A Novel Implant for Superior Pubic Ramus Fracture Fixation—Development and a Biomechanical Feasibility Study
Abstract
:1. Introduction
2. Materials and Methods
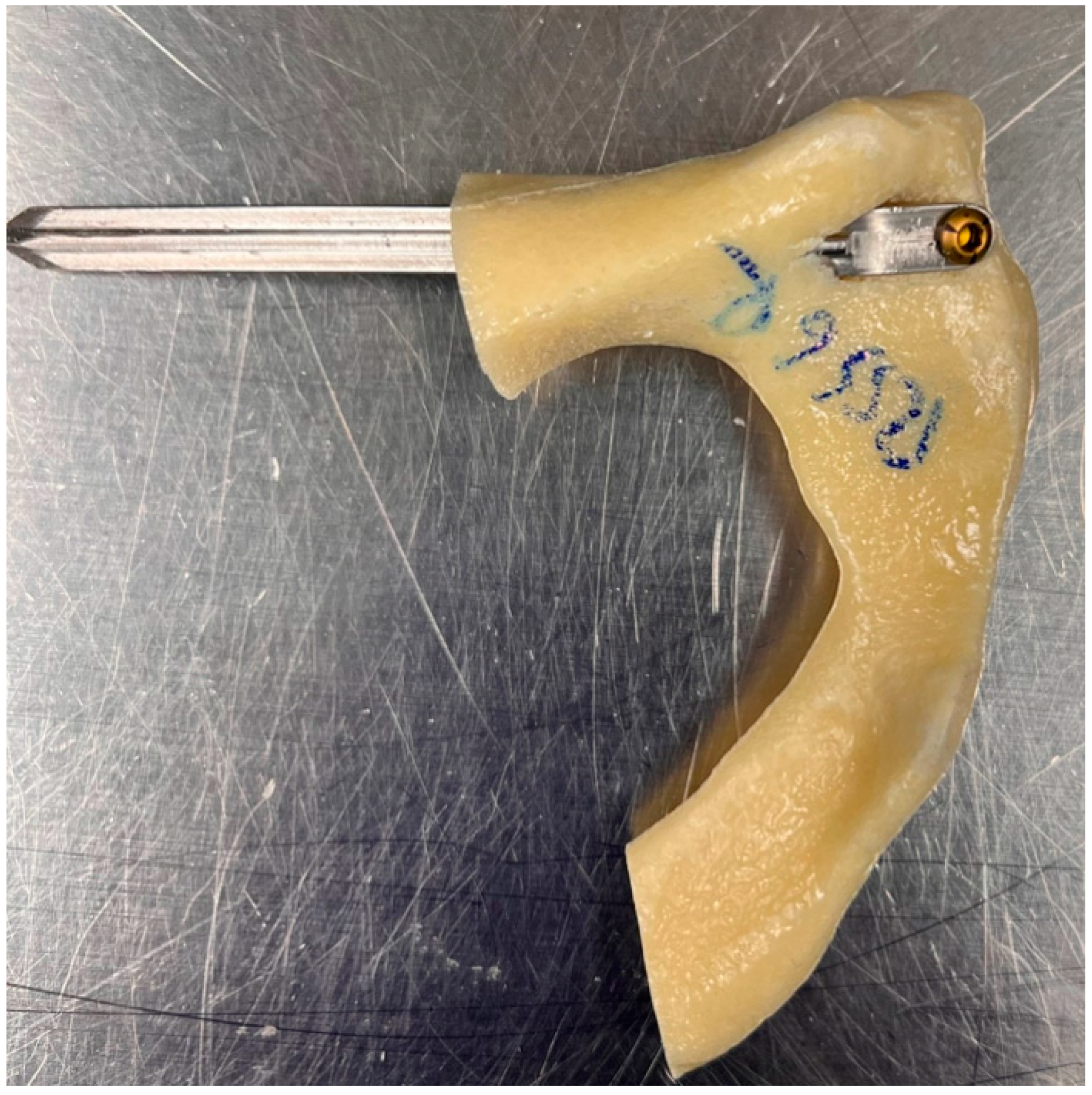
2.1. Features of the Novel Intramedullary Splinting Implant
2.2. Specimens Preparation
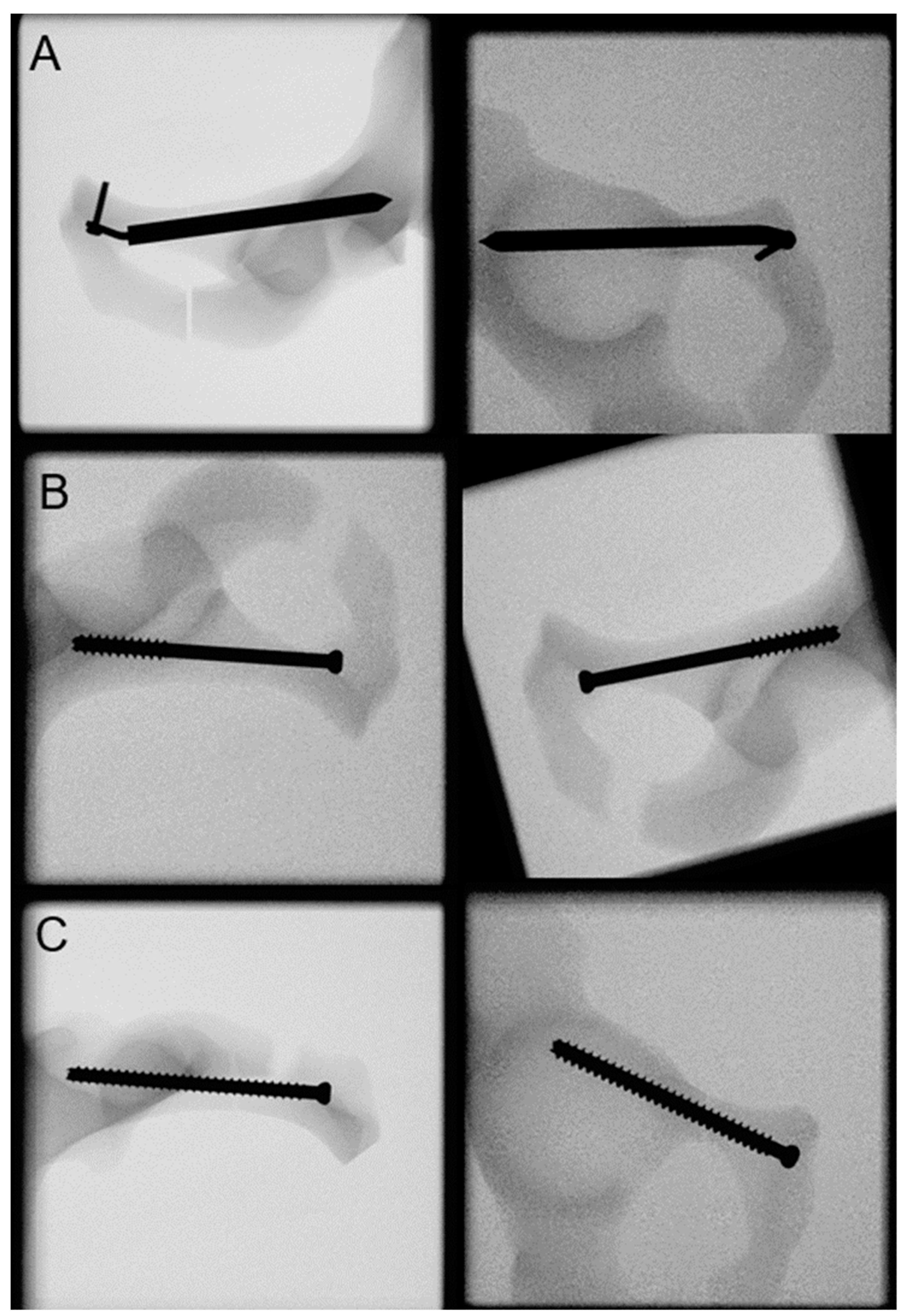
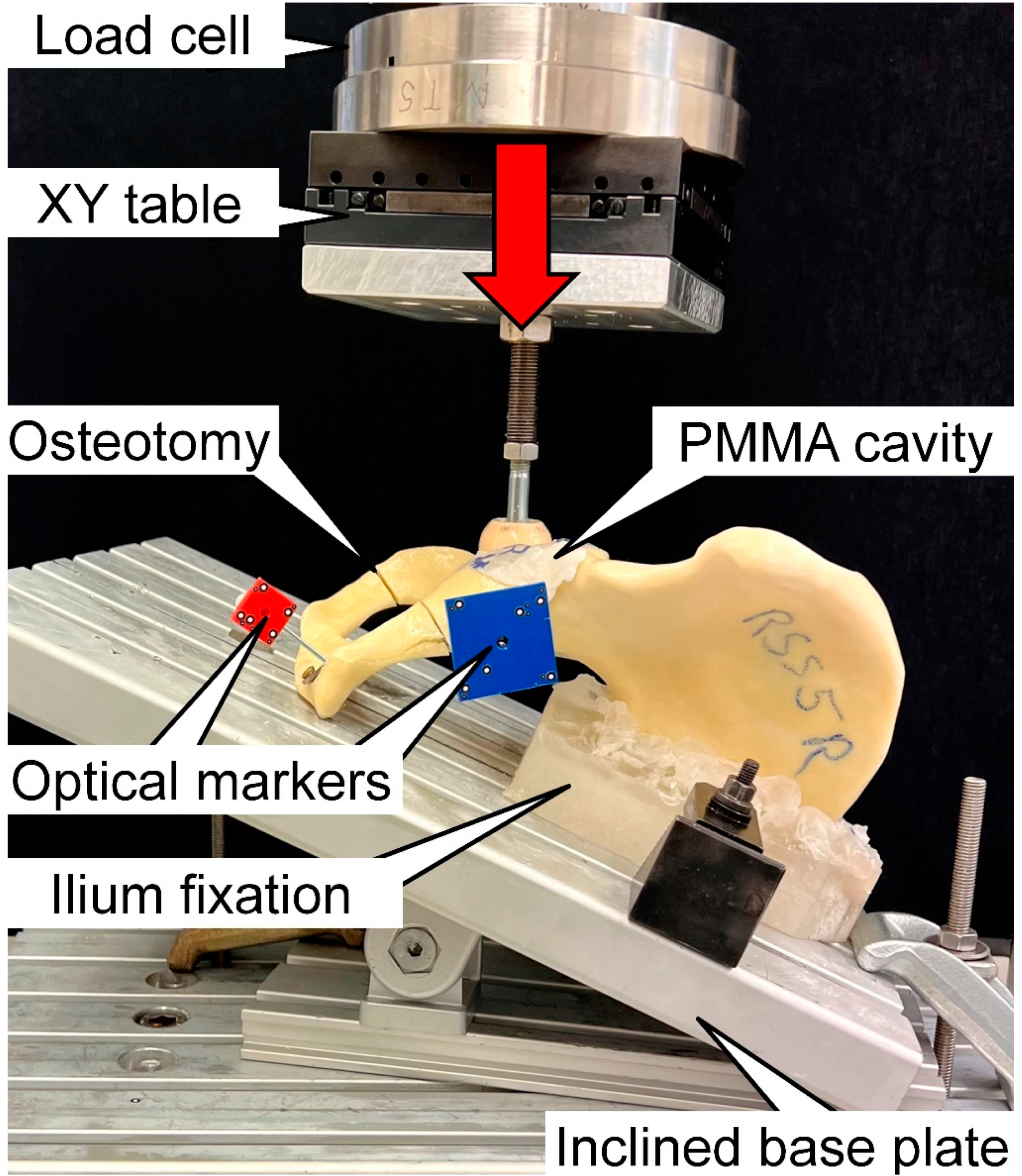
2.3. Biomechanical Testing
2.4. Data Acquisition and Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rommens, P.M.; Hopf, J.C.; Herteleer, M.; Devlieger, B.; Hofmann, A.; Wagner, D. Isolated pubic ramus fractures are serious adverse events for elderly persons: An observational study on 138 patients with fragility fractures of the pelvis type I (FFP type I). J. Clin. Med. 2020, 9, 2498. [Google Scholar] [CrossRef] [PubMed]
- Scheyerer, M.J.; Osterhoff, G.; Wehrle, S.; Wanner, G.A.; Simmen, H.P.; Werner, C.M. Detection of posterior pelvic injuries in fractures of the pubic rami. Injury 2012, 43, 1326–1329. [Google Scholar] [CrossRef]
- Van Dijk, W.; Poeze, M.; Van Helden, S.H.; Brink, P.R.G.; Verbruggen, J.P.A.M. Ten-year mortality among hospitalised patients with fractures of the pubic rami. Inj. Int. J. Care Inj. 2010, 41, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.M.F.; Robinson, C.M.; Keating, J.F. Fractures of the pubic rami: Epidemiology and five-year survival. J. Bone Jt. Surg. 2001, 83, 1141–1144. [Google Scholar] [CrossRef]
- Breuil, V.; Roux, C.H.; Testa, J.; Albert, C.; Chassang, M.; Brocq, O.; Euller-Ziegler, L. Outcome of osteoporotic pelvic fractures: An underestimated severity. Survey of 60 cases. Jt. Bone Spine 2008, 75, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Melton, L.J., III; Sampson, J.M.; Morrey, B.F.; Ilstrup, D.M. Epidemiologic features of pelvic fractures. Clin. Orthop. Relat. Res. 1981, 155, 43–47. [Google Scholar] [CrossRef]
- Kannus, P.; Palvanen, M.; Niemi, S.; Parkkari, J.; Järvinen, M. Epidemiology of osteoporotic pelvic fractures in elderly people in Finland: Sharp increase in 1970–1997 and alarming projections for the new millennium. Osteoporos. Int. 2000, 11, 443–448. [Google Scholar] [CrossRef]
- Li, C. Clinical comparative analysis on unstable pelvic fractures in the treatment with percutaneous sacroiliac screws and sacroiliac joint anterior plate fixation. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2704–2708. [Google Scholar] [PubMed]
- Gire, J.D.; Jiang, S.Y.; Gardner, M.J.; Bishop, J.A. Percutaneous versus open treatment of posterior pelvic ring injuries: Changes in practice patterns over time. J. Orthop. Trauma 2018, 32, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Starr, A.J.; Nakatani, T.; Reinert, C.M.; Cederberg, K. Superior pubic ramus fractures fixed with percutaneous screws: What predicts fixation failure? J. Orthop. Trauma 2008, 22, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Routt, M.C., Jr.; Nork, S.E.; Mills, W.J. Percutaneous fixation of pelvic ring disruptions. Clin. Orthop. Relat. Res. 2000, 375, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Routt, M.C., Jr.; Simonian, P.T.; Mills, W.J. Iliosacral screw fixation: Early complications of the percutaneous technique. J. Orthop. Trauma 1997, 11, 584–589. [Google Scholar] [CrossRef]
- Ivanov, P.A.; Zadneprovsky, N.N.; Nevedrov, A.V.; Kalensky, V.O. Pubic Rami Fractures Fixation by Interlocking Intramedually Nail: First Clinical Experience. Traumatol. Orthop. Russ. 2018, 24, 111–120. [Google Scholar] [CrossRef]
- Kuentscher, G. Intramedullary Splinting—a Brief History. In Proceedings of the Ciba Symposium, London, UK, 27–30 November 1962; pp. 50–54. [Google Scholar]
- Tarng, Y.W.; Lin, K.C.; Chen, C.F.; Yang, M.Y.; Chien, Y. The elastic stable intramedullary nails as an alternative treatment for adult humeral shaft fractures. J. Chin. Med. Assoc. 2021, 84, 644–649. [Google Scholar] [CrossRef]
- Tile, M. Acute pelvic fractures: I. Causation and classification. JAAOS J. Am. Acad. Orthop. Surg. 1996, 4, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, M.; West, L.R.; Bingham, R. AO Surgery Reference: A comprehensive guide for management of fractures. Br. J. Sport. Med. 2017, 51, 545–546. [Google Scholar] [CrossRef]
- Acklin, Y.P.; Zderic, I.; Grechenig, S.; Richards, R.G.; Schmitz, P.; Gueorguiev, B. Are two retrograde 3.5 mm screws superior to one 7.3 mm screw for anterior pelvic ring fixation in bones with low bone mineral density? Bone Jt. Res. 2017, 6, 8–13. [Google Scholar] [CrossRef]
- Morosato, F.; Traina, F.; Cristofolini, L. Standardization of hemipelvis alignment for in vitro biomechanical testing. J. Orthop. Res. 2018, 36, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, G.; Deuretzbacher, G.; Heller, M.; Graichen, F.; Rohlmann, A.; Strauss, J.; Duda, G.N. Hip contact forces and gait patterns from routine activities. J. Biomech. 2001, 34, 859–871. [Google Scholar] [CrossRef]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Permana, M.S.; Winarni, T.I.; van der Heide, E. Adopted walking condition for computational simulation approach on bearing of hip joint prosthesis: Review over the past 30 years. Heliyon 2022, 8, e12050. [Google Scholar] [CrossRef]
- Gueorguiev, B.; Ockert, B.; Schwieger, K.; Wähnert, D.; Lawson-Smith, M.; Windolf, M.; Stoffel, K. Angular stability potentially permits fewer locking screws compared with conventional locking in intramedullary nailed distal tibia fractures: A biomechanical study. J. Orthop. Trauma 2011, 25, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Windolf, M.; Muths, R.; Braunstein, V.; Gueorguiev, B.; Hänni, M.; Schwieger, K. Quantification of cancellous bone-compaction due to DHS® Blade insertion and influence upon cut-out resistance. Clin. Biomech. 2009, 24, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Marasco, S.; Saxena, P. Surgical rib fixation–Technical aspects. Injury 2015, 46, 929–932. [Google Scholar] [CrossRef]
- Bottlang, M.; Helzel, I.; Long, W.; Fitzpatrick, D.; Madey, S. Less-invasive stabilization of rib fractures by intramedullary fixation: A biomechanical evaluation. J. Trauma Acute Care Surg. 2010, 68, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Silva, F.B.; Kojima, K.E.; Joeris, A.; Silva, J.S.; Mattar, R., Jr. Single, superiorly placed reconstruction plate compared with flexible intramedullary nailing for midshaft clavicular fractures: A prospective, randomized controlled trial. JBJS 2015, 97, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Vialle, R.A.; Levassor, N.I.; Rillardon, L.U.; Templier, A.L.; Skalli, W.A.; Guigui, P.I. Radiographic analysis of the sagittal alignment and balance of the spine in asymptomatic subjects. J. Bone Jt. Surg. 2005, 87, 260–267. [Google Scholar] [CrossRef]
- Solomon, L.B.; Pohl, A.P.; Sukthankar, A.; Chehade, M.J. The subcristal pelvic external fixator: Technique, results, and rationale. J. Orthop. Trauma 2009, 23, 365–369. [Google Scholar] [CrossRef]
- Grewal, I.S.; Starr, A.J. What’s New in Percutaneous Pelvis Fracture Surgery? Orthop. Clin. 2020, 51, 317–324. [Google Scholar] [CrossRef]
- Ghanayem, A.J.; Wilber, J.H.; Lieberman, J.M.; Motta, A.O. The effect of laparotomy and external fixator stabilization on pelvic volume in an unstable pelvic injury. J. Trauma Acute Care Surg. 1995, 38, 396–401. [Google Scholar] [CrossRef]
- Gardner, M.P.; Chong, A.C.; Pollock, A.G.; Wooley, P.H. Mechanical evaluation of large-size fourth-generation composite femur and tibia models. Ann. Biomed. Eng. 2010, 38, 613–620. [Google Scholar] [CrossRef]
- Heiner, A.D. Structural properties of fourth-generation composite femurs and tibias. J. Biomech. 2008, 41, 3282–3284. [Google Scholar] [CrossRef]
- Zdero, R.; Olsen, M.; Bougherara, H.; Schemitsch, E.H. Cancellous bone screw purchase: A comparison of synthetic femurs, human femurs, and finite element analysis. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2008, 222, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Elfar, J.; Stanbury, S.; Menorca, R.M.G.; Reed, J.D. Composite bone models in orthopaedic surgery research and education. J. Am. Acad. Orthop. Surg. 2014, 22, 111. [Google Scholar] [PubMed]
- Camino Willhuber, G.; Zderic, I.; Gras, F.; Wahl, D.; Sancineto, C.; Barla, J.; Windolf, M.; Richards, R.G.; Gueorguiev, B. Analysis of sacro-iliac joint screw fixation: Does quality of reduction and screw orientation influence joint stability? A biomechanical study. Int. Orthop. 2016, 40, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Gardner, M.J.; Kendoff, D.; Ostermeier, S.; Citak, M.; Hüfner, T.; Krettek, C.; Nork, S.E. Sacroiliac joint compression using an anterior pelvic compressor: A mechanical study in synthetic bone. J. Orthop Trauma 2007, 21, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Yinger, K.; Scalise, J.; Olson, S.A.; Bay, B.K.; Finkemeier, C.G. Biomechanical comparison of posterior pelvic ring fixation. J. Orthop. Trauma 2003, 17, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Sahin, O.; Demirors, H.; Akgun, R.; Tuncay, I. Internal fixation of bilateral sacroiliac dislocation with transiliac locked plate: A biomechanical study on pelvic models. Acta Orthop. Traumatol. Turc. 2013, 47, 411–416. [Google Scholar] [CrossRef]
- Sagi, H.; Ordway, N.; DiPasquale, T. Biomechanical analysis of fixation for vertically unstable sacroiliac dislocations with iliosacral screws and symphyseal plating. J. Orthop. Trauma 2004, 18, 138–143. [Google Scholar] [CrossRef]
- Van Zwienen, C.M.; van den Bosch, E.W.; van Dijke, G.A.H.; Snijders, C.J.; van Vugt, A.B. Cyclic loading of sacroiliac screws in tile C pelvic fractures. J. Trauma Inj. Infect. Crit. Care 2005, 58, 1029–1034. [Google Scholar] [CrossRef]
- Perren, S.M. The biomechanics and biology of internal fixation using plates and nails. Orthopedics 1989, 12, 21–34. [Google Scholar] [CrossRef]
- McKibbin, B. The biology of fracture healing in long bones. J. Bone Jt. Surg. 1978, 60, 150–162. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Group | Cycles | p * | ||||
|---|---|---|---|---|---|---|---|
| 2000 | 4000 | 6000 | 8000 | 10,000 | |||
| Displacement [mm] | RIS | 0.29 ± 0.11 | 0.59 ± 0.23 | 1.11 ± 0.52 | 2.02 ± 1.35 | 2.39 ± 1.30 | 0.098 |
| p ** | 0.015 | ||||||
| RSP | 0.41 ± 0.21 | 0.78 ± 0.31 | 1.26 ± 0.41 | 1.78 ± 0.59 | 2.88 ± 1.49 | ||
| p ** | 0.045 | ||||||
| RSF | 0.16 ± 0.07 | 0.37 ± 0.14 | 0.66 ± 0.26 | 0.99 ± 0.38 | 1.40 ± 0.46 | ||
| p ** | <0.001 | ||||||
| Gap angle [°] | RIS | 0.26 ± 0.22 | 0.71 ± 0.41 | 1.40 ± 0.84 | 3.10 ± 3.32 | 3.93 ± 3.46 | 0.569 |
| p ** | 0.075 | ||||||
| RSP | 0.36 ± 0.21 | 0.64 ± 0.41 | 1.00 ± 0.44 | 1.36 ± 0.53 | 2.83 ± 2.36 | ||
| p ** | 0.164 | ||||||
| RSF | 0.37 ± 0.23 | 0.64 ± 0.40 | 1.08 ± 0.61 | 1.66 ± 0.79 | 2.45 ± 1.41 | ||
| p ** | 0.006 | ||||||
| Torsional displacement [°] | RIS | 0.93 ± 0.49 | 1.72 ± 1.05 | 3.56 ± 2.74 | 6.94 ± 7.74 | 7.94 ± 7.91 | 0.412 |
| p ** | 0.092 | ||||||
| RSP | 1.66 ± 0.83 | 3.09 ± 1.18 | 4.85 ± 1.27 | 7.25 ± 2.22 | 11.17 ± 6.74 | ||
| p ** | 0.081 | ||||||
| RSF | 0.53 ± 0.25 | 1.30 ± 0.59 | 2.65 ± 1.46 | 4.22 ± 2.28 | 6.32 ± 2.96 | ||
| p ** | 0.004 | ||||||
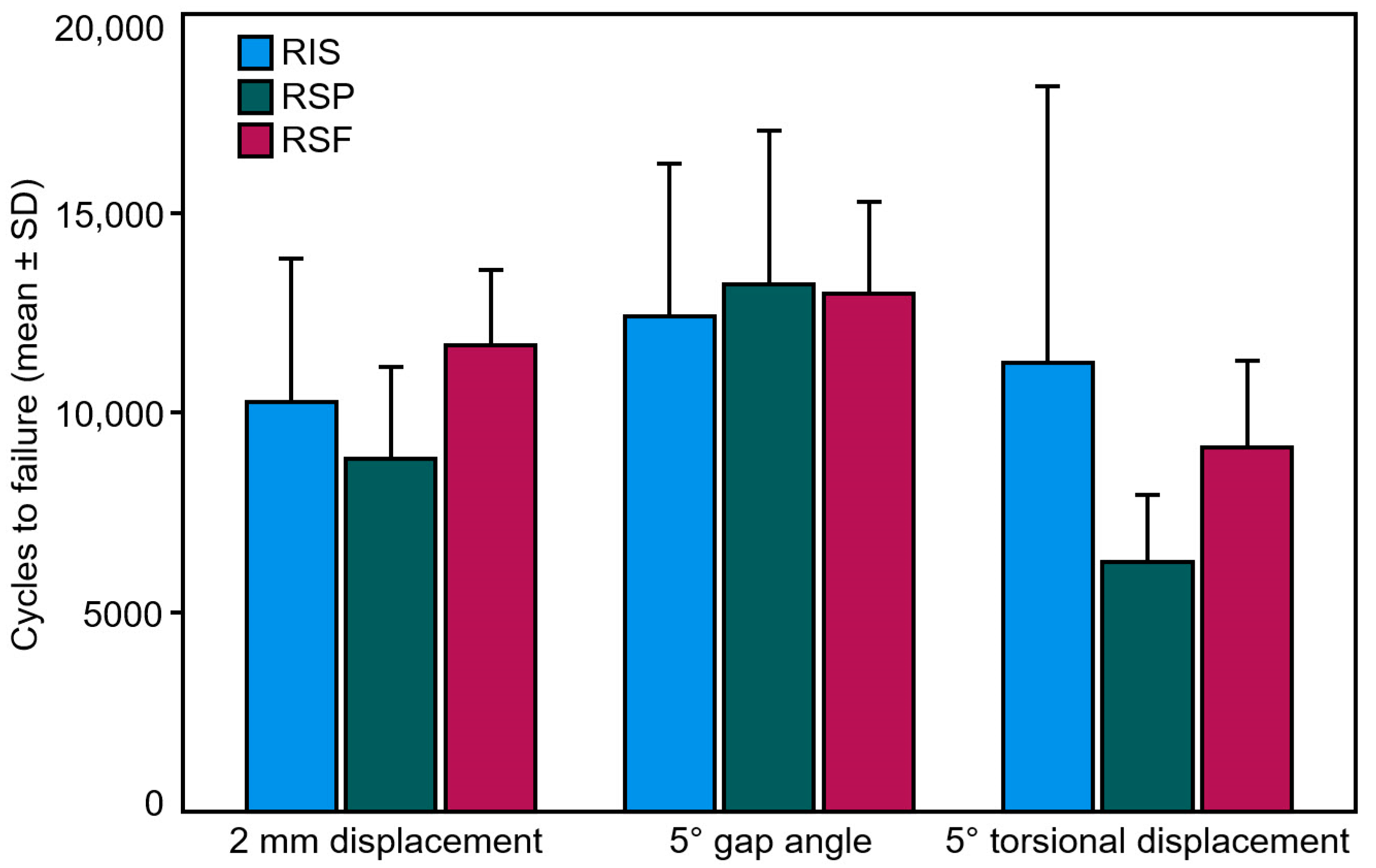
| Criterion | Group | Failure | p | |
|---|---|---|---|---|
| Cycles | Load [N] | |||
| 2 mm displacement | RIS | 10,279 ± 3590 | 1227.9 ± 359.0 | 0.257 |
| RSP | 8860 ± 2302 | 1086.0 ± 230.2 | ||
| RSF | 11,703 ± 1882 | 1370.3 ± 188.2 | ||
| 5° gap angle | RIS | 12,414 ± 3835 | 1441.4 ± 383.5 | 0.918 |
| RSP | 13,220 ± 3859 | 1522.0 ± 385.9 | ||
| RSF | 13,000 ± 2292 | 1500.0 ± 229.2 | ||
| 5° torsional displacement | RIS | 14,901 ± 4413 | 1690.1 ± 441.3 | 0.213 |
| RSP | 6254 ± 1686 | 825.4 ± 168.6 | ||
| RSF | 9145 ± 2163 | 1114.5 ± 216.3 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berk, T.; Zderic, I.; Caspar, J.; Schwarzenberg, P.; Pastor, T.; Halvachizadeh, S.; Makelov, B.; Richards, G.; Pape, H.-C.; Gueorguiev, B. A Novel Implant for Superior Pubic Ramus Fracture Fixation—Development and a Biomechanical Feasibility Study. Medicina 2023, 59, 740. https://doi.org/10.3390/medicina59040740
Berk T, Zderic I, Caspar J, Schwarzenberg P, Pastor T, Halvachizadeh S, Makelov B, Richards G, Pape H-C, Gueorguiev B. A Novel Implant for Superior Pubic Ramus Fracture Fixation—Development and a Biomechanical Feasibility Study. Medicina. 2023; 59(4):740. https://doi.org/10.3390/medicina59040740
Chicago/Turabian StyleBerk, Till, Ivan Zderic, Jan Caspar, Peter Schwarzenberg, Torsten Pastor, Sascha Halvachizadeh, Biser Makelov, Geoff Richards, Hans-Christoph Pape, and Boyko Gueorguiev. 2023. "A Novel Implant for Superior Pubic Ramus Fracture Fixation—Development and a Biomechanical Feasibility Study" Medicina 59, no. 4: 740. https://doi.org/10.3390/medicina59040740






