CT-Navigated Spinal Instrumentations–Three-Dimensional Evaluation of Screw Placement Accuracy in Relation to a Screw Trajectory Plan
Abstract
:1. Introduction
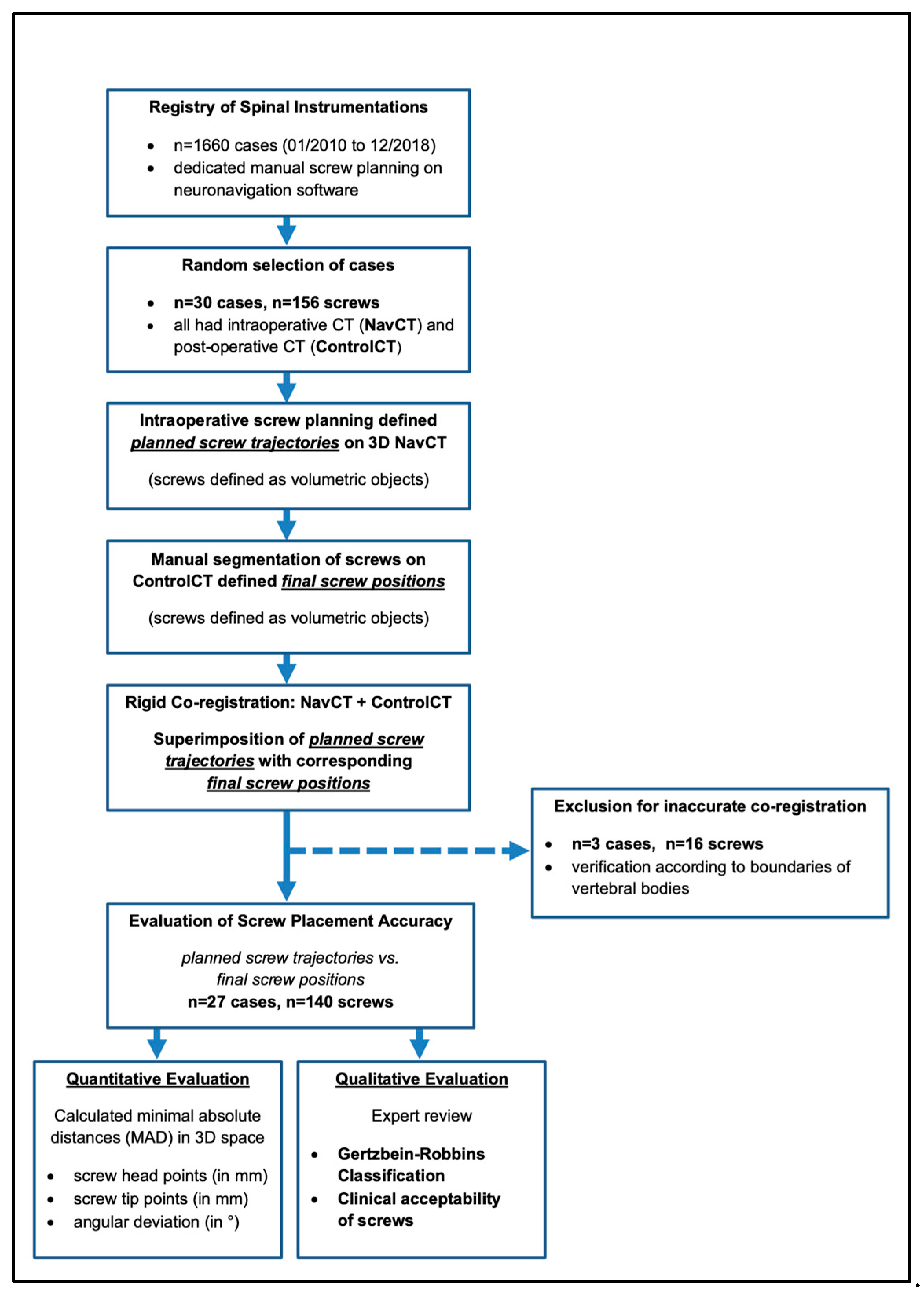
2. Materials and Methods
2.1. Patients and Study Design
2.2. Surgical Procedure for CT-Navigated Instrumentations
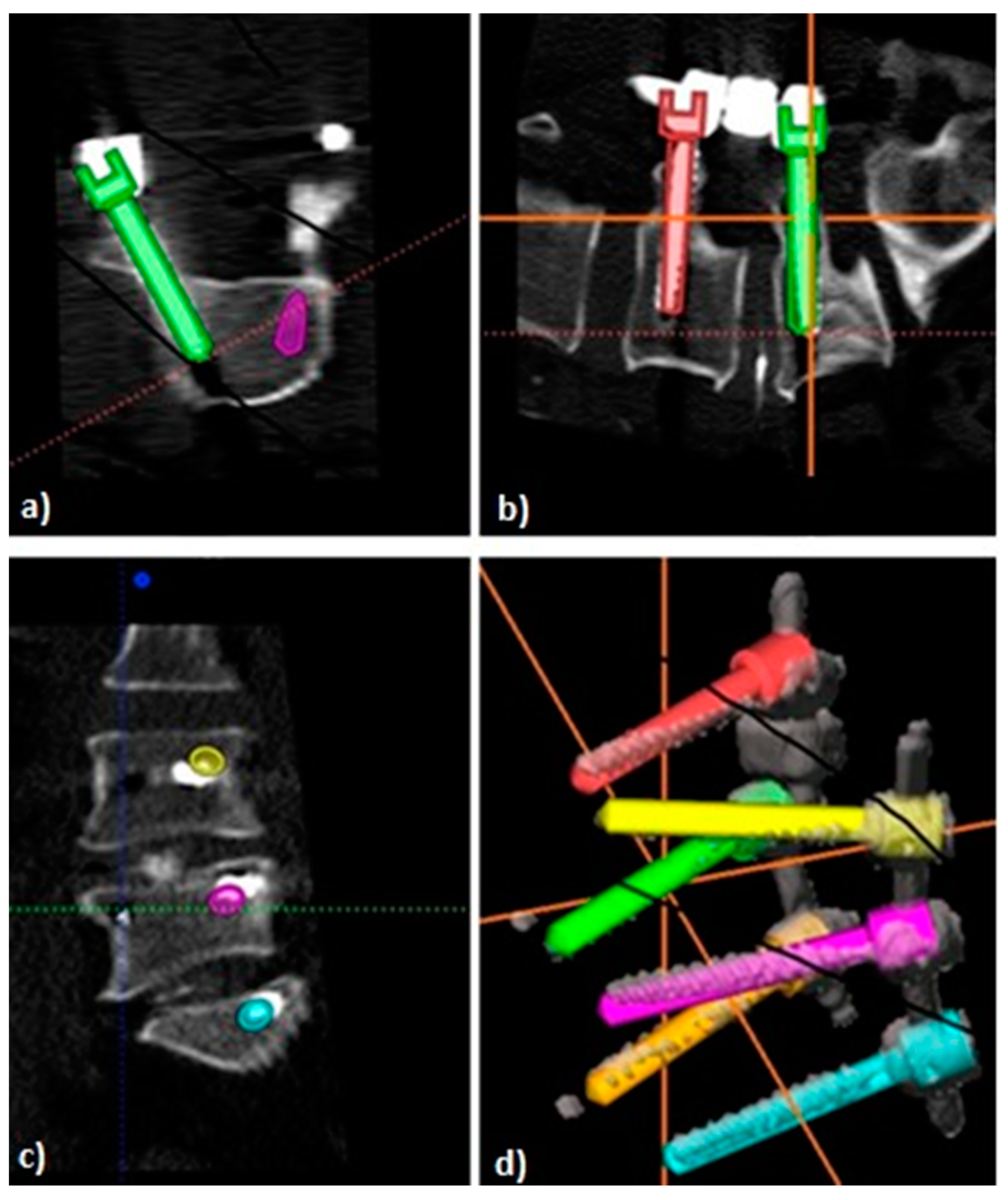
2.3. Postoperative Image Analysis
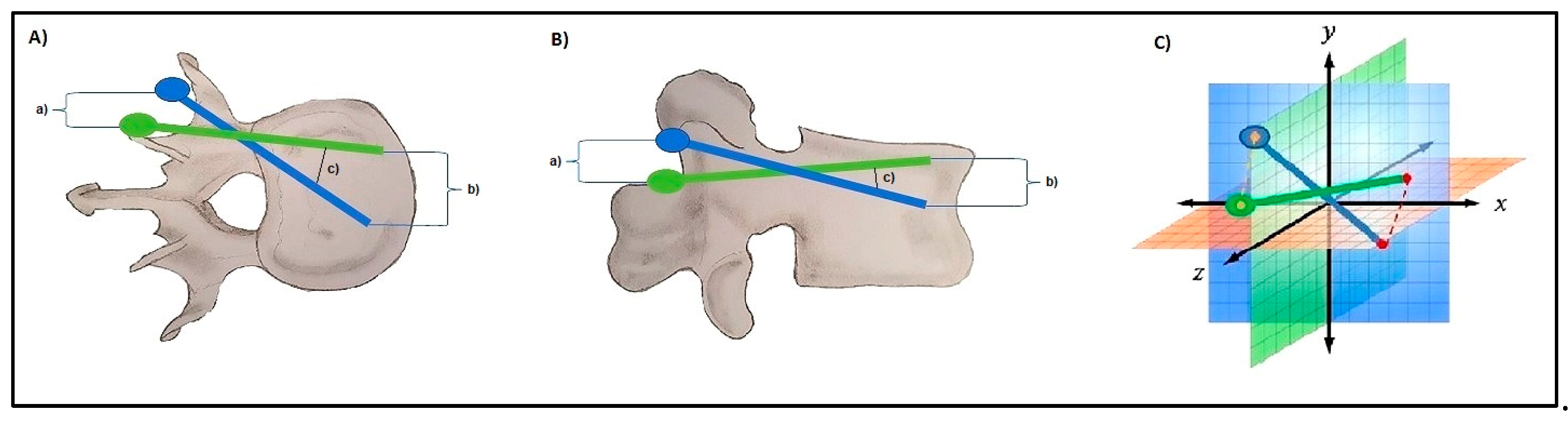
2.4. D-Quantitative Evaluation of Screw Positions
2.5. Qualitative Evaluation of Screw Positions Using Gertzbein–Robbins
2.6. Statistics
3. Results
3.1. Population
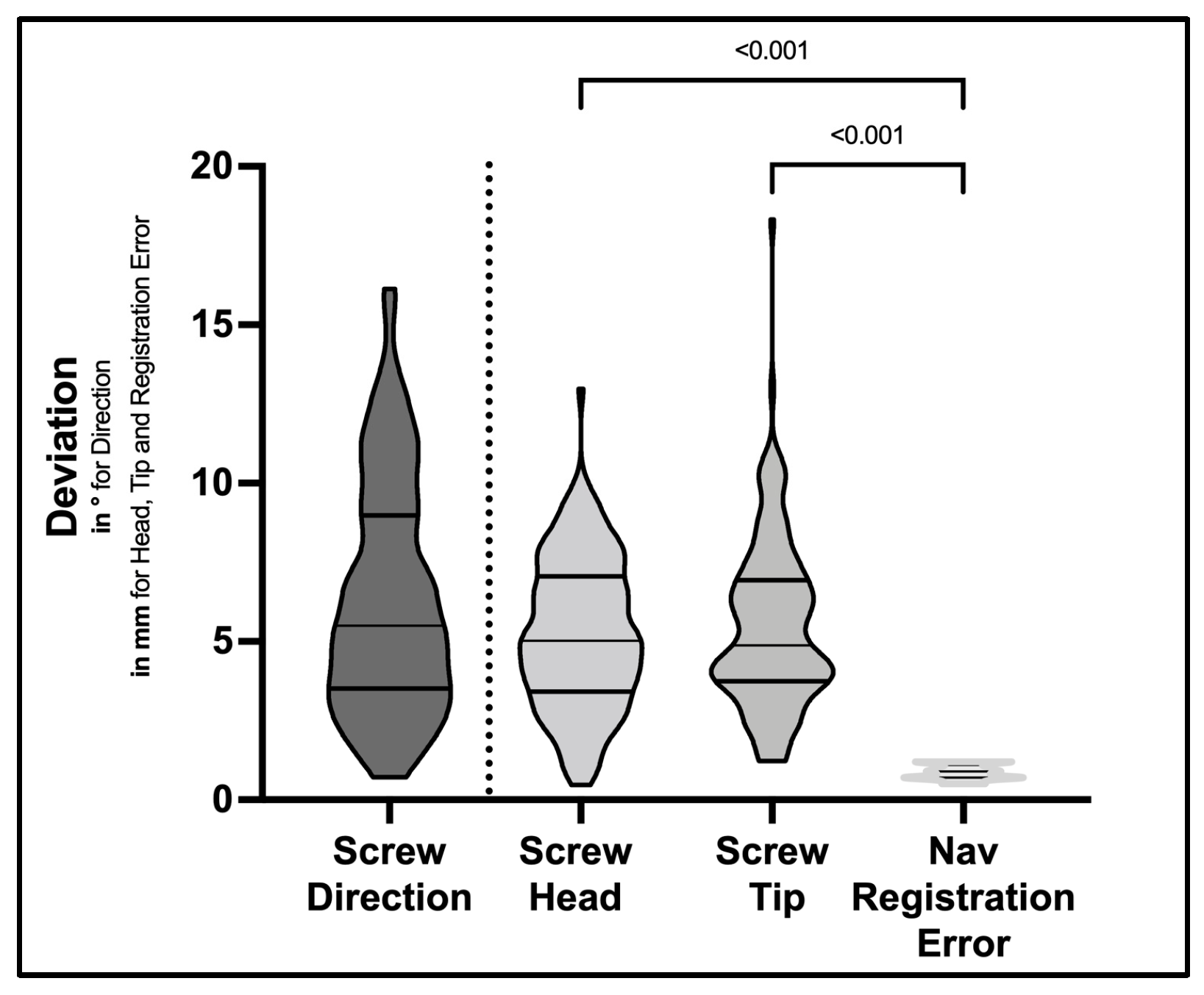
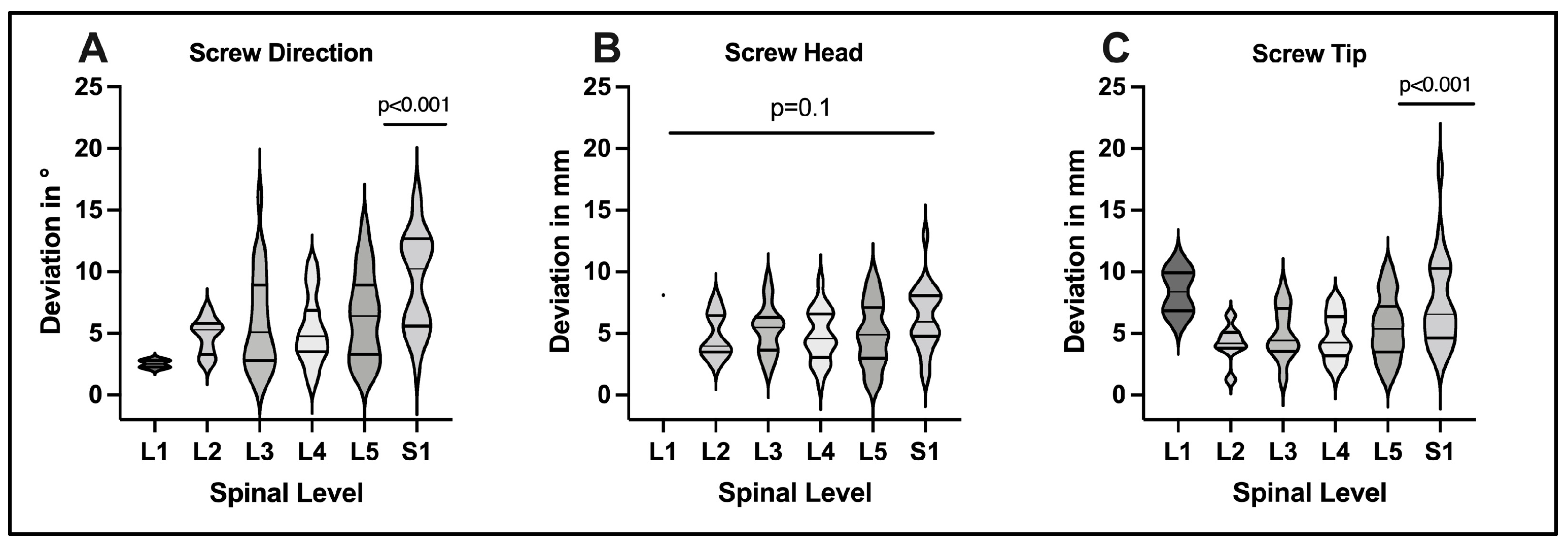
3.2. D-Quantitative Evaluation of Screw Positions
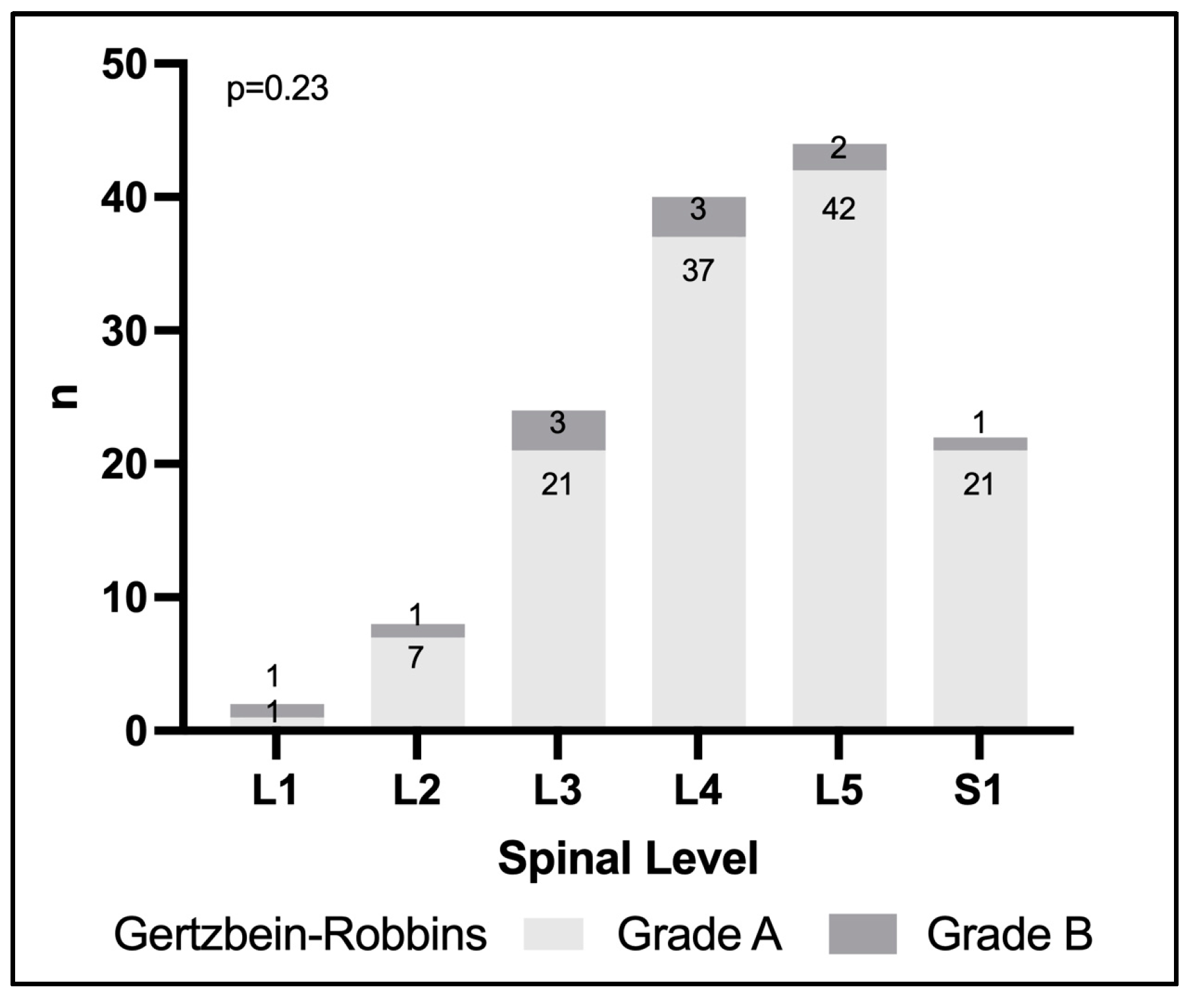
3.3. Evaluation of Screw Positions Using Gertzbein–Robbins
4. Discussion
4.1. Evaluation of Screw Accuracy
4.2. Technical Aspects of Screw Deviations
4.3. Clinical Importance of Screw Deviations
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Verma, R.; Krishan, S.; Haendlmayer, K.; Mohsen, A. Functional outcome of computer-assisted spinal pedicle screw placement: A systematic review and meta-analysis of 23 studies including 5992 pedicle screws. Eur. Spine J. 2010, 19, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.A.; Ledonio, C.G.; Hunt, M.A.; Siddiq, F.; Polly, D.W. Reliability of the planned pedicle screw trajectory versus the actual pedicle screw trajectory using intra-operative 3d ct and image guidance. Int. J. Spine Surg. 2016, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Attar, A.; Ugur, H.C.; Uz, A.; Tekdemir, I.; Egemen, N.; Genc, Y. Lumbar pedicle: Surgical anatomic evaluation and relationships. Eur. Spine J. 2001, 10, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Mason, A.; Paulsen, R.; Babuska, J.M.; Rajpal, S.; Burneikiene, S.; Nelson, E.L.; Villavicencio, A.T. The accuracy of pedicle screw placement using intraoperative image guidance systems: A systematic review. J. Neurosurg. Spine 2014, 20, 196–203. [Google Scholar] [CrossRef]
- Perna, F.; Borghi, R.; Pilla, F.; Stefanini, N.; Mazzotti, A.; Chehrassan, M. Pedicle screw insertion techniques: An update and review of the literature. Musculoskelet. Surg. 2016, 100, 165–169. [Google Scholar] [CrossRef]
- Ishak, B.; Younsi, A.; Wieckhusen, C.; Slonczewski, P.; Unterberg, A.W.; Kiening, K.L. Accuracy and revision rate of intraoperative computed tomography point-to-point navigation for lateral mass and pedicle screw placement: 11-year single-center experience in 1054 patients. Neurosurg. Rev. 2019, 42, 895–905. [Google Scholar] [CrossRef]
- Aoude, A.A.; Fortin, M.; Figueiredo, R.; Jarzem, P.; Ouellet, J.; Weber, M.H. Methods to determine pedicle screw placement accuracy in spine surgery: A systematic review. Eur. Spine J. 2015, 24, 990–1004. [Google Scholar] [CrossRef]
- Gertzbein, S.D.; Robbins, S.E. Accuracy of pedicular screw placement in vivo. Spine 1990, 15, 11–14. [Google Scholar] [CrossRef]
- Wiesner, L.; Kothe, R.; Rüther, W. Anatomic evaluation of two different techniques for the percutaneous insertion of pedicle screws in the lumbar spine. Spine 1999, 24, 1599–1603. [Google Scholar] [CrossRef]
- Rampersaud, Y.R.; Pik, J.H.T.; Salonen, D.; Farooq, S. Clinical accuracy of fluoroscopic computer-assisted pedicle screw fixation: A CT analysis. Spine 2005, 30, E183–E190. [Google Scholar] [CrossRef]
- Laudato, P.A.; Pierzchala, K.; Schizas, C. Pedicle Screw Insertion Accuracy Using O-Arm, Robotic Guidance, or Freehand Technique. Spine 2018, 43, E373–E378. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Pennington, Z.; Zhu, A.; Matsoukas, S.; Ahmed, A.K.; Ehresman, J.; Mahapatra, S.; Cottrill, E.; Sheppell, H.; Manbachi, A.; et al. Three-dimensional assessment of robot-assisted pedicle screw placement accuracy and instrumentation reliability based on a preplanned trajectory. J. Neurosurg. Spine 2020, 33, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Oertel, M.F.; Hobart, J.; Stein, M.; Schreiber, V.; Scharbrodt, W. Clinical and methodological precision of spinal navigation assisted by 3D intraoperative O-arm radiographic imaging. J. Neurosurg. Spine 2011, 14, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Shree Kumar, D.; Ampar, N.; Wee Lim, L. Accuracy and reliability of spinal navigation: An analysis of over 1000 pedicle screws. J. Orthop. 2020, 18, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, A.C.; Faulkner, A.R.; Bradley, Y.C.; Pasciak, A.S.; Barlow, P.; Gash, J.R.; Reid, W.S. Improved accuracy of minimally invasive transpedicular screw placement in the lumbar spine with 3-dimensional stereotactic image guidance: A comparative meta-analysis. J. Spinal Disord. Tech. 2015, 28, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, P.P.M.; Oh, J.Y.L.; Tan, M.; Nolan, C.P.; Yu, C.S.; Ling, J.M. Accuracy of Thoracolumbar Pedicle Screw Insertion Based on Routine Use of Intraoperative Imaging and Navigation. Asian Spine J. 2021, 15, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Van De Kelft, E.; Costa, F.; Van Der Planken, D.; Schils, F. A prospective multicenter registry on the accuracy of pedicle screw placement in the thoracic, lumbar, and sacral levels with the use of the O-arm imaging system and StealthStation Navigation. Spine 2012, 37, E1580–E1587. [Google Scholar] [CrossRef]
- Yang, B.P.; Wahl, M.M.; Idler, C.S. Percutaneous lumbar pedicle screw placement aided by computer-assisted fluoroscopy-based navigation: Perioperative results of a prospective, comparative, multicenter study. Spine 2012, 37, 2055–2060. [Google Scholar] [CrossRef]
- Kendlbacher, P.; Tkatschenko, D.; Czabanka, M.; Bayerl, S.; Bohner, G.; Woitzik, J.; Vajkoczy, P.; Hecht, N. Workflow and performance of intraoperative CT, cone-beam CT, and robotic cone-beam CT for spinal navigation in 503 consecutive patients. Neurosurg. Focus 2022, 52, E7. [Google Scholar] [CrossRef]
- Hecht, N.; Yassin, H.; Czabanka, M.; Föhre, B.; Arden, K.; Liebig, T.; Vajkoczy, P. Intraoperative Computed Tomography Versus 3D C-Arm Imaging for Navigated Spinal Instrumentation. Spine 2018, 43, 370–377. [Google Scholar] [CrossRef]
- Amato, V.; Giannachi, L.; Irace, C.; Corona, C. Accuracy of pedicle screw placement in the lumbosacral spine using conventional technique: Computed tomography postoperative assessment in 102 consecutive patients. J. Neurosurg. Spine 2010, 12, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.L.; McGirt, M.J.; Farber, S.H.; Amin, A.G.; Rick, A.-M.; Suk, I.; Bydon, A.; Sciubba, D.M.; Wolinsky, J.-P.; Gokaslan, Z.L.; et al. Accuracy of free-hand pedicle screws in the thoracic and lumbar spine: Analysis of 6816 consecutive screws. Neurosurgery 2011, 68, 170–178; discussion 178. [Google Scholar] [CrossRef]
- Crawford, B.D.; Nchako, C.M.; Rebehn, K.A.; Israel, H.; Place, H.M. Transpedicular Screw Placement Accuracy Using the O-Arm Versus Freehand Technique at a Single Institution. Glob. Spine J. 2022, 12, 447–451. [Google Scholar] [CrossRef]
- Ouchida, J.; Kanemura, T.; Satake, K.; Nakashima, H.; Segi, N.; Suzuki, K.; Imagama, S. True accuracy of percutaneous pedicle screw placement in thoracic and lumbar spinal fixation with a CT-based navigation system: Intraoperative and postoperative assessment of 763 percutaneous pedicle screws. J. Clin. Neurosci. 2020, 79, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Scheufler, K.-M.; Franke, J.; Eckardt, A.; Dohmen, H. Accuracy of image-guided pedicle screw placement using intraoperative computed tomography-based navigation with automated referencing. Part II: Thoracolumbar spine. Neurosurgery 2011, 69, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Waschke, A.; Walter, J.; Duenisch, P.; Reichart, R.; Kalff, R.; Ewald, C. CT-navigation versus fluoroscopy-guided placement of pedicle screws at the thoracolumbar spine: Single center experience of 4500 screws. Eur. Spine J. 2013, 22, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Cordemans, V.; Kaminski, L.; Banse, X.; Francq, B.G.; Detrembleur, C.; Cartiaux, O. Pedicle screw insertion accuracy in terms of breach and reposition using a new intraoperative cone beam computed tomography imaging technique and evaluation of the factors associated with these parameters of accuracy: A series of 695 screws. Eur. Spine J. 2017, 26, 2917–2926. [Google Scholar] [CrossRef] [PubMed]
- Tian, N.-F.; Huang, Q.-S.; Zhou, P.; Zhou, Y.; Wu, R.-K.; Lou, Y.; Xu, H.-Z. Pedicle screw insertion accuracy with different assisted methods: A systematic review and meta-analysis of comparative studies. Eur. Spine J. 2011, 20, 846–859. [Google Scholar] [CrossRef]
- Gelalis, I.D.; Paschos, N.K.; Pakos, E.E.; Politis, A.N.; Arnaoutoglou, C.M.; Karageorgos, A.C.; Ploumis, A.; Xenakis, T.A. Accuracy of pedicle screw placement: A systematic review of prospective in vivo studies comparing free hand, fluoroscopy guidance and navigation techniques. Eur. Spine J. 2012, 21, 247–255. [Google Scholar] [CrossRef]
- Liu, H.; Chen, W.; Liu, T.; Meng, B.; Yang, H. Accuracy of pedicle screw placement based on preoperative computed tomography versus intraoperative data set acquisition for spinal navigation system. J. Orthop. Surg. 2017, 25, 2309499017718901. [Google Scholar] [CrossRef] [Green Version]
- Du, J.P.; Wang, D.H.; Zhang, J.; Fan, Y.; Wu, Q.N.; Hao, D.J. Accuracy of Pedicle Screw Insertion Among 3 Image-Guided Navigation Systems: Systematic Review and Meta-Analysis. World Neurosurg. 2018, 109, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Perdomo-Pantoja, A.; Ishida, W.; Zygourakis, C.; Holmes, C.; Iyer, R.R.; Cottrill, E.; Theodore, N.; Witham, T.F.; Lo, S.-F.L. Accuracy of Current Techniques for Placement of Pedicle Screws in the Spine: A Comprehensive Systematic Review and Meta-Analysis of 51,161 Screws. World Neurosurg. 2019, 126, 664–678.e3. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Wang, W.; Chen, S.; Wu, K.; Wang, H. O-arm navigation versus C-arm guidance for pedicle screw placement in spine surgery: A systematic review and meta-analysis. Int. Orthop. 2020, 44, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Copay, A.G.; Subach, B.R.; Glassman, S.D.; Polly, D.W.; Schuler, T.C. Understanding the minimum clinically important difference: A review of concepts and methods. Spine J. 2007, 7, 541–546. [Google Scholar] [CrossRef]
- Jarvers, J.-S.; Schleifenbaum, S.; Pfeifle, C.; Oefner, C.; Edel, M.; von der Höh, N.; Heyde, C.-E. Comparison of three different screw trajectories in osteoporotic vertebrae: A biomechanical investigation. BMC Musculoskelet. Disord. 2021, 22, 418. [Google Scholar] [CrossRef]
- Widmer, J.; Fasser, M.-R.; Croci, E.; Spirig, J.; Snedeker, J.G.; Farshad, M. Individualized prediction of pedicle screw fixation strength with a finite element model. Comput. Methods Biomech. Biomed. Eng. 2020, 23, 155–167. [Google Scholar] [CrossRef]
- Scherer, M.; Kausch, L.; Ishak, B.; Norajitra, T.; Vollmuth, P.; Kiening, K.; Unterberg, A.; Maier-Hein, K.; Neumann, J.-O. Development and Validation of an Automated Planning Tool for Navigated Lumbosacral Pedicle Screws Using a Convolutional Neural Network. Spine J. 2022, 18, 1286–1291. [Google Scholar] [CrossRef]
| n (Cases) | 27 | |
| Age (years, median, IQR) | 69.0 (61.0–72.0) | |
| Weight (Kg, median, IQR) | 85.0 (76.0–95.0) | |
| Size (cm, median, IQR) | 170.0 (158.0–181.0) | |
| BMI (Kg/m2, median, IQR) | 29.5 (25.3–32.3) | |
| Diagnosis n (%) | Degenerative Spondylolisthesis°1 | 15 (55.5) |
| Degenerative Spondylolisthesis°2 | 1 (3.7) | |
| Spondylosis/Stenosis | 9 (33.3) | |
| Spondylodiscitis | 2 (7.4) | |
| ASA score n (%) | 2 | 15 (55.5) |
| 3 | 11 (40.7) | |
| NA | 1 (3.8) | |
| Nicotine abuse n (%) | no | 10 (37.0) |
| yes | 17 (63.0) | |
| Diabetes mellitus n (%) | no | 7 (25.9) |
| yes | 20 (74.1) | |
| Construct Length in Spinal Segments n (%) | 1 | 12 (44.4) |
| 2 | 10 (37.0) | |
| 3 | 3 (11.1) | |
| 4 | 2 (7.5) | |
| Screws Evaluated per Spinal Segment n (%) | L1 | 2 (1.4) |
| L2 | 8 (5.7) | |
| L3 | 24 (17.1) | |
| L4 | 40 (28.6) | |
| L5 | 44 (31.4) | |
| S1 | 22 (15.8) | |
| Gertzbein–Robbins Classification n (%) | A | 129 (92.1) |
| B | 11 (7.9) | |
| ≥C | 0 (0.0) |
| Grouped by Gertzbein–Robbins Classification | |||||
|---|---|---|---|---|---|
| Overall | A | B | p-Value | Test | |
| n | 140 | 129 | 11 | ||
| MAD Screw Direction (degrees, mean ± SD) | 6.3 ± 3.6 | 6.2 ± 3.5 | 7.5 ± 4.6 | 0.24 | t-Test |
| MAD Head Point (mm, mean ± SD) | 5.2 ± 2.4 | 5.1 ± 2.3 | 6.1 ± 3.0 | 0.20 | t-Test |
| MAD Tip Point (mm, mean ± SD) | 5.5 ± 2.7 | 5.4 ± 2.6 | 6.5 ± 2.8 | 0.19 | t-Test |
| Navigation registration error (mm, mean ± SD) | 0.87 ± 0.22 | 0.87 ± 0.23 | 0.89 ± 0.26 | 0.75 | t-Test |
| Age (Years, median, IQR) | 69.0 (61.0–72.0) | 69.0 (60.0–72.0) | 70.0 (69.0–78.0) | 0.056 | Kruskal–Wallis |
| BMI (Kg/m2, median, IQR) | 29.5 (25.3–32.3) | 29.4 (26.0–32.3) | 30.4 (25.0–31.5) | 0.914 | Kruskal–Wallis |
| Weight, (Kg, median, IQR) | 85.0 (76.0–95.0) | 83.0 (76.0–95.0) | 90.0 (77.0–95.0) | 0.052 | Kruskal–Wallis |
| Diagnosis (n, %) | |||||
| Degenerative Spondylolisthesis°1 | 70 (50.0) | 64 (49.6) | 6 (54.5) | 0.776 | X2-Test |
| Degenerative Spondylolisthesis°2 | 4 (2.9) | 4 (3.1) | |||
| Spondylosis/ Stenosis | 50 (35.7) | 47 (36.4) | 3 (27.3) | ||
| Spondylodiscitis | 16 (11.4) | 14 (10.9) | 2 (18.2) | ||
| (A) Original Articles | ||||||
|---|---|---|---|---|---|---|
| First Author (Year) | Imaging System | Navigation System | Number of Patients (n Screws) | Spine Region | Grading Method | Results |
| Scheufler (2011) | intraoperative CT | Brainlab | 55 (826) | T/L/S | Heary, Gertzbein–Robbins Python | 98.4% Heary I–III, 1.6% Heary IV 94.4% GR A, 4.6% GR B, 1% GR C |
| de Kelft (2012) | O-arm | Stealth Station | 353 (1922) | T/L/S | Qualitative: correct/misplaced | 95.7% of screws correctly placed |
| Waschke (2013) | intraoperative CT | Stealth Station | 505 (2422) | T/L/S | Modified Gertzbein–Robbins Python | 83.3% GR A, 11.2% GR B, 4.5% GR C, 1% GR D |
| Miller (2016) | O-arm | Stealth Station | 31 (240) | T/L/S | Quantitative: screw trajectory vs. midsagittal line and superior endplate | 2.17° ± 2.20° axial deviation 2.16° ± 2.24° sagittal deviation |
| Cordemans (2017) | ConeBeam CT | Brainlab | 118 (695) | T/L/S | Heary, Gertzbein–Robbins Python | Breach rate 11.7% GR, 15.4% Heary |
| Laudato (2018) | O-arm | O arm navigation unit | 25 (191) | T/L/S | Rampersaud criteria | 69.6% grade A, 4.2% Grade B |
| Hecht (2018) | intraoperative CT 3D C-Arm | Brainlab | 260 (1453) | C/T/L/S | Gertzbein–Robbins Python | 69% GR A, 25% GR B, 5% GR C, 1% GR D |
| Ishak (2019) | intraoperative CT | Stryker | (1384) | C/T/L/S | Gertzbein–Robbins Python | 92.6% GR A, 5.8% GR B, 1.4% GR C, 0.4% GR D, 0.7% GR E |
| Crawford (2020) | O-arm | O-arm navigation | 84 (454) | C/T/L/S | pedicle breach yes/no | pedicle breach in 55 (12.1%) |
| Kumar (2020) | O-arm | Stealth Station S7 | 219 (1152) | C/T/L/S | Gertzbein–Robbins Python | overall breach rate: 3.6% 2.29% GR B, 0.82% GR C, 96.4% accuracy of pedicle screw fixation |
| Ouchida (2020) | O-arm | Stealth Station S7 | 138 (763) | T/L/S | 3 points qualitative grading system | 9.7% of screws deviated at postop CT evaluation |
| Sundaram (2020) | O-arm | Stealth Station S7 | (2240) | T/L/S | Gertzbein–Robbins Python | accuracy 97.5%; overall breach rate 2.59%; major breach rate 0.94%, screw revision rate 0.7% |
| Kendlbacher (2022) | intraoperative CT, coneBeam CT, O-arm | Brainlab, Stealth Station S7 | 503 (2673) | C/T/L/S | Gertzbein–Robbins Python | 92.1% GR A + B, 7.9% GR C + D |
| (B) Meta-Analyses/Literature Reviews | ||||||
| First Author (Year) | Grading Method | Goal of the Article/Title | Central Finding | |||
| Tian (2011) | Pedicle screw violation (qualitative) | Pedicle screw insertion accuracy with different assisted methods: a systematic review and meta-analysis of comparative studies | pedicle breach less in CT navigated screws than in the conventional group (OR 95% CI 0.32–0.60) | |||
| Gelalis (2011) | Pedicle screw violation (qualitative, GR classification) | Accuracy of pedicle screw placement: a systematic review of prospective in vivo studies comparing free hand, fluoroscopy guidance and navigation techniques | violation in free hand technique range from 31 to 6%, vs. 11 to 0% with CT Navigation and 19 to 8% with fluoroscopy navigation | |||
| Liu (2017) | Qualitative | Accuracy of pedicle screw placement based on preoperative computed tomography versus intraoperative data set acquisition for spinal navigation system | higher accuracy in O-arm assisted screw insertion than in CT based navigation method (RR1.96; 95% CI 1.05–3.64; p = 0.03) | |||
| Du (2018) | 3 categories: (1) excellent (screw completely within pedicle), (2) acceptable (a portion of the screw outside the pedicle 1 mm), and (3) poor (a portion of the screw outside the pedicle > 1 mm) | Accuracy of pedicle screw insertion among 3 image-guided navigation systems: systematic review and meta-analysis | prevalence of pedicle violation lower in the 3D fluoroscopy navigation than the 2D fluoroscopy navigation and the CT navigation | |||
| Perdomo-Pantoja (2019) | Qualitative: cortical breach: minor (<4 mm) or major (4 mm) | Accuracy of current techniques for placement of pedicle screws in the spine: a comprehensive systematic review and meta-analysis of 51,161 screws | CT Navigation showed the highest pedicle screw placement accuracy (95.5%) | |||
| Feng (2020) | Qualitative | O-arm navigation versus C-arm guidance for pedicle screw placement in spine surgery: a systematic review and meta-analysis | accuracy with O-arm navigation higher than with C-arm fluoroscopy. | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gubian, A.; Kausch, L.; Neumann, J.-O.; Kiening, K.; Ishak, B.; Maier-Hein, K.; Unterberg, A.; Scherer, M. CT-Navigated Spinal Instrumentations–Three-Dimensional Evaluation of Screw Placement Accuracy in Relation to a Screw Trajectory Plan. Medicina 2022, 58, 1200. https://doi.org/10.3390/medicina58091200
Gubian A, Kausch L, Neumann J-O, Kiening K, Ishak B, Maier-Hein K, Unterberg A, Scherer M. CT-Navigated Spinal Instrumentations–Three-Dimensional Evaluation of Screw Placement Accuracy in Relation to a Screw Trajectory Plan. Medicina. 2022; 58(9):1200. https://doi.org/10.3390/medicina58091200
Chicago/Turabian StyleGubian, Arthur, Lisa Kausch, Jan-Oliver Neumann, Karl Kiening, Basem Ishak, Klaus Maier-Hein, Andreas Unterberg, and Moritz Scherer. 2022. "CT-Navigated Spinal Instrumentations–Three-Dimensional Evaluation of Screw Placement Accuracy in Relation to a Screw Trajectory Plan" Medicina 58, no. 9: 1200. https://doi.org/10.3390/medicina58091200






