Activity of Gallium Meso- and Protoporphyrin IX against Biofilms of Multidrug-Resistant Acinetobacter baumannii Isolates
Abstract
:1. Introduction
2. Results
2.1. Iron Sources Differentially Affect Bacterial Growth and Biofilm Formation of A. Baumannii In Vitro
2.2. Activity of Gallium Compounds on Planktonic Bacteria
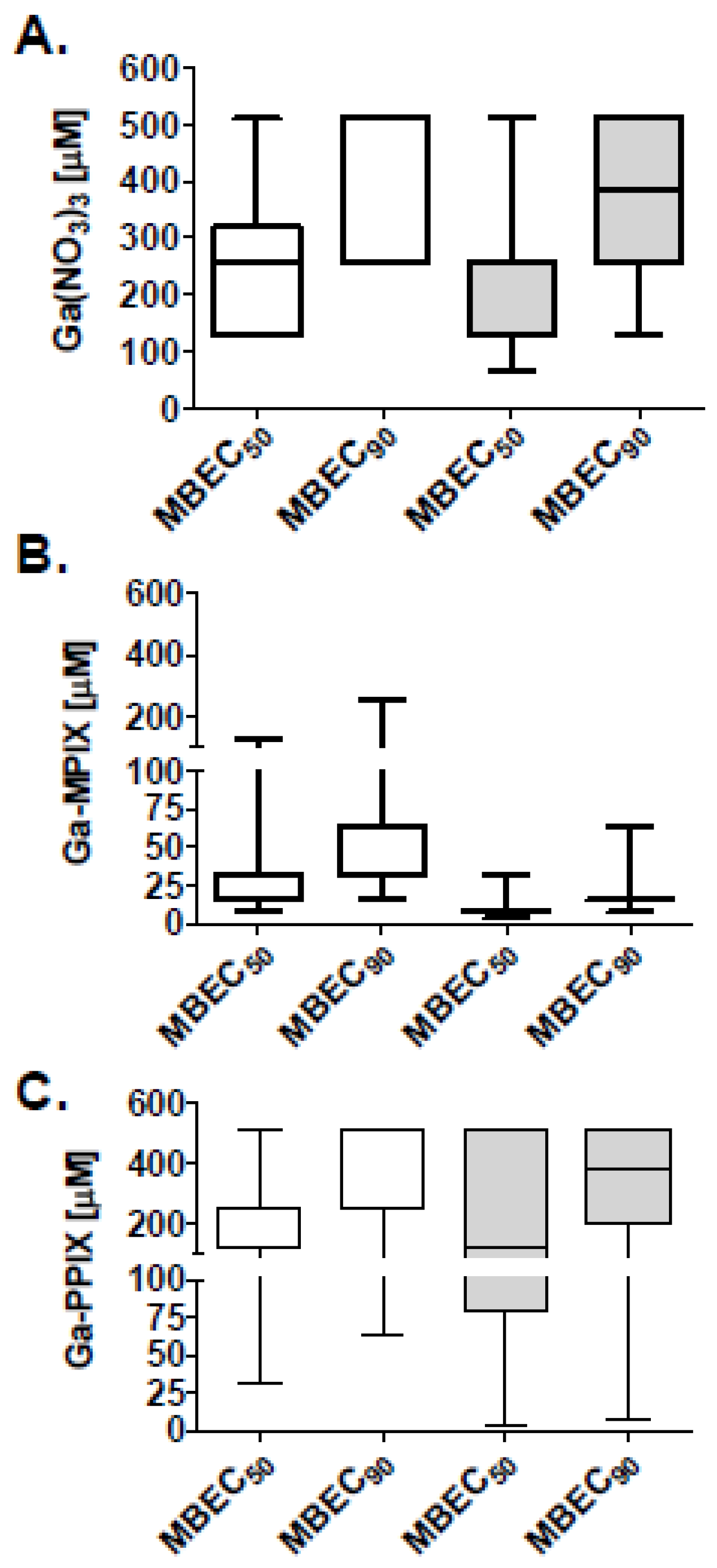
2.3. Antibiofilm Activity of Gallium Compounds
2.4. Assessment of the Cytotoxicity of Gallium Compounds
3. Discussion
4. Experimental Section
4.1. Reagents
4.2. Bacterial Strains and Culture Conditions
4.3. Effects of Iron Sources on Bacterial Growth and Biofilm Formation
4.4. Activity of Gallium Compounds on Planktonic Bacteria
4.5. Assessment of the Antibiofilm Activity of Gallium Compounds
4.6. Cellular Viability Assays
4.7. LDH Assays
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A


References
- McConnell, M.J.; Actis, L.; Pachon, J. Acinetobacter baumannii: Human infections, factors contributing to pathogenesis and animal models. FEMS Microbiol. Rev. 2013, 37, 130–155. [Google Scholar] [CrossRef] [PubMed]
- Vila, J.; Pachon, J. Therapeutic options for Acinetobacter baumannii infections. Expert Opin. Pharmacother. 2008, 9, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Wisplinghoff, H.; Edmond, M.B.; Pfaller, M.A.; Jones, R.N.; Wenzel, R.P.; Seifert, H. Nosocomial bloodstream infections caused by acinetobacter species in United States hospitals: Clinical features, molecular epidemiology, and antimicrobial susceptibility. Clin. Infect. Dis. 2000, 31, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Be, N.A.; Allen, J.E.; Brown, T.S.; Gardner, S.N.; McLoughlin, K.S.; Forsberg, J.A.; Kirkup, B.C.; Chromy, B.A.; Luciw, P.A.; Elster, E.A.; et al. Microbial profiling of combat wound infection through detection microarray and next-generation sequencing. J. Clin. Microbiol. 2014, 52, 2583–2594. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, J.H.; Murray, C.K.; Manring, M.M. Multidrug-resistant organisms in military wounds from Iraq and Afghanistan. Clin. Orthop. Relat. Res. 2008, 466, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Vento, T.J.; Cole, D.W.; Mende, K.; Calvano, T.P.; Rini, E.A.; Tully, C.C.; Zera, W.C.; Guymon, C.H.; Yu, X.; Cheatle, K.A.; et al. Multidrug-resistant gram-negative bacteria colonization of healthy us military personnel in the us and Afghanistan. BMC Infect. Dis. 2013, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Kempf, M.; Rolain, J.M. Emergence of resistance to carbapenems in Acinetobacter baumannii in Europe: Clinical impact and therapeutic options. Int. J. Antimicrob. Agents 2012, 39, 105–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez, F.; Hujer, A.M.; Hujer, K.M.; Decker, B.K.; Rather, P.N.; Bonomo, R.A. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2007, 51, 3471–3484. [Google Scholar] [CrossRef] [PubMed]
- Potron, A.; Poirel, L.; Nordmann, P. Emerging broad-spectrum resistance in Pseudomonas aeruginosa and Acinetobacter baumannii: Mechanisms and epidemiology. Int. J. Antimicrob. Agents 2015, 45, 568–585. [Google Scholar] [CrossRef] [PubMed]
- Goff, D.A.; Bauer, K.A.; Mangino, J.E. Bad bugs need old drugs: A stewardship program’s evaluation of minocycline for multidrug-resistant Acinetobacter baumannii infections. Clin. Infect. Dis. 2014, 59 (Suppl. 6), S381–S387. [Google Scholar] [CrossRef] [PubMed]
- Roca, I.; Espinal, P.; Vila-Farres, X.; Vila, J. The Acinetobacter baumannii oxymoron: Commensal hospital dweller turned pan-drug-resistant menace. Front. Microbiol. 2012, 3, 148. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Rojas, R.; Jimenez-Mejias, M.E.; Lepe, J.A.; Pachon, J. Acinetobacter baumannii resistant to colistin alters its antibiotic resistance profile: A case report from spain. J. Infect. Dis. 2011, 204, 1147–1148. [Google Scholar] [CrossRef] [PubMed]
- Rolain, J.M.; Roch, A.; Castanier, M.; Papazian, L.; Raoult, D. Acinetobacter baumannii resistant to colistin with impaired virulence: A case report from france. J. Infect. Dis. 2011, 204, 1146–1147. [Google Scholar] [CrossRef] [PubMed]
- Dallo, S.F.; Weitao, T. Insights into acinetobacter war-wound infections, biofilms, and control. Adv. Skin Wound Care 2010, 23, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Longo, F.; Vuotto, C.; Donelli, G. Biofilm formation in Acinetobacter baumannii. New Microbiol. 2014, 37, 119–127. [Google Scholar] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.G.; O’Toole, G.A. Innate and induced resistance mechanisms of bacterial biofilms. Curr. Top. Microbiol. Immunol. 2008, 322, 85–105. [Google Scholar] [PubMed]
- Mah, T.F.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Badave, G.K.; Kulkarni, D. Biofilm producing multidrug resistant Acinetobacter baumannii: An emerging challenge. J. Clin. Diagn. Res. JCDR 2015, 9, DC08–DC10. [Google Scholar] [PubMed]
- He, X.; Lu, F.; Yuan, F.; Jiang, D.; Zhao, P.; Zhu, J.; Cheng, H.; Cao, J.; Lu, G. Biofilm formation caused by clinical Acinetobacter baumannii isolates is associated with over-expression of the AdeFGH efflux pump. Antimicrob. Agents Chemother. 2015. [Google Scholar] [CrossRef] [PubMed]
- Espinal, P.; Marti, S.; Vila, J. Effect of biofilm formation on the survival of Acinetobacter baumannii on dry surfaces. J. Hosp. Infect. 2012, 80, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.; Deye, G.; Srinivasan, A.; Murray, C.; Moran, K.; Hulten, E.; Fishbain, J.; Craft, D.; Riddell, S.; Lindler, L.; et al. An outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus complex infection in the us military health care system associated with military operations in Iraq. Clin. Infect. Dis. 2007, 44, 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- Skaar, E.P. The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathog. 2010, 6, e1000949. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, C.W.; Beglin, M.S.; Actis, L.A. Detection and analysis of iron uptake components expressed by Acinetobacter baumannii clinical isolates. J. Clin. Microbiol. 2003, 41, 4188–4193. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, B.L.; Skaar, E.P. The contribution of nutrient metal acquisition and metabolism to Acinetobacter baumannii survival within the host. Front. Cell. Infect. Microbiol. 2013, 3, 95. [Google Scholar] [CrossRef] [PubMed]
- Zimbler, D.L.; Penwell, W.F.; Gaddy, J.A.; Menke, S.M.; Tomaras, A.P.; Connerly, P.L.; Actis, L.A. Iron acquisition functions expressed by the human pathogen Acinetobacter baumannii. Biometals 2009, 22, 23–32. [Google Scholar] [CrossRef] [PubMed]
- De Leseleuc, L.; Harris, G.; KuoLee, R.; Chen, W. In vitro and in vivo biological activities of iron chelators and gallium nitrate against Acinetobacter baumannii. Antimicrob. Agents Chemother. 2012, 56, 5397–5400. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.G.; Corey, B.W.; Si, Y.; Craft, D.W.; Zurawski, D.V. Antibacterial activities of iron chelators against common nosocomial pathogens. Antimicrob. Agents Chemother. 2012, 56, 5419–5421. [Google Scholar] [CrossRef] [PubMed]
- Bonchi, C.; Imperi, F.; Minandri, F.; Visca, P.; Frangipani, E. Repurposing of gallium-based drugs for antibacterial therapy. Biofactors 2014, 40, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Kelson, A.B.; Carnevali, M.; Truong-Le, V. Gallium-based anti-infectives: Targeting microbial iron-uptake mechanisms. Curr. Opin. Pharmacol. 2013, 13, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Chitambar, C.R. Medical applications and toxicities of gallium compounds. Int. J. Environ. Res. Public Health 2010, 7, 2337–2361. [Google Scholar] [CrossRef] [PubMed]
- Antunes, L.C.; Imperi, F.; Minandri, F.; Visca, P. In vitro and in vivo antimicrobial activities of gallium nitrate against multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2012, 56, 5961–5970. [Google Scholar] [CrossRef] [PubMed]
- Stojiljkovic, I.; Evavold, B.D.; Kumar, V. Antimicrobial properties of porphyrins. Expert Opin. Investig. Drugs 2001, 10, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Bozja, J.; Yi, K.; Shafer, W.M.; Stojiljkovic, I. Porphyrin-based compounds exert antibacterial action against the sexually transmitted pathogens neisseria gonorrhoeae and haemophilus ducreyi. Int. J. Antimicrob. Agents 2004, 24, 578–584. [Google Scholar] [CrossRef] [PubMed]
- De Leseleuc, L.; Harris, G.; KuoLee, R.; Xu, H.H.; Chen, W. Serum resistance, gallium nitrate tolerance and extrapulmonary dissemination are linked to heme consumption in a bacteremic strain of Acinetobacter baumannii. Int. J. Med. Microbiol. 2014, 304, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, E.D. Iron availability and infection. Biochim. Biophys. Acta 2009, 1790, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Ceri, H.; Olson, M.; Morck, D.; Storey, D.; Read, R.; Buret, A.; Olson, B. The mbec assay system: Multiple equivalent biofilms for antibiotic and biocide susceptibility testing. Methods Enzymol. 2001, 337, 377–385. [Google Scholar] [PubMed]
- Kaneko, Y.; Thoendel, M.; Olakanmi, O.; Britigan, B.E.; Singh, P.K. The transition metal gallium disrupts pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J. Clin. Investig. 2007, 117, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, L.R. Mechanisms of therapeutic activity for gallium. Pharmacol. Rev. 1998, 50, 665–682. [Google Scholar] [PubMed]
- Collery, P.; Keppler, B.; Madoulet, C.; Desoize, B. Gallium in cancer treatment. Crit. Rev. Oncol. Hematol. 2002, 42, 283–296. [Google Scholar] [CrossRef]
- Stojiljkovic, I.; Kumar, V.; Srinivasan, N. Non-iron metalloporphyrins: Potent antibacterial compounds that exploit haem/Hb uptake systems of pathogenic bacteria. Mol. Microbiol. 1999, 31, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Darmawan, E.T.; Zhang, M.; Zhang, L.; Bryers, J.D. Development of a poly(ether urethane) system for the controlled release of two novel anti-biofilm agents based on gallium or zinc and its efficacy to prevent bacterial biofilm formation. J. Control. Release 2013, 172, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Arivett, B.A.; Fiester, S.E.; Ohneck, E.J.; Penwell, W.F.; Kaufman, C.M.; Relich, R.F.; Actis, L.A. Antimicrobial activity of gallium protoporphyrin IX against Acinetobacter baumannii strains displaying different antibiotic resistance phenotypes. Antimicrob. Agents Chemother. 2015, 59, 7657–7665. [Google Scholar] [CrossRef] [PubMed]
- Imperi, F.; Antunes, L.C.; Blom, J.; Villa, L.; Iacono, M.; Visca, P.; Carattoli, A. The genomics of Acinetobacter baumannii: Insights into genome plasticity, antimicrobial resistance and pathogenicity. IUBMB Life 2011, 63, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhu, Y.; Yi, Y.; Lu, N.; Zhu, B.; Hu, Y. Comparative genomic analysis of Acinetobacter baumannii clinical isolates reveals extensive genomic variation and diverse antibiotic resistance determinants. BMC Genom. 2014, 15, 1163. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.E.; Hansen, L.A. Gallium nitrate. Ann. Pharmacother. 1992, 26, 354–362. [Google Scholar] [PubMed]
- Akers, K.S.; Mende, K.; Cheatle, K.A.; Zera, W.C.; Yu, X.; Beckius, M.L.; Aggarwal, D.; Li, P.; Sanchez, C.J.; Wenke, J.C.; et al. Biofilms and persistent wound infections in United States military trauma patients: A case-control analysis. BMC Infect. Dis. 2014, 14, 190. [Google Scholar] [CrossRef] [PubMed]
- Gales, A.C.; Reis, A.O.; Jones, R.N. Contemporary assessment of antimicrobial susceptibility testing methods for polymyxin B and colistin: Review of available interpretative criteria and quality control guidelines. J. Clin. Microbiol. 2001, 39, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Bronstein, P.A.; Filiatrault, M.J.; Myers, C.R.; Rutzke, M.; Schneider, D.J.; Cartinhour, S.W. Global transcriptional responses of pseudomonas syringae DC3000 to changes in iron bioavailability in vitro. BMC Microbiol. 2008, 8, 209. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Park, J.H.; Park, T.H.; Bronstein, P.A.; Schneider, D.J.; Cartinhour, S.W.; Shuler, M.L. Effect of iron concentration on the growth rate of Pseudomonas syringae and the expression of virulence factors in hrp-inducing minimal medium. Appl. Environ. Microbiol. 2009, 75, 2720–2726. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.J., Jr.; Mende, K.; Beckius, M.L.; Akers, K.S.; Romano, D.R.; Wenke, J.C.; Murray, C.K. Biofilm formation by clinical isolates and the implications in chronic infections. BMC Infect. Dis. 2013, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Coraca-Huber, D.C.; Fille, M.; Hausdorfer, J.; Pfaller, K.; Nogler, M. Staphylococcus aureus biofilm formation and antibiotic susceptibility tests on polystyrene and metal surfaces. J. Appl. Microbiol. 2012, 112, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.J., Jr.; Shiels, S.M.; Tennent, D.J.; Hardy, S.K.; Murray, C.K.; Wenke, J.C. Rifamycin derivatives are effective against staphylococcal biofilms in vitro and elutable from PMMA. Clin. Orthop. Relat. Res. 2015, 473, 2874–2884. [Google Scholar] [CrossRef] [PubMed]
- Barsoumian, A.; Sanchez, C.J.; Mende, K.; Tully, C.C.; Beckius, M.L.; Akers, K.S.; Wenke, J.C.; Murray, C.K. In vitro toxicity and activity of dakin’s solution, mafenide acetate, and amphotericin b on filamentous fungi and human cells. J. Orthop. Trauma 2013, 27, 428–436. [Google Scholar] [CrossRef] [PubMed]




| Isolate (A. baumannii) | Source | Specific Site | Phenotype a | Pulse Field Type (PFT) | Colistin Resistance b |
|---|---|---|---|---|---|
| AB 1 | Wound | Pelvis | MDR | 51 | N |
| AB 2 | Wound | Forearm | MDR | 50 | N |
| AB 3 | Wound | Forearm | MDR | 7 | N |
| AB 4 | Wound | Lower Extremity | MDR | 50 | N |
| AB 5 | Wound | Upper Extremity | MDR | 51 | N |
| AB 6 | Wound | Pelvis | MDR | 51 | N |
| AB 7 | Wound | Upper Extremity | MDR | 44 | N |
| AB 8 | Wound | Pelvis | MDR | 50 | N |
| AB 9 | Wound | Lower Extremity | MDR | 50 | N |
| AB 10 | Wound | Upper Extremity | MDR | 44 | N |
| AB 11 | Blood | --- | PDR | 49 | Y (MIC >256 µg/mL) |
| AB 12 | Wound | Lower Extremity | PDR | 44 | Y (MIC 12 µg/mL) |
| ATCC 17978 | Meningitis | Unknown | Non-MDR | Unknown | N |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, D.; Garcia, R.A.; Akers, K.S.; Mende, K.; Murray, C.K.; Wenke, J.C.; Sanchez, C.J. Activity of Gallium Meso- and Protoporphyrin IX against Biofilms of Multidrug-Resistant Acinetobacter baumannii Isolates. Pharmaceuticals 2016, 9, 16. https://doi.org/10.3390/ph9010016
Chang D, Garcia RA, Akers KS, Mende K, Murray CK, Wenke JC, Sanchez CJ. Activity of Gallium Meso- and Protoporphyrin IX against Biofilms of Multidrug-Resistant Acinetobacter baumannii Isolates. Pharmaceuticals. 2016; 9(1):16. https://doi.org/10.3390/ph9010016
Chicago/Turabian StyleChang, David, Rebecca A. Garcia, Kevin S. Akers, Katrin Mende, Clinton K. Murray, Joseph C. Wenke, and Carlos J. Sanchez. 2016. "Activity of Gallium Meso- and Protoporphyrin IX against Biofilms of Multidrug-Resistant Acinetobacter baumannii Isolates" Pharmaceuticals 9, no. 1: 16. https://doi.org/10.3390/ph9010016





