Angiogenesis: Managing the Culprits behind Tumorigenesis and Metastasis
Abstract
:1. Introduction
2. Targeting Tumor Vasculature as a Therapeutic Strategy
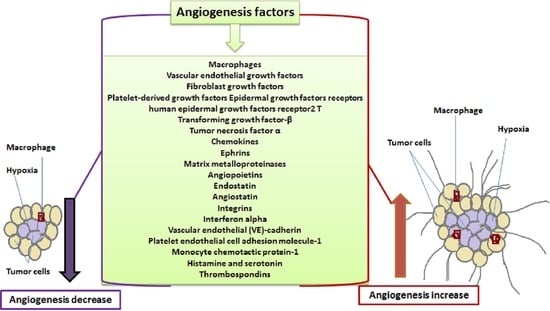
3. Molecular Mediators of Angiogenesis
3.1. Inflammatory Cells
3.2. Growth Factors
3.2.1. Vascular Endothelial Growth Factors (VEGFs)
3.2.2. Fibroblast Growth Factors (FGFs)
3.2.3. Platelet-Derived Growth Factors (PDGFs)
3.2.4. Epidermal Growth Factors Receptors (EGFR) and Human Epidermal Growth Factors Receptor2 (HER2)
3.2.5. Transforming Growth Factor-B (TGF-B)
3.2.6. Angiopoietins (Angs)
3.3. Cytokines and Chemokines
3.3.1. Tumor Necrosis Factor α (TNF-α)
3.3.2. Interferon Alpha (IFN-α)
3.3.3. Monocyte Chemotactic Protein-1 (MCP-1)
3.3.4. Hepatocyte Growth Factor (HGF) and C-Met
3.4. Membrane Protein and Adhesion Proteins
3.4.1. Ephrins (Eph)
3.4.2. Semaphorins
3.4.3. Integrins
3.4.4. Vascular Endothelial (VE)-Cadherin
3.4.5. Platelet Endothelial Cell Adhesion Molecule-1 (PECAM-1 or CD31)
3.5. Matrix Degrading Enzyme
Matrix Metalloproteinases (MMPs)
3.6. Small Mediators
3.6.1. Histamine and Serotonin
3.6.2. Endostatin
3.6.3. Angiostatin
3.6.4. Thrombospondins (TSPs)
3.6.5. Galectins (Gals)
3.7. MicroRNA (miRNAs)
4. Future Perspectives
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Folkman, J.; Merler, E.; Abernathy, C.; Williams, G. Isolation of a tumor factor responsible for angiogenesis. J. Exp. Med. 1971, 133, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.E.; Senger, D.R. Endothelial extracellular matrix biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ. Res. 2005, 97, 1093–1107. [Google Scholar] [CrossRef] [PubMed]
- Baldewijns, M.; Thijssen, V.; Van den Eynden, G.; Van Laere, S.; Bluekens, A.; Roskams, T.; Van Poppel, H.; De Bruine, A.; Griffioen, A.; Vermeulen, P. High-grade clear cell renal cell carcinoma has a higher angiogenic activity than low-grade renal cell carcinoma based on histomorphological quantification and qRT–PCR mRNA expression profile. Br. J. Cancer 2007, 96, 1888–1895. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, S.; Baluk, P.; Kaidoh, T.; Haskell, A.; Jain, R.K.; McDonald, D.M. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am. J. Pathol. 2002, 160, 985–1000. [Google Scholar] [CrossRef]
- Ruoslahti, E. Specialization of tumour vasculature. Nat. Rev. Cancer 2002, 2, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Monahan-Earley, R.; Dvorak, A.; Aird, W. Evolutionary origins of the blood vascular system and endothelium. J. Thromb. Haemost. 2013, 11, 46–66. [Google Scholar] [CrossRef] [PubMed]
- Koh, G.Y.; Joo, H.J.; Choi, D.K.; Park, J.-S.; Cho, S.W. Method for Forming Endothelial Cells. U.S. Patent Application 20150368617A1, 24 December 2015. [Google Scholar]
- Williams, P.A. Manipulating Endothelial Progenitor Cell Homing with Sphingosine-1-Phosphate for Terapeutic Angiogenesis; University of California: Davis, CA, USA, 2016. [Google Scholar]
- Yehya, A.H.; Asif, M.; Tan, Y.J.; Sasidharan, S.; Majid, A.M.A.; Oon, C.E. Broad spectrum targeting of tumor vasculature by medicinal plants: An updated review. J. Herb. Med. 2017, 9, 1–13. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Oon, C.E.; Bridges, E.; Sheldon, H.; Sainson, R.C.A.; Jubb, A.; Turley, H.; Leek, R.; Buffa, F.; Harris, A.L.; Li, J.L. Role of Delta-like 4 in Jagged1-induced tumour angiogenesis and tumour growth. Oncotarget 2017, 8, 40115–40131. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat. Rev. Drug Discov. 2011, 10, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Eelen, G.; de Zeeuw, P.; Simons, M.; Carmeliet, P. Endothelial cell metabolism in normal and diseased vasculature. Circ. Res. 2015, 116, 1231–1244. [Google Scholar] [CrossRef] [PubMed]
- Klemm, F.; Joyce, J.A. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol. 2015, 25, 198–213. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dabrosin, C.; Yin, X.; Fuster, M.M.; Arreola, A.; Rathmell, W.K.; Generali, D.; Nagaraju, G.P.; El-Rayes, B.; Ribatti, D.; et al. Broad targeting of angiogenesis for cancer prevention and therapy. Semin. Cancer Biol. 2015, 35, S224–S243. [Google Scholar] [CrossRef] [PubMed]
- Sun, W. Angiogenesis in metastatic colorectal cancer and the benefits of targeted therapy. J. Hematol. Oncol. 2012, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Siemann, D.W. The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by tumor-vascular disrupting agents. Cancer Treat. Rev. 2011, 37, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.R.; Capasso, M.; Hagemann, T. The tumor microenvironment at a glance. J. Cell Sci. 2012, 125, 5591–5596. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.C.; Jenkins, S.J.; Allen, J.E.; Taylor, P.R. Tissue-resident macrophages. Nat. Immunol. 2013, 14, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Jetten, N.; Verbruggen, S.; Gijbels, M.J.; Post, M.J.; De Winther, M.P.; Donners, M.M. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis 2014, 17, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.-Q.; Chen, G.; Zhang, W.; Xiong, X.-P.; Zhao, Y.; Liu, B.; Zhao, Y.-F. M2-polarized macrophages in keratocystic odontogenic tumor: Relation to tumor angiogenesis. Sci. Rep. 2015, 5, 15586. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.R.; Schmid, M.C. Macrophages as key drivers of cancer progression and metastasis. Mediat. Inflamm. 2017, 2017, 9624760. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.Y.; Kwon, S.M. Angiogenesis and its therapeutic opportunities. Mediat. Inflamm. 2013, 2013, 127170. [Google Scholar] [CrossRef] [PubMed]
- Olsson, A.-K.; Dimberg, A.; Kreuger, J.; Claesson-Welsh, L. VEGF receptor signalling? In control of vascular function. Nat. Rev. Mol. Cell Biol. 2006, 7, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Hicklin, D.J.; Ellis, L.M. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J. Clin. Oncol. 2005, 23, 1011–1027. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Nakayama, A.; van Lessen, M.; Yamamoto, H.; Hoffmann, S.; Drexler, H.C.; Itoh, N.; Hirose, T.; Breier, G.; Vestweber, D. Spatial regulation of VEGF receptor endocytosis in angiogenesis. Nat. Cell Biol. 2013, 15, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Terme, M.; Pernot, S.; Marcheteau, E.; Sandoval, F.; Benhamouda, N.; Colussi, O.; Dubreuil, O.; Carpentier, A.F.; Tartour, E.; Taieb, J. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res. 2013, 73, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Kubatka, P.; Kapinová, A.; Kello, M.; Kruzliak, P.; Kajo, K.; Výbohová, D.; Mahmood, S.; Murin, R.; Viera, T.; Mojžiš, J. Fruit peel polyphenols demonstrate substantial anti-tumour effects in the model of breast cancer. Eur. J. Nutr. 2016, 55, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.F.; Garcia, E.A.; Luz, M.A.M.; Pardal, F.; Rodrigues, M.; Longatto Filho, A. Clinicopathological correlation and prognostic significance of VEGF-A, VEGF-C, VEGFR-2 and VEGFR-3 expression in colorectal cancer. Cancer Genom. Proteom. 2013, 10, 55–67. [Google Scholar]
- Matsumoto, M.; Roufail, S.; Inder, R.; Caesar, C.; Karnezis, T.; Shayan, R.; Farnsworth, R.H.; Sato, T.; Achen, M.G.; Mann, G.B. Signaling for lymphangiogenesis via VEGFR-3 is required for the early events of metastasis. Clin. Exp. Metastasis 2013, 30, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Siemann, D.; Chaplin, D.; Horsman, M. Realizing the potential of vascular targeted therapy: The rationale for combining vascular disrupting agents and anti-angiogenic agents to treat cancer. Cancer Investig. 2017, 35, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qin, S.; Xu, J.; Xiong, J.; Wu, C.; Bai, Y.; Liu, W.; Tong, J.; Liu, Y.; Xu, R. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J. Clin. Oncol. 2016, 34, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.; Wang, M.; Gralow, J.; Dickler, M.; Cobleigh, M.; Perez, E.A.; Shenkier, T.; Cella, D.; Davidson, N.E. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N. Engl. J. Med. 2007, 357, 2666–2676. [Google Scholar] [CrossRef] [PubMed]
- Baird, A.; Esch, F.; Mormede, P.; Ueno, N.; Ling, N.; Bohlen, P.; Ying, S.; Wehrenberg, B.; Guillemin, R. Molecular characterization of fibroblast growth factor: Distribution and biological activities in various tissues. Recent Prog. Horm. Res. 1986, 42, 143–205. [Google Scholar] [PubMed]
- Cross, M.J.; Claesson-Welsh, L. FGF and VEGF function in angiogenesis: Signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol. Sci. 2001, 22, 201–207. [Google Scholar] [CrossRef]
- Greaves, N.S.; Ashcroft, K.J.; Baguneid, M.; Bayat, A. Current understanding of molecular and cellular mechanisms in fibroplasia and angiogenesis during acute wound healing. J. Dermatol. Sci. 2013, 72, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Presta, M.; Chiodelli, P.; Giacomini, A.; Rusnati, M.; Ronca, R. Fibroblast growth factors (FGFs) in cancer: FGF traps as a new therapeutic approach. Pharmacol. Ther. 2017, 179, 171–187. [Google Scholar] [CrossRef] [PubMed]
- Bendell, J.; Fuchs, C.; Voss, M.; Bauer, T.M.; Choueiri, T.K.; Drilon, A.; Thorn, K.; Wijayawardana, S.; Moser, B.; Uruñuela, A. Abstract CT090: A Phase 1b/2 Study of Ramucirumab in Combination with Emibetuzumab in Patients with Advanced Solid Tumors. In Proceedings of the AACR Annual Meeting, Washington, DC, USA, 1–5 April 2017. [Google Scholar]
- Jitariu, A.A.; Cimpean, A.M.; Kundnani, N.R.; Raica, M. State of the art paper Platelet-derived growth factors induced lymphangiogenesis: Evidence, unanswered questions and upcoming challenges. Arch. Med. Sci. 2015, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, A.A.; Siddik, Z.H. Platelet-derived growth factor (PDGF) signalling in cancer: Rapidly emergingsignalling landscape. Cell Biochem. Funct. 2015, 33, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.A.; Patel, K.D.; Medved, J.; Reiss, A.M.; Nishiyama, A. NG2 cells in white matter but not gray matter proliferate in response to PDGF. J. Neurosci. 2013, 33, 14558–14566. [Google Scholar] [CrossRef] [PubMed]
- Jurek, A.; Amagasaki, K.; Gembarska, A.; Heldin, C.-H.; Lennartsson, J. Negative and positive regulation of MAPK phosphatase 3 controls platelet-derived growth factor-induced Erk activation. J. Biol. Chem. 2009, 284, 4626–4634. [Google Scholar] [CrossRef] [PubMed]
- Heldin, C.-H. Targeting the PDGF signaling pathway in tumor treatment. Cell Commun. Signal. 2013, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Drevs, J.; Siegert, P.; Medinger, M.; Mross, K.; Strecker, R.; Zirrgiebel, U.; Harder, J.; Blum, H.; Robertson, J.; Jürgensmeier, J.M. Phase I clinical study of AZD2171, an oral vascular endothelial growth factor signaling inhibitor, in patients with advanced solid tumors. J. Clin. Oncol. 2007, 25, 3045–3054. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.-C.; Long, C.-Y.; Tian, Y.-F.; Wu, M.-P. Her-2/neu overexpression is associated with thrombospondin-1-related angiogenesis and thrombospondin-1-unrelated lymphangiogenesis in breast cancer. Gynecol. Minim. Invasive Ther. 2013, 2, 114–121. [Google Scholar] [CrossRef]
- Yewale, C.; Baradia, D.; Vhora, I.; Patil, S.; Misra, A. Epidermal growth factor receptor targeting in cancer: A review of trends and strategies. Biomaterials 2013, 34, 8690–8707. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Wu, W.; Wang, Z.; Li, C.; Lu, X.; Duan, H.; Zhou, J.; Wang, X.; Wan, P.; Song, Y. Comparative study of the effects of recombinant human epidermal growth factor and basic fibroblast growth factor on corneal epithelial wound healing and neovascularization in vivo and in vitro. Ophthalmic Res. 2013, 49, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Mross, K.; Drevs, J.; Müller, M.; Medinger, M.; Marmé, D.; Hennig, J.; Morgan, B.; Lebwohl, D.; Masson, E.; Ho, Y.-Y. Phase I clinical and pharmacokinetic study of PTK/ZK, a multiple VEGF receptor inhibitor, in patients with liver metastases from solid tumours. Eur. J. Cancer 2005, 41, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Chaudhuri, A.; Talmon, G.; Wisecarver, J.L.; Wang, J. TGF-beta suppresses VEGFA-mediated angiogenesis in colon cancer metastasis. PLoS ONE 2013, 8, e59918. [Google Scholar] [CrossRef] [PubMed]
- Distler, J.H.; Hirth, A.; Kurowska-Stolarska, M.; Gay, R.E.; Gay, S.; Distler, O. Angiogenic and angiostatic factors in the molecular control of angiogenesis. Q. J. Nucl. Med. 2003, 47, 149–161. [Google Scholar] [PubMed]
- Ucuzian, A.A.; Gassman, A.A.; East, A.T.; Greisler, H.P. Molecular mediators of angiogenesis. J. Burn Care Res. 2010, 31, 158–175. [Google Scholar] [CrossRef] [PubMed]
- Saenz, S.A.; Taylor, B.C.; Artis, D. Welcome to the neighborhood: Epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol. Rev. 2008, 226, 172–190. [Google Scholar] [CrossRef] [PubMed]
- Mehrad, B.; Keane, M.P.; Strieter, R.M. Chemokines as mediators of angiogenesis. Thromb. Haemost. 2007, 97, 755. [Google Scholar] [CrossRef] [PubMed]
- Sahin, H.; Borkham-Kamphorst, E.; Kuppe, C.; Zaldivar, M.M.; Grouls, C.; Al-samman, M.; Nellen, A.; Schmitz, P.; Heinrichs, D.; Berres, M.L. Chemokine Cxcl9 attenuates liver fibrosis-associated angiogenesis in mice. Hepatology 2012, 55, 1610–1619. [Google Scholar] [CrossRef] [PubMed]
- Passaro, C.; Borriello, F.; Vastolo, V.; Di Somma, S.; Scamardella, E.; Gigantino, V.; Franco, R.; Marone, G.; Portella, G. The oncolytic virus dl922-947 reduces IL-8/CXCL8 and MCP-1/CCL2 expression and impairs angiogenesis and macrophage infiltration in anaplastic thyroid carcinoma. Oncotarget 2016, 7, 1500. [Google Scholar] [CrossRef] [PubMed]
- Kroeze, K.L.; Boink, M.A.; Sampat-Sardjoepersad, S.C.; Waaijman, T.; Scheper, R.J.; Gibbs, S. Autocrine regulation of re-epithelialization after wounding by chemokine receptors CCR1, CCR10, CXCR1, CXCR2, and CXCR3. J. Investig. Dermatol. 2012, 132, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Guan, Z.; Chen, J.; Xie, H.; Yang, Z.; Fan, J.; Wang, X.; Li, L. CXCL5/CXCR2 axis promotes bladder cancer cell migration and invasion by activating PI3K/AKT-induced upregulation of MMP2/MMP9. Int. J. Oncol. 2015, 47, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Han, X.; Peng, J.; Qin, H.; Wang, Y. The role of CXC chemokines and their receptors in the progression and treatment of tumors. J. Mol. Histol. 2012, 43, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Datta, D.; Flaxenburg, J.A.; Laxmanan, S.; Geehan, C.; Grimm, M.; Waaga-Gasser, A.M.; Briscoe, D.M.; Pal, S. Ras-induced modulation of CXCL10 and its receptor splice variant CXCR3-B in MDA-MB-435 and MCF-7 cells: Relevance for the development of human breast cancer. Cancer Res. 2006, 66, 9509–9518. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, F.; Siekmann, A.F. The role of chemokines and their receptors in angiogenesis. Cell. Mol. Life Sci. 2011, 68, 2811–2830. [Google Scholar] [CrossRef] [PubMed]
- Keeley, E.C.; Mehrad, B.; Strieter, R.M. Chemokines as mediators of tumor angiogenesis and neovascularization. Exp. Cell Res. 2011, 317, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.-M.; Chung, A.C.; Lan, H.Y. Role of the TGF-β/BMP-7/Smad pathways in renal diseases. Clin. Sci. 2013, 124, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.; Lee, Y.F.; Ge, R. Novel endogenous angiogenesis inhibitors and their therapeutic potential. Acta Pharmacol. Sin. 2015, 36, 1177–1190. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.R.; Kang, S.K.; Kim, Y.S.; Lee, S.Y.; Hong, S.C.; Kim, E.C. TNF-α and LPS activate angiogenesis via VEGF and SIRT1 signalling in human dental pulp cells. Int. Endod. J. 2015, 48, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.A.; Giles, F.J.; Albitar, M.; Cortes, J.E.; Verstovsek, S.; Faderl, S.; O’brien, S.M.; Garcia-Manero, G.; Keating, M.J.; Pierce, S. Thalidomide therapy for myelofibrosis with myeloid metaplasia. Cancer 2006, 106, 1974–1984. [Google Scholar] [CrossRef] [PubMed]
- Von Marschall, Z.; Scholz, A.; Cramer, T.; Schäfer, G.; Schirner, M.; Öberg, K.; Wiedenmann, B.; Höcker, M.; Rosewicz, S. Effects of interferon alpha on vascular endothelial growth factor gene transcription and tumor angiogenesis. J. Nat. Cancer Inst. 2003, 95, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Indraccolo, S. Interferon-α as angiogenesis inhibitor: Learning from tumor models. Autoimmunity 2010, 43, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.H.; Ryu, J.; Han, K.H. Monocyte chemoattractant protein-1–induced angiogenesis is mediated by vascular endothelial growth factor-A. Blood 2005, 105, 1405. [Google Scholar] [CrossRef] [PubMed]
- Skibinski, G.; Skibinska, A.; James, K. The role of hepatocyte growth factor and its receptor c-met in interactions between lymphocytes and stromal cells in secondary human lymphoid organs. Immunology 2001, 102, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Merkulova-Rainon, T.; Han, Z.C.; Tobelem, G. HGF receptor up-regulation contributes to the angiogenic phenotype of human endothelial cells and promotes angiogenesis in vitro. Blood 2003, 101, 4816–4822. [Google Scholar] [CrossRef] [PubMed]
- Gallo, S.; Sala, V.; Gatti, S.; Crepaldi, T. HGF/Met axis in heart function and cardioprotection. Biomedicines 2014, 2, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; DeBusk, L.M.; Babichev, Y.O.; Dumont, D.J.; Lin, P.C. Hepatocyte growth factor mediates angiopoietin-induced smooth muscle cell recruitment. Blood 2006, 108, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Mira, A.; Morello, V.; Céspedes, M.V.; Perera, T.; Comoglio, P.M.; Mangues, R.; Michieli, P. Stroma-derived HGF drives metabolic adaptation of colorectal cancer to angiogenesis inhibitors. Oncotarget 2017, 8, 38193. [Google Scholar] [CrossRef] [PubMed]
- Pothula, S.P.; Xu, Z.; Goldstein, D.; Merrett, N.; Pirola, R.C.; Wilson, J.S.; Apte, M.V. Targeting the HGF/c-MET pathway: Stromal remodelling in pancreatic cancer. Oncotarget 2017, 8, 76722. [Google Scholar] [CrossRef] [PubMed]
- Lisabeth, E.M.; Falivelli, G.; Pasquale, E.B. Eph receptor signaling and ephrins. Cold Spring Harb. Perspect. Biol. 2013, 5, a009159. [Google Scholar] [CrossRef] [PubMed]
- Kullander, K.; Klein, R. Mechanisms and functions of Eph and ephrin signalling. Nat. Rev. Mol. Cell Biol. 2002, 3, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Mosch, B.; Reissenweber, B.; Neuber, C.; Pietzsch, J. Eph receptors and ephrin ligands: Important players in angiogenesis and tumor angiogenesis. J. Oncol. 2010, 2010, 135285. [Google Scholar] [CrossRef] [PubMed]
- Mross, K.; Frost, A.; Steinbild, S.; Hedbom, S.; Büchert, M.; Fasol, U.; Unger, C.; Krätzschmar, J.; Heinig, R.; Boix, O. A phase I dose–escalation study of regorafenib (BAY 73-4506), an inhibitor of oncogenic, angiogenic, and stromal kinases, in patients with advanced solid tumors. Clin. Cancer Res. 2012, 18, 2658–2667. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Giraudo, E. The role of semaphorins and their receptors in vascular development and cancer. Exp. Cell Res. 2013, 319, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Clarhaut, J.; Gemmill, R.M.; Potiron, V.A.; Ait-Si-Ali, S.; Imbert, J.; Drabkin, H.A.; Roche, J. ZEB-1, a repressor of the semaphorin 3F tumor suppressor gene in lung cancer cells. Neoplasia 2009, 11, IN2–IN5. [Google Scholar] [CrossRef]
- Singh, M.K.; Bhattacharya, D.; Chaudhuri, S.; Acharya, S.; Kumar, P.; Santra, P.; Basu, A.K.; Chaudhuri, S. T11TS inhibits glioma angiogenesis by modulation of MMPs, TIMPs, with related integrin αv and TGF-β1 expressions. Tumor Biol. 2014, 35, 2231–2246. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.T.; Liu, Y.; Kan, X.; Liu, M.; Lu, J.G. Cilengitide, a small molecule antagonist, targeted to integrin αν inhibits proliferation and induces apoptosis of laryngeal cancer cells in vitro. Eur. Arch. Oto-Rhino-Laryngol. 2014, 271, 2233–2240. [Google Scholar] [CrossRef] [PubMed]
- Notni, J.; Steiger, K.; Hoffmann, F.; Reich, D.; Kapp, T.G.; Rechenmacher, F.; Neubauer, S.; Kessler, H.; Wester, H.-J. Complementary, selective PET imaging of integrin subtypes α5β1 and αvβ3 using 68Ga-aquibeprin and 68Ga-avebetrin. J. Nucl. Med. 2016, 57, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Foubert, P.; Varner, J.A. Integrins in Tumor Angiogenesis and Lymphangiogenesis. In Integrin and Cell Adhesion Molecules; Shimaoka, M., Ed.; Humana Press: Totowa, NJ, USA, 2012; pp. 471–486. [Google Scholar]
- Wallez, Y.; Vilgrain, I.; Huber, P. Angiogenesis: The VE-Cadherin Switch. Trends Cardiovasc. Med. 2006, 16, 55–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aragon-Sanabria, V.; Pohler, S.E.; Eswar, V.J.; Bierowski, M.; Gomez, E.W.; Dong, C. VE-cadherin disassembly and cell contractility in the endothelium are necessary for barrier disruption induced by tumor cells. Sci. Rep. 2017, 7, 45835. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.M.; Hermanns, M.I.; Skrzynski, C.; Nesslinger, M.; Müller, K.-M.; Kirkpatrick, C.J. Expression of the endothelial markers PECAM-1, vWf, and CD34 in vivo and in vitro. Exp. Mol. Pathol. 2002, 72, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Baumann, C.I.; Bailey, A.S.; Li, W.; Ferkowicz, M.J.; Yoder, M.C.; Fleming, W.H. PECAM-1 is expressed on hematopoietic stem cells throughout ontogeny and identifies a population of erythroid progenitors. Blood 2004, 104, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Woodfin, A.; Voisin, M.-B.; Nourshargh, S. PECAM-1: A multi-functional molecule in inflammation and vascular biology. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2514. [Google Scholar] [CrossRef] [PubMed]
- Rundhaug, J.E. Matrix metalloproteinases and angiogenesis. J. Cell. Mol. Med. 2005, 9, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Takahashi, H.; Murai, Y.; Cui, Z.; Nomoto, K.; Niwa, H.; Tsuneyama, K.; Takano, Y. Expressions of MMP-2, MMP-9 and VEGF are closely linked to growth, invasion, metastasis and angiogenesis of gastric carcinoma. Anticancer Res. 2006, 26, 3579–3583. [Google Scholar] [PubMed]
- Qin, L.; Zhao, D.; Xu, J.; Ren, X.; Terwilliger, E.F.; Parangi, S.; Lawler, J.; Dvorak, H.F.; Zeng, H. The vascular permeabilizing factors histamine and serotonin induce angiogenesis through TR3/Nur77 and subsequently truncate it through thrombospondin-1. Blood 2013, 121, 2154–2164. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.H.; Lee, D.Y. Antiangiogenic actions of heparin derivatives for cancer therapy. Macromol. Res. 2016, 24, 767–772. [Google Scholar] [CrossRef]
- Venkatachalam, A. Effect of Mutant Endostatin and Kringle 5 Fusion Protein on Tumor Angiogenesis; ProQuest Dissertations Publishing: Ann Arbor, MI, USA, 2016. [Google Scholar]
- Wajih, N.; Sane, D.C. Angiostatin selectively inhibits signaling by hepatocyte growth factor in endothelial and smooth muscle cells. Blood 2003, 101, 1857–1863. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-W. Radiosensitization of Head & Neck Carcinoma Cells by Linifanib, A Receptor Tyrosine Kinase Inhibitor; Loma Linda University: Loma Linda, CA, USA, 2013. [Google Scholar]
- Peeters, C.F.; de Geus, L.-F.; Westphal, J.R.; de Waal, R.M.; Ruiter, D.J.; Wobbes, T.; Oyen, W.J.; Ruers, T.J. Decrease in circulating anti-angiogenic factors (angiostatin and endostatin) after surgical removal of primary colorectal carcinoma coincides with increased metabolic activity of liver metastases. Surgery 2005, 137, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, P. Thrombospondins function as regulators of angiogenesis. J. Cell Commun. Signal. 2009, 3, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Noh, Y.-H.; Matsuda, K.; Hong, Y.-K.; Kunstfeld, R.; Riccardi, L.; Koch, M.; Oura, H.; Dadras, S.S.; Streit, M.; Detmar, M. An N-terminal 80 kDa recombinant fragment of human thrombospondin-2 inhibits vascular endothelial growth factor induced endothelial cell migration in vitro and tumor growth and angiogenesis in vivo. J. Investig. Dermatol. 2003, 121, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Martin-Manso, G.; Pendrak, M.L.; Garfield, S.H.; Isenberg, J.S.; Roberts, D.D. Thrombospondin-1 inhibits VEGF receptor-2 signaling by disrupting its association with CD47. J. Biol. Chem. 2010, 285, 38923–38932. [Google Scholar] [CrossRef] [PubMed]
- Lawler, P.R.; Lawler, J. Molecular basis for the regulation of angiogenesis by thrombospondin-1 and-2. Cold Spring Harb. Perspect. Med. 2012, 2, a006627. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Huergo, S.P.; Blidner, A.G.; Rabinovich, G.A. Galectins: Emerging regulatory checkpoints linking tumor immunity and angiogenesis. Curr. Opin. Immunol. 2017, 45, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Etulain, J.; Negrotto, S.; Tribulatti, M.V.; Croci, D.O.; Carabelli, J.; Campetella, O.; Rabinovich, G.A.; Schattner, M. Control of angiogenesis by galectins involves the release of platelet-derived proangiogenic factors. PLoS ONE 2014, 9, e96402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kindt, N.; Journe, F.; Ghanem, G.E.; Saussez, S. Galectins and carcinogenesis: Their role in head and neck carcinomas and thyroid carcinomas. Int. J. Mol. Sci. 2017, 18, 2745. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Duckworth, C.; Fu, B.; Pritchard, D.M.; Rhodes, J.; Yu, L. Circulating galectins-2,-4 and-8 in cancer patients make important contributions to the increased circulation of several cytokines and chemokines that promote angiogenesis and metastasis. Br. J. Cancer 2014, 110, 741. [Google Scholar] [CrossRef] [PubMed]
- Varinska, L.; Kubatka, P.; Mojzis, J.; Zulli, A.; Gazdikova, K.; Zubor, P.; Büsselberg, D.; Caprnda, M.; Opatrilova, R.; Gasparova, I. Angiomodulators in cancer therapy: New perspectives. Biomed. Pharmacother. 2017, 89, 578–590. [Google Scholar] [CrossRef] [PubMed]
- Wdowiak, K.; Francuz, T.; Gallego-Colon, E.; Ruiz-Agamez, N.; Kubeczko, M.; Grochoła, I.; Wojnar, J. Galectin targeted therapy in oncology: Current knowledge and perspectives. Int. J. Mol. Sci. 2018, 19, 210. [Google Scholar] [CrossRef] [PubMed]
- Griffioen, A.W.; Thijssen, V.L. Galectins in tumor angiogenesis. Ann. Transl. Med. 2014, 2, 90. [Google Scholar] [PubMed]
- Lages, E.; Ipas, H.; Guttin, A.; Nesr, H.; Berger, F.; Issartel, J.-P. MicroRNAs: Molecular features and role in cancer. Front. Biosci. 2012, 17, 2508. [Google Scholar] [CrossRef]
- Cortés-Sempere, M.; de Cáceres, I.I. microRNAs as novel epigenetic biomarkers for human cancer. Clin. Transl. Oncol. 2011, 13, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Lou, W.; Liu, J.; Gao, Y.; Zhong, G.; Chen, D.; Shen, J.; Bao, C.; Xu, L.; Pan, J.; Cheng, J. MicroRNAs in cancer metastasis and angiogenesis. Oncotarget 2017, 8, 115787. [Google Scholar] [CrossRef] [PubMed]
- Van Beijnum, J.R.; Giovannetti, E.; Poel, D.; Nowak-Sliwinska, P.; Griffioen, A.W. miRNAs: Micro-managers of anticancer combination therapies. Angiogenesis 2017, 20, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lee, A.Y.W.; Wigg, J.P.; Peshavariya, H.; Liu, P.; Zhang, H. miR-126 regulation of angiogenesis in age-related macular degeneration in CNV mouse model. Int. J. Mol. Sci. 2016, 17, 895. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.-S.; Xu, M.; Song, J.-J.; Zhao, Z.-W.; Chen, M.-J.; Chen, W.-Q.; Tu, J.-F.; Yang, X.-M. Inhibition of microRNA-126 promotes the expression of Spred1 to inhibit angiogenesis in hepatocellular carcinoma after transcatheter arterial chemoembolization: In vivo study. Onco Targets Ther. 2016, 9, 4357. [Google Scholar] [PubMed]
- Beyer, S.; Fleming, J.; Meng, W.; Singh, R.; Haque, S.J.; Chakravarti, A. The Role of miRNAs in angiogenesis, invasion and metabolism and their therapeutic implications in gliomas. Cancers 2017, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Markus, R.; Chow, V.; Pan, Z.; Hanes, V. A phase I, randomized, single-dose study evaluating the pharmacokinetic equivalence of biosimilar ABP 215 and bevacizumab in healthy adult men. Cancer Chemother. Pharmacol. 2017, 80, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Hu-Lowe, D.D.; Zou, H.Y.; Grazzini, M.L.; Hallin, M.E.; Wickman, G.R.; Amundson, K.; Chen, J.H.; Rewolinski, D.A.; Yamazaki, S.; Wu, E.Y. Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin. Cancer Res. 2008, 14, 7272–7283. [Google Scholar] [CrossRef] [PubMed]
- McNamara, M.G.; Le, L.W.; Horgan, A.M.; Aspinall, A.; Burak, K.W.; Dhani, N.; Chen, E.; Sinaei, M.; Lo, G.; Kim, T.K. A Phase II trial of second-line axitinib following prior antiangiogenic therapy in advanced hepatocellular carcinoma. Cancer 2015, 121, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Sandler, A.; Gray, R.; Perry, M.C.; Brahmer, J.; Schiller, J.H.; Dowlati, A.; Lilenbaum, R.; Johnson, D.H. Paclitaxel–carboplatin alone or with bevacizumab for non–small-cell lung cancer. N. Engl. J. Med. 2006, 355, 2542–2550. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef] [PubMed]
- Sunwoo, J.B.; Chen, Z.; Dong, G.; Yeh, N.; Bancroft, C.C.; Sausville, E.; Adams, J.; Elliott, P.; Van Waes, C. Novel proteasome inhibitor PS-341 inhibits activation of nuclear factor-κB, cell survival, tumor growth, and angiogenesis in squamous cell carcinoma. Clin. Cancer Res. 2001, 7, 1419–1428. [Google Scholar] [PubMed]
- Roccaro, A.M.; Hideshima, T.; Raje, N.; Kumar, S.; Ishitsuka, K.; Yasui, H.; Shiraishi, N.; Ribatti, D.; Nico, B.; Vacca, A. Bortezomib mediates antiangiogenesis in multiple myeloma via direct and indirect effects on endothelial cells. Cancer Res. 2006, 66, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Corn, P.G.; Varkaris, A.; Li Ning Tapia, E.M.; Araujo, J.C.; Aparicio, A.; Tu, S.-M.; Zurita, A.J.; Efstathiou, E.; Qiao, W.; Wen, S. Modulation of soluble c-Met, bone turnover markers, angiogenic factors, and c-Met in men with mCRPC treated with cabozantinib. Am. Soc. Clin. Oncol. 2013, 31, 58. [Google Scholar] [CrossRef]
- Liu, J.; Barry, W.T.; Birrer, M.J.; Lee, J.-M.; Buckanovich, R.J.; Fleming, G.F.; Rimel, B.; Buss, M.K.; Nattam, S.R.; Hurteau, J. A randomized phase 2 trial comparing efficacy of the combination of the PARP inhibitor olaparib and the antiangiogenic cediranib against olaparib alone in recurrent platinum-sensitive ovarian cancer. Am. Soc. Clin. Oncol. 2014, 32, LBA5500. [Google Scholar] [CrossRef]
- Schoumacher, M.; Burbridge, M. Key roles of AXL and MER receptor tyrosine kinases in resistance to multiple anticancer therapies. Curr. Oncol. Rep. 2017, 19, 19. [Google Scholar] [CrossRef] [PubMed]
- Seront, E.; Rottey, S.; Sautois, B.; Kerger, J.; D’hondt, L.; Verschaeve, V.; Canon, J.-L.; Dopchie, C.; Vandenbulcke, J.; Whenham, N. Phase II study of everolimus in patients with locally advanced or metastatic transitional cell carcinoma of the urothelial tract: Clinical activity, molecular response, and biomarkers. Ann. Oncol. 2012, 23, 2663–2670. [Google Scholar] [CrossRef] [PubMed]
- Quintás-Cardama, A.; Kantarjian, H.M.; Manshouri, T.; Thomas, D.; Cortes, J.; Ravandi, F.; Garcia-Manero, G.; Ferrajoli, A.; Bueso-Ramos, C.; Verstovsek, S. Lenalidomide plus prednisone results in durable clinical, histopathologic, and molecular responses in patients with myelofibrosis. J. Clin. Oncol. 2009, 27, 4760–4766. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Cortes, J.; Verstovsek, S.; Mesa, R.A.; Thomas, D.; Lasho, T.L.; Hogan, W.J.; Litzow, M.R.; Allred, J.B.; Jones, D. Lenalidomide therapy in myelofibrosis with myeloid metaplasia. Blood 2006, 108, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Koyama, N.; Saito, K.; Nishioka, Y.; Yusa, W.; Yamamoto, N.; Yamada, Y.; Nokihara, H.; Koizumi, F.; Nishio, K.; Tamura, T. Pharmacodynamic change in plasma angiogenic proteins: A dose-escalation phase 1 study of the multi-kinase inhibitor lenvatinib. BMC Cancer 2014, 14, 530. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.-C.; DeBraud, F.; Bahleda, R.; Adamo, B.; Andre, F.; Dientsmann, R.; Delmonte, A.; Cereda, R.; Isaacson, J.; Litten, J. Phase I/IIa study evaluating the safety, efficacy, pharmacokinetics, and pharmacodynamics of lucitanib in advanced solid tumors. Ann. Oncol. 2014, 25, 2244–2251. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Barry, W.T.; Birrer, M.J.; Lee, J.-M.; Buckanovich, R.J.; Fleming, G.F.; Rimel, B.; Buss, M.K.; Nattam, S.R.; Hurteau, J. Overall survival and updated progression-free survival results from a randomized phase 2 trial comparing the combination of olaparib and cediranib against olaparib alone in recurrent platinum-sensitive ovarian cancer. Am. Soc. Clin. Oncol. 2017, 35, 5535. [Google Scholar]
- Tran, H.T.; Liu, Y.; Zurita, A.J.; Lin, Y.; Baker-Neblett, K.L.; Martin, A.-M.; Figlin, R.A.; Hutson, T.E.; Sternberg, C.N.; Amado, R.G. Prognostic or predictive plasma cytokines and angiogenic factors for patients treated with pazopanib for metastatic renal-cell cancer: A retrospective analysis of phase 2 and phase 3 trials. Lancet Oncol. 2012, 13, 827–837. [Google Scholar] [CrossRef]
- Palani, R.; Apperley, J.F.; Reid, A.; Foroni, L.; Deplano, S.; Milojkovic, D. Thyroid function abnormalities associated with ponatinib therapy in patients with chronic myeloid leukemia. Thyroid 2015, 25, 706–707. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.S.; Tomasek, J.; Yong, C.J.; Dumitru, F.; Passalacqua, R.; Goswami, C.; Safran, H.; dos Santos, L.V.; Aprile, G.; Ferry, D.R. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014, 383, 31–39. [Google Scholar] [CrossRef]
- Tamaskar, I.; Garcia, J.A.; Elson, P.; Wood, L.; Mekhail, T.; Dreicer, R.; Rini, B.I.; Bukowski, R.M. Antitumor effects of sunitinib or sorafenib in patients with metastatic renal cell carcinoma who received prior antiangiogenic therapy. J. Urol. 2008, 179, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, W.; Mesters, R.; Tinnefeld, H.; Loges, S.; Staib, P.; Dührsen, U.; Flasshove, M.; Ottmann, O.G.; Jung, W.; Cavalli, F. A phase 2 clinical study of SU5416 in patients with refractory acute myeloid leukemia. Blood 2003, 102, 2763–2767. [Google Scholar] [CrossRef] [PubMed]
- Giles, F.J.; Stopeck, A.T.; Silverman, L.R.; Lancet, J.E.; Cooper, M.A.; Hannah, A.L.; Cherrington, J.M.; O'Farrell, A.-M.; Yuen, H.A.; Louie, S.G. SU5416, a small molecule tyrosine kinase receptor inhibitor, has biologic activity in patients with refractory acute myeloid leukemia or myelodysplastic syndromes. Blood 2003, 102, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Moroney, J.; Fu, S.; Moulder, S.L.; Falchook, G.S.; Helgason, T.; Levenback, C.F.; Hong, D.S.; Naing, A.; Wheler, J.J.; Kurzrock, R. Phase I study of the anti-angiogenic antibody bevacizumab and the mTOR/hypoxia-inducible factor inhibitor temsirolimus combined with liposomal doxorubicin. Clin. Cancer Res. 2012. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.L.; Isakoff, S.J.; Klement, G.; Downing, S.R.; Chen, W.Y.; Hannagan, K.; Gelman, R.; Winer, E.P.; Burstein, H.J. Combination antiangiogenic therapy in advanced breast cancer: A phase 1 trial of vandetanib, a VEGFR inhibitor, and metronomic chemotherapy, with correlative platelet proteomics. Breast Cancer Res. Treat. 2012, 136, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Drevs, J.; Müller-Driver, R.; Wittig, C.; Fuxius, S.; Esser, N.; Hugenschmidt, H.; Konerding, M.A.; Allegrini, P.R.; Wood, J.; Hennig, J. PTK787/ZK 222584, a specific vascular endothelial growth factor-receptor tyrosine kinase inhibitor, affects the anatomy of the tumor vascular bed and the functional vascular properties as detected by dynamic enhanced magnetic resonance imaging. Cancer Res. 2002, 62, 4015–4022. [Google Scholar] [PubMed]
- Chen, H.; Modiano, M.; Neal, J.; Brahmer, J.; Rigas, J.; Jotte, R.; Leighl, N.; Riess, J.; Kuo, C.; Liu, L. A phase II multicentre study of ziv-aflibercept in combination with cisplatin and pemetrexed in patients with previously untreated advanced/metastatic non-squamous non-small cell lung cancer. Br. J. Cancer 2014, 110, 602–608. [Google Scholar] [CrossRef] [PubMed]

| No | Antiangiogenic Agent | Target/Targets | Cancer Type | References |
|---|---|---|---|---|
| 1 | ABP 215 | VEGF | Metastatic non-squamous NSCLC | [32,119] |
| 2 | Apatinib | VEGF and VEGFR-2 | Advanced or metastatic gastric cancer, advanced non-squamous non small cell lung cancer, colorectal cancer, metastatic esophageal cancer, advanced pancreatic cancer, advanced and metastatic breast cancer, metastatic renal cell carcinoma, and thyroid cancer Platinum-resistant or refractory ovarian cancer | [32,33] |
| 3 | Axitinib | VEGF-1, 2, and 3 | Renal cell carcinoma | [120,121] |
| 4 | Bevacizumab | VEGF | Metastatic colorectal cancer, non-squamous, non-small cell lung cancer and metastatic breast cancer | [34,122,123] |
| 5 | Bortezomib | NF-κB and VEGF | Multiple myeloma (MM) and mantle cell lymphoma | [124,125] |
| 6 | Cabozantinib | RET, MET, VEGFR-(1,2,and 3), KIT, TRKB, FMS-like tyrosine kinase-3(FLT3), AXL ROS1, TYRO3, and TIE-2 | Progressive, metastatic medullary thyroid cancer, Advanced renal cell carcinoma | [32,126] |
| 7 | Cediranib | VEGFR1, VEGFR2 PDGFR-β, and VEGFR-3 | Prostate, pancreatic, colon, breast, neck, renal cancers, ovarian and AML | [45,127] |
| 8 | Glesatinib | c-MET and AXL | Non-small cell lung cancer and head and neck squamous cell carcinoma | [128] |
| 9 | Emibetuzumab | FGF and HGF | Gastric cancer | [39] |
| 10 | Everolimus | mTOR | HER2-HR+ breast cancer Advanced renal cell carcinoma Pancreatic GI-NETNET Lung NET Subependymal giant cell astrocytoma | [32,129] |
| 11 | Lenalidomide | VEGF and Interleukin-6 | Multiple myeloma, primary myelofibrosis, and myeloid metastasis | [130,131] |
| 12 | Imatinib | VEGF, PDGF PDGF, SCF, c-kit, and BCR-ABL | Chronic myeloid leukemia (CML), gastrointestinal stromal tumor (GIST), and Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia | [123] |
| 13 | Lenvatinib | VEGFR-(1, 2, and 3), FGFR-(1, 2, 3, and 4), PDGFR-alpha, KIT, and RET | Differentiated thyroid cancer renal cell cancer | [32,132] |
| 14 | Lucitanib | VEGFR-(1, 2, 3) and FGFR-(1, 2) | Metastatic breast cancer | [32,133] |
| 15 | Olaparib | PARP and VEGFR | ovarian cancer | [134] |
| 16 | Pazopanib | VEGFR-1, -2, -3, PDGFR-alpha, PDGFR-beta, FGFR-1, -3, KIT, LTK, Lck, c-Fms | Advanced renal cell carcinoma Advanced soft tissue sarcoma | [32,135] |
| 17 | Ponatinib | ABL, VEGFR, PDGFR, FGFR, EPH receptors, SRC, KIT, RET, TIE2, FLT3 | Chronic myeloid leukemia Acute lymphoblastic leukemia | [32,136] |
| 18 | Ramucirumab | VEGFR-2 | Metastatic colorectal Metastatic NSCLC Advanced or metastatic gastric or gastroesophageal junction adenocarcinoma | [32,137] |
| 19 | Regorafenib | RET, VEGFR-1, -2, -3, KIT, PDGFR-alpha and beta, FGFR-1, -2, TIE2, DDR2, TrkA, Eph2A, RAF-1, BRAF and BRAFV600E, SAPK2, PTK5, Abl | Metastatic colorectal cancer locally advanced, unresectable, or metastatic GIST | [32,81] |
| 20 | Sorafenib | VEGFR-2 and -3, PDGFR-b, FLT3, and c-Kit VEGFR-1, -2, -3, PDGFR-beta, KIT, FLT3, RET, RET/PTC | Unresectable Hepatocellular carcinoma Advanced renal cell carcinoma Locally recurrent or metastatic, progressive, and differentiated thyroid carcinoma | [46] |
| 21 | Sunitinib | VEGFR-1, -2, -3, PDGFR-alpha and beta, KIT, FLT3, CSF-1R, RET | Advanced and metastatic renal cell carcinoma | [138] |
| 22 | SU5416 (Semaxinib) | VEGFR-(1 and 2), c-kit, and FLT3 | Advanced acute myeloid leukemia (AML) and myelodysplastic syndromes | [139,140] |
| 23 | Temsirolimus | mTOR | Advanced renal cell carcinoma | [141] |
| 24 | Thalidomide | TNF-α synthesis | AML myeloid metastasis | [68] |
| 25 | Vandetanib | VEGFR, EGFR, RET, BRK, TIE2, EPH receptor, SRC kinase | Symptomatic or progressive medullary thyroid cancer | [142] |
| 26 | Vatalanib | VEGFR and PDGFR tyrosine kinases | Breast, colorectal carcinoma, liver metastasis, AML, PMF, blast phase of chronic myelogenous leukemia, and myelodysplastic syndromes (MDS) 7374 | [50,143] |
| 27 | Ziv-aflibercept | VEGF-(A and B) and PIGF | Metastatic non-squamous non-small cell lung cancer | [144] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yehya, A.H.S.; Asif, M.; Petersen, S.H.; Subramaniam, A.V.; Kono, K.; Majid, A.M.S.A.; Oon, C.E. Angiogenesis: Managing the Culprits behind Tumorigenesis and Metastasis. Medicina 2018, 54, 8. https://doi.org/10.3390/medicina54010008
Yehya AHS, Asif M, Petersen SH, Subramaniam AV, Kono K, Majid AMSA, Oon CE. Angiogenesis: Managing the Culprits behind Tumorigenesis and Metastasis. Medicina. 2018; 54(1):8. https://doi.org/10.3390/medicina54010008
Chicago/Turabian StyleYehya, Ashwaq Hamid Salem, Muhammad Asif, Sven Hans Petersen, Ayappa V. Subramaniam, Koji Kono, Amin Malik Shah Abdul Majid, and Chern Ein Oon. 2018. "Angiogenesis: Managing the Culprits behind Tumorigenesis and Metastasis" Medicina 54, no. 1: 8. https://doi.org/10.3390/medicina54010008