Stromal Vascular Fraction Therapy for Knee Osteoarthritis: A Systematic Review
Abstract
:1. Introduction
- Addressing a Growing Global Health Issue: Knee OA is a widespread joint disease, increasingly prevalent due to aging populations and rising obesity rates. This research aims to tackle the growing burden of knee OA, which significantly affects the quality of life due to pain and reduced mobility.
- Symptom Management: Traditional treatments for knee OA, such as NSAIDs, corticosteroids, and joint replacement surgeries, often focus on symptom relief and come with various risks and limitations. In contrast, SVF therapy represents a paradigm shift towards addressing the underlying pathology of OA, offering the potential for tissue regeneration and disease modification, which could fundamentally alter the disease trajectory.
- Harnessing Regenerative Potential: The research into SVF therapy is at the forefront of regenerative medicine. SVF, derived from a patient’s own adipose tissue, contains a diverse mix of cells capable of exerting regenerative, immunomodulatory, and anti-inflammatory effects. This autologous nature minimizes the risk of immunogenic reactions, presenting a safer, personalized therapeutic option.
2. Materials and Methods
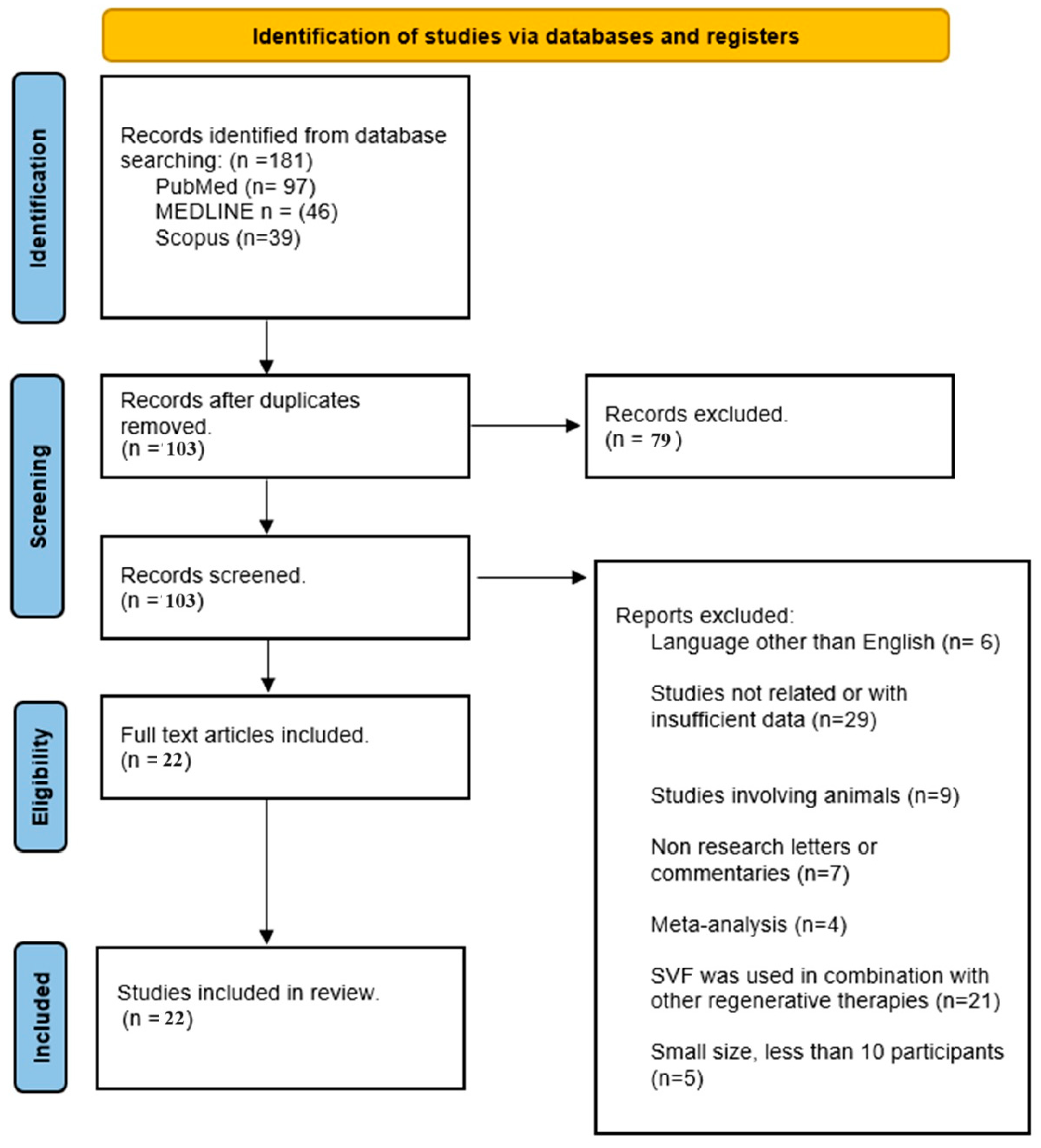
2.1. Search Strategy
2.1.1. Inclusion Criteria
2.1.2. Exclusion Criteria
2.2. Data Extraction and Quality Assessment
2.3. Quality Assessment and Risk of Bias Analysis
2.3.1. Search Strategy and Inclusion/Exclusion Criteria Strengths
2.3.2. Data Extraction and Quality Assessment Strengths
2.4. Ethical Considerations
3. Results
4. Discussion
4.1. Efficacy and Safety
4.2. Long-Term Efficacy
4.3. Adverse Events and Complications
4.4. Steps of SVF
4.5. Specificity of Response
4.6. Potential for Disease Modification
- Innovative Treatment Approach: SVF therapy represents a novel treatment strategy, moving beyond symptom management to potentially repairing and regenerating damaged knee tissues. This approach could revolutionize the way knee OA is treated, offering a more effective solution than current methods.
- Personalized Medicine: Since SVF therapy uses cells from the patient’s own body, it aligns with the principles of personalized medicine. This individualized approach may increase the treatment’s effectiveness and reduce the risk of adverse reactions compared to standard treatments.
- Long-Term Benefits: The regenerative potential of SVF therapy suggests that its benefits could be long-lasting, potentially slowing or even halting the progression of knee OA. This long-term improvement could reduce the overall healthcare burden associated with managing chronic knee conditions.
- Safety Profile: As an autologous treatment (using the patient’s own cells), SVF therapy is expected to have a favorable safety profile with minimal risk of immune reactions. This aspect is crucial in making the treatment a viable option for a broader range of patients.
4.7. Limitation of this Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Michael, J.W.; Schlüter-Brust, K.U.; Eysel, P. The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee. Dtsch Arztebl Int. 2010, 107, 152–162. [Google Scholar] [CrossRef]
- Kan, H.S.; Chan, P.K.; Chiu, K.Y.; Yan, C.H.; Yeung, S.S.; Ng, Y.L.; Shiu, K.W.; Ho, T. Non-surgical treatment of knee osteoarthritis. Hong Kong Med. J. 2019, 25, 127–133. [Google Scholar] [CrossRef]
- Skvortsov, D.; Kaurkin, S.; Prizov, A.; Altukhova, A.; Goncharov, E.; Nikitin, A. Gait analysis and knee joint kinematics before a and 6 months after of corrective valgus osteotomy at patients with medial knee arthritis. Int. Orthop. (SICOT) 2022, 46, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Krakowski, P.; Karpiński, R.; Jojczuk, M.; Nogalska, A.; Jonak, J. Knee MRI Underestimates the Grade of Cartilage Lesions. Appl. Sci. 2021, 11, 1552. [Google Scholar] [CrossRef]
- Carr, A.J.; Robertsson, O.; Graves, S.; Price, A.J.; Arden, N.K.; Judge, A.; Beard, D.J. Knee replacement. Lancet 2012, 379, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Agaverdiev, M.; Shamsov, B.; Mirzoev, S.; Vardikyan, A.; Ramirez, M.E.; Nurmukhametov, R.; Beilerli, A.; Zhang, B.; Gareev, I.; Pavlov, V. MiRNA regulated therapeutic potential of the stromal vascular fraction: Current clinical applications—A systematic review. Non-Coding RNA Res. 2023, 8, 146–154. [Google Scholar] [CrossRef]
- Yang, Y.; Lan, Z.; Yan, J.; Tang, Z.; Zhou, L.; Jin, D.; Jin, Q. Effect of intra-knee injection of autologous adipose stem cells or mesenchymal vascular components on short-term outcomes in patients with knee osteoarthritis: An updated meta-analysis of randomized controlled trials. Arthritis Res. Ther. 2023, 25, 147. [Google Scholar] [CrossRef]
- Reynoso, J.P.; De Jesus Encarnacion, M.; Nurmukhametov, R.; Melchenko, D.; Efe, I.E.; Goncharov, E.; Taveras, A.A.; Pena, I.J.R.; Montemurro, N. Anatomical Variations of the Sciatic Nerve Exit from the Pelvis and Its Relationship with the Piriformis Muscle: A Cadaveric Study. Neurol Int. 2022, 14, 894–902. [Google Scholar] [CrossRef]
- Rodriguez-Merchan, E.C. Autologous and Allogenic Utilization of Stromal Vascular Fraction and Decellularized Extracellular Matrices in Orthopedic Surgery: A Scoping Review. Arch. Bone Jt. Surg. 2022, 10, 827–832. [Google Scholar]
- Russo, A.; Condello, V.; Madonna, V.; Guerriero, M.; Zorzi, C. Autologous and micro-fragmented adipose tissue for the treatment of diffuse degenerative knee osteoarthritis. J. Exp. Orthop. 2017, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Labarre, K.W.; Zimmermann, G. Infiltration of the Hoffa’s fat pad with stromal vascular fraction in patients with osteoarthritis of the knee -Results after one year of follow-up. Bone Rep. 2022, 16, 101168. [Google Scholar] [CrossRef] [PubMed]
- Lapuente, J.P.; Dos-Anjos, S.; Blázquez-Martínez, A. Intra-articular infiltration of adipose-derived stromal vascular fraction cells slows the clinical progression of moderate-severe knee osteoarthritis: Hypothesis on the regulatory role of intra-articular adipose tissue. J. Orthop. Surg. Res. 2020, 15, 137. [Google Scholar] [CrossRef] [PubMed]
- Garza, J.R.; Campbell, R.E.; Tjoumakaris, F.P.; Freedman, K.B.; Miller, L.S.; Maria, D.S.; Tucker, B.S. Clinical Efficacy of Intra-articular Mesenchymal Stromal Cells for the Treatment of Knee Osteoarthritis: A Double-Blinded Prospective Randomized Controlled Clinical Trial. Am. J. Sports Med. 2020, 48, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Yokota, N.; Hattori, M.; Ohtsuru, T.; Otsuji, M.; Lyman, S.; Shimomura, K.; Nakamura, N. Comparative Clinical Outcomes After Intra-articular Injection With Adipose-Derived Cultured Stem Cells or Noncultured Stromal Vascular Fraction for the Treatment of Knee Osteoarthritis. Am. J. Sports Med. 2019, 47, 2577–2583. [Google Scholar] [CrossRef] [PubMed]
- Rothrauff, B.B.; Sasaki, H.; Kihara, S.; Overholt, K.J.; Gottardi, R.; Lin, H.; Fu, F.H.; Tuan, R.S.; Alexander, P.G. Point-of-Care Procedure for Enhancement of Meniscal Healing in a Goat Model Utilizing Infrapatellar Fat Pad-Derived Stromal Vascular Fraction Cells Seeded in Photocrosslinkable Hydrogel. Am. J. Sports Med. 2019, 47, 3396–3405. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.G.; Choi, Y.J.; Kwon, S.K.; Kim, Y.S.; Yeo, J.E. Clinical results and second-look arthroscopic findings after treatment with adipose-derived stem cells for knee osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2015, 23, 1308–1316. [Google Scholar] [CrossRef]
- Nguyen, P.D.; Tran, T.D.; Nguyen, H.T.; Vu, H.T.; Le, P.T.; Phan, N.L.-C.; Vu, N.B.; Phan, N.K.; Van Pham, P. Comparative Clinical Observation of Arthroscopic Microfracture in the Presence and Absence of a Stromal Vascular Fraction Injection for Osteoarthritis. Stem Cells Transl. Med. 2017, 6, 187–195. [Google Scholar] [CrossRef]
- Muñoz-Criado, I.; Meseguer-Ripolles, J.; Mellado-López, M.; Alastrue-Agudo, A.; Griffeth, R.J.; Forteza-Vila, J.; Cugat, R.; García, M.; Moreno-Manzano, V. Human Suprapatellar Fat Pad-Derived Mesenchymal Stem Cells Induce Chondrogenesis and Cartilage Repair in a Model of Severe Osteoarthritis. Stem Cells Int. 2017, 2017, 4758930. [Google Scholar] [CrossRef]
- Kim, Y.S.; Suh, D.S.; Tak, D.H.; Kwon, Y.B.; Koh, Y.G. Adipose-Derived Stromal Vascular Fractions Are Comparable With Allogenic Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells as a Supplementary Strategy of High Tibial Osteotomy for Varus Knee Osteoarthritis. Arthrosc. Sports Med. Rehabil. 2023, 5, e751–e764. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Oh, S.M.; Suh, D.S.; Tak, D.H.; Kwon, Y.B.; Koh, Y.G. Arthroscopic Implantation of Adipose-Derived Stromal Vascular Fraction Improves Cartilage Regeneration and Pain Relief in Patients With Knee Osteoarthritis. Arthrosc. Sports Med. Rehabil. 2023, 5, e707–e716. [Google Scholar] [CrossRef] [PubMed]
- Boada-Pladellorens, A.; Avellanet, M.; Pages-Bolibar, E.; Veiga, A. Stromal vascular fraction therapy for knee osteoarthritis: A systematic review. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720X221117879. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Oh, S.M.; Suh, D.S.; Tak, D.H.; Kwon, Y.B.; Koh, Y.G. Cartilage lesion size and number of stromal vascular fraction (SVF) cells strongly influenced the SVF implantation outcomes in patients with knee osteoarthritis. J. Exp. Orthop. 2023, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Yokota, N.; Lyman, S.; Hanai, H.; Shimomura, K.; Ando, W.; Nakamura, N. Clinical Safety and Effectiveness of Adipose-Derived Stromal Cell vs Stromal Vascular Fraction Injection for Treatment of Knee Osteoarthritis: 2-Year Results of Parallel Single-Arm Trials. Am. J. Sports Med. 2022, 50, 2659–2668. [Google Scholar] [CrossRef]
- Aletto, C.; Giordano, L.; Quaranta, M.; Zara, A.; Notarfrancesco, D.; Maffulli, N. Short-term results of intra-articular injections of stromal vascular fraction for early knee osteoarthritis. J. Orthop. Surg. Res. 2022, 17, 310. [Google Scholar] [CrossRef]
- Santoprete, S.; Marchetti, F.; Rubino, C.; Bedini, M.G.; Nasto, L.A.; Cipolloni, V.; Pola, E. Fresh autologous stromal tissue fraction for the treatment of knee osteoarthritis related pain and disability. Orthop Rev. 2021, 13, 9161. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, H.; He, B.; Fan, M.; Xiao, M.; Zhang, J.; Chen, D.; Tong, P.; Mao, Q. Mid-term prognosis of the stromal vascular fraction for knee osteoarthritis: A minimum 5-year follow-up study. Stem Cell Res. Ther. 2022, 13, 105. [Google Scholar] [CrossRef]
- Simunec, D.; Salari, H.; Meyer, J. Treatment of Grade 3 and 4 Osteoarthritis with Intraoperatively Separated Adipose Tissue-Derived Stromal Vascular Fraction: A Comparative Case Series. Cells 2020, 9, 2096. [Google Scholar] [CrossRef]
- Şahin, A.A.; Değirmenci, E.; Özturan, K.E.; Fırat, T.; Kükner, A. Effects of adipose tissue-derived stromal vascular fraction on osteochondral defects treated by hyaluronic acid-based scaffold: An experimental study. Jt. Dis. Relat. Surg. 2021, 32, 347–354. [Google Scholar] [CrossRef]
- Mehling, B.; Hric, M.; Salatkova, A.; Vetrak, R.; Santora, D.; Ovariova, M.; Mihalyova, R.; Manvelyan, M. A Retrospective Study of Stromal Vascular Fraction Cell Therapy for Osteoarthritis. J. Clin. Med. Res. 2020, 12, 747–751. [Google Scholar] [CrossRef]
- Hudetz, D.; Borić, I.; Rod, E.; Jeleč, Ž.; Kunovac, B.; Polašek, O.; Vrdoljak, T.; Plečko, M.; Skelin, A.; Polančec, D.; et al. Early results of intra-articular micro-fragmented lipoaspirate treatment in patients with late stages knee osteoarthritis: A prospective study. Croat. Med. J. 2019, 60, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Chen, J.; Zhang, S.; Zhao, C.; Bi, M.; Chen, X.; Bi, Q. Intra-articular injection of autologous adipose-derived stromal vascular fractions for knee osteoarthritis: A double-blind randomized self-controlled trial. Int. Orthop. 2019, 43, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.D.X.; Wu, C.M.; Dubey, N.K.; Deng, Y.H.; Su, C.W.; Pham, T.T.; Le, P.B.T.; Sestili, P.; Deng, W.-P. Time- and Kellgren⁻Lawrence Grade-Dependent Changes in Intra-Articularly Transplanted Stromal Vascular Fraction in Osteoarthritic Patients. Cells 2019, 8, 308. [Google Scholar] [CrossRef]
- Bora, P.; Majumdar, A.S. Adipose tissue-derived stromal vascular fraction in regenerative medicine: A brief review on biology and translation. Stem Cell Res. Ther. 2017, 8, 145. [Google Scholar] [CrossRef] [PubMed]
- Baer, P.C.; Geiger, H. Adipose-derived mesenchymal stromal/stem cells: Tissue localization, characterization, and heterogeneity. Stem Cells Int. 2012, 2012, 812693. [Google Scholar] [CrossRef]
- Francis, S.L.; Duchi, S.; Onofrillo, C.; Di Bella, C.; Choong, P.F.M. Adipose-Derived Mesenchymal Stem Cells in the Use of Cartilage Tissue Engineering: The Need for a Rapid Isolation Procedure. Stem Cells Int. 2018, 2018, 8947548. [Google Scholar] [CrossRef]
- Busato, A.; De Francesco, F.; Biswas, R.; Mannucci, S.; Conti, G.; Fracasso, G.; Conti, A.; Riccio, V.; Riccio, M.; Sbarbati, A. Simple and Rapid Non-Enzymatic Procedure Allows the Isolation of Structurally Preserved Connective Tissue Micro-Fragments Enriched with SVF. Cells 2020, 10, 36. [Google Scholar] [CrossRef]
- Guimarães-Camboa, N.; Cattaneo, P.; Sun, Y.; Moore-Morris, T.; Gu, Y.; Dalton, N.D.; Rockenstein, E.; Masliah, E.; Peterson, K.L.; Stallcup, W.B.; et al. Pericytes of Multiple Organs Do Not Behave as Mesenchymal Stem Cells In Vivo. Cell Stem Cell 2017, 20, 345–359. [Google Scholar] [CrossRef]
- Matsuo, F.S.; Cavalcanti de Araújo, P.H.; Mota, R.F.; Carvalho, A.J.R.; de Queiroz, M.S.; de Almeida, B.B.; Ferreira, K.C.; Metzner, R.J.M.; Ferrari, G.D.; Alberici, L.C.; et al. RANKL induces beige adipocyte differentiation in preadipocytes. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E866–E877. [Google Scholar] [CrossRef]
- Contreras, G.A.; Kabara, E.; Brester, J.; Neuder, L.; Kiupel, M. Macrophage infiltration in the omental and subcutaneous adipose tissues of dairy cows with displaced abomasum. J. Dairy Sci. 2015, 98, 6176–6187. [Google Scholar] [CrossRef]
- Dey, A.; Ni, Z.; Johnson, M.S.; Sedger, L.M. A multi-colour confocal microscopy method for identifying and enumerating macrophage subtypes and adherent cells in the stromal vascular fraction of human adipose. J. Immunol. Methods 2021, 491, 112988. [Google Scholar] [CrossRef] [PubMed]
- Dulong, J.; Loisel, S.; Rossille, D.; Léonard, S.; Bescher, N.; Bezier, I.; Latour, M.; Monvoisin, C.; Monnier, D.; Bertheuil, N.; et al. CD40L-expressing CD4+ T cells prime adipose-derived stromal cells to produce inflammatory chemokines. Cytotherapy 2022, 24, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Gulyaeva, O.; Dempersmier, J.; Sul, H.S. Genetic and epigenetic control of adipose development. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Mark, B.; Elliot, L.; Thomas, G.; Walter, O.; Jonathan, B.; Shawntae, D.; Sean, B. Prospective Study of Autologous Adipose Derived Stromal Vascular Fraction Containing Stem Cells for the Treatment of Knee Osteoarthritis. Int. J. Stem. Cell Res. Ther. 2019, 6, 064. [Google Scholar] [CrossRef]
- Pers, Y.M.; Quentin, J.; Ferreira, R.; Espinoza, F.; Abdellaoui, N.; Erkilic, N.; Green, M.; Dufourcq-Lopez, E.; Pullig, O.; Noth, U.; et al. Injection of Adipose-Derived Stromal Cells in the Knee of Patients with Severe Osteoarthritis has a Systemic Effect and Promotes an Anti-Inflammatory Phenotype of Circulating Immune Cells. Theranostics 2018, 8, 5519–5528. [Google Scholar] [CrossRef]
- Cho, H.; Kim, H.; Kim, Y.g.; Kim, K. Recent Clinical Trials in Adipose-derived Stem Cell Mediated Osteoarthritis Treatment. Biotechnol. Bioprocess Eng. 2019, 24, 839–853. [Google Scholar]
- Vargel, I.; Tuncel, A.; Baysal, N.; Hartuç-Çevik, I.; Korkusuz, F. Autologous Adipose-Derived Tissue Stromal Vascular Fraction (AD-tSVF) for Knee Osteoarthritis. Int. J. Mol. Sci. 2022, 23, 13517. [Google Scholar] [CrossRef]
- Pak, J.; Lee, J.H.; Pak, N.J.; Park, K.S.; Jeon, J.H.; Jeong, B.C.; Lee, S.H. Clinical Protocol of Producing Adipose Tissue-Derived Stromal Vascular Fraction for Potential Cartilage Regeneration. J. Vis. Exp. 2018, 139, e58363. [Google Scholar]
- Christian Lattermann, H.M. Norimasa Nakamura, Elizaveta Kon: Early Osteoarthritis State-of-the-Art Approaches to Diagnosis, Treatment and Controversies; Springer: Heidelberg, Germany, 2022. [Google Scholar]
- Maioli, M.; Rinaldi, S.; Santaniello, S.; Castagna, A.; Pigliaru, G.; Delitala, A.; Bianchi, F.; Tremolada, C.; Fontani, V.; Ventura, C. Radioelectric asymmetric conveyed fields and human adipose-derived stem cells obtained with a nonenzymatic method and device: A novel approach to multipotency. Cell Transpl. 2014, 23, 1489–1500. [Google Scholar] [CrossRef]
- Montemurro, N.; Pierozzi, E.; Inchingolo, A.M.; Pahwa, B.; De Carlo, A.; Palermo, A.; Scarola, R.; Dipalma, G.; Corsalini, M.; Inchingolo, A.D.; et al. New biograft solution, growth factors and bone regenerative approaches in neurosurgery, dentistry, and orthopedics: A review. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 7653–7664. [Google Scholar]
- Perdisa, F.; Gostynska, N.; Roffi, A.; Filardo, G.; Marcacci, M.; Kon, E. Adipose-Derived Mesenchymal Stem Cells for the Treatment of Articular Cartilage: A Systematic Review on Preclinical and Clinical Evidence. Stem Cells Int. 2015, 2015, 597652. [Google Scholar] [CrossRef]
- Aronowitz, J.A.; Ellenhorn, J.D.I. Adipose stromal vascular fraction isolation: A head-to-head comparison of four commercial cell separation systems. Plast. Reconstr. Surg. 2013, 132, 932e–939e. [Google Scholar] [CrossRef] [PubMed]
- Packer, J.D.; Chang, W.T.; Dragoo, J.L. The use of vibrational energy to isolate adipose-derived stem cells. Plast. Reconstr. Surg. Glob. Open 2018, 6, e1620. [Google Scholar] [CrossRef] [PubMed]
- Dragoo, J.L.; Chang, W. Arthroscopic Harvest of Adipose-Derived Mesenchymal Stem Cells from the Infrapatellar Fat Pad. Am. J. Sport. Med. 2017, 45, 3119–3127. [Google Scholar] [CrossRef] [PubMed]
- Aronowitz, J.A.; Lockhart, R.A.; Hakakian, C.S.; Birnbaum, Z.E. Adipose stromal vascular fraction isolation: A head-to-head comparison of 4 cell separation systems# 2. Ann. Plast. Surg. 2016, 77, 354–362. [Google Scholar] [PubMed]
- Domenis, R.; Lazzaro, L.; Calabrese, S.; Mangoni, D.; Gallelli, A.; Bourkoula, E.; Manini, I.; Bergamin, N.; Toffoletto, B.; Beltrami, C.A. Adipose tissue derived stem cells: In vitro and in vivo analysis of a standard and three commercially available cell-assisted lipotransfer techniques. Stem Cell Res. Ther. 2015, 6, 2. [Google Scholar] [CrossRef]
- Fang, C.; Patel, P.; Li, H.; Huang, L.T.; Wan, H.; Collins, S.; Connell, T.L.; Xu, H. Physical, biochemical, and biologic properties of fat graft processed via different methods. Plast. Reconstr. Surg. Glob. Open 2020, 8, e3010. [Google Scholar] [CrossRef]
- Tremolada, C.; Colombo, V.; Ventura, C. Adipose tissue and mesenchymal stem cells: State of the art and Lipogems® technology development. Curr. Stem Cell Rep. 2016, 2, 304–312. [Google Scholar] [CrossRef]
- Magnanelli, S.; Screpis, D.; Di Benedetto, P.; Natali, S.; Causero, A.; Zorzi, C. Open-wedge high tibial osteotomy associated with lipogems® intra-articular injection for the treatment of varus knee osteoarthritis–retrospective study. Acta Bio Med. Atenei Parm. 2020, 91, e2020022. [Google Scholar]
- Kavala, A.A.; Turkyilmaz, S. Autogenously derived regenerative cell therapy for venous leg ulcers. Arch. Med. Sci. Atheroscler. Dis. 2018, 3, e156–e163. [Google Scholar] [CrossRef] [PubMed]
- Lobascio, P.; Balducci, G.; Minafra, M.; Laforgia, R.; Fedele, S.; Conticchio, M.; Palasciano, N. Adipose-derived stem cells (MYSTEM® EVO Technology) as a treatment for complex transsphincteric anal fistula. Tech. Coloproctol. 2018, 22, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Stevens, H.P.; van Boxtel, J.; van Dijck, R.; van Dongen, J.A. Platelet Rich STROMA, the Combination of PRP and tSVF and Its Potential Effect on Osteoarthritis of the Knee. Appl. Sci. 2020, 10, 4691. [Google Scholar] [CrossRef]
- Copcu, H.E. Supercharged Mechanical Stromal-cell Transfer (MEST). Plast Reconstr. Surg. Glob. Open 2021, 9, e3552. [Google Scholar]
- Zocchi, M.L.; Facchin, F.; Pagani, A.; Bonino, C.; Sbarbati, A.; Conti, G.; Vindigni, V.; Bassetto, F. New perspectives in regenerative medicine and surgery: The bioactive composite therapies (BACTs). Eur. J. Plast. Surg. 2022, 45, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Roda, B.; Zia, S.; Vigliotta, I.; Zannini, C.; Alviano, F.; Bonsi, L.; Zattoni, A.; Reschiglian, P.; Gennai, A. Characterization of the Tissue and Stromal Cell Components of Micro-Superficial Enhanced Fluid Fat Injection (Micro-SEFFI) for Facial Aging Treatment. Aesthet Surg. J. 2020, 40, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.R.; Tiryaki, T.; Womack, H.A.; Canikyan, S.; Schlaudraff, K.U.; Scheflan, M. Cellular Optimization of Nanofat: Comparison of Two Nanofat Processing Devices in Terms of Cell Count and Viability. Aesthet Surg. J. Open Forum 2019, 1, ojz028. [Google Scholar] [CrossRef]
- Tiryaki, K.T.; Cohen, S.; Kocak, P.; Canikyan Turkay, S.; Hewett, S. In-Vitro Comparative Examination of the Effect of Stromal Vascular Fraction Isolated by Mechanical and Enzymatic Methods on Wound Healing. Aesthet Surg. J. 2020, 40, 1232–1240. [Google Scholar] [CrossRef]
- Sesé, B.; Sanmartín, J.M.; Ortega, B.; Matas-Palau, A.; Llull, R. Nanofat Cell Aggregates: A Nearly Constitutive Stromal Cell Inoculum for Regenerative Site-Specific Therapies. Plast Reconstr. Surg. 2019, 144, 1079–1088. [Google Scholar] [CrossRef]
- Caforio, M.; Nobile, C. Intra-Articular Administration of Autologous Purified Adipose Tissue Associated with Arthroscopy Ameliorates Knee Osteoarthritis Symptoms. J. Clin. Med. 2021, 10, 2053. [Google Scholar] [CrossRef]
- Ferguson, R.E.; Cui, X.; Fink, B.F.; Vasconez, H.C.; Pu, L.L. The viability of autologous fat grafts harvested with the LipiVage system: A comparative study. Ann. Plast Surg. 2008, 60, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Almhdie-Imjabbar, A.; Toumi, H.; Lespessailles, E. Radiographic Biomarkers for Knee Osteoarthritis: A Narrative Review. Life 2023, 13, 237. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, R. Knee joint osteoarthritis diagnosis based on selected acoustic signal discriminants using machine learning. Appl. Comput. Sci. 2022, 18, 71–85. [Google Scholar] [CrossRef]
- Montemurro, N.; Ortenzi, V.; Naccarato, G.A.; Perrini, P. Angioleiomyoma of the knee: An uncommon cause of leg pain. A systematic review of the literature. Interdiscip. Neurosurg. 2020, 22, 100877. [Google Scholar] [CrossRef]
- Krakowski, P.; Nogalski, A.; Jurkiewicz, A.; Karpiński, R.; Maciejewski, R.; Jonak, J. Comparison of Diagnostic Accuracy of Physical Examination and MRI in the Most Common Knee Injuries. Appl. Sci. 2019, 9, 4102. [Google Scholar] [CrossRef]

| Authors (Year) | Study Design | Sample Size | Clinical Outcomes Measured | Duration of Follow-Up | Adverse Events or Complications |
|---|---|---|---|---|---|
| Russo et al. [11] (2017) | Retrospective observational study | 40 | The safety and feasibility of using autologous and micro-fragmented adipose tissue. | 12 months | No relevant complications nor clinical worsening were recorded. |
| Labarre et al. [12] (2022) | Prospective study | 33 | SVF treatment has the potential to improve the quality of life of patients by improving joint function and mobility. | 1-year | No local adverse events were observed. |
| Lapuente et al. [13] (2020) | Retrospective and not controlled study | 50 | Intra-articular SVF infiltration for knee OA treatment is safe and effective. | 1-year | There were no serious adverse effects. |
| Garza et al. [14] (2020) | Randomized controlled trial | 39 | Intra-articular SVF injections can significantly decrease knee OA symptoms and pain at 6 months and at 1 year. | 6-month to 1-year | No adverse events. |
| Yokota et al. [15] (2019) | Cohort study | 42 | Both ASC and SVF resulted in clinical improvement in patients with knee OA. | 6 months | No major complications occurred in either group. |
| Rothrauff et al. [16] (2019) | Controlled study | 12 | When compared with tears left untreated or repaired with suture alone, augmented repairs demonstrated increased tissue formation in the meniscal tear site, as seen on MRI and macroscopically. Likewise, the neotissue of augmented repairs possessed a histological appearance more similar, although still inferior to healthy meniscus. | 6 months | No adverse events. |
| Koh et al. [17] (2015) | Prospective case series study | 30 | Almost all patients showed significant improvement in all clinical outcomes at the final follow-up examination. | 2-year | No major complications associated with arthroscopic lavage and liposuction. |
| Nguyen et al. [18] (2017) | Experimental | 30 | AM with SVF/PRP injection was effective for knee OA and had better long-term outcomes than AM alone. | 18 months | Included arterial hypertension, chest pain, dyspnea, and urinary retention. |
| Muñoz et al. [19] (2017) | Experimental | 24 | The supra- and infrapatellar fat pads of 24 patients with severe OA were resected during the surgical intervention for prosthetic implantation. | 3 months | No severe adverse events. |
| Kim et al. [20] (2023) | Retrospective comparative study | 156 | Improved clinical and radiologic outcomes and favorable cartilage regeneration were seen after surgery for varus knee OA in both SVF and hucb-MSC groups. | 33 months | Low complication. |
| Kim et al. [21] (2023) | Retrospective comparative study. | 97 | Significant correlations between the pain scores and MOCART scores were not observed in the SVF group until at the 6-month follow-up. | 12 months | No severe adverse events. |
| Boada-Pladellorens et al. [22] (2022) | Retrospective | 239 | SVF was considered a safe treatment for knee OA and could hold promise in terms of pain, functionality, and improvement of anatomical structure. | 6 to 24 months | Minor adverse effects. |
| Kim et al. [23] (2023) | Retrospective study | 43 | Showed an encouraging improvement in pain levels and cartilage regeneration after SVF implantation in patients with knee OA. | 12 months | Low complication. |
| Yokota et al. [24] (2022) | Cohort study | 80 | ASC and SVF injections substantially improved knee pain and function. | 24 months | No severe adverse events. |
| Aletto et al. [25] (2022) | Prospective study | 123 | Intra-articular knee injection of SVF is safe and effective to ameliorate the clinical and functional scores. | 6 months | No complications were observed in the patients treated. |
| Santoprete et al. [26] (2022) | Retrospective | 84 | SVF injection into the knee joint of patients with OA resulted in symptomatic improvement at short-term follow-up. | 7.9 months | The only complication noted was knee joint swelling lasting for less than 7 days after the injection in 7% of the patients. |
| Zhang et al. [27] (2022) | Retrospective | 126 | The cartilage volume was reduced in both the SVF and control groups at 5 years but reduced less in the SVF group. | 5-year | No complications. |
| Simunec et al. [28] (2020) | Retrospective | 12 | Intra-articular injection of SVF is a safe and effective technique for the management of knee OA. Prior to an invasive artificial joint replacement, the treatment of arthritic knee joints with the intraarticular injection of autologous adipose tissue-derived SVF should be considered a regenerative treatment option. | 12 months | No serious adverse events or unwanted side effects related to the SVF treatment were observed or reported. |
| Şahin et al. [29] (2021) | Experimental | 18 | Adipose-derived SVF improved healing of osteochondral defects treated with MF and HA-based scaffolds. | No complications. | |
| Mehling et al. [30] (2020) | Retrospective | 350 | Subjects with stage III arthritis showed better results after SVF cell therapy. | 3, 6, and 12 months | No complications. |
| Hudetz et al. [31] (2019) | Prospective, non-randomized study | 20 | Suggests that intra-articular injection of microfragmented adipose tissue decreases clinical symptoms in patients with late-stage knee OA, with no observed adverse events. | 12 months | No complications. |
| Hong et al. [32] (2019) | Randomized Controlled Trial | 16 | Autologous treatment of SVF derived from adipose tissue is safe and can effectively relieve pain. | 12-months | No severe adverse events. |
| Tran et al. [33] (2019) | Open-label, single-center, non-randomized, placebo-controlled, phase I/II clinical trial | 33 | SVF therapy is more effective in patients with KL grade 3 OA compared to patients with KL grade 2 OA. | 24 months | No severe adverse events. |
| Type of Cells | Functions | Authors, Year [Ref.] |
|---|---|---|
| Mesenchymal Progenitor/Stem Cells | Capacity to perform self-renewal and differentiation into specific cell lineages and to support maintenance of other cells via paracrine secretion. | Spees et al., 2018 [36] |
| Lymphocytes | Participate in both innate and adaptive immune responses with multiple effect or functions. Produce antibodies, direct cell-mediated killing of virus-infected and/or tumor cells, and regulate immune responses. | Busato et al., 2020 [37] |
| Smooth Muscle Cells | Display involuntary contractile activity to control the diameter, wall movement, and wall stiffness of specific organs. | Guimarães, 2017 [38] |
| Adipose tissue-derived Stem Cells | Secrete growth factors, cytokines, and antioxidant factors into a microenvironment, regulating intracellular signaling pathways in neighboring cells. Protective outcome via inflammatory and immunomodulatory effects. | Bora et al., 2017 [34] |
| Preadipocytes | Promote growth of adipose tissue by differentiating into mature and metabolically active adipocytes. Proliferating preadipocytes may also exhibit phagocytic activity towards microorganisms and behave similarly to macrophage-like cells. | Matsuo et al., 2020 [39] |
| Mφ2 Macrophage | The type 2 macrophage (Mφ2) is produced by the type 2 T helper immune response and takes on an anti-inflammatory role, typically characterized by an increase in the production of interleukins (IL-4, IL-5, IL-9, and IL-13). It is also directly involved in regenerative and tissue repair processes that occur after injuries. | Contreras et al., 2015 [40]; Dey et al., 2021 [41] |
| T Cells | As components of the adaptive immune system with major importance, these cells are responsible for eliminating infected host cells, activating other immune cells and secreting cytokines that further regulate immune responses. | Dulong et al., 2022 [42] |
| Endothelial Precursor Cells and Endothelial Cells | Differentiate into functional endothelial cells and sustain vasculo genesis by incorporating themselves into the injured endothelium with the formation of functional blood vessels and through the local secretion of pro-angiogenic factors with a paracrine effect on the cells that form the vessel. Play a critical role in vascular homeostasis as well as physiological or pathological processes such as thrombosis, inflammation, and vascular wall remodeling. Resting endothelial cells control blood flow and the passage of protein from blood into tissues, as well as inhibiting inflammation and preventing coagulation. | Gulyaeva et al., 2019 [43] |
| Regulation of pro-inflammatory molecules | Decreases IL-1b and IL-6 levels. [39] |
| Hyaline cartilage extracellular matrix | Increases glycosaminoglycan level. [40] |
| Triggering of IL-1Ra | Reduces the catabolic effect of IL-1. [41] |
| Increasing of ADAMTS-4 and -5 | Provides tissue balance (homeostasis). [43] |
| Anti-inflammatory | Reduces tissue swelling (edema). [44] |
| Anti-apoptotic | Reduces and stops programmed cell death. [44] |
| Increasing of TIMPs-1, -3, and -4 metalloproteinases | Provides tissue balance (homeostasis). [45] |
| Conventional | Modified Approach | |
|---|---|---|
| Obtaining adipose tissue | - Abdominal fat. - Reusable Sorenson type lipoaspiration cannula. - Klein’s Translumination solution: Modified. - Klein solution (500 mL isotonic, 20 mL lidocaine, 2% epinephrine, 2 mL bicarbonate). - 50 mL Luer-Lock syringe | - Abdominal fat. - Disposable/Re-usable Coleman style cannula. - Klein’s Translumination solution: Modified. - Klein solution (500 mL isotonic, 20 mL lidocaine, 2% epinephrine, and 2 mL bicarbonate). - 50 mL Luer-Lock syringe. |
| Mechanical separation/shredding | - Shredding of tissue by shaking with glass ball (shaking time and strength depend on the user). | - Separation by the effect of gravity in a screw form mechanical separator at standard power and time. |
| Pre-filtration | - Polyethylene filtration in a 100 micrometer porous polyethylene bag. | - Filtration with the effect of gravity in the 100-micrometer porous device whose base will be supported by a metallic or polymeric cage. |
| Washing | [-] | - Washing in the device. |
| Final filtration | -Filtration on 10 micrometer porous polyethylene filters in 10 mL syringes. | - Final filtration with the rise of adipose tissue and SVF to the solution surface in serum within the device. |
| Collection of SVF/adipose tissue | - Available in an equivalent system. | - Proximal adipose tissue and SVF separation reservoir. |
| Cell counting and characterization | - Cell counting, determination of viability, determination of cell characteristics, and histochemical identification. | - Cell counting, determination of viability, determination of cell characteristics, and histochemical identification. |
| Product | Company | Article |
|---|---|---|
| Cha-Station | Somnotec http://www.somnotec.net | [53] |
| Octagone D200 | Endecotts Ltd. https://www.endecotts.com | [54] |
| AdiPrep | Harvest http://www.harvest.co.kr/clinician/clinician-home/adiprep/advantages/quality.html | [55] |
| Lipokit | Medi-Khan http://www.medikanint.com | [56,57] |
| Puregraft 250 | Puregraft LLC http://www.puregraft.com | [58] |
| Lipogems | Lipogems http://understandlipogems.com | [59,60] |
| MyStem | MyStem LLC https://mystem.eu/ | [61,62] |
| Arthrex SVF | https://www.arthrex.com/orthobiologics | [63] |
| Adinizer | BSL http://biosl.com/?ckattempt=1 | [64] |
| Microlyser | Tlab https://tlab.com.tr/en/products/microlyzer-svf-kit/ | [65] |
| SEFFIE | Advanced-Maes http://www.advanced-maes.com/ | [66] |
| LIPOCUBE | STEMC https://lipocube.com/ | [67,68] |
| Q-Graft | Human Med AG https://www.humanmed.com/en/products/q-graft/ | [28] |
| Tulip Nanotransfer | Tulip Medical https://tulipmedical.com/ | [69] |
| Lipocell | Tissyou https://www.tissyou.com/portfolio_page/lipocell/ | [70] |
| LipiVage | Genesis Biosystems https://www.genesisbiosystems.com/lipivagesystem-autologous-fat-transfer/ | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goncharov, E.N.; Koval, O.A.; Nikolaevich Bezuglov, E.; Encarnacion Ramirez, M.d.J.; Engelgard, M.; Igorevich, E.I.; Saporiti, A.; Valentinovich Kotenko, K.; Montemurro, N. Stromal Vascular Fraction Therapy for Knee Osteoarthritis: A Systematic Review. Medicina 2023, 59, 2090. https://doi.org/10.3390/medicina59122090
Goncharov EN, Koval OA, Nikolaevich Bezuglov E, Encarnacion Ramirez MdJ, Engelgard M, Igorevich EI, Saporiti A, Valentinovich Kotenko K, Montemurro N. Stromal Vascular Fraction Therapy for Knee Osteoarthritis: A Systematic Review. Medicina. 2023; 59(12):2090. https://doi.org/10.3390/medicina59122090
Chicago/Turabian StyleGoncharov, Evgeniy Nikolaevich, Oleg Aleksandrovich Koval, Eduard Nikolaevich Bezuglov, Manuel de Jesus Encarnacion Ramirez, Mikhail Engelgard, Eremin Ilya Igorevich, Alessandra Saporiti, Konstantin Valentinovich Kotenko, and Nicola Montemurro. 2023. "Stromal Vascular Fraction Therapy for Knee Osteoarthritis: A Systematic Review" Medicina 59, no. 12: 2090. https://doi.org/10.3390/medicina59122090







