Nanosized Drug Delivery Systems in Gastrointestinal Targeting: Interactions with Microbiota
Abstract
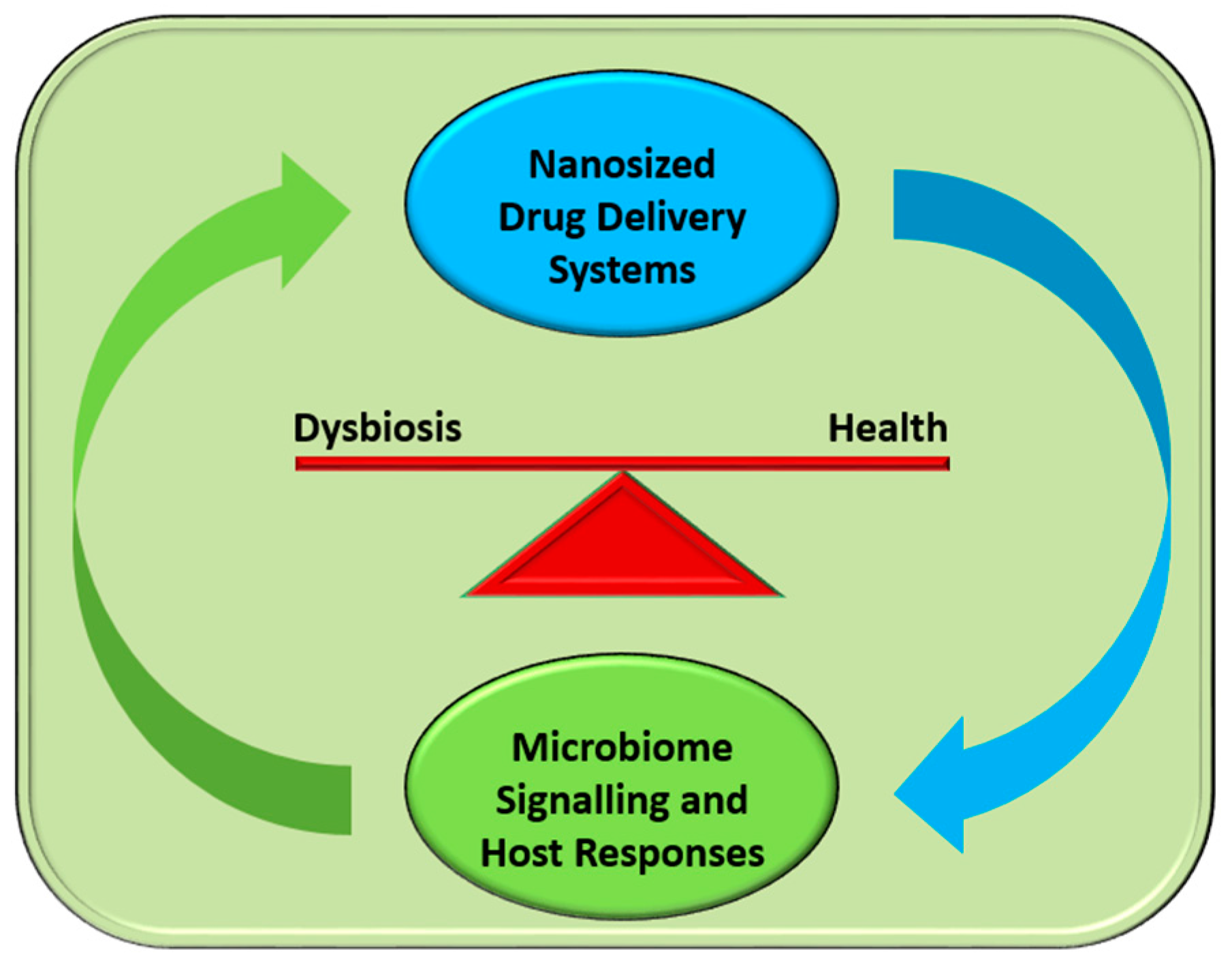
:1. Introduction
2. Gut Microbiota, Health, and Disease
2.1. The Complexity of the Human Microbiome
2.2. The Role of Microbiome Homeostasis: Dysbiosis and Disease
3. Gut Pathogens and Gastrointestinal Infections and Treatment
3.1. Most Common Infections
3.2. Dysbiosis: Treating the Imbalance
4. Antimicrobial Nanosystems for Gastrointestinal Drug Delivery–Impact on Microbiota
4.1. Types
4.1.1. Metal Nanoparticles
4.1.2. Carbon-Based Nanomaterials
4.1.3. Polymeric Nanoparticles
4.2. Mechanisms and Behavior in the Gastrointestinal Tract
4.2.1. Absorption
4.2.2. Dissolution
5. Conclusions and Perspectives
Acknowledgments
Conflicts of Interest
References
- Murthy, S.K. Nanoparticles in modern medicine: State of the art and future challenges. Int. J. Nanomed. 2007, 2, 129–141. [Google Scholar]
- Bergin, I.L.; Witzmann, F.A. Nanoparticle toxicity by the gastrointestinal route: Evidence and knowledge gaps. Int. Biomed. Nanosci. Nanotechnol. 2013, 3, 163–210. [Google Scholar] [CrossRef] [PubMed]
- Ensign, L.M.; Cone, R.; Hanes, J. Oral drug delivery with polymeric nanoparticles: The gastrointestinal mucus barriers. Adv. Drug Deliv. Rev. 2012, 64, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Holban, A.M.; Grumezescu, A.M.; Gestal, M.C.; Mogoantǎ, L.; Mogoşanu, G.D. Novel drug delivery magnetite nano-systems used in antimicrobial therapy. Curr. Org. Chem. 2014, 18, 185–191. [Google Scholar] [CrossRef]
- Stan, M.S.; Constanda, S.; Grumezescu, V.; Andronescu, E.; Ene, A.M.; Holban, A.M.; Vasile, B.S.; Mogoantă, L.; Bălşeanu, T.-A.; Mogoşanu, G.D. Thin coatings based on ZnO@C18-usnic acid nanoparticles prepared by MAPLE inhibit the development of Salmonella enterica early biofilm growth. Appl. Surf. Sci. 2016, 374, 318–325. [Google Scholar] [CrossRef]
- Nagao-Kitamoto, H.; Kitamoto, S.; Kuffa, P.; Kamada, N. Pathogenic role of the gut microbiota in gastrointestinal diseases. Intest. Res. 2016, 14, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E.; Fröhlich, E.F. Cytotoxicity of nanoparticles contained in food on intestinal cells and the gut microbiota. Int. J. Mol. Sci. 2016, 17, 509. [Google Scholar] [CrossRef] [PubMed]
- The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [Green Version]
- Human Microbiome Project Consortium. A framework for human microbiome research. Nature 2012, 486, 215–221. [Google Scholar] [Green Version]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ley, R.E.; Peterson, D.A.; Gordon, J.I. Ecological and Evolutionary Forces Shaping Microbial Diversity in the Human Intestine. Cell 2006, 124, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Willing, B.P.; Singh, V.P.; Proctor, S. Koch’s postulates, microbial dysbiosis and inflammatory bowel disease. Clin. Microbiol. Infect. 2016, 22, 594–599. [Google Scholar]
- Ley, R.E.; Lozupone, C.A.; Hamady, M.; Knight, R.; Gordon, J.I. Worlds within worlds: Evolution of the vertebrate gut microbiota. Nat. Rev. Micro. 2008, 6, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Zmora, N.; Levy, M.; Elinav, E. The microbiome and innate immunity. Nature 2016, 535, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Althani, A.A.; Marei, H.E.; Hamdi, W.S.; Nasrallah, G.K.; El Zowalaty, M.E.; Al Khodor, S.; Al-Asmakh, M.; Abdel-Aziz, H.; Cenciarelli, C. Human Microbiome and its Association With Health and Diseases. J. Cell. Physiol. 2016, 231, 1688–1694. [Google Scholar] [CrossRef] [PubMed]
- Karavolos, M.H. Host microbe interactions: A licence to interfere? Curr. Pharm. Biotechnol. 2015, 16, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Osborn, D.A.; Sinn, J.K.H. Probiotics in infants for prevention of allergic disease and food hypersensitivity. Cochrane Database Syst. Rev. 2007, 4. [Google Scholar] [CrossRef]
- Mimee, M.; Citorik, R.J.; Lu, T.K. Microbiome therapeutics—Advances and challenges. Adv. Drug Deliv. Rev. 2016, 105, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Wilding, L.A.; Bassis, C.M.; Walacavage, K.; Hashway, S.; Leroueil, P.R.; Morishita, M.; Maynard, A.D.; Philbert, M.A.; Bergin, I.L. Repeated dose (28-day) administration of silver nanoparticles of varied size and coating does not significantly alter the indigenous murine gut microbiome. Nanotoxicology 2016, 10, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.A.; Chun-Hsun, H.; Jia-You, F. Nanomedical Strategies for Targeting Skin Microbiomes. Curr. Drug Metab. 2015, 16, 255–271. [Google Scholar]
- Bloomfield, L.E.; Riley, T.V. Epidemiology and Risk Factors for Community-Associated Clostridium difficile Infection: A Narrative Review. Infect. Dis. Ther. 2016, 5, 231–251. [Google Scholar] [CrossRef] [PubMed]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J. Multistate Point-Prevalence Survey of Health Care–Associated Infections. New Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- van Opstal, E.; Kolling, G.L.; Moore, J.H.; Coquery, C.M.; Wade, N.S.; Loo, W.M.; Bolick, D.T.; Shin, J.H.; Erickson, L.D.; Warren, C.A. Vancomycin Treatment Alters Humoral Immunity and Intestinal Microbiota in an Aged Mouse Model of Clostridium difficile Infection. J. Infect. Dis. 2016, 214, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Jellbauer, S.; Perez Lopez, A.; Behnsen, J.; Gao, N.; Nguyen, T.; Murphy, C.; Edwards, R.A.; Raffatellu, M. Beneficial effects of sodium phenylbutyrate administration during infection with salmonella enterica serovar typhimurium. Infect. Immun. 2016, 84, 2639–2652. [Google Scholar] [CrossRef] [PubMed]
- Ziklo, N.; Huston, W.M.; Hocking, J.S.; Timms, P. Chlamydia trachomatis genital tract infections: When host immune response and the microbiome collide. Trends Microbiol. 2016, 24, 750–765. [Google Scholar] [CrossRef] [PubMed]
- Nasioudis, D.; Linhares, I.M.; Ledger, W.J.; Witkin, S.S. Bacterial vaginosis: A critical analysis of current knowledge. BJOG 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, L.; Wolfe, A. The urinary microbiota: A paradigm shift for bladder disorders? Curr. Opin. Obstet. Gynecol. 2016, 28, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Khoruts, A.; Sadowsky, M.J. Understanding the mechanisms of faecal microbiota transplantation. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Sbahi, H.; Palma, J.A.D. Faecal microbiota transplantation: Applications and limitations in treating gastrointestinal disorders. BMJ Open Gastroenterol. 2016, 3, e000087. [Google Scholar] [CrossRef] [PubMed]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut Microbiota in Health and Disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [PubMed]
- Manges, A.R.; Steiner, T.S.; Wright, A.J. Fecal microbiota transplantation for the intestinal decolonization of extensively antimicrobial-resistant opportunistic pathogens: A review. Infect. Dis. 2016, 48, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Ngwuluka, N.C.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Modi, G.; Pillay, V. An optimized gastroretentive nanosystem for the delivery of levodopa. Int. J. Pharm. 2015, 494, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Liakos, I.; Grumezescu, A.M.; Holban, A.M. Magnetite nanostructures as novel strategies for anti-infectious therapy. Molecules 2014, 19, 12710–12726. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.N.; Ho, H.O.; Yu, C.Y.; Sheu, M.T. Development of swelling/floating gastroretentive drug delivery system based on a combination of hydroxyethyl cellulose and sodium carboxymethyl cellulose for Losartan and its clinical relevance in healthy volunteers with CYP2C9 polymorphism. Euro. J. Pharm. Sci. 2010, 39, 82–89. [Google Scholar]
- Choi, S.K.; Myc, A.; Silpe, J.E.; Sumit, M.; Wong, P.T.; McCarthy, K.; Desai, A.M.; Thomas, T.P.; Kotlyar, A.; Holl, M.M.B. Dendrimer-Based Multivalent Vancomycin Nanoplatform for Targeting the Drug-Resistant Bacterial Surface. ACS Nano 2013, 7, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Holban, A.M.; Gestal, M.C.; Grumezescu, A.M. Control of biofilm-associated infections by signaling molecules and nanoparticles. Int. J. Pharm. 2016, 510, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Khlebtsov, N.; Dykman, L. Biodistribution and toxicity of engineered gold nanoparticles: A review of in vitro and in vivo studies. Chem. Soc. Rev. 2011, 40, 1647–1671. [Google Scholar] [CrossRef] [PubMed]
- Maliszewska, I. Effects of the biogenic gold nanoparticles on microbial community structure and activities. Ann. Microbiol. 2016, 66, 785–794. [Google Scholar] [CrossRef]
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium dioxide nanoparticles: A review of current toxicological data. Part. Fibre Toxicol. 2013, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Kubacka, A.; Diez, M.S.; Rojo, D.; Bargiela, R.; Ciordia, S.; Zapico, I.; Albar, J.P.; Barbas, C.; Martins dos Santos, V.A.; Fernandez-Garcia, M.; et al. Understanding the antimicrobial mechanism of TiO2-based nanocomposite films in a pathogenic bacterium. Sci. Rep. 2014, 4, 4134. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.T.; Al-Khedhairy, A.A.; Musarrat, J. ZnO and TiO2 nanoparticles as novel antimicrobial agents for oral hygiene: A review. J. Nanopart. Res. 2015, 17, 276. [Google Scholar] [CrossRef]
- López-Moreno, M.L.; de la Rosa, G.; Hernández-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. XAS Corroboration of the Uptake and Storage of CeO2 Nanoparticles and Assessment of their Differential Toxicity in Four Edible Plant Species. J. Agric. Food Chem. 2010, 58, 3689–3693. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.A.; Marcus, I.M.; Guysi, R.L.; Walker, S.L. Metal oxide nanoparticles induce minimal phenotypic changes in a model colon gut microbiota. Environ. Eng. Sci. 2015, 32, 602–612. [Google Scholar] [CrossRef]
- Kolosnjaj-Tabi, J.; Hartman, K.B.; Boudjemaa, S.; Ananta, J.S.; Morgan, G.; Szwarc, H.; Wilson, L.J.; Moussa, F. In vivo behavior of large doses of ultrashort and full-length single-walled carbon nanotubes after oral and intraperitoneal administration to Swiss mice. ACS Nano 2010, 4, 1481–1492. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, B.; Gao, D.; Guan, M.; Zheng, L.; Ouyang, H.; Chai, Z.; Zhao, Y.; Feng, W. Broad-spectrum antibacterial activity of carbon nanotubes to human gut bacteria. Small 2013, 9, 2735–2746. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.W.; James, J.T.; McCluskey, R.; Arepalli, S.; Hunter, R.L. A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Crit. Rev. Toxicol. 2006, 36, 189–217. [Google Scholar] [CrossRef] [PubMed]
- Madureira, A.R.; Pereira, A.; Pintado, M. Current state on the development of nanoparticles for use against bacterial gastrointestinal pathogens. Focus on chitosan nanoparticles loaded with phenolic compounds. Carbohydr. Polym. 2015, 130, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Coll. Surf. B: Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Nie, X.; Zou, M.; Shi, Y.; Cheng, G. Recent advances in materials for extended-release antibiotic delivery system. J. Antibiot. 2011, 64, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Jena, P.K.; Singh, S.; Prajapati, B.; Nareshkumar, G.; Mehta, T.; Seshadri, S. Impact of targeted specific antibiotic delivery for gut microbiota modulation on high-fructose-fed rats. Appl. Biochem. Biotechnol. 2014, 172, 3810–3826. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.M.; Noble, M.L.; Garty, S.; Ma, H.; Bryers, J.D.; Shen, T.T.; Ratner, B.D. Sustained release of antibiotic from poly(2-hydroxyethyl methacrylate) to prevent blinding infections after cataract surgery. Biomaterials 2009, 30, 5675–5681. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, F.; Hosseini-Nasr, M.; Rad-Malekshahi, M.; Samadi, N.; Atyabi, F.; Dinarvand, R. Preparation and antibacterial activity evaluation of rifampicin-loaded poly lactide-co-glycolide nanoparticles. Nanomed.: Nanotechnol. Biol. Med. 2007, 3, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, G.; Nokhodchi, A.; Barzegar-Jalali, M.; Lotfipour, F.; Adibkia, K.; Ehyaei, N.; Valizadeh, H. Physicochemical and anti-bacterial performance characterization of clarithromycin nanoparticles as colloidal drug delivery system. Coll. Surf. B: Biointerfaces 2011, 88, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Vukomanovic, M.; Skapin, S.D.; Poljansek, I.; Zagar, E.; Kralj, B.; Ignjatovic, N.; Uskokovic, D. Poly(d,l-lactide-co-glycolide)/hydroxyapatite core-shell nanosphere. Part 2: Simultaneous release of a drug and a prodrug (clindamycin and clindamycin phosphate). Coll. Surf. B: Biointerfaces 2011, 82, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.; Moreira, R.G.; Castell-Perez, E. Poly (d,l-lactide-co-glycolide) (PLGA) nanoparticles with entrapped trans-cinnamaldehyde and eugenol for antimicrobial delivery applications. J. Food Sci. 2011, 76, N16–N24. [Google Scholar] [CrossRef] [PubMed]
- Pagonis, T.C.; Chen, J.; Fontana, C.R.; Devalapally, H.; Ruggiero, K.; Song, X.; Foschi, F.; Dunham, J.; Skobe, Z.; Yamazaki, H.; et al. Nanoparticle-based endodontic antimicrobial photodynamic therapy. J. Endod. 2010, 36, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Soosan Abdollahi, F.L. PLGA- and PLA-Based polymeric nanoparticles for antimicrobial drug delivery. Biomed. Int. 2012, 3, 1–11. [Google Scholar]
- Kluin, O.S.; van der Mei, H.C.; Busscher, H.J.; Neut, D. A surface-eroding antibiotic delivery system based on poly-(trimethylene carbonate). Biomaterials 2009, 30, 4738–4742. [Google Scholar] [CrossRef] [PubMed]
- Neut, D.; Kluin, O.S.; Crielaard, B.J.; van der Mei, H.C.; Busscher, H.J.; Grijpma, D.W. A biodegradable antibiotic delivery system based on poly-(trimethylene carbonate) for the treatment of osteomyelitis. Acta Orthop. 2009, 80, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Yang, J.; Xue, J. Characterization of antimicrobial poly(lactic acid)/poly(trimethylene carbonate) films with cinnamaldehyde. J. Mater. Sci. 2015, 50, 1150–1158. [Google Scholar] [CrossRef]
- Mosqueira, V.C.F.; Loiseau, P.M.; Bories, C.; Legrand, P.; Devissaguet, J.-P.; Barratt, G. Efficacy and pharmacokinetics of intravenous nanocapsule formulations of halofantrine in plasmodium berghei-Infected mice. Antimicrob. Agents Chemother. 2004, 48, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Salmaso, S.; Elvassore, N.; Bertucco, A.; Lante, A.; Caliceti, P. Nisin-loaded poly-l-lactide nano-particles produced by CO2 anti-solvent precipitation for sustained antimicrobial activity. Int. J. Pharm. 2004, 287, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Fu, X. Naturally derived materials-based cell and drug delivery systems in skin regeneration. J. Controll. Release 2010, 142, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Anal, A.K.; Stevens, W.F.; Remunan-Lopez, C. Ionotropic cross-linked chitosan microspheres for controlled release of ampicillin. Int. J. Pharm. 2006, 312, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Abbott, L.C.; Maynard, A.D. Exposure assessment approaches for engineered nanomaterials. Risk Anal. 2010, 30, 1634–1644. [Google Scholar] [CrossRef] [PubMed]
- Frohlich, E.; Roblegg, E. Models for oral uptake of nanoparticles in consumer products. Toxicology 2012, 291, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Jani, P.; Halbert, G.W.; Langridge, J.; Florence, A.T. Nanoparticle uptake by the rat gastrointestinal mucosa: Quantitation and particle size dependency. J. Pharm. Pharmacol. 1990, 42, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; Germolec, D.R.; Weaver, J.L. Evaluation of nanoparticle immunotoxicity. Nat. Nanotechnol. 2009, 4, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Mwilu, S.K.; El Badawy, A.M.; Bradham, K.; Nelson, C.; Thomas, D.; Scheckel, K.G.; Tolaymat, T.; Ma, L.; Rogers, K.R. Changes in silver nanoparticles exposed to human synthetic stomach fluid: Effects of particle size and surface chemistry. Sci. Total Environ. 2013, 447, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.S.; Kang, B.C.; Lee, J.K.; Jeong, J.; Che, J.H.; Seok, S.H. Comparative absorption, distribution, and excretion of titanium dioxide and zinc oxide nanoparticles after repeated oral administration. Part. Fibre Toxicol. 2013, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Loretz, B.; Bernkop-Schnürch, A. In vitro cytotoxicity testing of non-thiolated and thiolated chitosan nanoparticles for oral gene delivery. Nanotoxicology 2007, 1, 139–148. [Google Scholar] [CrossRef]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karavolos, M.; Holban, A. Nanosized Drug Delivery Systems in Gastrointestinal Targeting: Interactions with Microbiota. Pharmaceuticals 2016, 9, 62. https://doi.org/10.3390/ph9040062
Karavolos M, Holban A. Nanosized Drug Delivery Systems in Gastrointestinal Targeting: Interactions with Microbiota. Pharmaceuticals. 2016; 9(4):62. https://doi.org/10.3390/ph9040062
Chicago/Turabian StyleKaravolos, Michail, and Alina Holban. 2016. "Nanosized Drug Delivery Systems in Gastrointestinal Targeting: Interactions with Microbiota" Pharmaceuticals 9, no. 4: 62. https://doi.org/10.3390/ph9040062




