent in acute diarrhea in young children. J. Pediatr. Gastroenterol. Nutr. 1997, 24, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Saxelin, M. Probiotic formulations and applications, the current probiotics market, and changes in the marketplace: A European perspective. Clin. Infect Dis. 2008, 46, S76–S79. [Google Scholar] [CrossRef] [PubMed]
- Hatakka, K.; Savilahti, E.; Ponka, A.; Meurman, J.H.; Poussa, T.; Näse, L.; Saxelin, M.; Korpela, R. Effect of long term consumption of probiotic milk on infections in children attending day care centres: Double blind, randomised trial. BMJ 2001, 322, 1327. [Google Scholar] [CrossRef] [PubMed]
- Prilassnig, M.; Wenisch, C.; Daxboeck, F.; Feierl, G. Are probiotics detectable in human feces after oral uptake by healthy volunteers? Wien. Klin. Wochenschr. 2007, 119, 456–462. [Google Scholar] [CrossRef]
- Saxelin, M.; Pessi, T.; Salminen, S. Fecal recovery following oral administration of Lactobacillus Strain GG (ATCC 53103) in gelatine capsules to healthy volunteers. Int. J. Food Microbiol. 1995, 25, 199–203. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).