Abstract
Background and Objectives: Numerous studies have indicated that antibiotics may adversely affect testicular and sperm function. As an alternative to penicillin, vancomycin is a glycopeptide antibiotic developed to treat resistant strains of Staphylococcus aureus. A few studies have suggested that vancomycin could cause testicular toxicity and apoptosis. Vancomycin, however, has not been investigated in terms of its mechanism of causing testicular toxicity. Materials and Methods: An experiment was conducted to investigate the effects of resveratrol (20 mg/kg, oral gavage) against vancomycin (200 mg/kg, i.p.) on the testicular function of Wistar rats for one week (7 days). There were three subgroups of animals. First, saline (i.p.) was administered to the control group. Then, in the second group, vancomycin was administered. Finally, vancomycin and resveratrol were administered in combination in the third group. Results: After seven days of vancomycin treatment, testosterone levels, sperm counts, and sperm motility were significantly reduced, but resveratrol attenuated the effects of vancomycin and restored the testosterone levels, sperm counts, and sperm motility to normal. In the presence of resveratrol, the vancomycin effects were attenuated, and the luteinizing hormone and follicular hormone levels were normalized after seven days of treatment with vancomycin. Histologically, vancomycin administration for seven days caused damage to testicular tissues and reduced the thickness of the basal lamina. However, the resveratrol administration with vancomycin prevented vancomycin’s toxic effects on testicular tissue. Conclusion: Resveratrol showed potential protective effects against vancomycin-induced testicular toxicity in Wistar rats.
1. Introduction
There has been a growing body of research indicating that antibiotics may adversely affect sperm and testicular function [1,2,3,4]. Generally, vancomycin is used for infections caused by Methicillin-resistant Staphylococcus aureus (MRSA) and for patients allergic to penicillin or cephalosporins [5,6]. It has been reported that higher vancomycin trough concentrations should be achieved because MRSA-related infections require higher minimum inhibitory concentrations due to the difficulty in penetrating vulnerable sites such as the lungs, brain, and bone [7,8,9]. However, vancomycin treatment is associated with kidney- and liver-related side effects [10,11,12,13], making it unsuitable for patients with impaired renal and hepatic function [10,11].
There has been significant evidence that various antibiotics can impair the motility of sperm, reduce the weight of the reproductive organs, and cause apoptosis in the testes, eventually leading to testicular failure [12]. A large body of research has shown that the use of antibiotics can disrupt the normal functioning of the male reproductive system by interfering with the hormones that regulate spermatogenesis and sperm motility. Additionally, antibiotics can damage sperm cells, leading to reduced sperm count and motility, which can eventually cause infertility [2,13,14,15]. Several recent studies have reported that vancomycin may adversely affect the testes and cause apoptosis in some cases. [16,17]. It is still unclear how vancomycin causes testicular toxicity. It has been shown that vancomycin increases oxidative stress, such as elevated lipid peroxide levels, and reduces antioxidant enzymes, such as glutathione (GSH) and mitochondrial damage [18,19]. Furthermore, vancomycin stimulates reactive oxygen species (ROS) and inhibits DNA synthesis [20,21]. The presence of ROS is physiologically essential in semen; however, higher levels of ROS production exceed the natural sperm antioxidant ability to prevent ROS damage [22]. Thus, higher levels of free radicals can reduce spermiogenesis resulting in loss of motility and DNA damage in the sperm nucleus [23].
Resveratrol (3,4′,5-trihydroxy-trans-stilbene) is a natural polyphenolic compound found in vegetables such as grapes, berries, and peanuts. Resveratrol has continuously been reported to have a growing number of benefits. For example, cumulative reports have suggested that resveratrol has potential benefits as an anti-inflammatory, antidiabetic, anticancer, and protective effect against cardiovascular disease. Moreover, resveratrol could improve stress resistance, extend human lifespans, and prevent the progression of many illnesses, including cancer, cardiovascular disease, and ischemic injuries [24,25,26]. As a result, resveratrol’s antioxidant properties have effectively protected cells from hydrogen-peroxide-induced oxidative stress and UV-irradiation-induced cell death after pretreatment with resveratrol [27,28,29]. Furthermore, in pharmaceutical products, resveratrol can delay the oxidation of lipids, reduce the toxic byproducts of oxidation, and extend the shelf life while maintaining the nutritional quality [30,31]. The structure of resveratrol is similar to that of estradiol, which suggests that it may play a similar role in the testes [32]. In humans and domestic animals, resveratrol has been shown to improve sperm quality [33]. This appears to be possible as a result of its ability to pass through the blood–testis barrier and impart its protective properties to the testes [34]. The use of resveratrol to treat infertility in vivo has been found to be effective. Resveratrol has been found to be effective in treating men afflicted with dyszoospermia because it ameliorated the effect induced by 2,5-hexanedione on spermatogenesis [32]. It has been shown that oral administration of resveratrol and coenzyme Q10 protects against radiation-induced spermatogenesis injuries, suggesting that the combination may be beneficial for promoting male fertility [35]. Even though considerable research has been conducted, it is unclear what role resveratrol plays in male reproductive function.
Therefore, many attempts have been implemented to reduce antibiotics’ effects on spermatogenesis [36,37]. It is well known that resveratrol is an antioxidant that can scavenge ROS, preventing the damage of cells in tissues. There is promising evidence that antioxidants effectively preserve spermatogenesis in an animal model and treat male factor infertility. Several studies have shown that vancomycin can cause testicular atrophy and impaired sperm quality in animals and humans. However, limited information about vancomycin’s effect on men with reproductive disorders is available. This study aimed to determine whether high doses of vancomycin induce testicular or spermatotoxicity in rats and to investigate whether resveratrol might have a modulatory effect on the development of testicular damage induced by high doses of vancomycin.
2. Material and Methods
2.1. Drugs
During the study, resveratrol was obtained from ProHealth in the USA (B094XH3W98), mixed with saline (0.9% NaCl) as suspension, and given to animals immediately, and vancomycin was obtained from Medis in Tunisia (AMM12/96860), dissolved in saline (0.9% NaCl). The dose of resveratrol was selected based on several studies that used 20 mg/kg to show its antioxidant and anti-inflammatory effects [38,39,40]; the vancomycin dose was selected based on its ability to produce toxic effects on several organs, including testicular tissues [41,42,43].
2.2. Animals
Twenty-one adult male Wistar rats weighing 160–200 g (7 weeks old) were used in the study. The animals were housed in plastic cages in a humidity-controlled room under a 12 h light/12 h dark schedule for the experiment. Daily monitoring was performed to ensure the wellbeing of the animals, who had access to food and water ad libitum. All animals were obtained from the King Abdulaziz University animal house.
2.3. Experimental Design
The experiment was conducted for eight days. The rats were divided into three groups: (1) control group (n = 7), rats injected with saline (i.p) for seven days; (2) vancomycin group (n = 7), rats injected with vancomycin (200 mg i.p) for seven days; (3) vancomycin + resveratrol group (n = 7), rats injected with vancomycin (200 mg i.p) and resveratrol (20 mg/kg, oral gavage) for seven days (Table 1). On day eight, all rats were euthanized using CO2; then, serum and tissue samples were collected. The blood samples were collected from their retroorbital plexus and tail vain. For the separation of serum from plasma, a blood sample was collected and centrifuged at 3000× g for 15 min at 4 °C. For testing, all samples were centrifuged and analyzed immediately.

Table 1.
Experimental groups for the resveratrol and vancomycin administration.
2.4. Determination of Testosterone, Follicle Stimulating Hormone (FSH), and Luteinizing Hormone (LH) by ELISA
Serum samples were collected on day 8. Each sample was centrifuged, and each animal’s serum was analyzed separately. The ELISA technique was used to determine the serum levels of testosterone, FSH, and LH according to the manufacturer’s protocol (MyBioSource, Inc San Diego, CA 92195-3308, USA).
2.5. Determination of the Sperm Motility and Counts
The male rats were euthanized by CO2 inhalation. The sperm samples were diluted with physiological solution (10 μL), pipetted with TL-HEPES solution containing 3 mg/mL bovine serum albumin, and then used to buffer HEPES-buffered Tyrode lactate (TL-HEPES) solution. The cauda epididymis was cut at several points at 37 °C to allow the sperm to flow out. The sperm motility percentage and sperm count (Cells/mm3) were obtained using computer-assisted semen analysis to measure the sperm motility and forward motility with the SpermVision™ CASA System (MiniTub, Tiefenbach, Germany). Following Zemjanis’ method, the sperm motility of rats was measured within 2–4 min of sacrifice [44].
2.6. Histological Examination
The testicular tissues were used for histopathological assessment and prepared in 10% formalin solution for two days. The tissue was embedded in paraffin blocks and stained with Hematoxylin and Eosin (H&E). The thickness of the slices was between 3 and 5 mm. An OMAX 3 MP Digital Compound Microscope was used to observe the stained sections at various magnifications and to take photographic micrographs. Under a light microscope at ×400, 20 seminiferous tubules from each animal section were evaluated for histomorphometric changes. In addition, histopathological changes in the testes were examined.
2.7. Statistical Analysis
The serum testosterone, follicle-stimulating hormone, luteinizing hormone, and sperm motility were analyzed using a one-way analysis of variance (ANOVA) followed by Tukey’s post hoc tests. The data were analyzed using Prism version 9.4.1 (p-value < 0.05).
3. Results
3.1. Body Weight
There was no significant difference in the body weight of all the treated groups compared to the controls on day 1. However, repeated measure two-way ANOVA analysis showed a significant main effect in days, as shown in the ANOVA table [F (1, 6) = 178.5, p < 0.0001], treatment [F (2, 12) = 4.026, p = 0.0459], the days x treatment [F (2, 12) = 126.1, p < 0.0001] (Figure 1). In addition, multiple comparison tests using the Tukey post hoc test revealed a significant reduction in body weight in the vancomycin group on day 8 compared to the vancomycin group on day 1 (p < 0.0001). Moreover, there was a reduction in body weight in the vancomycin group compared to the control group on day 8 (p < 0.0001). However, there was an increase in the body weight of the vancomycin + resveratrol group compared to the vancomycin group (p < 0.0001).
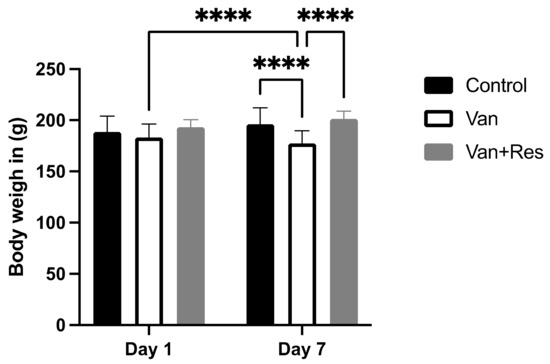
Figure 1.
The effect of the resveratrol and vancomycin administration on the animals’ body weights during the experiment. **** p < 0.0001.
3.2. Testosterone, Follicle Stimulating Hormone, and Luteinizing Hormone in the Blood Levels
The testosterone level in the blood was determined on day 8 of the experiment. One-way ANOVA revealed significant changes in the level of testosterone (ng/dl) between the treatment groups, as shown in the ANOVA table [F (2, 18) = 17.02, p < 0.0001, Figure 2]. Further analysis using Tukey’s multiple comparison tests showed a significant reduction in the testosterone levels in the vancomycin group compared to the control group (p = 0.0002) and the vancomycin + resveratrol group (p = 0.0003). However, no significant changes were found between the control group and the vancomycin + resveratrol group (p = 0.9977).
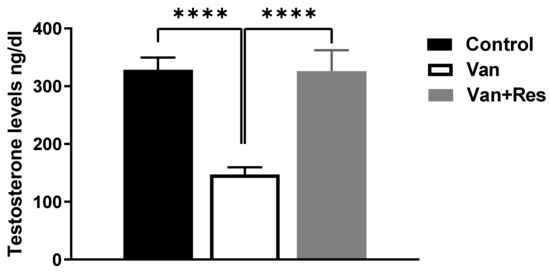
Figure 2.
The effect of the resveratrol and vancomycin administration on the testosterone during the experiment. **** p < 0.0001.
The FSH level in the blood was determined on day 8 of the experiment. One-way ANOVA revealed significant changes in the level of FSH (mIU/mL) between the treatment groups, as shown in the ANOVA table [F (2, 18) = 41.43, p < 0.0001, Figure 3]. Further analysis using Tukey’s multiple comparison tests presented a significant increase in the FSH levels in the vancomycin group compared to the control group (p < 0.0001) and the vancomycin + resveratrol group (p < 0.0001). However, no significant changes were found between the control group and the vancomycin + resveratrol group (p = 0.8106).
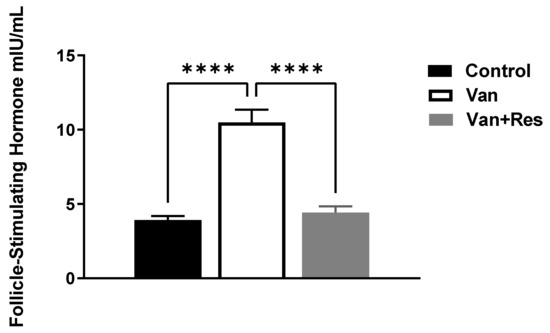
Figure 3.
The effect of the resveratrol and vancomycin administration on the follicle-stimulating hormone during the experiment. **** p < 0.0001.
The LH level in the blood was determined on day 8 of the experiment. One-way ANOVA revealed significant changes in the level of LH (mIU/mL) between the treatment groups, as shown in the ANOVA table [F (2, 18) = 50.58, p < 0.0001, Figure 4]. Further analysis using Tukey’s multiple comparison tests showed a significant increase in the LH levels in the vancomycin group compared to the control group (p < 0.0001) and the vancomycin + resveratrol group (p < 0.0001). However, no significant changes were found between the control group and the vancomycin + resveratrol group (p = 0.8745).
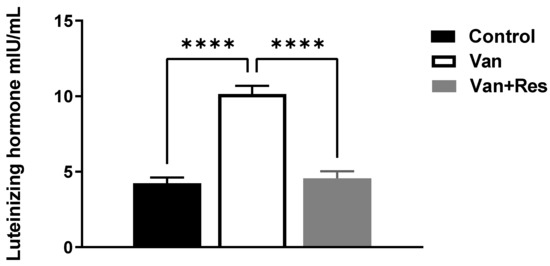
Figure 4.
The effect of the resveratrol and vancomycin administration on the luteinizing hormone during the experiment. **** p < 0.0001.
3.3. Sperm Motility and Counts
The sperm motility was determined on day 8 of the experiment. One-way ANOVA revealed significant changes in the sperm motility between the treatment groups, as shown in the ANOVA table [F (2, 18) = 21.70, p < 0.0001, Figure 5]. Further analysis using Tukey’s multiple comparison tests showed a significant reduction in the sperm motility in the vancomycin group compared to the control group (p < 0.001) and the vancomycin + resveratrol group (p < 0.0001). However, no significant changes were found between the control group and the vancomycin + resveratrol group (p = 0.9314).
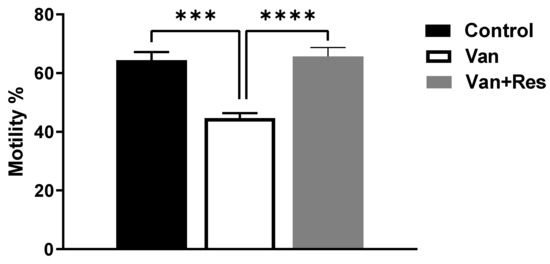
Figure 5.
The effect of the resveratrol and vancomycin administration on the motility during the experiment. *** p < 0.001, **** p < 0.0001.
The sperm counts were determined on day 8 of the experiment. One-way ANOVA revealed significant changes in the sperm counts (Cells/mm3) between the treatment groups, as shown in the ANOVA table [F (2, 18) = 29.55, p < 0.0001, Figure 6]. Further analysis using Tukey’s multiple comparison tests showed a significant reduction in the sperm motility in the vancomycin group compared to the control group (p < 0.0001) and the vancomycin + resveratrol group (p = 0.0001). However, no significant changes were found between the control group and the vancomycin + resveratrol group (p = 0.1148).
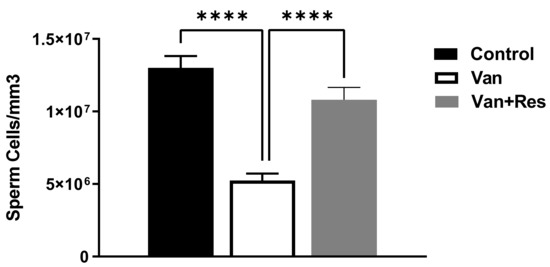
Figure 6.
The effect of the resveratrol and vancomycin administration on the sperm during the experiment. **** p < 0.0001.
3.4. Histological Examinations of the Testicles
The histology of the testicles was not significantly different between the rats given daily saline alone and the controls (Figure 7A–C). Hence, it was found that normal spermatogenesis had taken place, that the Sertoli cells had been preserved well, and that the tubular basement membrane had been clearly defined. Furthermore, the interstitial space between the tubules and the Leydig cells also appeared intact. However, the vancomycin-treated group showed a significant difference in the histology of the testes, where the seminiferous tubules were observed to be swallowed up completely. The tubular basement membranes of the seminiferous tubules were identified in other areas of the section. While most germ cells, including highly differentiated germ cells and deformed sperm, were degenerating, a small percentage were flourishing. It is also important to note that the ground substance within the interstitium partially disappeared and was replaced by fibroblasts and inflammatory cells. There was an improvement in these toxic effects in the group treated with vancomycin and resveratrol.
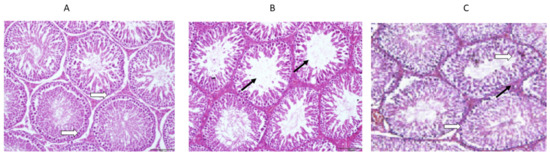
Figure 7.
(A) The effect of the normal saline administration during the experiment on the histological sections of rat testes was a normal morphology with a normal thickness of the basal lamina (white arrows). (B) The effect of the vancomycin administration on the histological sections of rat testes was thinning and splitting of the basal lamina, with atrophy and widely separated seminiferous tubules (black arrows). (C) The effect of the resveratrol and vancomycin administration on the histological sections of rat testes was a normal thickness of the basal lamina (white arrows) and normal seminiferous tubules (black arrows).
4. Discussion
A growing concern has been raised over the possibility that antibiotics may adversely affect human fertility [3,13,45]. Vancomycin is considered one of the most common antibiotics used globally for treating severe Gram-positive infections caused by meticillin-resistant S aureus (MRSA) [5,46]. Nevertheless, vancomycin has long been recognized as one of the most commonly encountered drugs that induces nephrotoxicity and hepatotoxicity. In addition to its toxic effects, vancomycin can also cause testicular toxicity [43]. Hence, the present study was conducted to investigate the protective effects of resveratrol against the toxic effects of vancomycin on the testicular functions of male Wistar rats, through analysis of the histopathological and biochemical profiles.
The present study demonstrated the toxicological effects of vancomycin in male Wistar rats. There was a significant decrease in the serum testosterone levels after administering vancomycin. Low intratesticular testosterone concentrations may result in germ cell degeneration due to vancomycin exposure. It has been suggested that the testosterone level in the testes is essential for spermatogenesis and maintaining the seminiferous tubules’ structural morphology and physiology [47,48,49]. Vancomycin administration was associated with the degeneration of germ cells, including highly differentiated germ cells and deformed sperm. Moreover, changes in the testes’ morphological characteristics in the vancomycin treatment groups were observed. Epithelium, tubular shrinkage, and atrophy were manifestations of these changes.
In addition, several studies have demonstrated that the sperm count and motility are the most valuable indicators of male fertility. Research shows that the sperm count and motility are positively associated with pregnancy rates. Based on the findings of our study, the vancomycin-induced structural damage to rat testicular tissues resulted in a severe reduction in the sperm count and motility. Furthermore, it has been suggested that administering vancomycin can lead to various biochemical malfunctions [50,51]. Unfortunately, there is a lack of understanding the mechanisms through which vancomycin produces these effects. There is, however, evidence that reactive oxygen species (ROS) are involved. In fact, the rats exposed to vancomycin showed an elevation in oxidative stress caused by a reduction in antioxidant enzymes, such as the glutathione levels, coupled with an increase in the lipid peroxide levels, which resulted in oxidative stress in the animals [16,52]. Other antibiotics have shown similar patterns regarding testicular dysfunction. In vivo and in vitro, it has been well established that gentamicin is capable of causing ROS formation and oxidative damage. In the testes of rats treated with gentamicin, a similar reduction in enzymatic and nonenzymatic antioxidant activity was observed, along with increased lactoperoxidase levels [53]. As a result of the treatment with gentamicin, the MDA concentrations increased, and the GSH levels decreased. Consequently, the oxidative stress caused by the gentamicin treatment could be linked to increased lactoperoxidase levels and decreased antioxidant activity and GSH levels in the testes [54]. Therefore, it is essential to monitor antibiotic use to minimize the potential risks.
Moreover, there was a significant increase in the serum LH and FSH levels after administering vancomycin. The LH level was also significantly higher in the treatment group, indicating that the hypophyseal–pituitary axis was affected [55]. In fact, male interstitial cells produce testosterone in response to LH stimulation [56]. Additionally, the FSH level was significantly higher than that of the controls. It has been reported that there is a direct interaction between FSH and Sertoli cells; therefore, FSH binds to its receptor and stimulates its signaling pathway, which leads to Sertoli cell differentiation [57]. It is possible that an elevated level of FSH indicates abnormal spermatogenesis and may indicate testicular failure [58]. Conversely, a low level of testosterone and raised FSH and LH levels have been associated with insufficient sperm production by the testicles [59]. As a result of these changes, this study suggests that using vancomycin in high doses is likely to lead to infertility.
Known for its antioxidant properties, resveratrol is a natural polyphenolic compound present in vegetables such as grapes, berries, and peanuts [60]. In recent years, researchers have extensively studied the antiaging properties of resveratrol and its potential to help prevent aging-related diseases, such as Alzheimer’s and diabetes [61,62]. Furthermore, resveratrol may play a role in preventing heart disease and stroke [63] and improving cognitive function [64]. According to cumulative reports, resveratrol has potential anti-inflammatory and protective effects against cancer [65]. Moreover, resveratrol could improve stress resistance, extend human lifespans, and prevent the progression of many illnesses [66]. Further, it has recently been shown that in vivo treatment with resveratrol prevents oxidative stress in the testes of hyperthyroid rats and rats treated with a chemotherapy drug [33,67]. Although resveratrol appears to have an antioxidant effect on male reproduction, its exact mechanism of action is unknown. As part of this study, resveratrol was examined for its protective effects against damage caused by vancomycin on rats’ spermatozoa. The protective effects of resveratrol on the glutathione levels have been reported, particularly those conducted in a testicular ischemia model. Furthermore, resveratrol stimulates spermatogenesis and testicular regeneration in adults [32]. In addition, rats treated with resveratrol produced more spermatozoa [68]. A similar increase in spermatozoa production was observed in the resveratrol groups compared to the vancomycin groups. In the present study, we observed that resveratrol prevented the sperm reduction caused by vancomycin, suggesting that it has antioxidant properties. The oxidative stress produced by vancomycin, including hydroxyl radicals, can be scavenged effectively by resveratrol [69]. However, several possible molecular mechanisms could explain the effects of resveratrol. It has been reported that resveratrol maintains the integrity of mitochondrial membranes and provides sufficient energy to spermatogonial stem cells through modulating the SIRT1 protein and deacetylating FOXO1 in vitro [70]. Moreover, a study conducted in rats showed that resveratrol increased the expression of sirtuin-1, neuronal nitric oxide synthase (nNOS), decreased the rate of cell death, and stimulated the differentiation of germ cells in rats [35,71,72,73]. However, the mechanisms of action of resveratrol remain unclear, even though it protects spermatozoa against oxidative stress.
5. Conclusions
In conclusion, it is becoming increasingly apparent that antibiotics may adversely affect human fertility. Vancomycin was investigated in this study to understand the possible protective effects of resveratrol on vancomycin-induced testicular toxicity. This study investigated the protective effects of resveratrol against vancomycin’s toxic effects on the testicular functions of adult male Wistar rats through analyzing the histopathological and biochemical profiles.
Funding
This research received no external funding.
Institutional Review Board Statement
The Biomedical Committee of Research Ethics at the Faculty of Medicine at Umm Al-Qura University approved the study (Approval No. HAPO-02-K-012-2022-06-1127; approval date 12 June 2022).
Informed Consent Statement
This work has not been published previously, and it is not under consideration for publication elsewhere. The author has approved this manuscript and the responsible authorities where the research was conducted tacitly and explicitly. It will not be published elsewhere in the same form, in English or in any other language, including electronically without the written consent of the copyright holder.
Data Availability Statement
The original data will be avalaible upon request from the author.
Conflicts of Interest
The author declares no conflict of interest.
References
- Manson, J.M.; Zolna, L.E.; Kang, Y.J.; Johnson, C.M. Effects of cefonicid and other cephalosporin antibiotics on male sexual development in rats. Antimicrob. Agents Chemother. 1987, 31, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Farombi, E.O.; Ugwuezunmba, M.C.; Ezenwadu, T.T.; Oyeyemi, M.O.; Ekor, M. Tetracycline-induced reproductive toxicity in male rats: Effects of vitamin C and N-acetylcysteine. Exp. Toxicol. Pathol. 2008, 60, 77–85. [Google Scholar] [CrossRef]
- Sakai, K.; Ideta-Otsuka, M.; Saito, H.; Hiradate, Y.; Hara, K.; Igarashi, K.; Tanemura, K. Effects of doxorubicin on sperm DNA methylation in mouse models of testicular toxicity. Biochem. Biophys. Res. Commun. 2018, 498, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Karaman, M.; Budak, H.; Ciftci, M. Amoxicillin and gentamicin antibiotics treatment adversely influence the fertility and morphology through decreasing the Dazl gene expression level and increasing the oxidative stress. Arch. Physiol. Biochem. 2019, 125, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Marsot, A.; Boulamery, A.; Bruguerolle, B.; Simon, N. Vancomycin: A review of population pharmacokinetic analyses. Clin. Pharmacokinet. 2012, 51, 1–13. [Google Scholar] [CrossRef]
- Appelbaum, P.C. The emergence of vancomycin-intermediate and vancomycin-resistant Staphylococcus aureus. Clin. Microbiol. Infect. 2006, 12 (Suppl. S1), 16–23. [Google Scholar] [CrossRef]
- Lodise, T.P.; Patel, N.; Lomaestro, B.M.; Rodvold, K.A.; Drusano, G.L. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin. Infect. Dis. 2009, 49, 507–514. [Google Scholar] [CrossRef]
- Bosso, J.A.; Nappi, J.; Rudisill, C.; Wellein, M.; Bookstaver, P.B.; Swindler, J.; Mauldin, P.D. Relationship between vancomycin trough concentrations and nephrotoxicity: A prospective multicenter trial. Antimicrob. Agents Chemother. 2011, 55, 5475–5479. [Google Scholar] [CrossRef]
- Van Hal, S.J.; Paterson, D.L.; Lodise, T.P. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob. Agents Chemother. 2013, 57, 734–744. [Google Scholar] [CrossRef]
- Brunetti, L.; Song, J.H.; Suh, D.; Kim, H.J.; Seong, Y.H.; Lee, D.S.; Lee, S.M.; Suh, D.C. The risk of vancomycin toxicity in patients with liver impairment. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 13. [Google Scholar] [CrossRef]
- Chu, Y.; Luo, Y.; Qu, L.; Zhao, C.; Jiang, M. Application of vancomycin in patients with varying renal function, especially those with augmented renal clearance. Pharm. Biol. 2016, 54, 2802–2806. [Google Scholar] [CrossRef] [PubMed]
- Abeer, M. The protective effect of some natural antioxidants against azithromycin induced testicular dysfunction in rats. Am. Int. J. Contemp. Sci. Res. 2015, 2, 39–40. [Google Scholar]
- Kim, S.H.; Lee, I.C.; Baek, H.S.; Shin, I.S.; Moon, C.; Kim, S.H.; Yun, W.K.; Nam, K.H.; Kim, H.C.; Kim, J.C. Melatonin prevents gentamicin-induced testicular toxicity and oxidative stress in rats. Andrologia 2014, 46, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.; ElHawary, N.; Mohamed, S.; Hashim, N.; Saleh, S.; Bakeer, M.; Sawiress, F. Effect of stem cell therapy on gentamicin induced testicular dysfunction in rats. J. Health Med. Inform. 2017, 8, 1000263. [Google Scholar]
- Tahri, A.; Ksouda, K.; Kallel, R.; Daoud, S.; Boudawara, T.; Zeghal, K.M.; Sahnoun, Z. A carbapenem antibiotic imipenem/cilastatin induces an oxidative stress-status and gonadotoxic effects in «wistar» rats. Biomed. Pharmacother. 2017, 95, 308–316. [Google Scholar] [CrossRef]
- Caglayan, C.; Taslimi, P.; Demir, Y.; Kucukler, S.; Kandemir, F.M.; Gulcin, I. The effects of zingerone against vancomycin-induced lung, liver, kidney and testis toxicity in rats: The behavior of some metabolic enzymes. J. Biochem. Mol. Toxicol. 2019, 33, e22381. [Google Scholar] [CrossRef]
- Naidu, E.C.S.; Olojede, S.O.; Lawal, S.K.; Peter, A.I.; Akang, E.A.; Azu, O.O. Effects of vancomycin linoleic acid nanoparticles on male reproductive indices of Sprague-Dawley rats. Artif. Cells Nanomed. Biotechnol. 2021, 49, 587–595. [Google Scholar] [CrossRef]
- Yu, P.; Luo, J.; Song, H.; Qian, T.; He, X.; Fang, J.; Dong, W.; Bian, X. N-acetylcysteine Ameliorates Vancomycin-induced Nephrotoxicity by Inhibiting Oxidative Stress and Apoptosis in the in vivo and in vitro Models. Int. J. Med. Sci. 2022, 19, 740–752. [Google Scholar] [CrossRef]
- Zhou, P.; Xia, D.; Xia, Y.; Zhang, H.; Wang, Y.; Tang, T.; Xu, S. Synergistic effect of vancomycin and l-homocarnosine alleviates Staphylococcus aureus-induced osteomyelitis in rats. Biomed. Pharmacother. 2019, 111, 31–35. [Google Scholar] [CrossRef]
- Beauchamp, D.; Pellerin, M.; Gourde, P.; Pettigrew, M.; Bergeron, M.G. Effects of daptomycin and vancomycin on tobramycin nephrotoxicity in rats. Antimicrob. Agents Chemother. 1990, 34, 139–147. [Google Scholar] [CrossRef]
- Nishino, Y.; Takemura, S.; Minamiyama, Y.; Hirohashi, K.; Tanaka, H.; Inoue, M.; Okada, S.; Kinoshita, H. Inhibition of vancomycin-induced nephrotoxicity by targeting superoxide dismutase to renal proximal tubule cells in the rat. Redox Rep. 2002, 7, 317–319. [Google Scholar] [CrossRef]
- Guthrie, H.D.; Welch, G.R. Effects of reactive oxygen species on sperm function. Theriogenology 2012, 78, 1700–1708. [Google Scholar] [CrossRef] [PubMed]
- Sanocka, D.; Kurpisz, M. Reactive oxygen species and sperm cells. Reprod. Biol. Endocrinol. 2004, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Fong, H.H.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Bradamante, S.; Barenghi, L.; Villa, A. Cardiovascular protective effects of resveratrol. Cardiovasc. Drug Rev. 2004, 22, 169–188. [Google Scholar] [CrossRef]
- Sinha, K.; Chaudhary, G.; Gupta, Y.K. Protective effect of resveratrol against oxidative stress in middle cerebral artery occlusion model of stroke in rats. Life Sci. 2002, 71, 655–665. [Google Scholar] [CrossRef]
- Marques, F.Z.; Markus, M.A.; Morris, B.J. Resveratrol: Cellular actions of a potent natural chemical that confers a diversity of health benefits. Int. J. Biochem. Cell Biol. 2009, 41, 2125–2128. [Google Scholar] [CrossRef]
- Konyalioglu, S.; Armagan, G.; Yalcin, A.; Atalayin, C.; Dagci, T. Effects of resveratrol on hydrogen peroxide-induced oxidative stress in embryonic neural stem cells. Neural Regen. Res. 2013, 8, 485–495. [Google Scholar] [CrossRef]
- Means, J.C.; Gerdes, B.C.; Koulen, P. Distinct Mechanisms Underlying Resveratrol-Mediated Protection from Types of Cellular Stress in C6 Glioma Cells. Int. J. Mol. Sci. 2017, 18, 1521. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant properties of resveratrol: A structure–activity insight. Innov. Food Sci. Emerg. Technol. 2010, 11, 210–218. [Google Scholar] [CrossRef]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant Polyphenols as Antioxidant and Antibacterial Agents for Shelf-Life Extension of Meat and Meat Products: Classification, Structures, Sources, and Action Mechanisms. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1243–1268. [Google Scholar] [CrossRef]
- Juan, M.E.l.; González-Pons, E.; Munuera, T.; Ballester, J.; Rodríguez-Gil, J.E.; Planas, J.M. trans-Resveratrol, a natural antioxidant from grapes, increases sperm output in healthy rats. J. Nutr. 2005, 135, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Ourique, G.M.; Finamor, I.A.; Saccol, E.M.; Riffel, A.P.; Pes, T.S.; Gutierrez, K.; Goncalves, P.B.; Baldisserotto, B.; Pavanato, M.A.; Barreto, K.P. Resveratrol Improves Sperm Motility, Prevents Lipid Peroxidation and Enhances Antioxidant Defences in the Testes of Hyperthyroid Rats. Reprod Toxicol 2013, 37, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Aitken, S.; Roman, S. Antioxidative system and oxidative stress in the testis. In Molecular Mechanisms in Spermatogenesis; Cheng, C.Y., Ed.; Landes Bioscience: New York, NY, USA, 2008. [Google Scholar]
- Najafi, M.; Cheki, M.; Amini, P.; Javadi, A.; Shabeeb, D.; Musa, A.E. Evaluating the protective effect of resveratrol, Q10, and alpha-lipoic acid on radiation-induced mice spermatogenesis injury: A histopathological study. Int. J. Reprod. BioMedicine 2019, 17, 907. [Google Scholar] [CrossRef] [PubMed]
- Quiles, J.L.; Huertas, J.R.; Battino, M.; Mataix, J.; Ramirez-Tortosa, M.C. Antioxidant nutrients and adriamycin toxicity. Toxicology 2002, 180, 79–95. [Google Scholar] [CrossRef]
- Kalender, Y.; Yel, M.; Kalender, S. Doxorubicin hepatotoxicity and hepatic free radical metabolism in rats. The effects of vitamin E and catechin. Toxicology 2005, 209, 39–45. [Google Scholar] [CrossRef]
- Prabhakar, O. Cerebroprotective effect of resveratrol through antioxidant and anti-inflammatory effects in diabetic rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2013, 386, 705–710. [Google Scholar] [CrossRef]
- Fathy, S.M.; Abdelkader, I.Y. Effect of resveratrol on the inflammatory status and oxidative stress in thymus gland and spleen of sulfoxaflor-treated rats. Environ. Toxicol. 2021, 36, 1326–1337. [Google Scholar] [CrossRef]
- Al-Baqami, N.M.; Hamza, R.Z. Protective effect of resveratrol against hepatotoxicity of cadmium in male rats: Antioxidant and histopathological approaches. Coatings 2021, 11, 594. [Google Scholar] [CrossRef]
- He, J.; Xu, W.; Zheng, X.; Zhao, B.; Ni, T.; Yu, P.; Deng, S.; Pan, X.; Chen, E.; Mao, E. Vitamin C reduces vancomycin-related nephrotoxicity through the inhibition of oxidative stress, apoptosis, and inflammation in mice. Ann. Transl. Med. 2021, 9, 1319. [Google Scholar] [CrossRef]
- Güzel, S.; Şahinoğullari, Z.U.; Canacankatan, N.; Antmen, Ş.E.; Kibar, D.; Bayrak, G. The ameliorating effect of silymarin against vancomycin-induced apoptosis and inflammation in rat liver. J. Res. Pharm. 2019, 23, 719–728. [Google Scholar] [CrossRef]
- Kandemir, Ö.; AKSU, E.H. The effects of vancomycin on sperm motility and testicular inflammation in rats. Vet. Sci. Pract. 2022, 17, 41–44. [Google Scholar] [CrossRef]
- Zemjanis, R. Diagnostic and therapeutic techniques in animal reproduction. In Diagnostic and Therapeutic Techniques in Animal Reproduction; The Williams & Wilkins Co.: Baltimore, MD, USA, 1970. [Google Scholar]
- Timermans, A.; Vazquez, R.; Otero, F.; Gosalvez, J.; Johnston, S.; Fernandez, J.L. Antibiotic toxicity on human spermatozoa assessed using the sperm DNA fragmentation dynamic assay. Andrologia 2022, 54, e14328. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.; Yang, S.; Rao, X. Vancomycin resistant Staphylococcus aureus infections: A review of case updating and clinical features. J. Adv. Res. 2020, 21, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, R.M.; Maddocks, S.; Millar, M.; Kerr, J.B.; Saunders, P.T.; McKinnell, C. Testosterone and spermatogenesis. Identification of stage-specific, androgen-regulated proteins secreted by adult rat seminiferous tubules. J. Androl. 1992, 13, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.H. Molecular mechanisms of testosterone action in spermatogenesis. Steroids 2009, 74, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.H. Testosterone signaling and the regulation of spermatogenesis. Spermatogenesis 2011, 1, 116–120. [Google Scholar] [CrossRef]
- Bellos, I.; Daskalakis, G.; Pergialiotis, V. Relationship of vancomycin trough levels with acute kidney injury risk: An exposure-toxicity meta-analysis. J. Antimicrob. Chemother. 2020, 75, 2725–2734. [Google Scholar] [CrossRef] [PubMed]
- Zamoner, W.; Prado, I.R.S.; Balbi, A.L.; Ponce, D. Vancomycin dosing, monitoring and toxicity: Critical review of the clinical practice. Clin. Exp. Pharmacol. Physiol. 2019, 46, 292–301. [Google Scholar] [CrossRef]
- Qu, S.; Dai, C.; Lang, F.; Hu, L.; Tang, Q.; Wang, H.; Zhang, Y.; Hao, Z. Rutin Attenuates Vancomycin-Induced Nephrotoxicity by Ameliorating Oxidative Stress, Apoptosis, and Inflammation in Rats. Antimicrob. Agents Chemother. 2019, 63, e01545-18. [Google Scholar] [CrossRef]
- Aly, H. Testicular toxicity of gentamicin in adult rats: Ameliorative effect of lycopene. Hum. Exp. Toxicol. 2019, 38, 1302–1313. [Google Scholar] [CrossRef] [PubMed]
- Aly, H.A.; Hassan, M.H. Potential testicular toxicity of gentamicin in adult rats. Biochem. Biophys. Res. Commun. 2018, 497, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Kreider, K.E. Anatomy and Physiology of the Hypothalamic-Piuitary Axis. In Advanced Practice in Endocrinology Nursing; Springer: Cham, Switzerland, 2019; pp. 231–243. [Google Scholar]
- Oduwole, O.O.; Huhtaniemi, I.T.; Misrahi, M. The Roles of Luteinizing Hormone, Follicle-Stimulating Hormone and Testosterone in Spermatogenesis and Folliculogenesis Revisited. Int. J. Mol. Sci. 2021, 22, 12735. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.M.; Li, Z.F.; Yang, W.X.; Tan, F.Q. Follicle-stimulating hormone signaling in Sertoli cells: A licence to the early stages of spermatogenesis. Reprod. Biol. Endocrinol. 2022, 20, 97. [Google Scholar] [CrossRef] [PubMed]
- Keskin, M.; Budak, S.; Zeyrek, T.; Çelik, O.; Mertoglu, O.; Yoldas, M.; Ilbey, Y. The relationship between serum hormone levels (follicle-stimulating hormone, luteinizing hormone, total testosterone) and semen parameters. Arch. Ital. Urol. Androl. 2015, 87, 194. [Google Scholar] [CrossRef] [PubMed]
- Oduwole, O.O.; Peltoketo, H.; Huhtaniemi, I.T. Role of Follicle-Stimulating Hormone in Spermatogenesis. Front. Endocrinol. 2018, 9, 763. [Google Scholar] [CrossRef]
- Tian, B.; Liu, J. Resveratrol: A review of plant sources, synthesis, stability, modification and food application. J. Sci. Food Agric. 2020, 100, 1392–1404. [Google Scholar] [CrossRef]
- Rao, Y.L.; Ganaraja, B.; Joy, T.; Pai, M.M.; Ullal, S.D.; Murlimanju, B.V. Neuroprotective effects of resveratrol in Alzheimer’s disease. Front. Biosci. Elite 2020, 12, 139–149. [Google Scholar]
- Jeyaraman, M.M.; Al-Yousif, N.S.; Mann, A.S.; Dolinsky, V.W.; Rabbani, R.; Zarychanski, R.; Abou-Setta, A.M. Resveratrol for adults with type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2020, 2020, CD011919. [Google Scholar] [CrossRef]
- Dyck, G.J.; Raj, P.; Zieroth, S.; Dyck, J.R.; Ezekowitz, J.A. The effects of resveratrol in patients with cardiovascular disease and heart failure: A narrative review. Int. J. Mol. Sci. 2019, 20, 904. [Google Scholar] [CrossRef]
- Tosatti, J.A.G.; Fontes, A.F.d.S.; Caramelli, P.; Gomes, K.B. Effects of resveratrol supplementation on the cognitive function of patients with Alzheimer’s disease: A systematic review of randomized controlled trials. Drugs Aging 2022, 39, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Carter, L.G.; D’Orazio, J.A.; Pearson, K.J. Resveratrol and cancer: Focus on in vivo evidence. Endocr. Relat. Cancer 2014, 21, R209–R225. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Yulug, E.; Turedi, S.; Alver, A.; Turedi, S.; Kahraman, C. Effects of resveratrol on methotrexate-induced testicular damage in rats. Sci. World J. 2013, 2013, 489659. [Google Scholar] [CrossRef]
- Reddy, K.P.; Madhu, P.; Reddy, P.S. Protective effects of resveratrol against cisplatin-induced testicular and epididymal toxicity in rats. Food Chem. Toxicol. 2016, 91, 65–72. [Google Scholar] [CrossRef]
- Leonard, S.S.; Xia, C.; Jiang, B.H.; Stinefelt, B.; Klandorf, H.; Harris, G.K.; Shi, X. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem. Biophys. Res. Commun. 2003, 309, 1017–1026. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, Y.; Shen, Q.; Zhou, Z.; Liu, W.; Hua, J. Resveratrol changes spermatogonial stem cells (SSCs) activity and ameliorates their loss in busulfan-induced infertile mouse. Oncotarget 2016, 7, 82085. [Google Scholar] [CrossRef]
- Uguralp, S.; Usta, U.; Mizrak, B. Resveratrol may reduce apoptosis of rat testicular germ cells after experimental testicular torsion. Eur. J. Pediatr. Surg. 2005, 15, 333–336. [Google Scholar] [CrossRef]
- Revel, A.; Raanani, H.; Younglai, E.; Xu, J.; Rogers, I.; Han, R.; Savouret, J.F.; Casper, R.F. Resveratrol, a natural aryl hydrocarbon receptor antagonist, protects lung from DNA damage and apoptosis caused by benzo[a]pyrene. J. Appl. Toxicol. 2003, 23, 255–261. [Google Scholar] [CrossRef]
- Pasquariello, R.; Verdile, N.; Brevini, T.A.L.; Gandolfi, F.; Boiti, C.; Zerani, M.; Maranesi, M. The Role of Resveratrol in Mammalian Reproduction. Molecules 2020, 25, 4554. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).