Effects of Silymarin-Loaded Nanoparticles on HT-29 Human Colon Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Nano-SLM
2.2. Characterization of Nano-SLM
2.3. Drug Entrapment Efficiency
2.4. In Vitro Drug Release
2.5. Experimental Design
2.6. Cell Viability
2.7. Clonogenicity Assay
2.8. Annexin V-FITC/Propidium Iodide Apoptosis Assay
2.9. Statistical Analysis
3. Results
3.1. Characterization of Nano-SLM
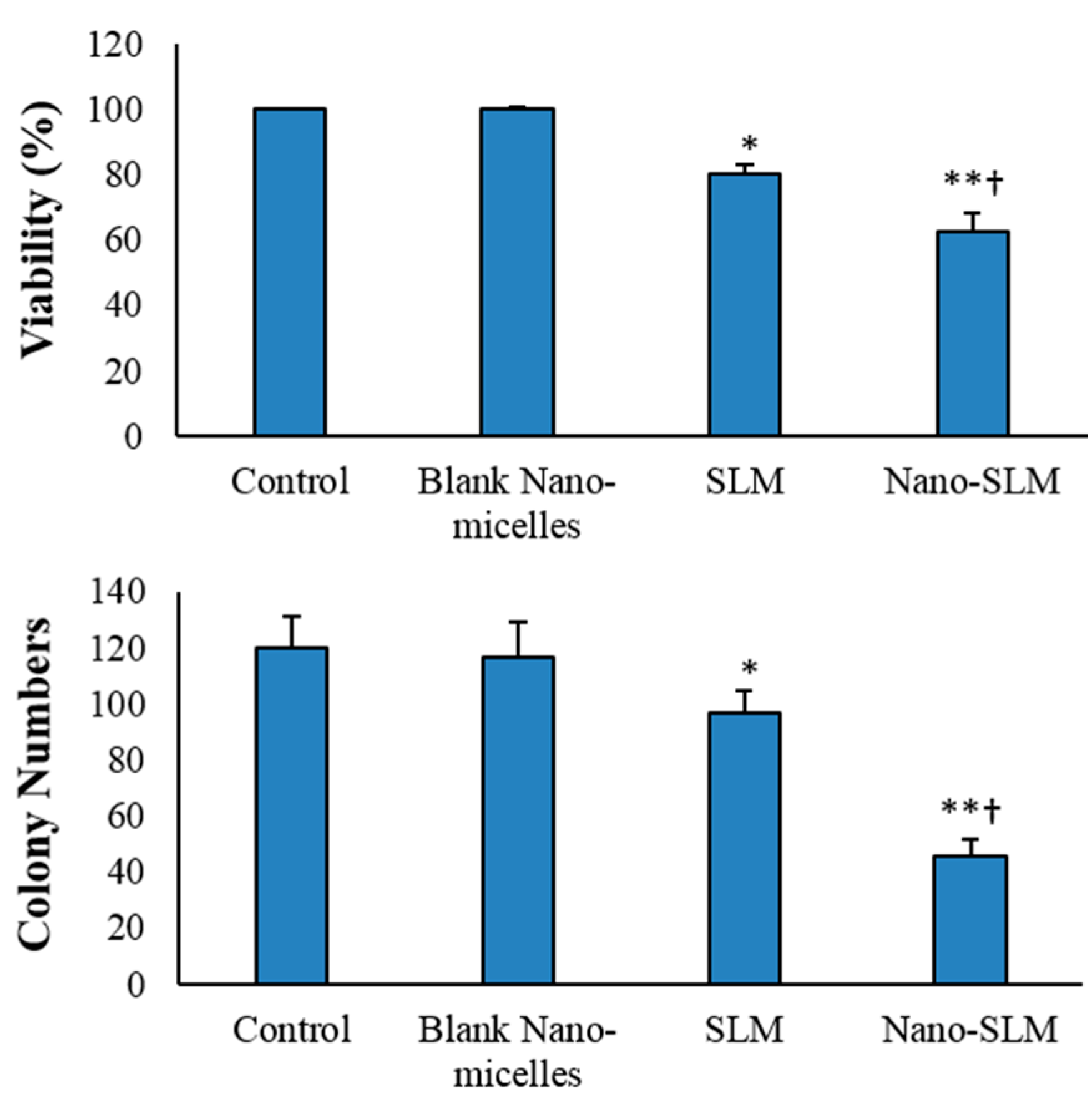
3.2. Cell Viability and Proliferation
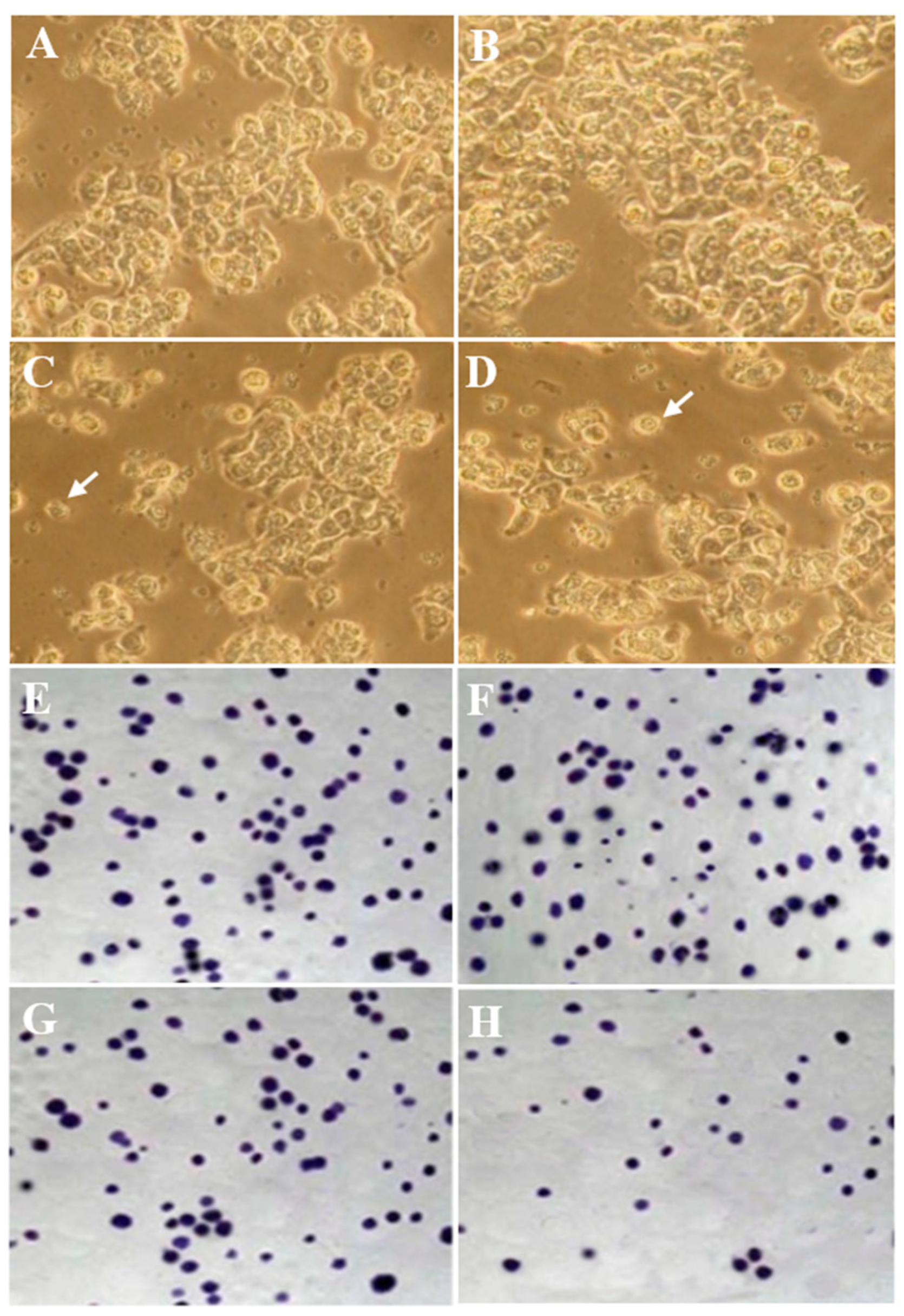
3.3. Morphology Evaluation
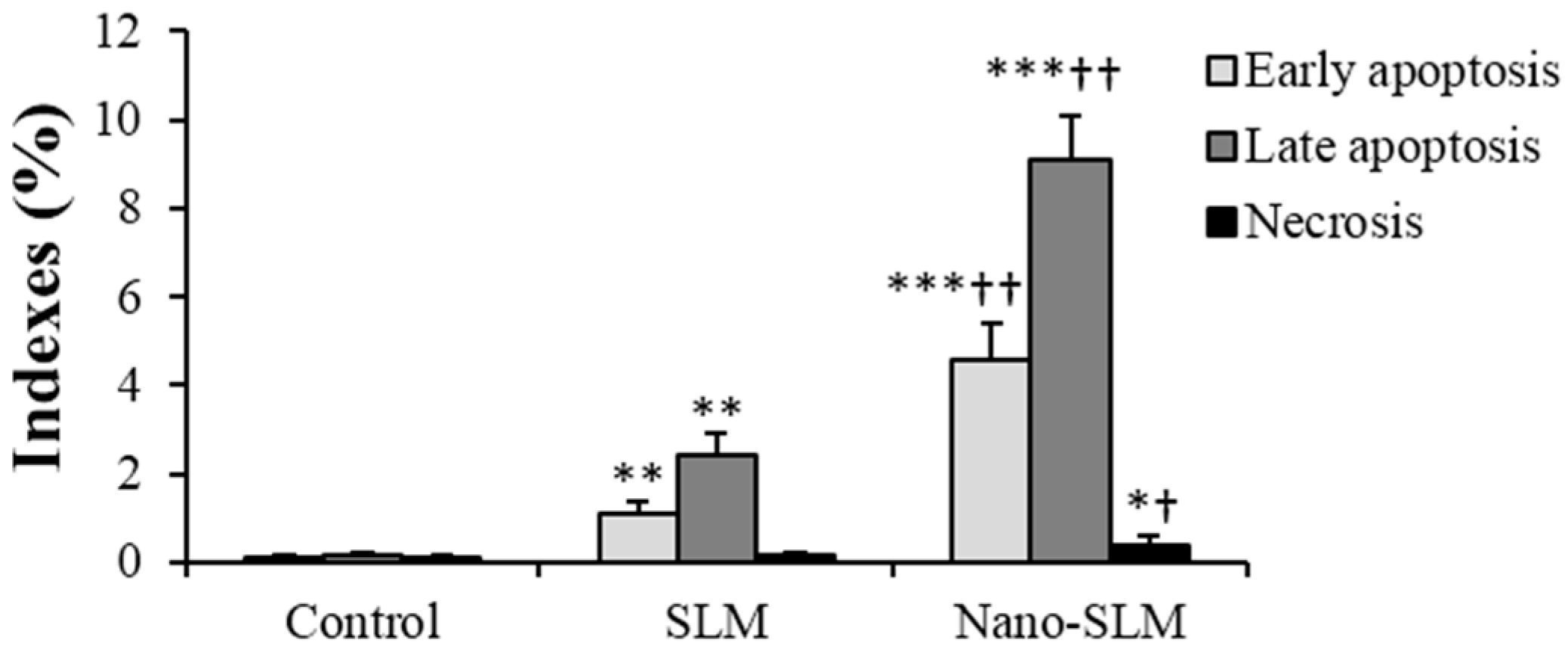
3.4. Annexin V-FITC/Propidium Iodide Apoptosis Assay
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Haggar, F.A.; Boushey, R.P. Colorectal cancer epidemiology: Incidence, mortality, survival, and risk factors. Clin. Colon Rectal Surg. 2009, 22, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Hayat, M.J.; Howlader, N.; Reichman, M.E.; Edwards, B.K. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) program. Oncologist 2007, 12, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Mendoza, N.; Madrigal-Santillán, E.; Morales-González, A.; Esquivel-Soto, J.; Esquivel-Chirino, C.; García-Luna, M.; González-Rubio, Y.; Morales-González, J.A. Hepatoprotective effect of silymarin. World J. Hepatol. 2014, 6, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, C.; Wadhwa, R.; Deep, G.; Biedermann, D.; Gažák, R.; Křen, V.; Agarwal, R. Anti-cancer efficacy of Silybin derivatives—A structure-activity relationship. PLoS ONE 2013, 8, e60074. [Google Scholar] [CrossRef] [PubMed]
- Javed, S.; Kohli, K.; Ali, M. Reassessing bioavailability of silymarin. Altern. Med. Rev. 2011, 16, 239–249. [Google Scholar] [PubMed]
- Snima, K.S.; Arunkumar, P.; Jayakumar, R.; Lakshmanan, V. Silymarin encapsulated poly (d,l-lactic-co-glycolic acid) nanoparticles: A prospective candidate for prostate cancer therapy. J. Biomed. Nanotechnol. 2014, 10, 559–570. [Google Scholar] [CrossRef] [PubMed]
- El-Samaligy, M.S.; Afifi, N.N.; Mahmoud, E.A. Evaluation of hybrid liposomes-encapsulated silymarin regarding physical stability and in vivo performance. Int. J. Pharm. 2006, 319, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhao, Y.; Zhang, Y.; Dang, B.; Liu, Y.; Feng, N. Enhanced oral bioavailability of silymarin using liposomes containing a bile salt: Preparation by supercritical fluid technology and evaluation in vitro and in vivo. Int. J. Nanomed. 2015, 10, 6633–6644. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhao, Y.; Feng, N.; Zhang, Y.; Liu, Y.; Dang, B. Improved dissolution and bioavailability of silymarin delivered by a solid dispersion prepared using supercritical fluids. Asian J. Pharm. Sci. 2015, 10, 194–202. [Google Scholar] [CrossRef]
- Li, X.; Huang, Y.; Chen, X.; Zhou, Y.; Zhang, Y.; Li, P.; Liu, Y.; Sun, Y.; Zhao, J.; Wang, F. Selfassembly and characterization of Pluronic P105 micelles for liver-targeted delivery of silybin. J. Drug Target. 2009, 17, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Khan, M.A.; Burgessa, D.J. A two-stage reverse dialysis in vitro dissolution testing method for passive targeted liposomes. Int. J. Pharm. 2012, 426, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Pandey, H.; Rani, R.; Agarwal, V. Liposome and Their Applications in Cancer Therapy. Braz. Arch. Boil. Technol. 2016, 59, 1–10. [Google Scholar] [CrossRef]
- Paolino, D.; Cosco, D.; Gaspari, M.; Celano, M.; Wolfram, J.; Voce, P.; Puxeddu, E.; Filetti, S.; Celia, C.; Ferrari, M.; et al. Targeting the thyroid gland with thyroid-stimulating hormone (TSH)-nanoliposomes. Biomaterials 2014, 35, 7101–7109. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.S.; Patanam, T.; Mobli, K.; Celia, C.; Zage, P.E.; Bean, A.J.; Tasciotti, E. Etoposide-loaded immunoliposomes as active targeting agents for GD2-positive malignancies. Cancer Biol. Ther. 2014, 15, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Celia, C.; Ferrati, S.; Bansal, S.; van de Ven, A.L.; Ruozi, B.; Zabre, E.; Hosali, S.; Paolino, D.; Sarpietro, M.G.; Fine, D.; et al. Sustained zero-order release of intact ultra-stable drug-loaded liposomes from an implantable nanochannel delivery system. Adv. Healthc. Mater. 2014, 3, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Paolino, D.; Cosco, D.; Racanicchi, L.; Trapasso, E.; Celia, C.; Iannone, M.; Puxeddu, E.; Costante, G.; Filetti, S.; Russo, D.; et al. Gemcitabine-loaded PEGylated unilamellar liposomes vs. GEMZAR: Biodistribution, pharmacokinetic features and in vivo antitumor activity. J. Control Release 2010, 144, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Florence, A.T.; Attwood, D. Physicochemical Principles of Pharmacy, 6th ed.; McMillan: London, UK, 2006; Chapter 2. [Google Scholar]
- Elmowafy, M.; Viitala, T.; Ibrahim, H.M.; Abu-Elyazid, S.K.; Samy, A.; Kassem, A.; Yliperttula, M. Silymarin loaded liposomes for hepatic targeting: In vitro evaluation and HepG2 drug uptake. Eur. Pharm. Sci. 2013, 50, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, K.; Subramanian, G.S.; Mallayasamy, S.R.; Averineni, R.K.; Reddy, M.S.; Udupa, N. A study of rivastigmine liposomes for delivery into the brain through intranasal route. Acta Pharm. 2008, 58, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Shivhare, U.; Ambulkar, D.; Mathur, V.; Bhusari, K.; Godbole, M. Formulation and evaluation of pentoxifylline liposome formulation. Dig. J. Nanomater. Biostruct. 2009, 4, 857–862. [Google Scholar]
- Soleimani, M.; Khorsandi, L.; Atashi, A.; Nejaddehbashi, F. Chondrogenic differentiation of human umbilical cord blood-derived unrestricted somatic stem cells on a 3D beta-tricalcium phosphate-alginate-gelatin scaffold. Cell J. 2014, 16, 43–52. [Google Scholar] [PubMed]
- Khorsandi, L.; Saki, G.; Bavarsad, N.; Mombeini, M. Silymarin induces a multi-targeted cell death process in the human colon cancer cell line HT-29. Biomed. Pharmacother. 2017, 94, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Yazdi Rouholamini, S.E.; Moghassemi, S.; Maharat, Z.; Hakamivala, A.; Kashanian, S.; Omidfar, K. Effect of silibinin-loaded nano-niosomal coated with trimethyl chitosan on miRNAs expression in 2D and 3D models of T47D breast cancer cell line. Artif. Cells Nanomed. Biotechnol. 2017, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mao, W.; Lock, L.L.; Tang, J.; Sui, M.; Sun, W.; Cui, H.; Xu, D.; Shen, Y. The Role of Micelle Size in Tumor Accumulation, Penetration, and Treatment. ACS Nano 2015, 9, 7195–7206. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.G.; Ming, L.C.; Lee, K.S.; Yuen, K.H. Influence of the encapsulation efficiency and size of liposome on the oral bioavailability of griseofulvin-loaded liposomes. Pharmaceutics 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Sui, W.; Yin, C.; Kong, X. Micellar solubilization and in vitro release of silymarin in the self-aggregates of an amphiphilic derivative of chitosan polymers and organic chemistry. Macromol. Symp. 2010, 297, 147–153. [Google Scholar] [CrossRef]
- Fan, L.; Ma, Y.; Liu, Y.; Zheng, D.; Huang, G. Silymarin induces cell cycle arrest and apoptosis in ovarian cancer cells. Eur. J. Pharmacol. 2014, 743, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, G.; Lo Muzio, L.; Elinos-Báez, C.M.; Jagan, S.; Augustine, T.A.; Kamaraj, S.; Anandakumar, P.; Devaki, T. Silymarin inhibited proliferation and induced apoptosis in hepatic cancer cells. Cell Prolif. 2009, 42, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Huang, Z.; Kim, D.J.; Kim, S.H.; Kim, M.O.; Lee, S.Y.; Xie, H.; Park, S.J.; Kim, J.Y.; Kundu, J.K.; et al. Direct targeting of MEK1/2 and RSK2 by silybin induces cell-cycle arrest and inhibits melanoma cell growth. Cancer Prev. Res. (Phila) 2013, 6, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Imai-Sumida, M.; Chiyomaru, T.; Majid, S.; Saini, S.; Nip, H.; Dahiya, R.; Tanaka, Y.; Yamamura, S. Silibinin suppresses bladder cancer through down-regulation of actin cytoskeleton and PI3K/Akt signaling pathways. Oncotarget 2017, 8, 92032–92042. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yu, J.; Wang, C. Silibinin promotes the apoptosis of gastric cancer BGC823 cells through caspase pathway. J. BUON 2017, 22, 1148–1153. [Google Scholar] [PubMed]
- Ham, J.; Lim, W.; Bazer, F.W.; Song, G. Silibinin stimluates apoptosis by inducing generation of ROS and ER stress in human choriocarcinoma cells. J. Cell. Physiol. 2018, 233, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.S.; Oh, S.; Jang, B.; Yu, H.J.; Shin, J.A.; Cho, N.P.; Yang, I.H.; Won, D.H.; Kwon, H.J.; Hong, S.D.; et al. Silymarin and its active component silibinin act as novel therapeutic alternatives for salivary gland cancer by targeting the ERK1/2-Bim signaling cascade. Cell. Oncol. (Dordr.) 2017, 40, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gu, N.; Xue, C.; Li, B.R. Plant flavonoid taxifolin inhibits the growth, migration and invasion of human osteosarcoma cells. Mol. Med. Rep. 2018, 17, 3239–3245. [Google Scholar] [CrossRef] [PubMed]
- Alzaharna, M.; Alqouqa, I.; Cheung, H.Y. Taxifolin synergizes Andrographolide-induced cell death by attenuation of autophagy and augmentation of caspase dependent and independent cell death in HeLa cells. PLoS ONE 2017, 12, e0171325. [Google Scholar] [CrossRef] [PubMed]
- Manigandan, K.; Manimaran, D.; Jayaraj, R.L.; Elangovan, N.; Dhivya, V.; Kaphle, A. Taxifolin curbs NF-κB-mediated Wnt/β-catenin signaling via up-regulating Nrf2 pathway in experimental colon carcinogenesis. Biochimie 2015, 119, 103–112. [Google Scholar] [CrossRef] [PubMed]
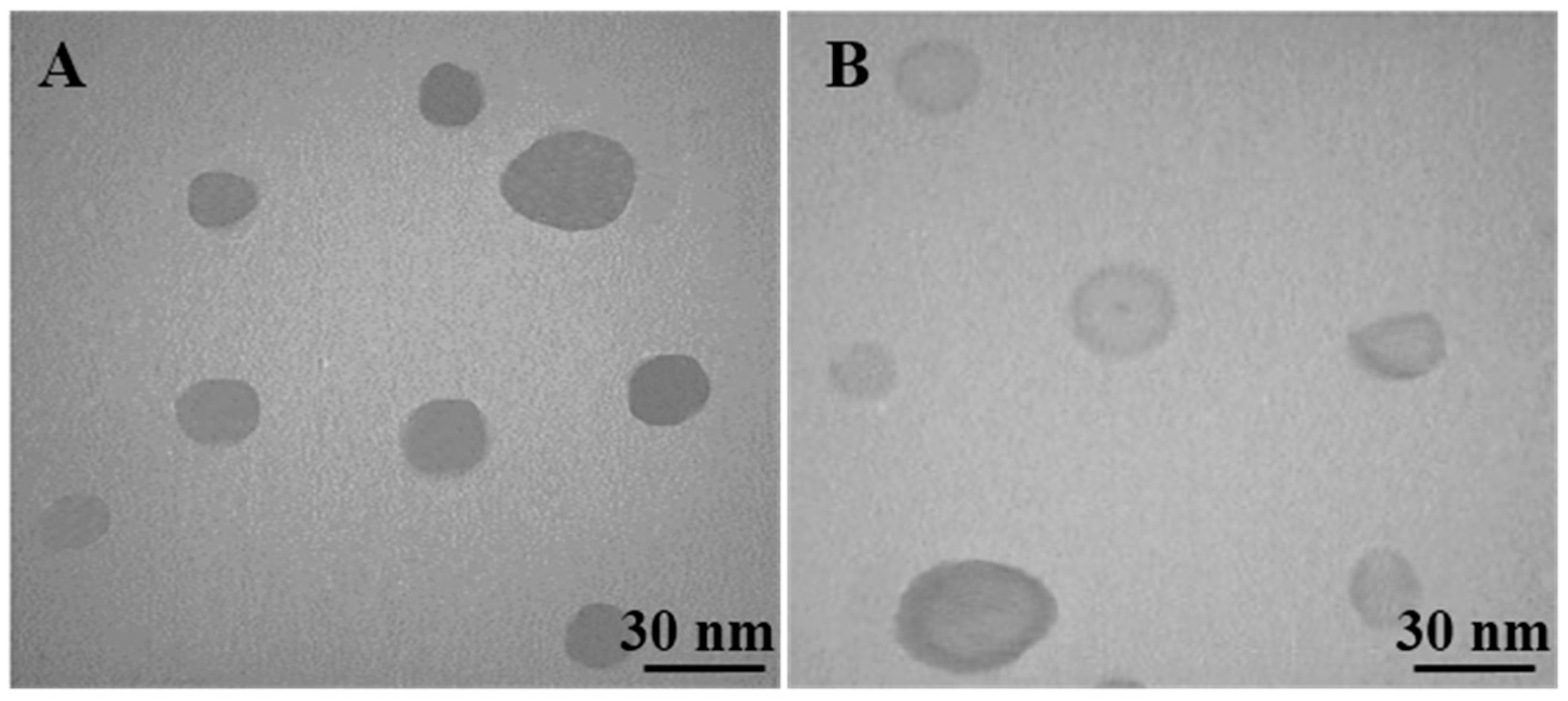


| Groups | Particle Size | PDI | Zeta Potential |
|---|---|---|---|
| nm | Mv | ||
| Nano-SLM | 26.5 ± 4.3 | 0.53 ± 0.03 | −23.5 ± 0.6 |
| Blank micelles | 23.4 ± 3.9 | 0.56 ± 0.04 | −23.6 ± 0.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mombeini, M.; Saki, G.; Khorsandi, L.; Bavarsad, N. Effects of Silymarin-Loaded Nanoparticles on HT-29 Human Colon Cancer Cells. Medicina 2018, 54, 1. https://doi.org/10.3390/medicina54010001
Mombeini M, Saki G, Khorsandi L, Bavarsad N. Effects of Silymarin-Loaded Nanoparticles on HT-29 Human Colon Cancer Cells. Medicina. 2018; 54(1):1. https://doi.org/10.3390/medicina54010001
Chicago/Turabian StyleMombeini, Maryam, Ghasem Saki, Layasadat Khorsandi, and Neda Bavarsad. 2018. "Effects of Silymarin-Loaded Nanoparticles on HT-29 Human Colon Cancer Cells" Medicina 54, no. 1: 1. https://doi.org/10.3390/medicina54010001





