TRP Channels in Skin Biology and Pathophysiology
Abstract
:1. Introduction to TRP Channels
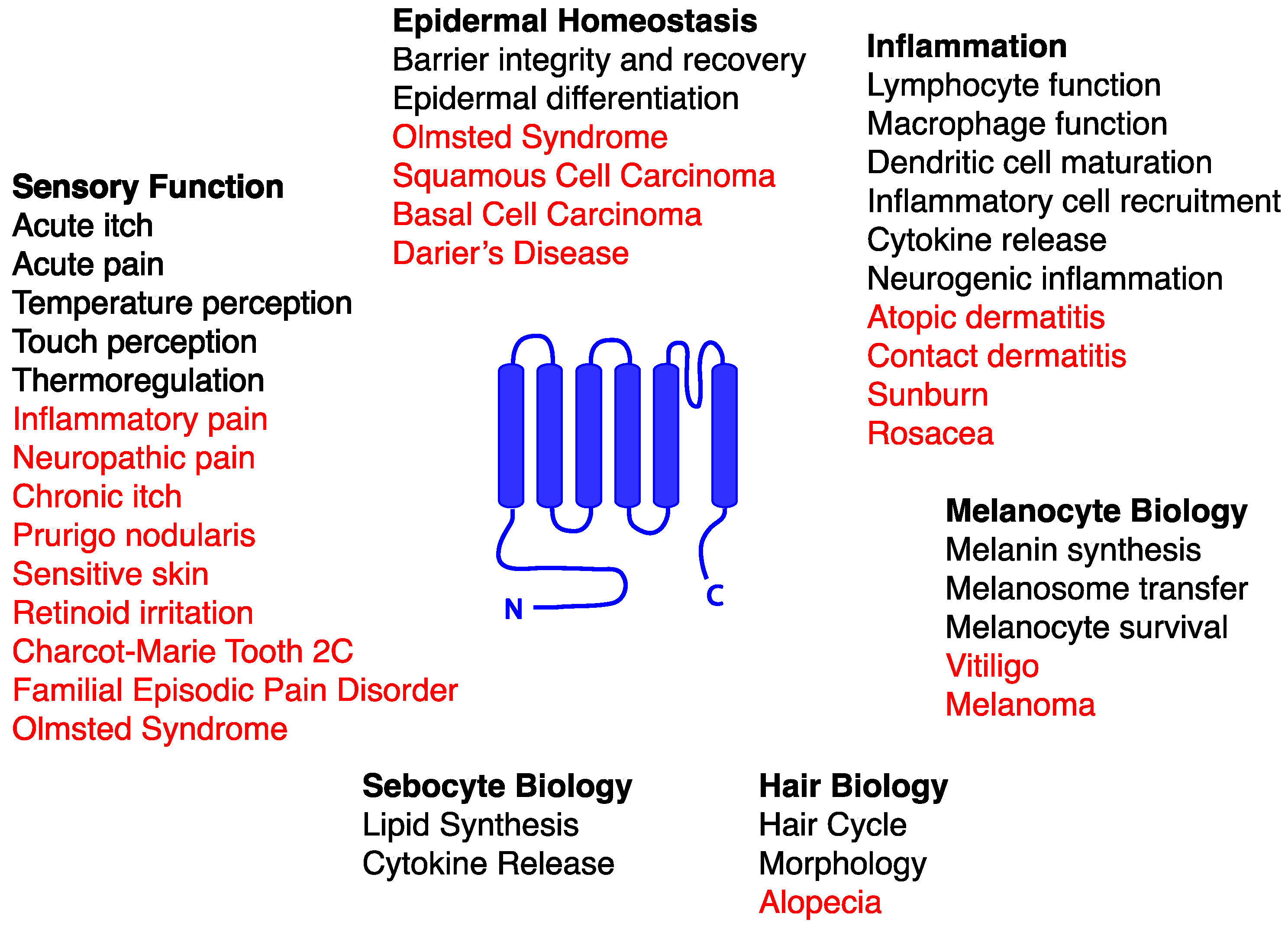
2. Contributions of TRP Channels to Skin Biology and Pathophysiology
2.1. TRPC Channels and Skin
2.1.1. TRPC Channels and Keratinocyte Differentiation
2.1.2. TRPC Channels in Darier’s Disease
2.1.3. TRPC Channels and Sensory Function
2.2. TRPV1 and Skin
2.2.1. TRPV1 in Pain, Itch, and Neurogenic Inflammation
2.2.2. TRPV1 Expression in Keratinocytes
2.2.3. TRPV1 in Epidermal Homeostasis and Dermatitis
2.2.4. TRPV1 and Ultraviolet Radiation
2.2.5. TRPV1 Epidermal Upregulation in Human Skin Diseases
2.2.6. TRPV1 and Skin Cancer
2.2.7. TRPV1 in Skin Appendages
2.3. TRPV2 and Skin
2.3.1. TRPV2 and Sensory Function
2.3.2. TRPV2 and Immune Cell Function
2.4. TRPV3 and Skin
2.4.1. TRPV3 and Cutaneous Temperature Sensation
2.4.2. TRPV3 and Epidermal Homeostasis and Hair Development
2.4.3. TRPV3 and Skin Pathology
2.5. TRPV4 and Skin
2.5.1. TRPV4 and Epidermal Barrier Function
2.5.2. TRPV4 and Skin Cancer
2.5.3. TRPV4 and Sensory Function
2.6. TRPV6 and Skin
TRPV6 and Keratinocyte Differentiation
2.7. TRPA1 and Skin
2.7.1. TRPA1 and Cutaneous Pain Sensation
2.7.2. TRPA1 and Itch
2.7.3. TRPA1 and Inflammation
2.7.4. TRPA1 and Barrier Function
2.7.5. Loci of TRPA1 Action in Its Cutaneous Functions
2.8. TRPM1 and Skin
2.8.1. TRPM1 and Melanocytes
2.8.2. TRPM1 and Melanoma
2.9. TRPM2 and Skin
2.9.1. TRPM2 and Cutaneous Pain Sensation
2.9.2. TRPM2 and Melanoma
2.10. TRPM3 and Skin
TRPM3 and Cutaneous Pain Sensation
2.11. TRPM4 and Skin
TRPM4 and Lymphocytes
2.12. TRPM7 and Skin
TRPM7 and Melanocytes
2.13. TRPM8 and Skin
2.13.1. TRPM8 and Cutaneous Cold and Pain Sensation
2.13.2. TRPM8 and Epidermal Homeostasis
2.13.3. TRPM8 and Melanoma
2.14. TRPML3 and Skin
TRPML3 and Melanocytes
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Caterina, M.J.; Julius, D. The vanilloid receptor: A molecular gateway to the pain pathway. Annu. Rev. Neurosci. 2001, 24, 487–517. [Google Scholar] [CrossRef] [PubMed]
- Montell, C.; Birnbaumer, L.; Flockerzi, V.; Bindels, R.J.; Bruford, E.A.; Caterina, M.J.; Clapham, D.E.; Harteneck, C.; Heller, S.; Julius, D.; et al. A unified nomenclature for the superfamily of TRP cation channels. Mol. Cell 2002, 9, 229–231. [Google Scholar] [CrossRef]
- Ramsey, I.S.; Delling, M.; Clapham, D.E. An introduction to TRP channels. Annu. Rev. Physiol. 2006, 68, 619–647. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Owsianik, G.; Voets, T.; Peters, J.A. Transient receptor potential cation channels in disease. Physiol. Rev. 2007, 87, 165–217. [Google Scholar] [CrossRef] [PubMed]
- Agarwala, M.K.; George, R.; Pramanik, R.; McGrath, J.A. Olmsted syndrome in an Indian male with a new de novo mutation in TRPV3. Br. J. Dermatol. 2016, 174, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Cao, E.; Liao, M.; Cheng, Y.; Julius, D. TRPV1 structures in distinct conformations reveal activation mechanisms. Nature 2013, 504, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Huynh, K.W.; Cohen, M.R.; Jiang, J.; Samanta, A.; Lodowski, D.T.; Zhou, Z.H.; Moiseenkova-Bell, V.Y. Structure of the full-length TRPV2 channel by cryo-EM. Nat. Commun. 2016, 7, 11130. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Cao, E.; Julius, D.; Cheng, Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 2013, 504, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Zubcevic, L.; Herzik, M.A., Jr.; Chung, B.C.; Liu, Z.; Lander, G.C.; Lee, S.Y. Cryo-electron microscopy structure of the TRPV2 ion channel. Nat. Struct. Mol. Biol. 2016, 23, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, C.E.; Armache, J.P.; Gao, Y.; Cheng, Y.; Julius, D. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature 2015, 525, 552. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J. TRP channel cannabinoid receptors in skin sensation, homeostasis, and inflammation. ACS Chem. Neurosci. 2014, 5, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Lumpkin, E.A.; Caterina, M.J. Mechanisms of sensory transduction in the skin. Nature 2007, 445, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Yuspa, S.H.; Hennings, H.; Tucker, R.W.; Jaken, S.; Kilkenny, A.E.; Roop, D.R. Signal transduction for proliferation and differentiation in keratinocytes. Ann. N. Y. Acad. Sci. 1988, 548, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Bonifas, J.M.; Beech, J.; Bench, G.; Shigihara, T.; Ogawa, H.; Ikeda, S.; Mauro, T.; Epstein, E.H., Jr. Mutations in ATP2C1, encoding a calcium pump, cause Hailey-Hailey disease. Nat. Genet. 2000, 24, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Sakuntabhai, A.; Dhitavat, J.; Burge, S.; Hovnanian, A. Mosaicism for ATP2A2 mutations causes segmental darier’s disease. J. Investig. Dermatol. 2000, 115, 1144–1147. [Google Scholar] [CrossRef] [PubMed]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Barr, T.P.; Hou, Q.; Dib-Hajj, S.D.; Black, J.A.; Albrecht, P.J.; Petersen, K.; Eisenberg, E.; Wymer, J.P.; Rice, F.L.; et al. Voltage-gated sodium channel expression in rat and human epidermal keratinocytes: Evidence for a role in pain. Pain 2008, 139, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.S.; Hildner, K.; Murphy, K.M.; Allen, P.M. TRPM4 differentially regulates Th1 and Th2 function by altering calcium signaling and NFAT localization. J. Immunol. 2010, 185, 2836–2846. [Google Scholar] [CrossRef] [PubMed]
- Hanack, C.; Moroni, M.; Lima, W.C.; Wende, H.; Kirchner, M.; Adelfinger, L.; Schrenk-Siemens, K.; Tappe-Theodor, A.; Wetzel, C.; Kuich, P.H.; et al. GABA blocks pathological but not acute TRPV1 pain signals. Cell 2015, 160, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Kiselyov, K.; Shin, D.M.; Kim, J.Y.; Yuan, J.P.; Muallem, S. TRPC channels: Interacting proteins. Handb. Exp. Pharmacol. 2007, 559–574. [Google Scholar] [CrossRef]
- Beck, B.; Lehen’kyi, V.; Roudbaraki, M.; Flourakis, M.; Charveron, M.; Bordat, P.; Polakowska, R.; Prevarskaya, N.; Skryma, R. TRPC channels determine human keratinocyte differentiation: New insight into basal cell carcinoma. Cell Calcium 2008, 43, 492–505. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Fatherazi, S.; Presland, R.B.; Belton, C.M.; Izutsu, K.T. TRPC channel expression during calcium-induced differentiation of human gingival keratinocytes. J. Dermatol. Sci. 2005, 40, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Lehen’kyi, V.; Beck, B.; Polakowska, R.; Charveron, M.; Bordat, P.; Skryma, R.; Prevarskaya, N. TRPV6 is a Ca2+ entry channel essential for Ca2+-induced differentiation of human keratinocytes. J. Biol. Chem. 2007, 282, 22582–22591. [Google Scholar] [CrossRef] [PubMed]
- Woelfle, U.; Laszczyk, M.N.; Kraus, M.; Leuner, K.; Kersten, A.; Simon-Haarhaus, B.; Scheffler, A.; Martin, S.F.; Muller, W.E.; Nashan, D.; et al. Triterpenes promote keratinocyte differentiation in vitro, ex vivo and in vivo: A role for the transient receptor potential canonical (subtype) 6. J. Investig. Dermatol. 2010, 130, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Takagi, A.; Kamijo, M.; Ikeda, S. Darier disease. J. Dermatol. 2016, 43, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Sakuntabhai, A.; Ruiz-Perez, V.; Carter, S.; Jacobsen, N.; Burge, S.; Monk, S.; Smith, M.; Munro, C.S.; O’Donovan, M.; Craddock, N.; et al. Mutations in ATP2A2, encoding a Ca2+ pump, cause darier disease. Nat. Genet. 1999, 21, 271–277. [Google Scholar] [PubMed]
- Gordon-Smith, K.; Jones, L.A.; Burge, S.M.; Munro, C.S.; Tavadia, S.; Craddock, N. The neuropsychiatric phenotype in darier disease. Br. J. Dermatol. 2010, 163, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Castori, M.; Barboni, L.; Duncan, P.J.; Paradisi, M.; Laino, L.; De Bernardo, C.; Robinson, D.O.; Grammatico, P. Darier disease, multiple bone cysts, and aniridia due to double de novo heterozygous mutations in ATP2A2 and PAX6. Am. J. Med. Genet. A 2009, 149A, 1768–1772. [Google Scholar] [CrossRef] [PubMed]
- Pani, B.; Cornatzer, E.; Cornatzer, W.; Shin, D.M.; Pittelkow, M.R.; Hovnanian, A.; Ambudkar, I.S.; Singh, B.B. Up-regulation of transient receptor potential canonical 1 (TRPC1) following sarco(endo)plasmic reticulum Ca2+ ATPase 2 gene silencing promotes cell survival: A potential role for TRPC1 in darier’s disease. Mol. Biol. Cell 2006, 17, 4446–4458. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhang, J.R.; Wang, Y.; Li, C.L.; Lu, D.; Guan, S.M.; Chen, J. Effects of a non-selective TRPC channel blocker, SKF-96365, on melittin-induced spontaneous persistent nociception and inflammatory pain hypersensitivity. Neurosci. Bull. 2012, 28, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Alessandri-Haber, N.; Dina, O.A.; Chen, X.; Levine, J.D. TRPC1 and TRPC6 channels cooperate with TRPV4 to mediate mechanical hyperalgesia and nociceptor sensitization. J. Neurosci. 2009, 29, 6217–6228. [Google Scholar] [CrossRef] [PubMed]
- Alkhani, H.; Ase, A.R.; Grant, R.; O’Donnell, D.; Groschner, K.; Seguela, P. Contribution of TRPC3 to store-operated calcium entry and inflammatory transductions in primary nociceptors. Mol. Pain 2014, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, K.; Lennerz, J.K.; Hein, A.; Link, A.S.; Kaczmarek, J.S.; Delling, M.; Uysal, S.; Pfeifer, J.D.; Riccio, A.; Clapham, D.E. Transient receptor potential cation channel, subfamily C, member 5 (TRPC5) is a cold-transducer in the peripheral nervous system. Proc. Natl. Acad. Sci. USA 2011, 108, 18114–18119. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Li, Y.; Pan, X.; Zhang, P.; LaMotte, R.H.; Ma, C. Transient receptor potential canonical 3 (TRPC3) is required for IgG immune complex-induced excitation of the rat dorsal root ganglion neurons. J. Neurosci. 2012, 32, 9554–9562. [Google Scholar] [CrossRef] [PubMed]
- Garrison, S.R.; Dietrich, A.; Stucky, C.L. TRPC1 contributes to light-touch sensation and mechanical responses in low-threshold cutaneous sensory neurons. J. Neurophysiol. 2012, 107, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.Z.; Gu, Q.; Wang, C.; Colton, C.K.; Tang, J.; Kinoshita-Kawada, M.; Lee, L.Y.; Wood, J.D.; Zhu, M.X. 2-aminoethoxydiphenyl borate is a common activator of TRPV1, TRPV2, and TRPV3. J. Biol. Chem. 2004, 279, 35741–35748. [Google Scholar] [CrossRef] [PubMed]
- Gunthorpe, M.J.; Chizh, B.A. Clinical development of TRPV1 antagonists: Targeting a pivotal point in the pain pathway. Drug Discov. Today 2009, 14, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Leffler, A.; Malmberg, A.B.; Martin, W.J.; Trafton, J.; Petersen-Zeitz, K.R.; Koltzenburg, M.; Basbaum, A.I.; Julius, D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000, 288, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.B.; Gray, J.; Gunthorpe, M.J.; Hatcher, J.P.; Davey, P.T.; Overend, P.; Harries, M.H.; Latcham, J.; Clapham, C.; Atkinson, K.; et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 2000, 405, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Chizh, B.A.; O’Donnell, M.B.; Napolitano, A.; Wang, J.; Brooke, A.C.; Aylott, M.C.; Bullman, J.N.; Gray, E.J.; Lai, R.Y.; Williams, P.M.; et al. The effects of the TRPV1 antagonist SB-705498 on TRPV1 receptor-mediated activity and inflammatory hyperalgesia in humans. Pain 2007, 132, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Gavva, N.R.; Tamir, R.; Qu, Y.; Klionsky, L.; Zhang, T.J.; Immke, D.; Wang, J.; Zhu, D.; Vanderah, T.W.; Porreca, F.; et al. AMG 9810 [(E)-3-(4-t-butylphenyl)-N-(2,3-dihydrobenzo[b][1,4] dioxin-6-yl)acrylamide], a novel vanilloid receptor 1 (TRPV1) antagonist with antihyperalgesic properties. J. Pharmacol. Exp. Ther. 2005, 313, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Puttfarcken, P.S.; Han, P.; Joshi, S.K.; Neelands, T.R.; Gauvin, D.M.; Baker, S.J.; Lewis, L.G.; Bianchi, B.R.; Mikusa, J.P.; Koenig, J.R.; et al. A-995662 [(R)-8-(4-methyl-5-(4-(trifluoromethyl)phenyl)oxazol-2-ylamino)-1,2,3,4-tetrahydr onaphthalen-2-ol], a novel, selective TRPV1 receptor antagonist, reduces spinal release of glutamate and CGRP in a rat knee joint pain model. Pain 2010, 150, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Ghilardi, J.R.; Rohrich, H.; Lindsay, T.H.; Sevcik, M.A.; Schwei, M.J.; Kubota, K.; Halvorson, K.G.; Poblete, J.; Chaplan, S.R.; Dubin, A.E.; et al. Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain. J. Neurosci. 2005, 25, 3126–3131. [Google Scholar] [CrossRef] [PubMed]
- Niiyama, Y.; Kawamata, T.; Yamamoto, J.; Furuse, S.; Namiki, A. SB366791, a TRPV1 antagonist, potentiates analgesic effects of systemic morphine in a murine model of bone cancer pain. Br. J. Anaesth. 2009, 102, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Hong, S.; Cui, M.; Sharma, P.K.; Lee, J.; Choi, S. Transient receptor potential vanilloid type 1 antagonists: A patent review (2011–2014). Expert Opin. Ther. Pat. 2015, 25, 291–318. [Google Scholar] [CrossRef] [PubMed]
- Rami, H.K.; Gunthorpe, M.J. The therapeutic potential of TRPV1 (VR1) antagonists: Clinical answers await. Drug Disc. Today Ther. Strateg. 2004. [Google Scholar] [CrossRef]
- McGaraughty, S.; Chu, K.L.; Faltynek, C.R.; Jarvis, M.F. Systemic and site-specific effects of a-425619, a selective TRPV1 receptor antagonist, on wide dynamic range neurons in CFA-treated and uninjured rats. J. Neurophysiol. 2006, 95, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Cevikbas, F.; Wang, X.; Akiyama, T.; Kempkes, C.; Savinko, T.; Antal, A.; Kukova, G.; Buhl, T.; Ikoma, A.; Buddenkotte, J.; et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1. J. Allergy Clin. Immunol. 2014, 133, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Shim, W.S.; Tak, M.H.; Lee, M.H.; Kim, M.; Kim, M.; Koo, J.Y.; Lee, C.H.; Kim, M.; Oh, U. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J. Neurosci. 2007, 27, 2331–2337. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Barry, D.M.; Liu, X.Y.; Yin, S.; Munanairi, A.; Meng, Q.T.; Cheng, W.; Mo, P.; Wan, L.; Liu, S.B.; et al. Facilitation of TRPV4 by TRPV1 is required for itch transmission in some sensory neuron populations. Sci. Signal. 2016, 9, ra71. [Google Scholar] [CrossRef] [PubMed]
- Geppetti, P.; Nassini, R.; Materazzi, S.; Benemei, S. The concept of neurogenic inflammation. BJU Int. 2008, 101 (Suppl. 3), 2–6. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Koizumi, S.; Fuziwara, S.; Denda, S.; Inoue, K.; Denda, M. Functional vanilloid receptors in cultured normal human epidermal keratinocytes. Biochem. Biophys. Res. Commun. 2002, 291, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.K.; Lee, H.; Caterina, M.J. Warm temperatures activate TRPV4 in mouse 308 keratinocytes. J. Biol. Chem. 2003, 278, 32037–32046. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.K.; Lee, H.; Mizuno, A.; Suzuki, M.; Caterina, M.J. TRPV3 and TRPV4 mediate warmth-evoked currents in primary mouse keratinocytes. J. Biol. Chem. 2004, 279, 21569–21575. [Google Scholar] [CrossRef] [PubMed]
- Southall, M.D.; Li, T.; Gharibova, L.S.; Pei, Y.; Nicol, G.D.; Travers, J.B. Activation of epidermal vanilloid receptor-1 induces release of proinflammatory mediators in human keratinocytes. J. Pharmacol. Exp. Ther. 2003, 304, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.W.; Seo, J.A.; Jeong, Y.S.; Bae, I.H.; Jang, W.H.; Lee, J.; Kim, S.Y.; Shin, S.S.; Woo, B.Y.; Lee, K.W.; et al. TRPV1 antagonist can suppress the atopic dermatitis-like symptoms by accelerating skin barrier recovery. J. Dermatol. Sci 2011, 62, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Li, W.H.; Lee, Y.M.; Kim, J.Y.; Kang, S.; Kim, S.; Kim, K.H.; Park, C.H.; Chung, J.H. Transient receptor potential vanilloid-1 mediates heat-shock-induced matrix metalloproteinase-1 expression in human epidermal keratinocytes. J. Investig. Dermatol. 2007, 127, 2328–2335. [Google Scholar] [CrossRef] [PubMed]
- Stander, S.; Moormann, C.; Schumacher, M.; Buddenkotte, J.; Artuc, M.; Shpacovitch, V.; Brzoska, T.; Lippert, U.; Henz, B.M.; Luger, T.A.; et al. Expression of vanilloid receptor subtype 1 in cutaneous sensory nerve fibers, mast cells, and epithelial cells of appendage structures. Exp. Dermatol. 2004, 13, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Denda, M.; Sokabe, T.; Fukumi-Tominaga, T.; Tominaga, M. Effects of skin surface temperature on epidermal permeability barrier homeostasis. J. Investig. Dermatol. 2007, 127, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.M.; Park, Y.H. Development of PAC-14028, a novel transient receptor potential vanilloid type 1 (TRPV1) channel antagonist as a new drug for refractory skin diseases. Arch. Pharm. Res. 2012, 35, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.W.; Seo, J.A.; Jang, W.H.; Koh, H.J.; Bae, I.H.; Park, Y.H.; Lim, K.M. Antipruritic effects of TRPV1 antagonist in murine atopic dermatitis and itching models. J. Investig. Dermatol. 2011, 131, 1576–1579. [Google Scholar] [CrossRef] [PubMed]
- Banvolgyi, A.; Palinkas, L.; Berki, T.; Clark, N.; Grant, A.D.; Helyes, Z.; Pozsgai, G.; Szolcsanyi, J.; Brain, S.D.; Pinter, E. Evidence for a novel protective role of the vanilloid TRPV1 receptor in a cutaneous contact allergic dermatitis model. J. Neuroimmunol. 2005, 169, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Kim, Y.K.; Kim, K.H.; Park, S.J.; Kim, S.J.; Chung, J.H. A novel role for the TRPV1 channel in UV-induced matrix metalloproteinase (MMP)-1 expression in HaCaT cells. J. Cell. Physiol. 2009, 219, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Kang, S.M.; Lee, S.R.; Kong, K.H.; Lee, J.Y.; Kim, E.J.; Chung, J.H. Inhibitory effects of TRPV1 blocker on UV-induced responses in the hairless mice. Arch. Dermatol. Res. 2011, 303, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Sulk, M.; Seeliger, S.; Aubert, J.; Schwab, V.D.; Cevikbas, F.; Rivier, M.; Nowak, P.; Voegel, J.J.; Buddenkotte, J.; Steinhoff, M. Distribution and expression of non-neuronal transient receptor potential (TRPV) ion channels in rosacea. J. Investig. Dermatol. 2012, 132, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Han, S.B.; Kim, H.; Cho, S.H.; Lee, J.D.; Chung, J.H.; Kim, H.S. Transient receptor potential vanilloid-1 in epidermal keratinocytes may contribute to acute pain in herpes zoster. Acta Derm. Venereol. 2016, 96, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Ehnis-Perez, A.; Torres-Alvarez, B.; Cortes-Garcia, D.; Hernandez-Blanco, D.; Fuentes-Ahumada, C.; Castanedo-Cazares, J.P. Relationship between transient receptor potential vanilloid-1 expression and the intensity of sensitive skin symptoms. J. Cosmet. Dermatol. 2016, 15, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Li, D.G.; Du, H.Y.; Gerhard, S.; Imke, M.; Liu, W. Inhibition of TRPV1 prevented skin irritancy induced by phenoxyethanol. A preliminary in vitro and in vivo study. Int. J. Cosmet. Sci. 2016. [Google Scholar] [CrossRef]
- Yin, S.; Luo, J.; Qian, A.; Du, J.; Yang, Q.; Zhou, S.; Yu, W.; Du, G.; Clark, R.B.; Walters, E.T.; et al. Retinoids activate the irritant receptor TRPV1 and produce sensory hypersensitivity. J. Clin. Investig. 2013, 123, 3941–3951. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.K.; Bode, A.M.; Byun, S.; Song, N.R.; Lee, H.J.; Lee, K.W.; Dong, Z. Cocarcinogenic effect of capsaicin involves activation of EGFR signaling but not TRPV1. Cancer Res. 2010, 70, 6859–6869. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Bode, A.M.; Zhu, F.; Liu, K.; Zhang, J.; Kim, M.O.; Reddy, K.; Zykova, T.; Ma, W.Y.; Carper, A.L.; et al. TRPV1-antagonist AMG9810 promotes mouse skin tumorigenesis through EGFR/akt signaling. Carcinogenesis 2011, 32, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Bodo, E.; Biro, T.; Telek, A.; Czifra, G.; Griger, Z.; Toth, B.I.; Mescalchin, A.; Ito, T.; Bettermann, A.; Kovacs, L.; et al. A hot new twist to hair biology: Involvement of vanilloid receptor-1 (VR1/TRPV1) signaling in human hair growth control. Am. J. Pathol. 2005, 166, 985–998. [Google Scholar] [CrossRef]
- Biro, T.; Bodo, E.; Telek, A.; Geczy, T.; Tychsen, B.; Kovacs, L.; Paus, R. Hair cycle control by vanilloid receptor-1 (TRPV1): Evidence from TRPV1 knockout mice. J. Investig. Dermatol. 2006, 126, 1909–1912. [Google Scholar] [CrossRef] [PubMed]
- Toth, B.I.; Benko, S.; Szollosi, A.G.; Kovacs, L.; Rajnavolgyi, E.; Biro, T. Transient receptor potential vanilloid-1 signaling inhibits differentiation and activation of human dendritic cells. FEBS Lett. 2009, 583, 1619–1624. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, M.; Zhang, Y.Q.; Mashima, H.; Li, L.; Shibata, H.; Kojima, I. Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-i. Nat. Cell Biol. 1999, 1, 165–170. [Google Scholar] [PubMed]
- Caterina, M.J.; Rosen, T.A.; Tominaga, M.; Brake, A.J.; Julius, D. A capsaicin receptor homologue with a high threshold for noxious heat. Nature 1999, 398, 436–441. [Google Scholar] [PubMed]
- Penna, A.; Juvin, V.; Chemin, J.; Compan, V.; Monet, M.; Rassendren, F.A. Pi3-kinase promotes TRPV2 activity independently of channel translocation to the plasma membrane. Cell Calcium 2006, 39, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Neeper, M.P.; Liu, Y.; Hutchinson, T.L.; Lubin, M.L.; Flores, C.M. TRPV2 is activated by cannabidiol and mediates CGRP release in cultured rat dorsal root ganglion neurons. J. Neurosci. 2008, 28, 6231–6238. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.; Kim, K.Y.; Yoo, S.; Lee, S.H.; Hwang, S.W. Transient receptor potential V2 expressed in sensory neurons is activated by probenecid. Neurosci. Lett. 2007, 425, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Muraki, K.; Iwata, Y.; Katanosaka, Y.; Ito, T.; Ohya, S.; Shigekawa, M.; Imaizumi, Y. TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ. Res. 2003, 93, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, K.; Murayama, N.; Ono, K.; Ishizaki, Y.; Tominaga, M. TRPV2 enhances axon outgrowth through its activation by membrane stretch in developing sensory and motor neurons. J. Neurosci. 2010, 30, 4601–4612. [Google Scholar] [CrossRef] [PubMed]
- Park, U.; Vastani, N.; Guan, Y.; Raja, S.N.; Koltzenburg, M.; Caterina, M.J. TRP vanilloid 2 knock-out mice are susceptible to perinatal lethality but display normal thermal and mechanical nociception. J. Neurosci. 2011, 31, 11425–11436. [Google Scholar] [CrossRef] [PubMed]
- Neeper, M.P.; Liu, Y.; Hutchinson, T.L.; Wang, Y.; Flores, C.M.; Qin, N. Activation properties of heterologously expressed mammalian TRPV2: Evidence for species dependence. J. Biol. Chem. 2007, 282, 15894–15902. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.R.; Johnson, W.M.; Pilat, J.M.; Kiselar, J.; DeFrancesco-Lisowitz, A.; Zigmond, R.E.; Moiseenkova-Bell, V.Y. Nerve growth factor regulates transient receptor potential vanilloid 2 via extracellular signal-regulated kinase signaling to enhance neurite outgrowth in developing neurons. Mol. Cell. Biol. 2015, 35, 4238–4252. [Google Scholar] [CrossRef] [PubMed]
- Santoni, G.; Farfariello, V.; Liberati, S.; Morelli, M.B.; Nabissi, M.; Santoni, M.; Amantini, C. The role of transient receptor potential vanilloid type-2 ion channels in innate and adaptive immune responses. Front. Immunol. 2013, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, K.; Sasano, T.; Tojo, K.; Namekata, I.; Kurokawa, J.; Sawada, N.; Suganami, T.; Kamei, Y.; Tanaka, H.; Tajima, N.; et al. Role of transient receptor potential vanilloid 2 in LPS-induced cytokine production in macrophages. Biochem. Biophys. Res. Commun. 2010, 398, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Link, T.M.; Park, U.; Vonakis, B.M.; Raben, D.M.; Soloski, M.J.; Caterina, M.J. TRPV2 has a pivotal role in macrophage particle binding and phagocytosis. Nat. Immunol. 2010, 11, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, M.; Nakagawa, Y.; Tanaka, S.; Kojima, I. Chemotactic peptide fmetleuphe induces translocation of the TRPV2 channel in macrophages. J. Cell. Physiol. 2007, 210, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Szollosi, A.G.; Olah, A.; Toth, I.B.; Papp, F.; Czifra, G.; Panyi, G.; Biro, T. Transient receptor potential vanilloid-2 mediates the effects of transient heat shock on endocytosis of human monocyte-derived dendritic cells. FEBS Lett. 2013, 587, 1440–1445. [Google Scholar] [CrossRef] [PubMed]
- Compan, V.; Baroja-Mazo, A.; Lopez-Castejon, G.; Gomez, A.I.; Martinez, C.M.; Angosto, D.; Montero, M.T.; Herranz, A.S.; Bazan, E.; Reimers, D.; et al. Cell volume regulation modulates NLRP3 inflammasome activation. Immunity 2012, 37, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, M.; Kojima, I. Translocation of calcium-permeable TRPV2 channel to the podosome: Its role in the regulation of podosome assembly. Cell Calcium 2012, 51, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Liberati, S.; Morelli, M.B.; Amantini, C.; Farfariello, V.; Santoni, M.; Conti, A.; Nabissi, M.; Cascinu, S.; Santoni, G. Loss of TRPV2 homeostatic control of cell proliferation drives tumor progression. Cells 2014, 3, 112–128. [Google Scholar] [CrossRef] [PubMed]
- Morelli, M.B.; Liberati, S.; Amantini, C.; Nabiss, M.; Santoni, M.; Farfariello, V.; Santoni, G. Expression and function of the transient receptor potential ion channel family in the hematologic malignancies. Curr. Mol. Pharmacol. 2013, 6, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Peier, A.M.; Reeve, A.J.; Andersson, D.A.; Moqrich, A.; Earley, T.J.; Hergarden, A.C.; Story, G.M.; Colley, S.; Hogenesch, J.B.; McIntyre, P.; et al. A heat-sensitive TRP channel expressed in keratinocytes. Science 2002, 296, 2046–2049. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.D.; Gunthorpe, M.J.; Kelsell, R.E.; Hayes, P.D.; Reilly, P.; Facer, P.; Wright, J.E.; Jerman, J.C.; Walhin, J.P.; Ooi, L.; et al. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 2002, 418, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ramsey, I.S.; Kotecha, S.A.; Moran, M.M.; Chong, J.A.; Lawson, D.; Ge, P.; Lilly, J.; Silos-Santiago, I.; Xie, Y.; et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 2002, 418, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.K.; Lee, H.; Mizuno, A.; Suzuki, M.; Caterina, M.J. 2-aminoethoxydiphenyl borate activates and sensitizes the heat-gated ion channel TRPV3. J. Neurosci. 2004, 24, 5177–5182. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.; Yoo, S.; Yang, T.J.; Cho, H.; Hwang, S.W. Farnesyl pyrophosphate is a novel pain-producing molecule via specific activation of TRPV3. J. Biol. Chem. 2010, 285, 19362–19371. [Google Scholar] [CrossRef] [PubMed]
- Moqrich, A.; Hwang, S.W.; Earley, T.J.; Petrus, M.J.; Murray, A.N.; Spencer, K.S.; Andahazy, M.; Story, G.M.; Patapoutian, A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 2005, 307, 1468–1472. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Delling, M.; Jun, J.C.; Clapham, D.E. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat. Neurosci. 2006, 9, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.Z.; Xiao, R.; Wang, C.; Gao, N.; Colton, C.K.; Wood, J.D.; Zhu, M.X. Potentiation of TRPV3 channel function by unsaturated fatty acids. J. Cell. Physiol. 2006, 208, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.S.; Tannert, A.; Schaefer, M. Cholesterol sensitises the transient receptor potential channel TRPV3 to lower temperatures and activator concentrations. Cell Calcium 2014, 55, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Karttunen, S.; Duffield, M.; Scrimgeour, N.R.; Squires, L.; Lim, W.L.; Dallas, M.L.; Scragg, J.L.; Chicher, J.; Dave, K.A.; Whitelaw, M.L.; et al. Oxygen-dependent hydroxylation by fih regulates the TRPV3 ion channel. J. Cell Sci. 2015, 128, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Mandadi, S.; Sokabe, T.; Shibasaki, K.; Katanosaka, K.; Mizuno, A.; Moqrich, A.; Patapoutian, A.; Fukumi-Tominaga, T.; Mizumura, K.; Tominaga, M. TRPV3 in keratinocytes transmits temperature information to sensory neurons via atp. Pflugers Arch. 2009, 458, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. TRPV3 regulates nitric oxide synthase-independent nitric oxide synthesis in the skin. Nat. Commun. 2011, 2, 369. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.M.; Lee, H.; Chung, M.K.; Park, U.; Yu, Y.Y.; Bradshaw, H.B.; Coulombe, P.A.; Walker, J.M.; Caterina, M.J. Overexpressed transient receptor potential vanilloid 3 ion channels in skin keratinocytes modulate pain sensitivity via prostaglandin E2. J. Neurosci. 2008, 28, 13727–13737. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.M.; Li, X.; Yu, Y.; Wang, J.; Caterina, M.J. TRPV3 and TRPV4 ion channels are not major contributors to mouse heat sensation. Mol. Pain 2011, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Marics, I.; Malapert, P.; Reynders, A.; Gaillard, S.; Moqrich, A. Acute heat-evoked temperature sensation is impaired but not abolished in mice lacking TRPV1 and TRPV3 channels. PLoS ONE 2014, 9, e99828. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Jin, J.; Hu, L.; Shen, D.; Dong, X.P.; Samie, M.A.; Knoff, J.; Eisinger, B.; Liu, M.L.; Huang, S.M.; et al. TRP channel regulates EGFR signaling in hair morphogenesis and skin barrier formation. Cell 2010, 141, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Borbiro, I.; Lisztes, E.; Toth, B.I.; Czifra, G.; Olah, A.; Szollosi, A.G.; Szentandrassy, N.; Nanasi, P.P.; Peter, Z.; Paus, R.; et al. Activation of transient receptor potential vanilloid-3 inhibits human hair growth. J. Investig. Dermatol. 2011, 131, 1605–1614. [Google Scholar] [CrossRef] [PubMed]
- Broad, L.M.; Mogg, A.J.; Eberle, E.; Tolley, M.; Li, D.L.; Knopp, K.L. TRPV3 in drug development. Pharmaceuticals 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto-Kasai, E.; Imura, K.; Yasui, K.; Shichijou, M.; Oshima, I.; Hirasawa, T.; Sakata, T.; Yoshioka, T. TRPV3 as a therapeutic target for itch. J. Investig. Dermatol. 2012, 132, 2109–2112. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, P.; Wan, E.; Holdcroft, A.; Facer, P.; Davis, J.B.; Smith, G.D.; Bountra, C.; Anand, P. Increased capsaicin receptor TRPV1 in skin nerve fibres and related vanilloid receptors TRPV3 and TRPV4 in keratinocytes in human breast pain. BMC Womens Health 2005, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.O.; Cho, Y.S.; Park, S.Y.; Kwak, I.S.; Choi, M.G.; Chung, B.Y.; Park, C.W.; Lee, J.Y. Increased activity of TRPV3 in keratinocytes in hypertrophic burn scars with postburn pruritus. Wound Repair Regen. 2016, 24, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Aijima, R.; Wang, B.; Takao, T.; Mihara, H.; Kashio, M.; Ohsaki, Y.; Zhang, J.Q.; Mizuno, A.; Suzuki, M.; Yamashita, Y.; et al. The thermosensitive TRPV3 channel contributes to rapid wound healing in oral epithelia. FASEB J. 2015, 29, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, M.; Yoshioka, T.; Matsutani, T.; Hikita, I.; Suzuki, M.; Oshima, I.; Tsukahara, K.; Arimura, A.; Horikawa, T.; Hirasawa, T.; et al. Association of a mutation in TRPV3 with defective hair growth in rodents. J. Investig. Dermatol. 2006, 126, 2664–2672. [Google Scholar] [CrossRef] [PubMed]
- Imura, K.; Yoshioka, T.; Hikita, I.; Tsukahara, K.; Hirasawa, T.; Higashino, K.; Gahara, Y.; Arimura, A.; Sakata, T. Influence of TRPV3 mutation on hair growth cycle in mice. Biochem. Biophys. Res. Commun. 2007, 363, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Tian, J.; Tang, J.; Zhu, M.X. The TRPV3 mutation associated with the hairless phenotype in rodents is constitutively active. Cell Calcium 2008, 43, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Imura, K.; Yoshioka, T.; Hirasawa, T.; Sakata, T. Role of TRPV3 in immune response to development of dermatitis. J. Inflamm. (Lond.) 2009, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Chen, Q.; Lee, M.; Cao, X.; Zhang, J.; Ma, D.; Chen, L.; Hu, X.; Wang, H.; Wang, X.; et al. Exome sequencing reveals mutations in TRPV3 as a cause of olmsted syndrome. Am. J. Hum. Genet. 2012, 90, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Wilson, N.J.; Cole, C.; Milstone, L.M.; Kiszewski, A.E.; Hansen, C.D.; O’Toole, E.A.; Schwartz, M.E.; McLean, W.H.; Smith, F.J. Expanding the phenotypic spectrum of olmsted syndrome. J. Investig. Dermatol. 2015, 135, 2879–2883. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zeng, K.; Zhang, X.; Chen, Q.; Wu, J.; Li, H.; Zhou, Y.; Glusman, G.; Roach, J.; Etheridge, A.; et al. A gain-of-function mutation in TRPV3 causes focal palmoplantar keratoderma in a Chinese family. J. Investig. Dermatol. 2015, 135, 907–909. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, W.; Choe, Y.; Marti-Renom, M.A.; Bell, A.M.; Denis, C.S.; Sali, A.; Hudspeth, A.J.; Friedman, J.M.; Heller, S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 2000, 103, 525–535. [Google Scholar] [CrossRef]
- Strotmann, R.; Harteneck, C.; Nunnenmacher, K.; Schultz, G.; Plant, T.D. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat. Cell Biol. 2000, 2, 695–702. [Google Scholar] [PubMed]
- Wissenbach, U.; Bodding, M.; Freichel, M.; Flockerzi, V. TRP12, a novel TRP related protein from kidney. FEBS Lett. 2000, 485, 127–134. [Google Scholar] [CrossRef]
- Watanabe, H.; Vriens, J.; Prenen, J.; Droogmans, G.; Voets, T.; Nilius, B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 2003, 424, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Guler, A.D.; Lee, H.; Iida, T.; Shimizu, I.; Tominaga, M.; Caterina, M. Heat-evoked activation of the ion channel, TRPV4. J. Neurosci. 2002, 22, 6408–6414. [Google Scholar] [PubMed]
- Kida, N.; Sokabe, T.; Kashio, M.; Haruna, K.; Mizuno, Y.; Suga, Y.; Nishikawa, K.; Kanamaru, A.; Hongo, M.; Oba, A.; et al. Importance of transient receptor potential vanilloid 4 (TRPV4) in epidermal barrier function in human skin keratinocytes. Pflugers Arch. 2012, 463, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Akazawa, Y.; Yuki, T.; Yoshida, H.; Sugiyama, Y.; Inoue, S. Activation of TRPV4 strengthens the tight-junction barrier in human epidermal keratinocytes. Skin Pharmacol. Physiol. 2013, 26, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Fusi, C.; Materazzi, S.; Minocci, D.; Maio, V.; Oranges, T.; Massi, D.; Nassini, R. Transient receptor potential vanilloid 4 (TRPV4) is downregulated in keratinocytes in human non-melanoma skin cancer. J. Investig. Dermatol. 2014, 134, 2408–2417. [Google Scholar] [CrossRef] [PubMed]
- Alessandri-Haber, N.; Joseph, E.; Dina, O.A.; Liedtke, W.; Levine, J.D. TRPV4 mediates pain-related behavior induced by mild hypertonic stimuli in the presence of inflammatory mediator. Pain 2005, 118, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Alessandri-Haber, N.; Yeh, J.J.; Boyd, A.E.; Parada, C.A.; Chen, X.; Reichling, D.B.; Levine, J.D. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron 2003, 39, 497–511. [Google Scholar] [CrossRef]
- Liedtke, W.; Friedman, J.M. Abnormal osmotic regulation in TRPV4 −/− mice. Proc. Natl. Acad. Sci. USA 2003, 100, 13698–13703. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Mizuno, A.; Kodaira, K.; Imai, M. Impaired pressure sensation in mice lacking TRPV4. J. Biol. Chem. 2003, 278, 22664–22668. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kanju, P.; Fang, Q.; Lee, S.H.; Parekh, P.K.; Lee, W.; Moore, C.; Brenner, D.; Gereau, R.W.T.; Wang, F.; et al. TRPV4 is necessary for trigeminal irritant pain and functions as a cellular formalin receptor. Pain 2014, 155, 2662–2672. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Williams, S.H.; McNulty, A.L.; Hong, J.H.; Lee, S.H.; Rothfusz, N.E.; Parekh, P.K.; Moore, C.; Gereau, R.W., IV; Taylor, A.B.; et al. Temporomandibular joint pain: A critical role for TRPV4 in the trigeminal ganglion. Pain 2013, 154, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Segond von Banchet, G.; Boettger, M.K.; Konig, C.; Iwakura, Y.; Brauer, R.; Schaible, H.G. Neuronal IL-17 receptor upregulates TRPV4 but not TRPV1 receptors in DRG neurons and mediates mechanical but not thermal hyperalgesia. Mol. Cell. Neurosci. 2013, 52, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Alessandri-Haber, N.; Dina, O.A.; Yeh, J.J.; Parada, C.A.; Reichling, D.B.; Levine, J.D. Transient receptor potential vanilloid 4 is essential in chemotherapy-induced neuropathic pain in the rat. J. Neurosci. 2004, 24, 4444–4452. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, C.; Wang, Z.J. Proteinase-activated receptor 2 sensitizes transient receptor potential vanilloid 1, transient receptor potential vanilloid 4, and transient receptor potential ankyrin 1 in paclitaxel-induced neuropathic pain. Neuroscience 2011, 193, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.L.; Wang, Y.H.; Ning, L.P.; Zhang, Y.; Ge, H.Y.; Jiang, H.; Wang, R.; Yue, S.W. Involvement of TRPV4-NO-cGMP-PKG pathways in the development of thermal hyperalgesia following chronic compression of the dorsal root ganglion in rats. Behav. Brain Res. 2010, 208, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Grant, A.D.; Cottrell, G.S.; Amadesi, S.; Trevisani, M.; Nicoletti, P.; Materazzi, S.; Altier, C.; Cenac, N.; Zamponi, G.W.; Bautista-Cruz, F.; et al. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J. Physiol. 2007, 578 Pt 3, 715–733. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.; Cevikbas, F.; Pasolli, H.A.; Chen, Y.; Kong, W.; Kempkes, C.; Parekh, P.; Lee, S.H.; Kontchou, N.A.; Yeh, I.; et al. Uvb radiation generates sunburn pain and affects skin by activating epidermal TRPV4 ion channels and triggering endothelin-1 signaling. Proc. Natl. Acad. Sci. USA 2013, 110, E3225–3234. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Ivanov, M.; Nagamine, M.; Davoodi, A.; Carstens, M.I.; Ikoma, A.; Cevikbas, F.; Kempkes, C.; Buddenkotte, J.; Steinhoff, M.; et al. Involvement of TRPV4 in serotonin-evoked scratching. J. Investig. Dermatol. 2016, 136, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fang, Q.; Wang, Z.; Zhang, J.Y.; MacLeod, A.S.; Hall, R.P.; Liedtke, W.B. Transient receptor potential vanilloid 4 ion channel functions as a pruriceptor in epidermal keratinocytes to evoke histaminergic itch. J. Biol. Chem. 2016, 291, 10252–10262. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Iida, T.; Mizuno, A.; Suzuki, M.; Caterina, M.J. Altered thermal selection behavior in mice lacking transient receptor potential vanilloid 4. J. Neurosci. 2005, 25, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- Auer-Grumbach, M.; Olschewski, A.; Papic, L.; Kremer, H.; McEntagart, M.E.; Uhrig, S.; Fischer, C.; Frohlich, E.; Balint, Z.; Tang, B.; et al. Alterations in the ankyrin domain of TRPV4 cause congenital distal SMA, scapuloperoneal SMA and HMSN2C. Nat. Genet. 2010, 42, 160–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, H.X.; Klein, C.J.; Yan, J.; Shi, Y.; Wu, Y.; Fecto, F.; Yau, H.J.; Yang, Y.; Zhai, H.; Siddique, N.; et al. Scapuloperoneal spinal muscular atrophy and CMT2C are allelic disorders caused by alterations in TRPV4. Nat. Genet. 2010, 42, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Landoure, G.; Zdebik, A.A.; Martinez, T.L.; Burnett, B.G.; Stanescu, H.C.; Inada, H.; Shi, Y.; Taye, A.A.; Kong, L.; Munns, C.H.; et al. Mutations in TRPV4 cause charcot-marie-tooth disease type 2C. Nat. Genet. 2010, 42, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Lehen’kyi, V.; Vandenberghe, M.; Belaubre, F.; Julie, S.; Castex-Rizzi, N.; Skryma, R.; Prevarskaya, N. Acceleration of keratinocyte differentiation by transient receptor potential vanilloid (TRPV6) channel activation. J. Eur. Acad. Dermatol. Venereol. 2011, 25 (Suppl. 1), 12–18. [Google Scholar] [CrossRef] [PubMed]
- Bianco, S.D.; Peng, J.B.; Takanaga, H.; Suzuki, Y.; Crescenzi, A.; Kos, C.H.; Zhuang, L.; Freeman, M.R.; Gouveia, C.H.; Wu, J.; et al. Marked disturbance of calcium homeostasis in mice with targeted disruption of the TRPV6 calcium channel gene. J. Bone Miner. Res. 2007, 22, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.B.; Zhuang, L.; Berger, U.V.; Adam, R.M.; Williams, B.J.; Brown, E.M.; Hediger, M.A.; Freeman, M.R. CAT1 expression correlates with tumor grade in prostate cancer. Biochem. Biophys. Res. Commun. 2001, 282, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Appendino, G.; Owsianik, G. The transient receptor potential channel TRPA1: From gene to pathophysiology. Pflugers Arch. 2012, 464, 425–458. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Shim, H.S.; Wang, X.; Montell, C. Control of thermotactic behavior via coupling of a TRP channel to a phospholipase C signaling cascade. Nat. Neurosci. 2008, 11, 871–873. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, M.; Brennan, K.M.; Tayler, T.D.; Phelps, P.O.; Patapoutian, A.; Garrity, P.A. The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Genes Dev. 2005, 19, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Viswanath, V.; Story, G.M.; Peier, A.M.; Petrus, M.J.; Lee, V.M.; Hwang, S.W.; Patapoutian, A.; Jegla, T. Opposite thermosensor in fruitfly and mouse. Nature 2003, 423, 822–823. [Google Scholar] [CrossRef] [PubMed]
- Gracheva, E.O.; Ingolia, N.T.; Kelly, Y.M.; Cordero-Morales, J.F.; Hollopeter, G.; Chesler, A.T.; Sanchez, E.E.; Perez, J.C.; Weissman, J.S.; Julius, D. Molecular basis of infrared detection by snakes. Nature 2010, 464, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Story, G.M.; Peier, A.M.; Reeve, A.J.; Eid, S.R.; Mosbacher, J.; Hricik, T.R.; Earley, T.J.; Hergarden, A.C.; Andersson, D.A.; Hwang, S.W.; et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003, 112, 819–829. [Google Scholar] [CrossRef]
- Moparthi, L.; Survery, S.; Kreir, M.; Simonsen, C.; Kjellbom, P.; Hogestatt, E.D.; Johanson, U.; Zygmunt, P.M. Human TRPA1 is intrinsically cold- and chemosensitive with and without its N-terminal ankyrin repeat domain. Proc. Natl. Acad. Sci. USA 2014, 111, 16901–16906. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lee, J.; Ro, J.Y.; Chung, M.K. Warmth suppresses and desensitizes damage-sensing ion channel TRPA1. Mol. Pain 2012, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Moparthi, L.; Kichko, T.I.; Eberhardt, M.; Hogestatt, E.D.; Kjellbom, P.; Johanson, U.; Reeh, P.W.; Leffler, A.; Filipovic, M.R.; Zygmunt, P.M. Human TRPA1 is a heat sensor displaying intrinsic u-shaped thermosensitivity. Sci. Rep. 2016, 6, 28763. [Google Scholar] [CrossRef] [PubMed]
- Miyake, T.; Nakamura, S.; Zhao, M.; So, K.; Inoue, K.; Numata, T.; Takahashi, N.; Shirakawa, H.; Mori, Y.; Nakagawa, T.; et al. Cold sensitivity of TRPA1 is unveiled by the prolyl hydroxylation blockade-induced sensitization to ROS. Nat. Commun. 2016, 7, 12840. [Google Scholar] [CrossRef] [PubMed]
- Meseguer, V.; Alpizar, Y.A.; Luis, E.; Tajada, S.; Denlinger, B.; Fajardo, O.; Manenschijn, J.A.; Fernandez-Pena, C.; Talavera, A.; Kichko, T.; et al. TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat. Commun. 2014, 5, 3125. [Google Scholar] [CrossRef] [PubMed]
- Park, C.K.; Xu, Z.Z.; Berta, T.; Han, Q.; Chen, G.; Liu, X.J.; Ji, R.R. Extracellular micrornas activate nociceptor neurons to elicit pain via TLR7 and TRPA1. Neuron 2014, 82, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Kerstein, P.C.; del Camino, D.; Moran, M.M.; Stucky, C.L. Pharmacological blockade of TRPA1 inhibits mechanical firing in nociceptors. Mol. Pain 2009, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Kwan, K.Y.; Glazer, J.M.; Corey, D.P.; Rice, F.L.; Stucky, C.L. TRPA1 modulates mechanotransduction in cutaneous sensory neurons. J. Neurosci. 2009, 29, 4808–4819. [Google Scholar] [CrossRef] [PubMed]
- Lennertz, R.C.; Kossyreva, E.A.; Smith, A.K.; Stucky, C.L. TRPA1 mediates mechanical sensitization in nociceptors during inflammation. PLoS ONE 2012, 7, e43597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Chen, J.; Faltynek, C.R.; Moreland, R.B.; Neelands, T.R. Transient receptor potential A1 mediates an osmotically activated ion channel. Eur. J. Neurosci. 2008, 27, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Bautista, D.M.; Jordt, S.E.; Nikai, T.; Tsuruda, P.R.; Read, A.J.; Poblete, J.; Yamoah, E.N.; Basbaum, A.I.; Julius, D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 2006, 124, 1269–1282. [Google Scholar] [CrossRef] [PubMed]
- McNamara, C.R.; Mandel-Brehm, J.; Bautista, D.M.; Siemens, J.; Deranian, K.L.; Zhao, M.; Hayward, N.J.; Chong, J.A.; Julius, D.; Moran, M.M.; et al. TRPA1 mediates formalin-induced pain. Proc. Natl. Acad. Sci. USA 2007, 104, 13525–13530. [Google Scholar] [CrossRef] [PubMed]
- del Camino, D.; Murphy, S.; Heiry, M.; Barrett, L.B.; Earley, T.J.; Cook, C.A.; Petrus, M.J.; Zhao, M.; D’Amours, M.; Deering, N.; et al. TRPA1 contributes to cold hypersensitivity. J. Neurosci. 2010, 30, 15165–15174. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.A.; Gentry, C.; Light, E.; Vastani, N.; Vallortigara, J.; Bierhaus, A.; Fleming, T.; Bevan, S. Methylglyoxal evokes pain by stimulating TRPA1. PLoS ONE 2013, 8, e77986. [Google Scholar] [CrossRef]
- Nassini, R.; Gees, M.; Harrison, S.; De Siena, G.; Materazzi, S.; Moretto, N.; Failli, P.; Preti, D.; Marchetti, N.; Cavazzini, A.; et al. Oxaliplatin elicits mechanical and cold allodynia in rodents via TRPA1 receptor stimulation. Pain 2011, 152, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.A.; Filipovic, M.R.; Gentry, C.; Eberhardt, M.; Vastani, N.; Leffler, A.; Reeh, P.; Bevan, S. Streptozotocin stimulates the ion channel TRPA1 directly: Involvement of peroxynitrite. J. Biol. Chem. 2015, 290, 15185–15196. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, A.; Hukkanen, M.; Saarnilehto, M.; Chapman, H.; Kuokkanen, K.; Wei, H.; Viisanen, H.; Akerman, K.E.; Lindstedt, K.; Pertovaara, A. Inhibiting TRPA1 ion channel reduces loss of cutaneous nerve fiber function in diabetic animals: Sustained activation of the TRPA1 channel contributes to the pathogenesis of peripheral diabetic neuropathy. Pharmacol. Res. 2012, 65, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Kremeyer, B.; Lopera, F.; Cox, J.J.; Momin, A.; Rugiero, F.; Marsh, S.; Woods, C.G.; Jones, N.G.; Paterson, K.J.; Fricker, F.R.; et al. A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome. Neuron 2010, 66, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Babes, A.; Sauer, S.K.; Moparthi, L.; Kichko, T.I.; Neacsu, C.; Namer, B.; Filipovic, M.; Zygmunt, P.M.; Reeh, P.W.; Fischer, M.J. Photosensitization in porphyrias and photodynamic therapy involves TRPA1 and TRPV1. J. Neurosci. 2016, 36, 5264–5278. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.R.; Gerhold, K.A.; Bifolck-Fisher, A.; Liu, Q.; Patel, K.N.; Dong, X.; Bautista, D.M. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat. Neurosci. 2011, 14, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.R.; The, L.; Batia, L.M.; Beattie, K.; Katibah, G.E.; McClain, S.P.; Pellegrino, M.; Estandian, D.M.; Bautista, D.M. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 2013, 155, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Lieu, T.; Jayaweera, G.; Zhao, P.; Poole, D.P.; Jensen, D.; Grace, M.; McIntyre, P.; Bron, R.; Wilson, Y.M.; Krappitz, M.; et al. The bile acid receptor TGR5 activates the TRPA1 channel to induce itch in mice. Gastroenterology 2014, 147, 1417–1428. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.R.; Nelson, A.M.; Batia, L.; Morita, T.; Estandian, D.; Owens, D.M.; Lumpkin, E.A.; Bautista, D.M. The ion channel TRPA1 is required for chronic itch. J. Neurosci. 2013, 33, 9283–9294. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.H.; Oh, S.Y.; Lu, J.; Lou, H.; Myers, A.C.; Zhu, Z.; Zheng, T. TRPA1-dependent pruritus in IL-13-induced chronic atopic dermatitis. J. Immunol. 2013, 191, 5371–5382. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Escalera, J.; Balakrishna, S.; Fan, L.; Caceres, A.I.; Robinson, E.; Sui, A.; McKay, M.C.; McAlexander, M.A.; Herrick, C.A.; et al. TRPA1 controls inflammation and pruritogen responses in allergic contact dermatitis. FASEB J. 2013, 27, 3549–3563. [Google Scholar] [CrossRef] [PubMed]
- Saarnilehto, M.; Chapman, H.; Savinko, T.; Lindstedt, K.; Lauerma, A.I.; Koivisto, A. Contact sensitizer 2,4-dinitrochlorobenzene is a highly potent human TRPA1 agonist. Allergy 2014, 69, 1424–1427. [Google Scholar] [CrossRef] [PubMed]
- Caceres, A.I.; Brackmann, M.; Elia, M.D.; Bessac, B.F.; del Camino, D.; D’Amours, M.; Witek, J.S.; Fanger, C.M.; Chong, J.A.; Hayward, N.J.; et al. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc. Natl. Acad. Sci. USA 2009, 106, 9099–9104. [Google Scholar] [CrossRef] [PubMed]
- Denda, M.; Tsutsumi, M.; Goto, M.; Ikeyama, K.; Denda, S. Topical application of TRPA1 agonists and brief cold exposure accelerate skin permeability barrier recovery. J. Investig. Dermatol. 2010, 130, 1942–1945. [Google Scholar] [CrossRef] [PubMed]
- Uta, D.; Furue, H.; Pickering, A.E.; Rashid, M.H.; Mizuguchi-Takase, H.; Katafuchi, T.; Imoto, K.; Yoshimura, M. TRPA1-expressing primary afferents synapse with a morphologically identified subclass of substantia gelatinosa neurons in the adult rat spinal cord. Eur. J. Neurosci. 2010, 31, 1960–1973. [Google Scholar] [CrossRef] [PubMed]
- Chiu, I.M.; Heesters, B.A.; Ghasemlou, N.; Von Hehn, C.A.; Zhao, F.; Tran, J.; Wainger, B.; Strominger, A.; Muralidharan, S.; Horswill, A.R.; et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nature 2013, 501, 52–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinter, E.; Helyes, Z.; Szolcsanyi, J. Inhibitory effect of somatostatin on inflammation and nociception. Pharmacol. Ther. 2006, 112, 440–456. [Google Scholar] [CrossRef] [PubMed]
- Zappia, K.J.; Garrison, S.R.; Palygin, O.; Weyer, A.D.; Barabas, M.E.; Lawlor, M.W.; Staruschenko, A.; Stucky, C.L. Mechanosensory and atp release deficits following keratin14-cre-mediated TRPA1 deletion despite absence of TRPA1 in murine keratinocytes. PLoS ONE 2016, 11, e0151602. [Google Scholar] [CrossRef] [PubMed]
- Bellono, N.W.; Kammel, L.G.; Zimmerman, A.L.; Oancea, E. Uv light phototransduction activates transient receptor potential a1 ion channels in human melanocytes. Proc. Natl. Acad. Sci. USA 2013, 110, 2383–2388. [Google Scholar] [CrossRef] [PubMed]
- Oehler, B.; Scholze, A.; Schaefer, M.; Hill, K. TRPA1 is functionally expressed in melanoma cells but is not critical for impaired proliferation caused by allyl isothiocyanate or cinnamaldehyde. Naunyn Schmiedebergs Arch. Pharmacol. 2012, 385, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Oancea, E.; Wicks, N.L. TRPM1: New trends for an old TRP. Adv. Exp. Med. Biol. 2011, 704, 135–145. [Google Scholar] [PubMed]
- Miller, A.J.; Du, J.; Rowan, S.; Hershey, C.L.; Widlund, H.R.; Fisher, D.E. Transcriptional regulation of the melanoma prognostic marker melastatin (TRPM1) by MITF in melanocytes and melanoma. Cancer Res. 2004, 64, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Carlson, J.A.; Slominski, A. Role of TRPM in melanocytes and melanoma. Exp. Dermatol. 2012, 21, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.; Markandeya, Y.; Maddodi, N.; Dhingra, A.; Vardi, N.; Balijepalli, R.C.; Setaluri, V. Metabotropic glutamate receptor 6 signaling enhances TRPM1 calcium channel function and increases melanin content in human melanocytes. Pigment Cell Melanoma Res. 2013, 26, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Mansuri, M.S.; Singh, M.; Begum, R. miRNA signatures and transcriptional regulation of their target genes in vitiligo. J. Dermatol. Sci 2016, 84, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Bellone, R.R.; Brooks, S.A.; Sandmeyer, L.; Murphy, B.A.; Forsyth, G.; Archer, S.; Bailey, E.; Grahn, B. Differential gene expression of TRPM1, the potential cause of congenital stationary night blindness and coat spotting patterns (LP) in the Appaloosa horse (Equus caballus). Genetics 2008, 179, 1861–1870. [Google Scholar] [CrossRef] [PubMed]
- Oancea, E.; Vriens, J.; Brauchi, S.; Jun, J.; Splawski, I.; Clapham, D.E. TRPM1 forms ion channels associated with melanin content in melanocytes. Sci. Signal. 2009, 2, ra21. [Google Scholar] [CrossRef] [PubMed]
- Kunisada, T.; Lu, S.Z.; Yoshida, H.; Nishikawa, S.; Nishikawa, S.; Mizoguchi, M.; Hayashi, S.; Tyrrell, L.; Williams, D.A.; Wang, X.; et al. Murine cutaneous mastocytosis and epidermal melanocytosis induced by keratinocyte expression of transgenic stem cell factor. J. Exp. Med. 1998, 187, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Levy, C.; Khaled, M.; Iliopoulos, D.; Janas, M.M.; Schubert, S.; Pinner, S.; Chen, P.H.; Li, S.; Fletcher, A.L.; Yokoyama, S.; et al. Intronic miR-211 assumes the tumor suppressive function of its host gene in melanoma. Mol. Cell 2010, 40, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; McNaughton, P.A. The TRPM2 ion channel is required for sensitivity to warmth. Nature 2016, 536, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Held, K.; Voets, T.; Vriens, J. TRPM3 in temperature sensing and beyond. Temperature (Austin) 2015, 2, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Vriens, J.; Held, K.; Janssens, A.; Toth, B.I.; Kerselaers, S.; Nilius, B.; Vennekens, R.; Voets, T. Opening of an alternative ion permeation pathway in a nociceptor TRP channel. Nat. Chem. Biol. 2014, 10, 188–195. [Google Scholar] [CrossRef] [PubMed]
- McNeill, M.S.; Paulsen, J.; Bonde, G.; Burnight, E.; Hsu, M.Y.; Cornell, R.A. Cell death of melanophores in zebrafish TRPM7 mutant embryos depends on melanin synthesis. J. Investig. Dermatol. 2007, 127, 2020–2030. [Google Scholar] [CrossRef] [PubMed]
- Bautista, D.M.; Siemens, J.; Glazer, J.M.; Tsuruda, P.R.; Basbaum, A.I.; Stucky, C.L.; Jordt, S.E.; Julius, D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 2007, 448, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Colburn, R.W.; Lubin, M.L.; Stone, D.J., Jr.; Wang, Y.; Lawrence, D.; D’Andrea, M.R.; Brandt, M.R.; Liu, Y.; Flores, C.M.; Qin, N. Attenuated cold sensitivity in TRPM8 null mice. Neuron 2007, 54, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Dhaka, A.; Murray, A.N.; Mathur, J.; Earley, T.J.; Petrus, M.J.; Patapoutian, A. TRPM8 is required for cold sensation in mice. Neuron 2007, 54, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.K.; Caterina, M.J. TRP channel knockout mice lose their cool. Neuron 2007, 54, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Bromm, B.; Scharein, E.; Darsow, U.; Ring, J. Effects of menthol and cold on histamine-induced itch and skin reactions in man. Neurosci. Lett. 1995, 187, 157–160. [Google Scholar] [CrossRef]
- Shapovalov, G.; Gkika, D.; Devilliers, M.; Kondratskyi, A.; Gordienko, D.; Busserolles, J.; Bokhobza, A.; Eschalier, A.; Skryma, R.; Prevarskaya, N. Opiates modulate thermosensation by internalizing cold receptor TRPM8. Cell Rep. 2013, 4, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Asuthkar, S.; Demirkhanyan, L.; Sun, X.; Elustondo, P.A.; Krishnan, V.; Baskaran, P.; Velpula, K.K.; Thyagarajan, B.; Pavlov, E.V.; Zakharian, E. The TRPM8 protein is a testosterone receptor: II. Functional evidence for an ionotropic effect of testosterone on TRPM8. J. Biol. Chem. 2015, 290, 2670–2688. [Google Scholar] [CrossRef] [PubMed]
- Asuthkar, S.; Elustondo, P.A.; Demirkhanyan, L.; Sun, X.; Baskaran, P.; Velpula, K.K.; Thyagarajan, B.; Pavlov, E.V.; Zakharian, E. The TRPM8 protein is a testosterone receptor: I. Biochemical evidence for direct TRPM8-testosterone interactions. J. Biol. Chem. 2015, 290, 2659–2669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Barritt, G.J. Evidence that TRPM8 is an androgen-dependent Ca2+ channel required for the survival of prostate cancer cells. Cancer Res. 2004, 64, 8365–8373. [Google Scholar] [CrossRef] [PubMed]
- Bidaux, G.; Borowiec, A.S.; Gordienko, D.; Beck, B.; Shapovalov, G.G.; Lemonnier, L.; Flourakis, M.; Vandenberghe, M.; Slomianny, C.; Dewailly, E.; et al. Epidermal TRPM8 channel isoform controls the balance between keratinocyte proliferation and differentiation in a cold-dependent manner. Proc. Natl. Acad. Sci. USA 2015, 112, E3345–E3354. [Google Scholar] [CrossRef] [PubMed]
- Denda, M.; Tsutsumi, M.; Denda, S. Topical application of TRPM8 agonists accelerates skin permeability barrier recovery and reduces epidermal proliferation induced by barrier insult: Role of cold-sensitive TRP receptors in epidermal permeability barrier homoeostasis. Exp. Dermatol. 2010, 19, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Park, N.H.; Na, Y.J.; Choi, H.T.; Cho, J.C.; Lee, H.K. Activation of transient receptor potential melastatin 8 reduces ultraviolet B-induced prostaglandin E2 production in keratinocytes. J. Dermatol. 2013, 40, 919–922. [Google Scholar] [CrossRef] [PubMed]
- Mergler, S.; Derckx, R.; Reinach, P.S.; Garreis, F.; Bohm, A.; Schmelzer, L.; Skosyrski, S.; Ramesh, N.; Abdelmessih, S.; Polat, O.K.; et al. Calcium regulation by temperature-sensitive transient receptor potential channels in human uveal melanoma cells. Cell Signal. 2014, 26, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, H.; Ugawa, S.; Ueda, T.; Morita, A.; Shimada, S. TRPM8 activation suppresses cellular viability in human melanoma. Am. J. Physiol. Cell Physiol. 2008, 295, C296–C301. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Delling, M.; Li, L.; Dong, X.; Clapham, D.E. Activating mutation in a mucolipin transient receptor potential channel leads to melanocyte loss in varitint-waddler mice. Proc. Natl. Acad. Sci. USA 2007, 104, 18321–18326. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caterina, M.J.; Pang, Z. TRP Channels in Skin Biology and Pathophysiology. Pharmaceuticals 2016, 9, 77. https://doi.org/10.3390/ph9040077
Caterina MJ, Pang Z. TRP Channels in Skin Biology and Pathophysiology. Pharmaceuticals. 2016; 9(4):77. https://doi.org/10.3390/ph9040077
Chicago/Turabian StyleCaterina, Michael J., and Zixuan Pang. 2016. "TRP Channels in Skin Biology and Pathophysiology" Pharmaceuticals 9, no. 4: 77. https://doi.org/10.3390/ph9040077




