Effect of the Combination of Probiotics and Korean Red Ginseng on Diabetic Wound Healing Exposed to Diesel Exhaust Particles(DEPs)
Abstract
:1. Introduction
2. Materials and Methods
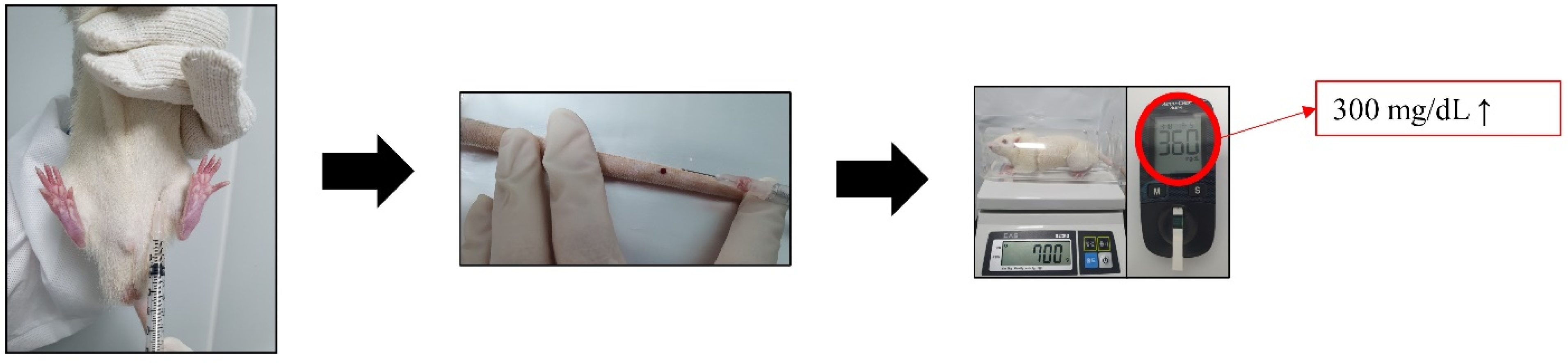
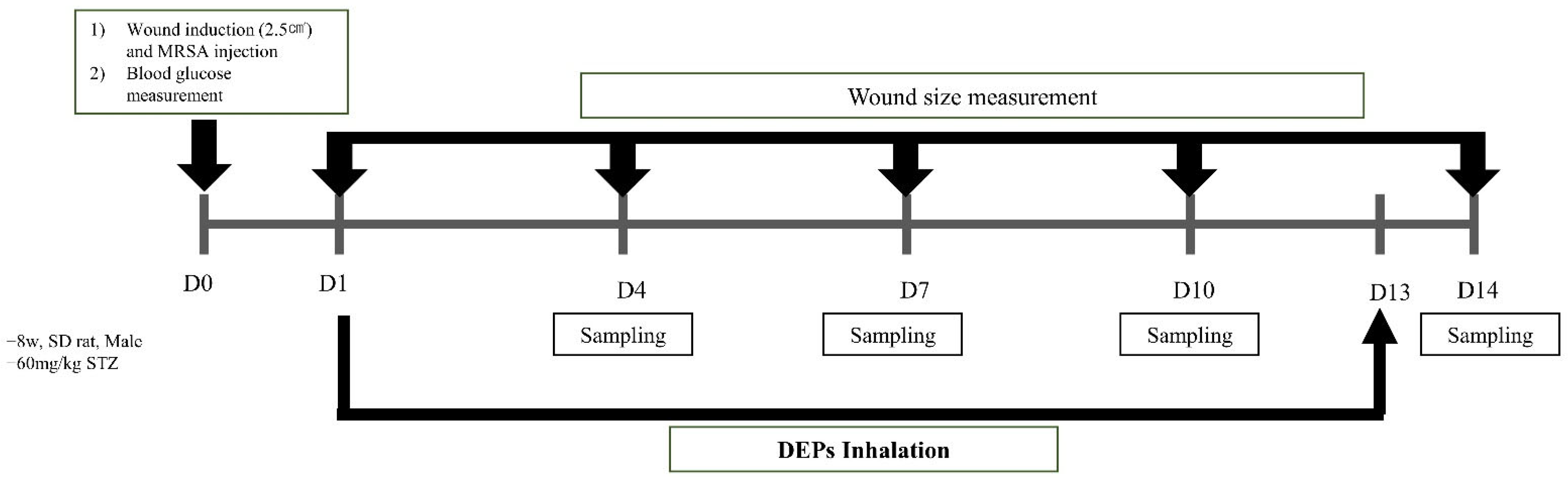
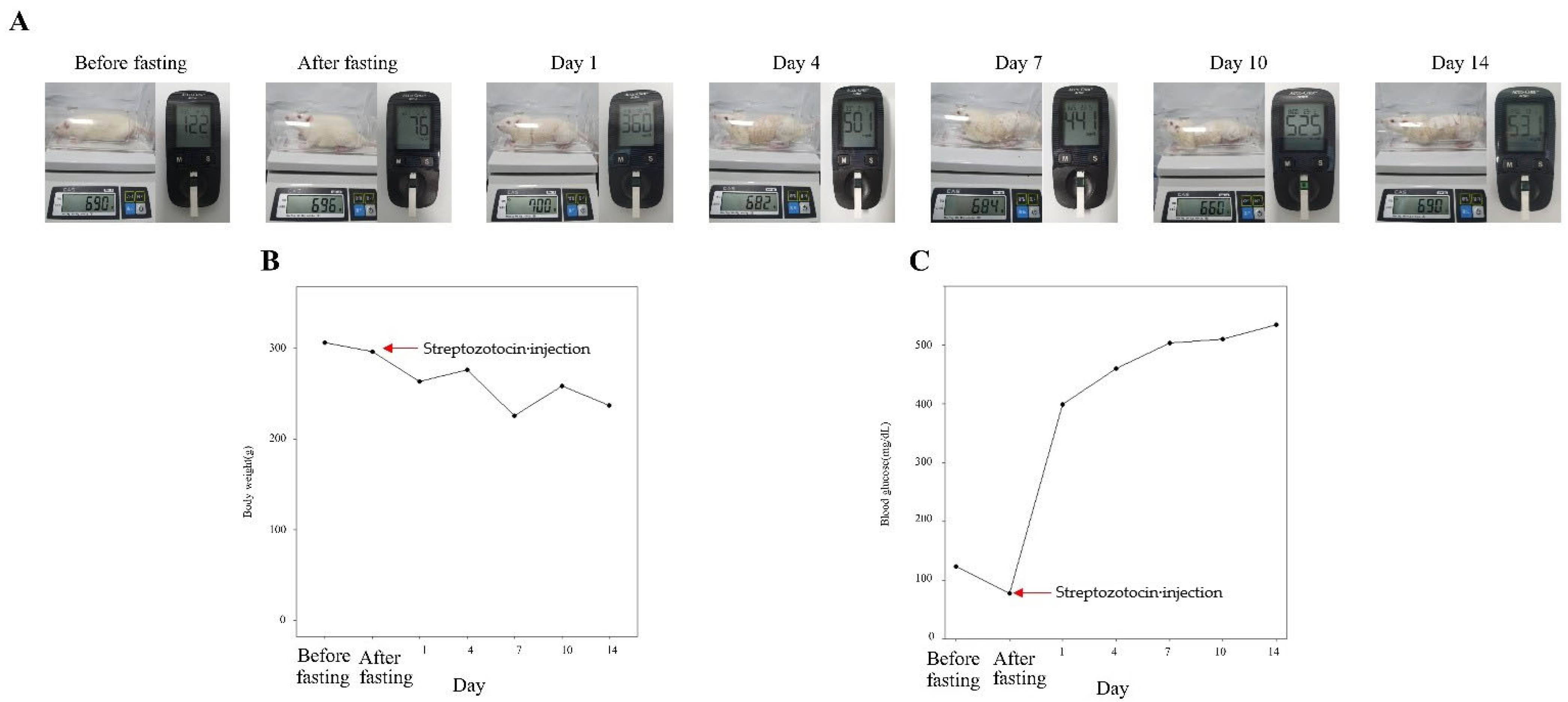
2.1. Animal and Induction of Diabetes
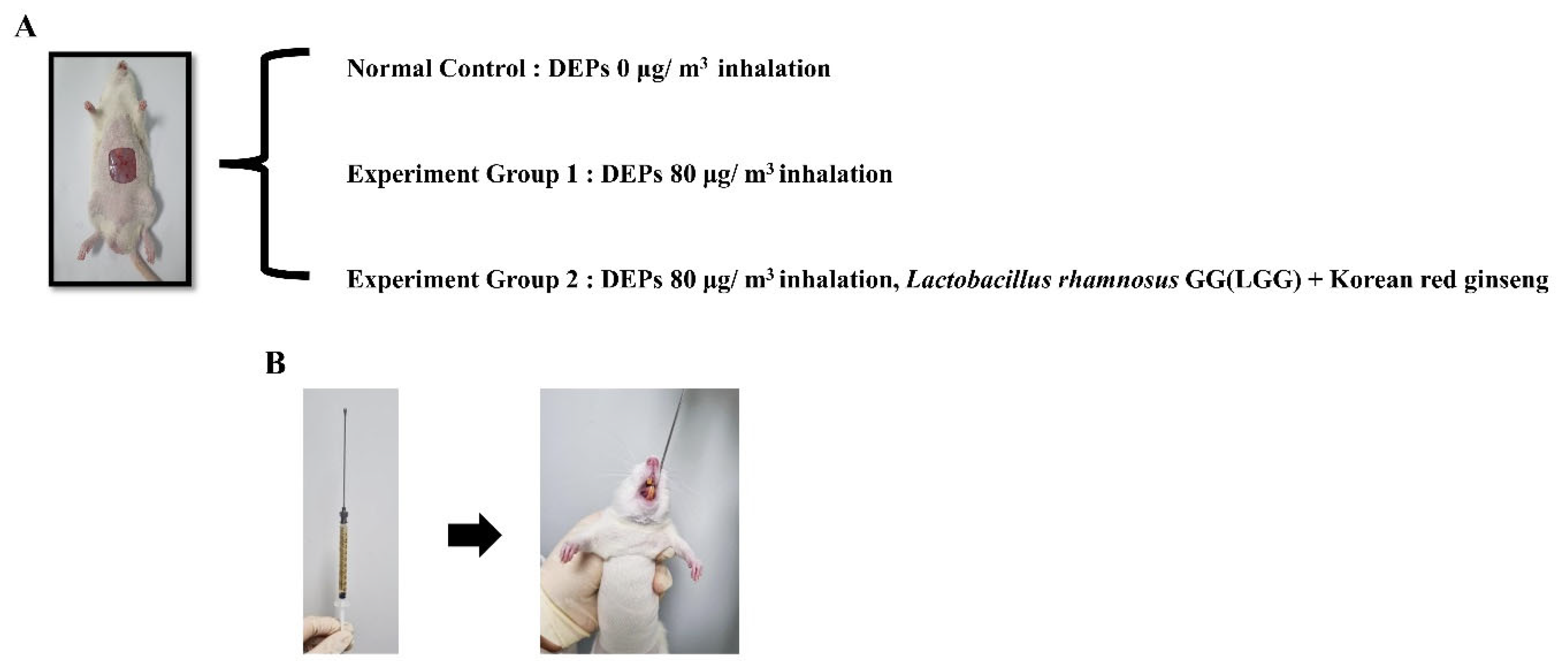
2.2. Methicillin-Resistant S. aureus (MRSA) Infection Wound Induction and DEP Inhalation
2.3. Treatment with a Combination of Probiotics and KRG
2.4. Skin Biopsy
2.5. Wound Size Measurement
2.6. Western Blotting
2.7. Histology Analysis of Hematoxylin and Eosin (H&E) Staining
2.8. Histology Analysis of Immunohistochemistry (IHC) Staining
2.9. Statistical Analysis
3. Results
3.1. Weight and Blood Glucose Measurement
3.2. Confirmation of Wound Size in Animal Diabetic Wound Model Exposed to PM 2.5
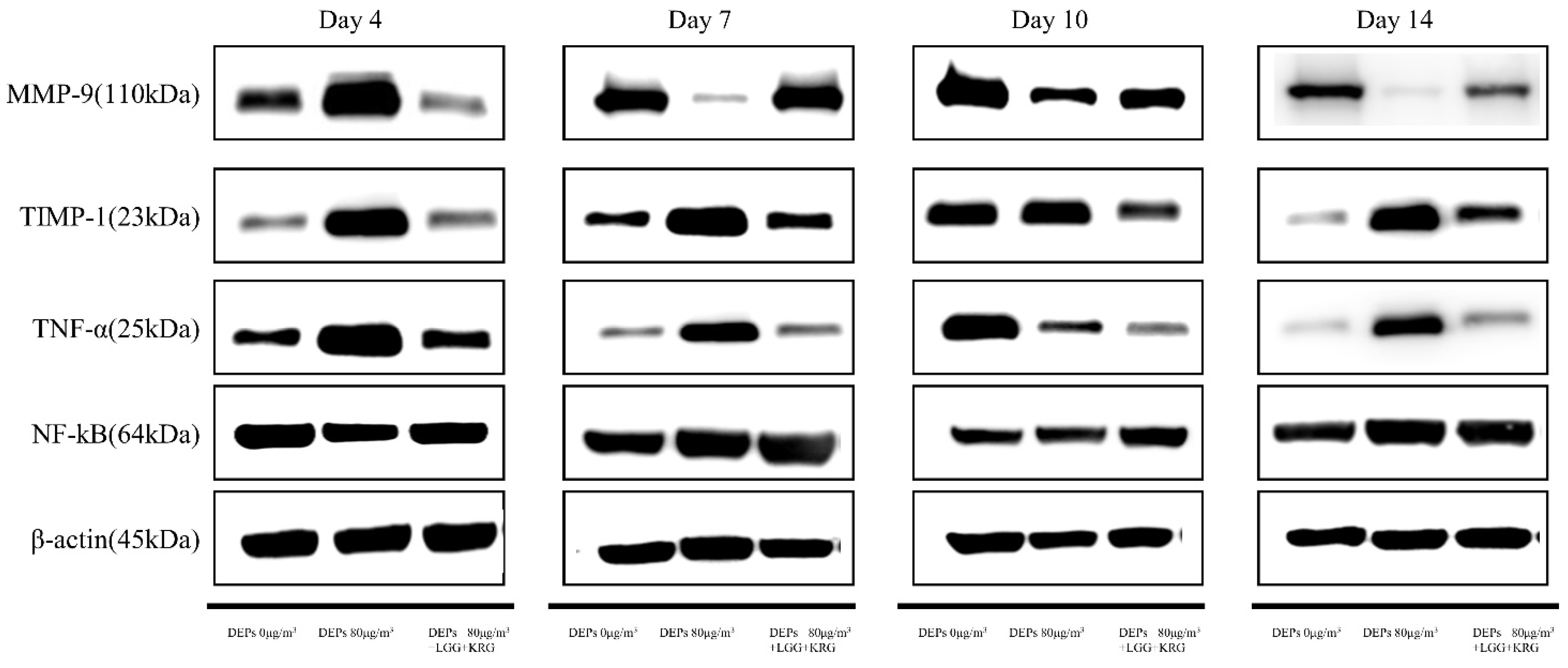
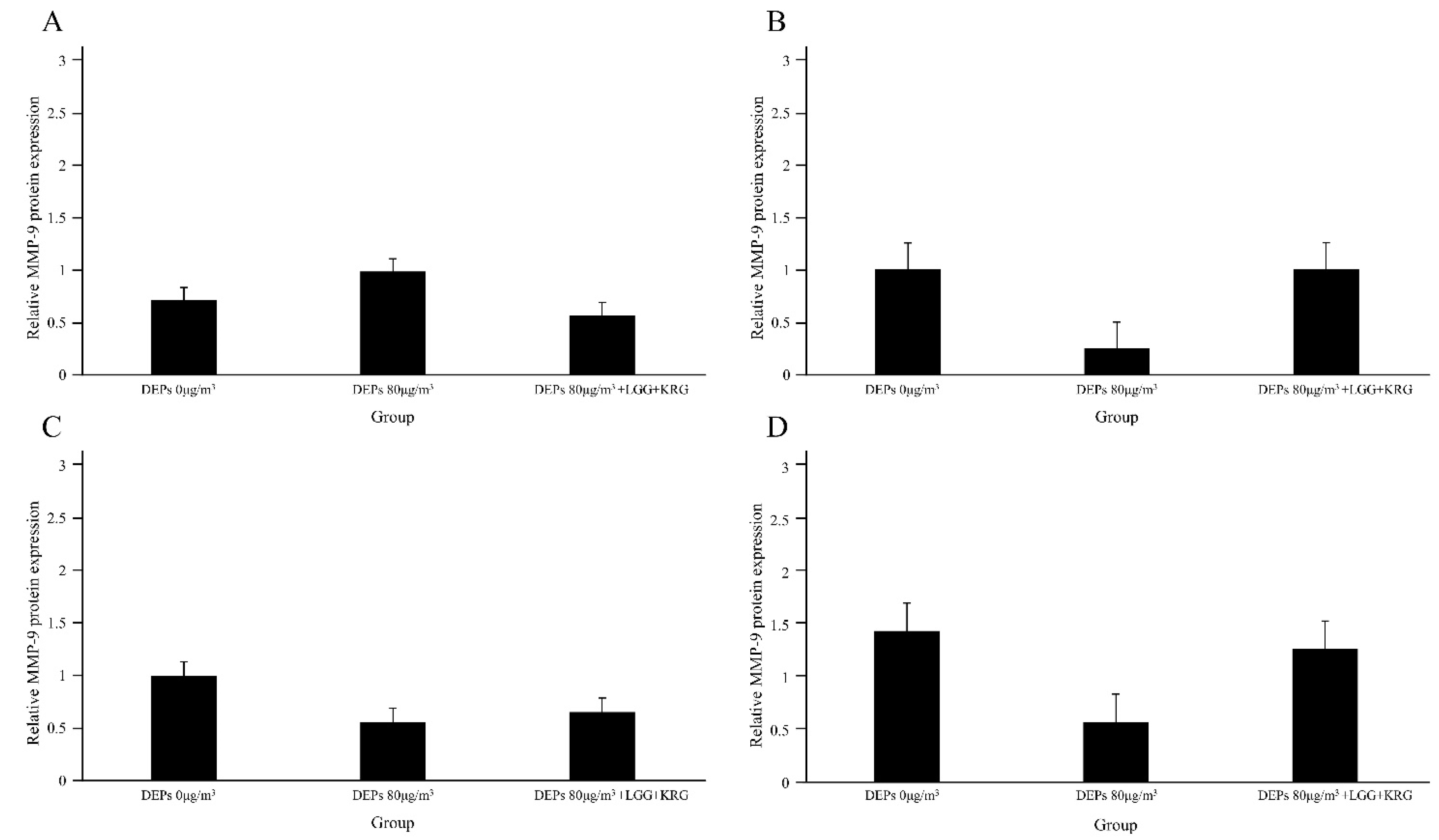
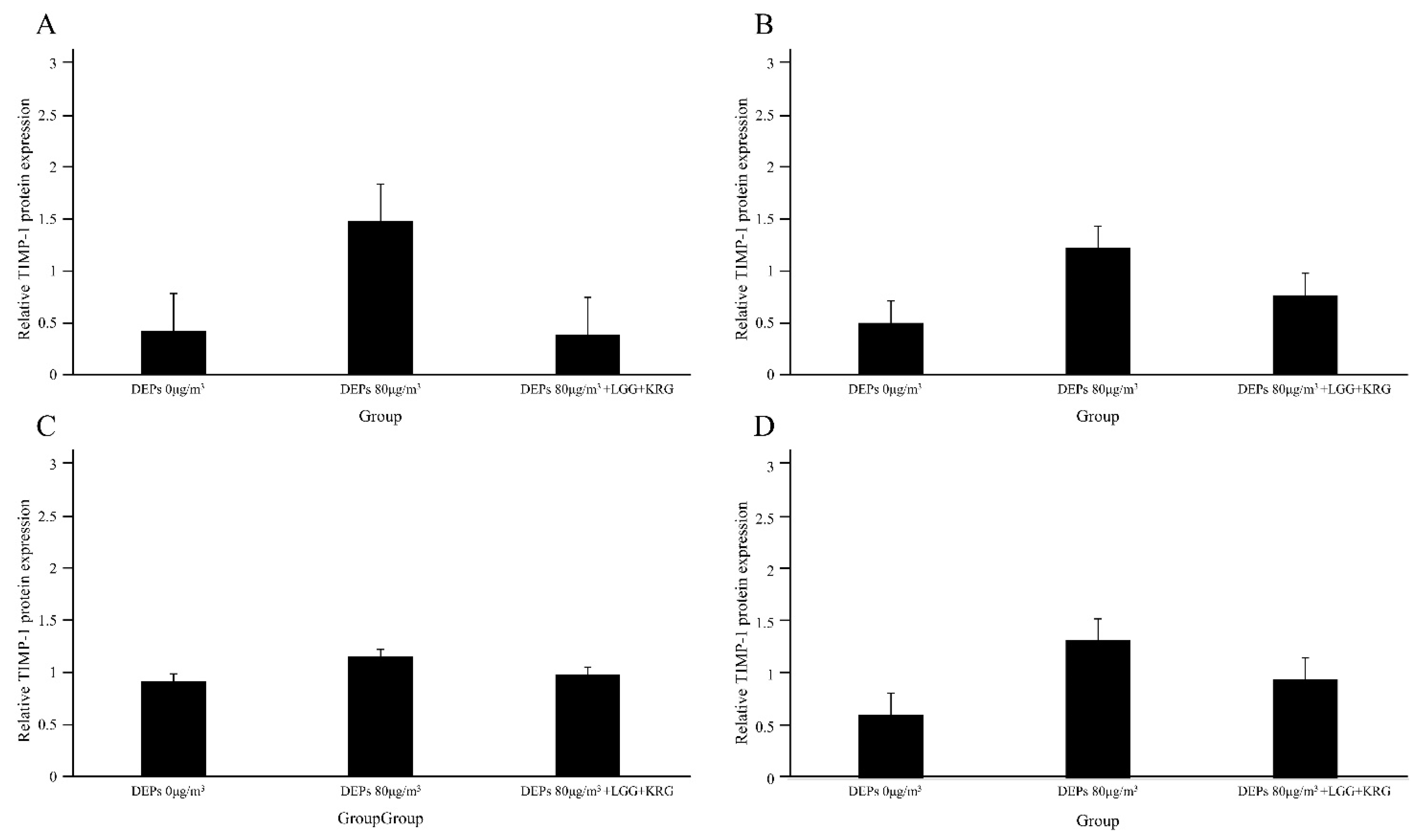
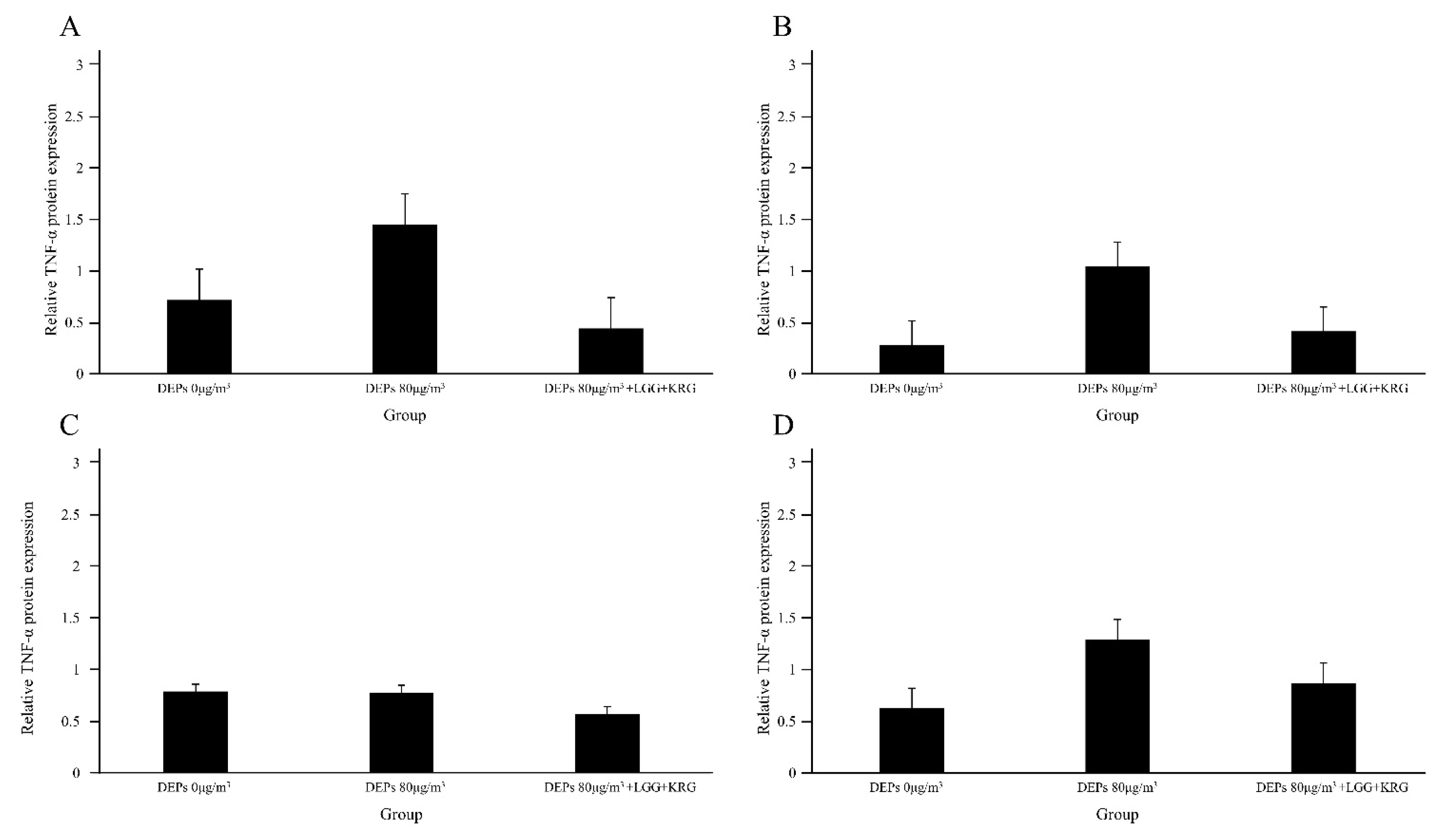
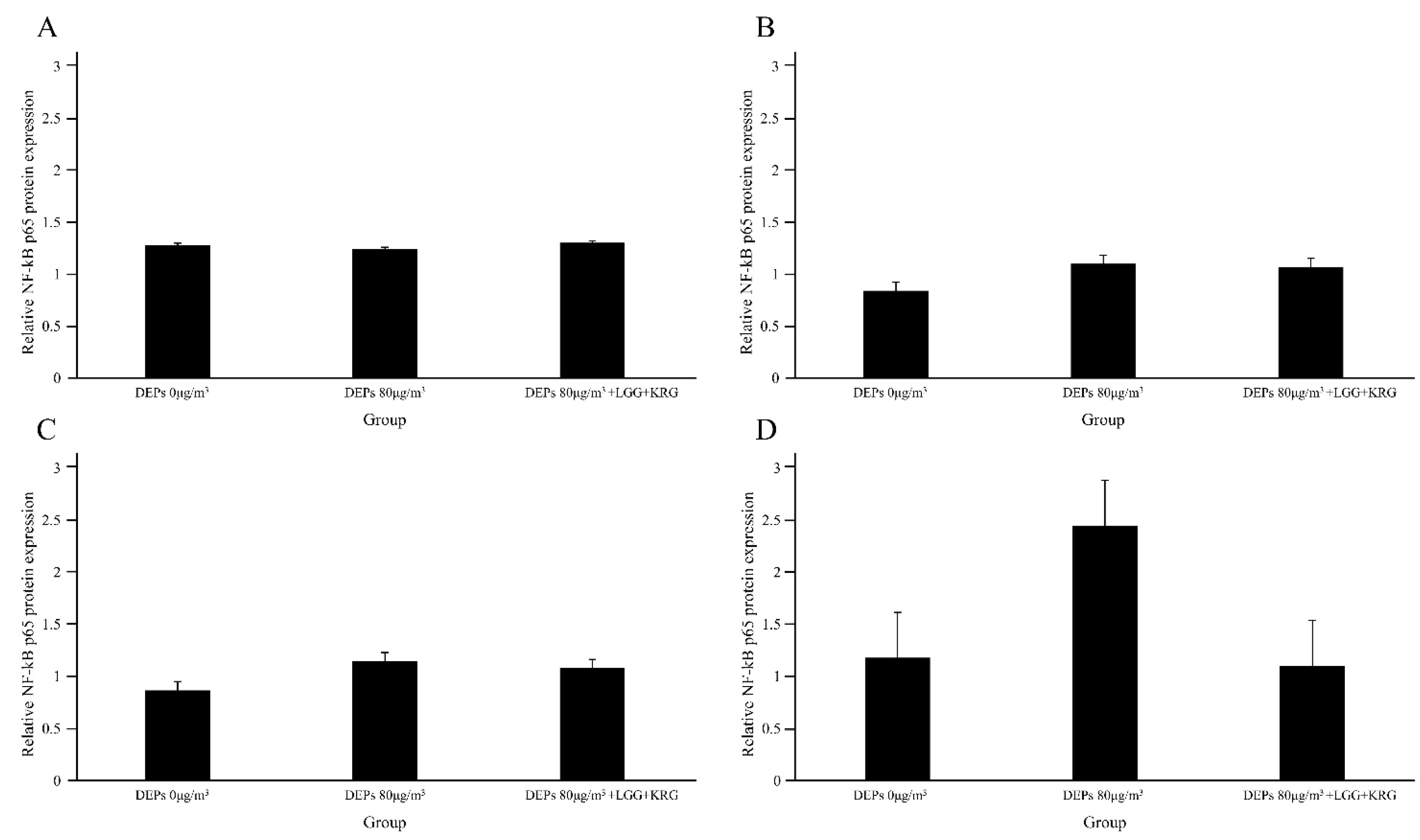
3.3. Western Blot
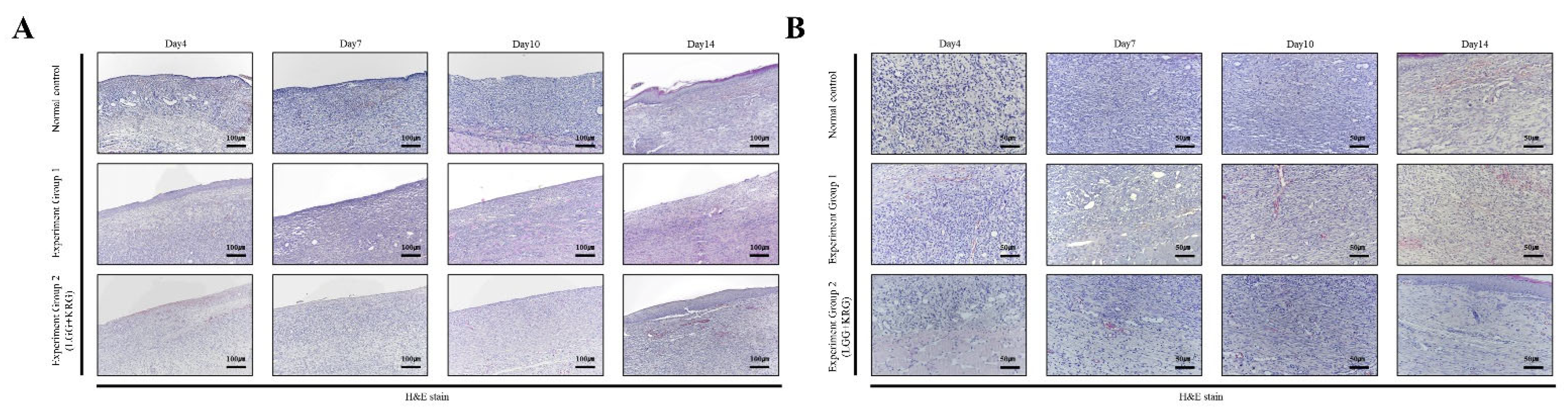
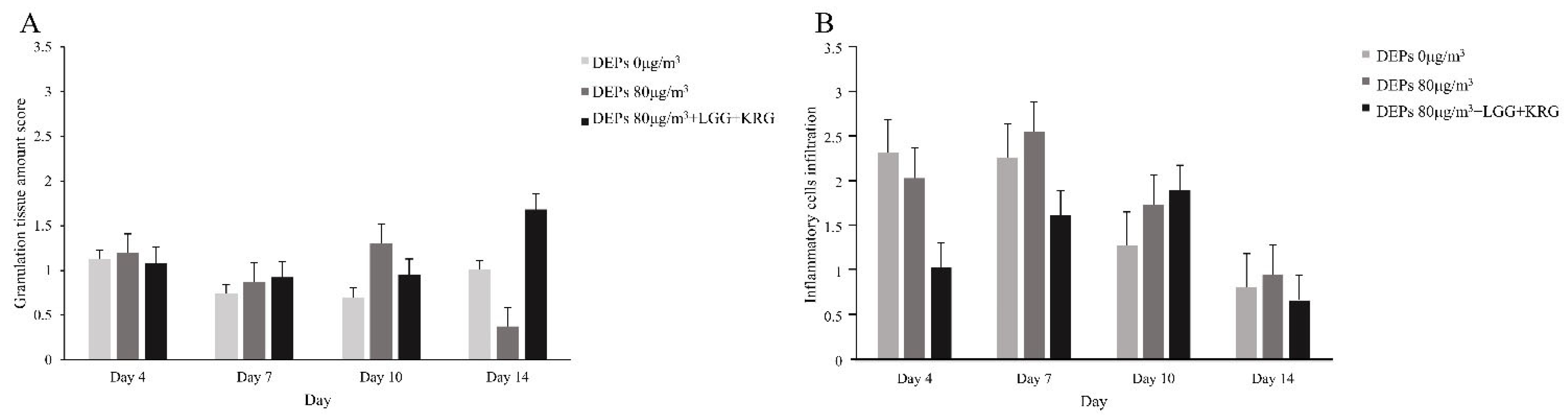
3.4. Hematoxylin and Eosin (H&E) Staining
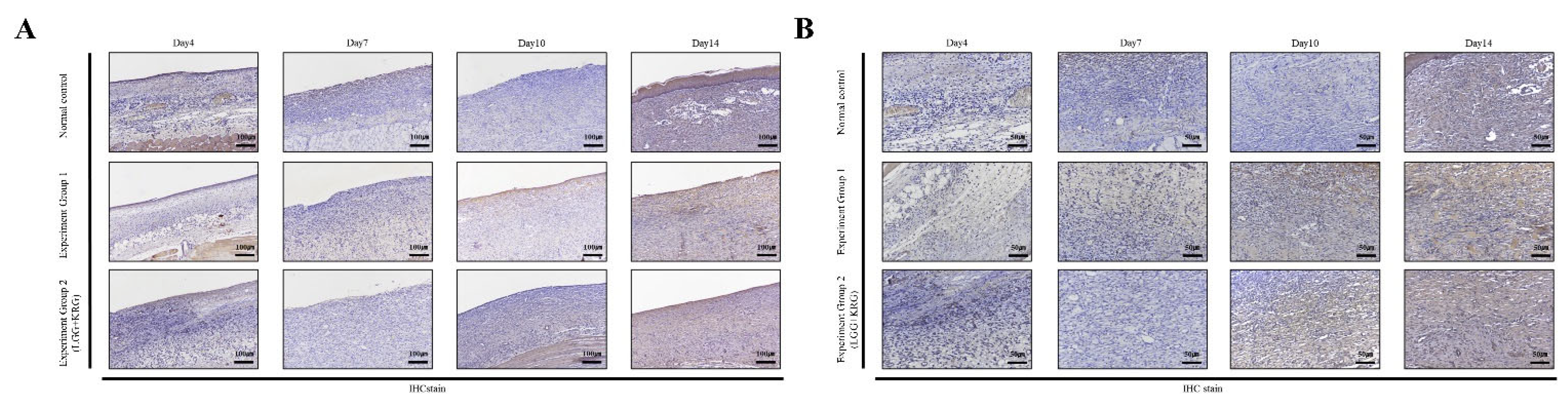
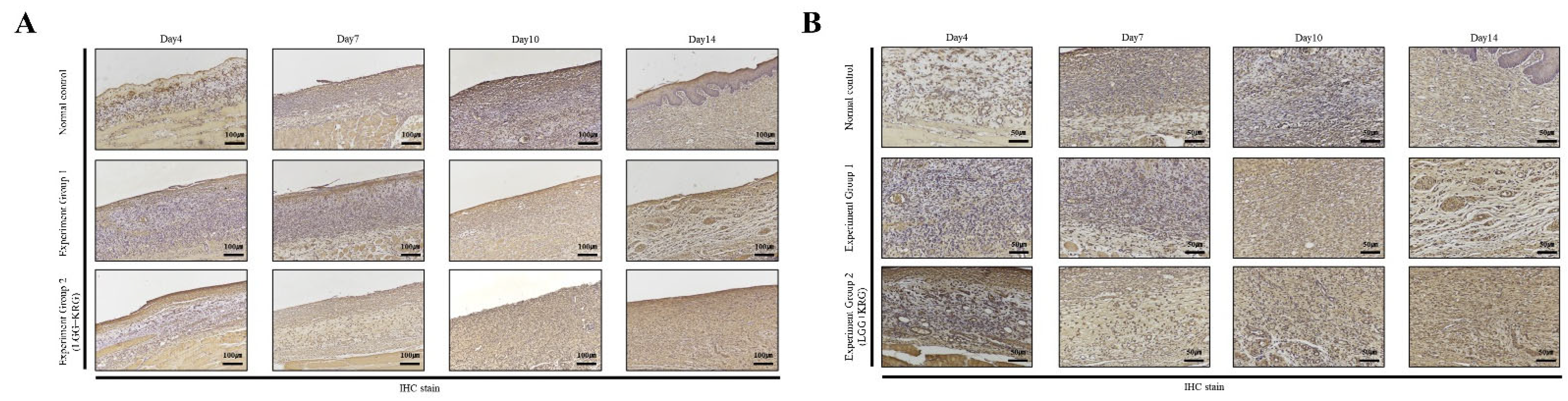
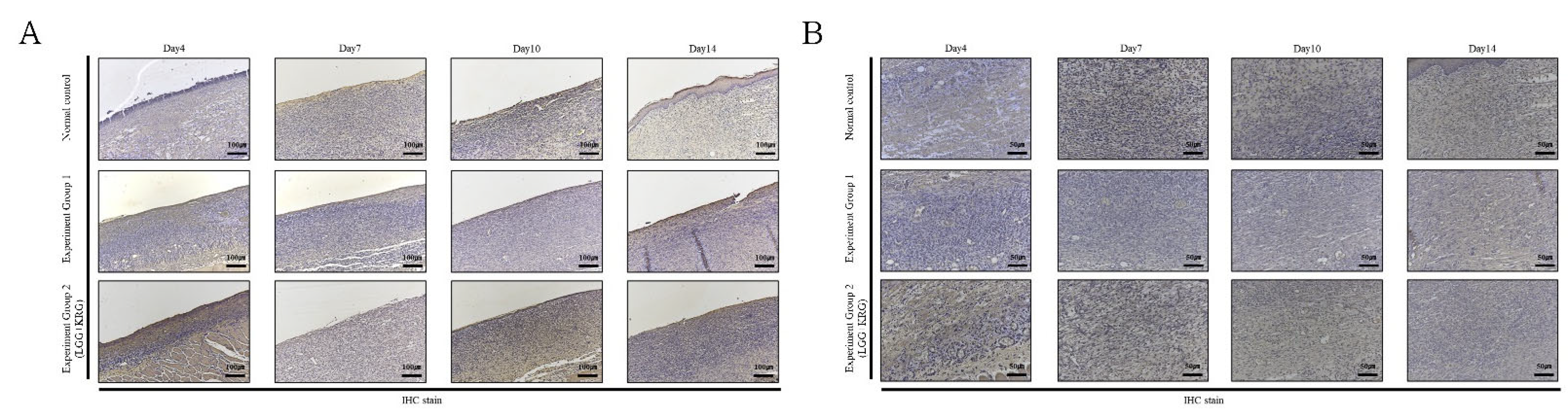
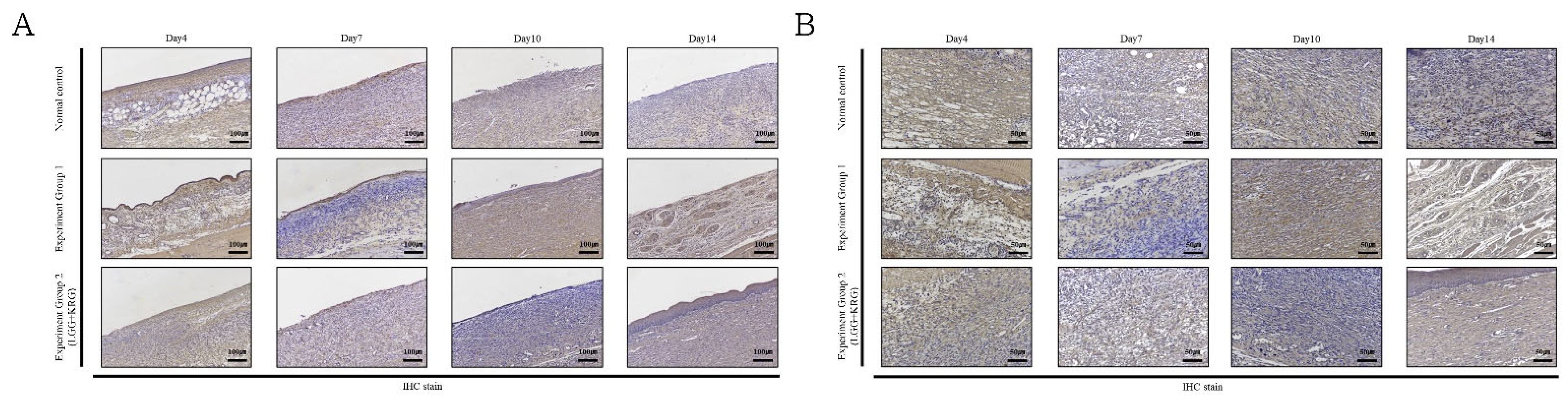
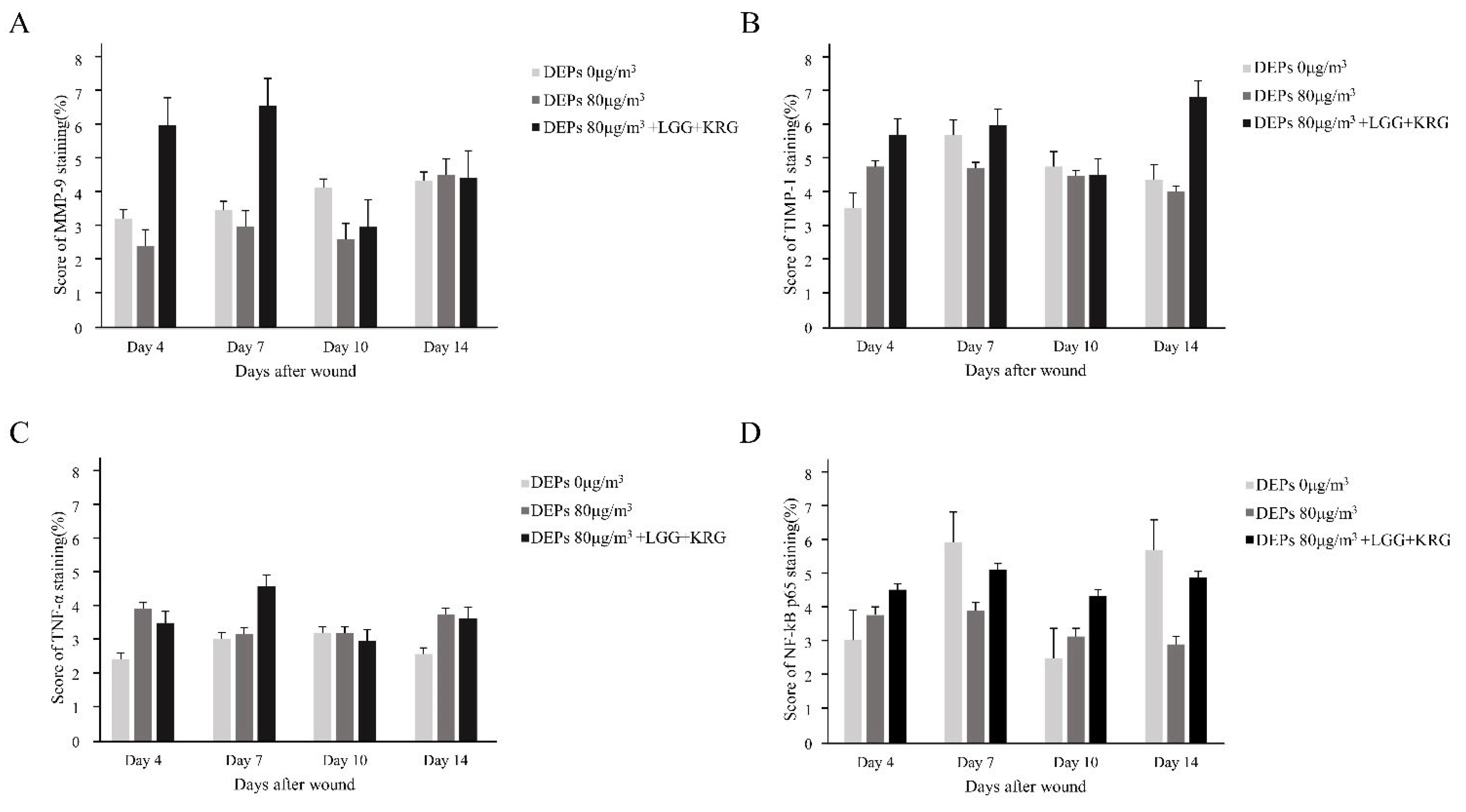
3.5. Immunohistochemistry (IHC) Staining
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Cho, N.H.; Shaw, J.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.; Ohlrogge, A.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Lu, J.; Jing, Y.; Tang, S.; Zhu, D.; Bi, Y. Global epidemiology of diabetic foot ulceration: A systematic review and meta-analysis (dagger). Ann. Med. 2017, 49, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.C.; Chhatbar, K.C.; Kashikar, A.; Mehndiratta, A. Diabetic foot. Bmj 2017, 359, j5064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanolkar, M.; Bain, S.; Stephens, J. The diabetic foot. QJM Int. J. Med. 2008, 101, 685–695. [Google Scholar] [CrossRef]
- Riedl, M.; Diaz-Sanchez, D. Biology of diesel exhaust effects on respiratory function. J. Allergy Clin. Immunol. 2005, 115, 221–228, quiz 229. [Google Scholar] [CrossRef]
- Park, J.; Park, E.H.; Schauer, J.J.; Yi, S.-M.; Heo, J. Reactive oxygen species (ROS) activity of ambient fine particles (PM2.5) measured in Seoul, Korea. Environ. Int. 2018, 117, 276–283. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Jayawardena, T.U.; Sanjeewa, K.K.A.; Wang, L.; Jeon, Y.J.; Lee, W.W. Anti-inflammatory potential of alginic acid from Sargassum horneri against urban aerosol-induced inflammatory responses in keratinocytes and macrophages. Ecotoxicol. Environ. Saf. 2018, 160, 24–31. [Google Scholar] [CrossRef]
- Ellis, S.; Lin, E.J.; Tartar, D. Immunology of Wound Healing. Curr. Dermatol. Rep. 2018, 7, 350–358. [Google Scholar] [CrossRef] [Green Version]
- Schierle, C.F.; De la Garza, M.; Mustoe, T.A.; Galiano, R.D. Staphylococcal biofilms impair wound healing by delaying reepithelialization in a murine cutaneous wound model. Wound Repair. Regen. 2009, 17, 354–359. [Google Scholar] [CrossRef]
- Prabhakara, R.; Harro, J.M.; Leid, J.G.; Keegan, A.D.; Prior, M.L.; Shirtliff, M.E. Suppression of the inflammatory immune response prevents the development of chronic biofilm infection due to methicillin-resistant Staphylococcus aureus. Infect. Immun. 2011, 79, 5010–5018. [Google Scholar] [CrossRef] [Green Version]
- Cremon, C.; Barbaro, M.R.; Ventura, M.; Barbara, G. Pre- and probiotic overview. Curr. Opin. Pharmacol. 2018, 43, 87–92. [Google Scholar] [CrossRef]
- Hacini-Rachinel, F.; Gheit, H.; Le Luduec, J.-B.; Dif, F.; Nancey, S.; Kaiserlian, D. Oral probiotic control skin inflammation by acting on both effector and regulatory T cells. PLoS ONE 2009, 4, e4903. [Google Scholar] [CrossRef] [Green Version]
- Lukic, J.; Chen, V.; Strahinic, I.; Begovic, J.; Lev-Tov, H.; Davis, S.C.; Tomic-Canic, M.; Pastar, I. Probiotics or pro-healers: The role of beneficial bacteria in tissue repair. Wound Repair. Regen. 2017, 25, 912–922. [Google Scholar] [CrossRef]
- Lam, E.K.; Yu, L.; Wong, H.P.; Wu, W.K.; Shin, V.Y.; Tai, E.K.; So, W.H.; Woo, P.C.; Cho, C.H. Probiotic Lactobacillus rhamnosus GG enhances gastric ulcer healing in rats. Eur. J. Pharmacol. 2007, 565, 171–179. [Google Scholar] [CrossRef]
- Jung, C.H.; Seog, H.M.; Choi, I.W.; Choi, H.D.; Cho, H.Y. Effects of wild ginseng (Panax ginseng C.A. Meyer) leaves on lipid peroxidation levels and antioxidant enzyme activities in streptozotocin diabetic rats. J. Ethnopharmacol. 2005, 98, 245–250. [Google Scholar] [CrossRef]
- Kim, Y.S.; Cho, I.H.; Jeong, M.J.; Jeong, S.J.; Nah, S.Y.; Cho, Y.S.; Kim, S.H.; Go, A.; Kim, S.E.; Kang, S.S.; et al. Therapeutic effect of total ginseng saponin on skin wound healing. J. Ginseng Res. 2011, 35, 360–367. [Google Scholar] [CrossRef] [Green Version]
- Park, K.S.; Park, D.H. The effect of Korean Red Ginseng on full-thickness skin wound healing in rats. J. Ginseng Res. 2019, 43, 226–235. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, C.; Graves, D.T. Abnormal cell responses and role of TNF-alpha in impaired diabetic wound healing. Biomed. Res. Int. 2013, 2013, 754802. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Saqib, U.; Sarkar, S.; Suk, K.; Mohammad, O.; Baig, M.S.; Savai, R. Phytochemicals as modulators of M1-M2 macrophages in inflammation. Oncotarget 2018, 9, 17937–17950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Z.; Shi, F.; Zhou, Z.; Sun, F.; Sun, M.H.; Sun, Q.; Chen, L.; Li, D.; Jiang, C.Y.; Zhao, R.Z.; et al. M1 macrophage mediated increased reactive oxygen species (ROS) influence wound healing via the MAPK signaling in vitro and in vivo. Toxicol. Appl. Pharmacol. 2019, 366, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.R.; Sultan, M.T.; Park, H.J.; Lee, J.M.; Ju, H.W.; Lee, O.J.; Lee, D.J.; Kaplan, D.L.; Park, C.H. NF-kappaB signaling is key in the wound healing processes of silk fibroin. Acta Biomater. 2018, 67, 183–195. [Google Scholar] [CrossRef]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef] [Green Version]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef]
- Mirza, R.; Koh, T.J. Dysregulation of monocyte/macrophage phenotype in wounds of diabetic mice. Cytokine 2011, 56, 256–264. [Google Scholar] [CrossRef]
- Liu, C.; Ying, Z.; Harkema, J.; Sun, Q.; Rajagopalan, S. Epidemiological and experimental links between air pollution and type 2 diabetes. Toxicol. Pathol. 2013, 41, 361–373. [Google Scholar] [CrossRef] [Green Version]
- Lawal, A.O.; Folorunso, I.M.; Iwaloye, O. Morin hydrate protects type-2-diabetic wistar rats exposed to diesel exhaust particles from inflammation and oxidative stress. J. Diabetes Metab. Disord. 2022, 21, 805–816. [Google Scholar] [CrossRef]
- Nemmar, A.; Subramaniyan, D.; Yasin, J.; Ali, B.H. Impact of experimental type 1 diabetes mellitus on systemic and coagulation vulnerability in mice acutely exposed to diesel exhaust particles. Part. Fibre Toxicol. 2013, 10, 14. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.S.; Sung, I.H.; Lim, J.Y.; Kyun, A.H.; Yeo, E.D.; Lee, S.G.; Lee, Y.K. Evaluation of the Effects of Air Pollutants on Diabetic Wounds. Wounds 2017, 29, 65–70. [Google Scholar]
- Kim, K.; Kim, H.Y. Korean red ginseng stimulates insulin release from isolated rat pancreatic islets. J. Ethnopharmacol. 2008, 120, 190–195. [Google Scholar] [CrossRef]
- Park, J.-K.; Shim, J.-Y.; Cho, A.-R.; Cho, M.-R.; Lee, Y.-J. Korean red ginseng protects against mitochondrial damage and intracellular inflammation in an animal model of type 2 diabetes mellitus. J. Med. Food 2018, 21, 544–550. [Google Scholar] [CrossRef]
- Kimura, Y.; Sumiyoshi, M.; Kawahira, K.; Sakanaka, M. Effects of ginseng saponins isolated from Red Ginseng roots on burn wound healing in mice. Br. J. Pharmacol. 2006, 148, 860–870. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Liu, J.; Xia, Y.; Ai, L. Probiotics-based interventions for diabetes mellitus: A review. Food Biosci. 2021, 43, 101172. [Google Scholar] [CrossRef]
- Lim, S.-M.; Jeong, J.-J.; Woo, K.H.; Han, M.J.; Kim, D.-H. Lactobacillus sakei OK67 ameliorates high-fat diet–induced blood glucose intolerance and obesity in mice by inhibiting gut microbiota lipopolysaccharide production and inducing colon tight junction protein expression. Nutr. Res. 2016, 36, 337–348. [Google Scholar] [CrossRef]
- Tsiouris, C.G.; Tsiouri, M.G. Human microflora, probiotics and wound healing. Wound Med. 2017, 19, 33–38. [Google Scholar] [CrossRef]
- Pinto, D.; Marzani, B.; Minervini, F.; Calasso, M.; Giuliani, G.; Gobbetti, M.; De Angelis, M. Plantaricin A synthesized by Lactobacillus plantarum induces in vitro proliferation and migration of human keratinocytes and increases the expression of TGF-β1, FGF7, VEGF-A and IL-8 genes. Peptides 2011, 32, 1815–1824. [Google Scholar] [CrossRef]
- Ghassib, I.; Chen, Z.; Zhu, J.; Wang, H.L. Use of IL-1 beta, IL-6, TNF-alpha, and MMP-8 biomarkers to distinguish peri-implant diseases: A systematic review and meta-analysis. Clin. Implant. Dent. Relat. Res. 2019, 21, 190–207. [Google Scholar] [CrossRef] [Green Version]
- Madlener, M.; Parks, W.C.; Werner, S. Matrix metalloproteinases (MMPs) and their physiological inhibitors (TIMPs) are differentially expressed during excisional skin wound repair. Exp. Cell Res. 1998, 242, 201–210. [Google Scholar] [CrossRef]
- Nemmar, A.; Al-Salam, S.; Subramaniyan, D.; Yasin, J.; Yuvaraju, P.; Beegam, S.; Ali, B.H. Influence of experimental type 1 diabetes on the pulmonary effects of diesel exhaust particles in mice. Toxicol. Lett. 2013, 217, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.-W.; Li, H.; Guedez, L.; Wingfield, P.T.; Diaz, T.; Salloum, R.; Wei, B.-y.; Stetler-Stevenson, W.G. TIMP-2 mediated inhibition of angiogenesis: An MMP-independent mechanism. Cell. 2003, 114, 171–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, A.H.; Edwards, D.R.; Murphy, G. Metalloproteinase inhibitors: Biological actions and therapeutic opportunities. J. Cell Sci. 2002, 115, 3719–3727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, C.-C.E.; Wu, C.-S.; Huang, S.-M.; Wu, I.-H.; Chen, G.-S. High-glucose environment enhanced oxidative stress and increased interleukin-8 secretion from keratinocytes: New insights into impaired diabetic wound healing. Diabetes 2013, 62, 2530–2538. [Google Scholar] [CrossRef] [Green Version]


| Target Gene | Company | Dilution | Size |
|---|---|---|---|
| Anti NF-κB p65 | Abcam | 1:300 | 64 kDa |
| Anti TNF-A | Santa Cruz Biotechnology | 1:500 | 25 kDa |
| Anti MMP-9 | Abcam | 1:500 | 92 kDa |
| Anti TIMP-1 | Santa Cruz Biotechnology | 1:1000 | 23 kDa |
| Anti B-actin | Cell Signaling | 1:5000 | 45 kDa |
| Judgement Element | Standard | Score |
|---|---|---|
| 1) Granulation tissue of formation | None (not formation) | 0 |
| 2) Epidermis formation | Scant | 1 |
| 3) Keratin layer formation | Moderate | 2 |
| 4) Collagen deposition | Abundant | 3 |
| Judgement Element | Standard | Score |
|---|---|---|
| Inflammatory cells infiltration | None (no necrosis and inflammation) | 0 |
| Slight inflammation (not clusters and only exist in cell form) | 1 | |
| Moderate inflammation (when small clusters are formed and inflammatory cells are visible) | 2 | |
| Abundant (when large clusters are formed and many inflammatory cells are visible) | 3 |
| Judgement Element | Standard | Score |
|---|---|---|
| Dyeing intensity | None | 0 |
| Slight | 1 | |
| Moderate | 2 | |
| Heavy | 3 | |
| Cell staining rate (%) | 0% | 0 |
| 1–25% | 1 | |
| 26–50% | 2 | |
| 51–75% | 3 | |
| 76–100% | 4 |
| Experimental Groups | Wound Closure Rate (%) | |||||
|---|---|---|---|---|---|---|
| Period of Treatment | ||||||
| Day 1 | Day 4 | Day 7 | Day 10 | Day 14 | N | |
| Normal control (0 µg/m3) | 100 | 77.12 ± 1.27 | 56.75 ± 16.61 | 22.31 ± 7.64 | 16.20 ± 7.60 | 3 |
| Group 1 (80 µg/m3) | 100 | 61.55 ± 31.69 | 32.17 ± 21.94 | 21.06 ± 15.16 | 11.82 ± 5.15 | 3 |
| Group 2 (80 µg/m3,LGG + KRG) | 100 | 86.06 ± 8.97 | 70.34 ± 5.09 | 44.47 ± 1.52 | 18.82 ± 3.35 | 3 |
| Experimental Groups | Relative Protein Expression (%) | |||||
|---|---|---|---|---|---|---|
| Period of Treatment | ||||||
| Gene | Day 4 | Day 7 | Day 10 | Day 14 | N | |
| Normal control (0 µg/m3) | MMP-9 | 0.71 ± 0.59 | 1.01 ± 0.31 | 1.00 ± 0.18 | 1.45 ± 0.13 | 3 |
| TIMP-1 | 0.42 ± 0.24 | 0.50 ± 0.03 | 0.92 ± 0.34 | 0.62 ± 0.46 | 3 | |
| TNF-a | 0.72 ± 0.35 | 0.2 ± 0.06 | 0.79 ± 0.41 | 0.63 ± 0.46 | 3 | |
| NF-kB | 1.28 ± 0.20 | 0.84 ± 0.37 | 0.87 ± 0.28 | 1.18 ± 0.40 | 3 | |
| Group 1 (80 µg/m3) | MMP-9 | 0.99 ± 0.23 | 0.25 ± 0.26 | 0.56 ± 0.51 | 0.58 ± 0.46 | 3 |
| TIMP-1 | 1.48 ± 0.82 | 1.22 ± 0.04 | 1.15 ± 0.20 | 1.33 ± 0.26 | 3 | |
| TNF-a | 1.45 ± 0.96 | 1.05 ± 0.17 | 0.78 ± 0.33 | 1.29 ± 0.40 | 3 | |
| NF-kB | 1.24 ± 0.32 | 1.10 ± 0.49 | 1.15 ± 0.59 | 2.45 ± 1.00 | 3 | |
| Group 2 (80 µg/m3,LGG + KRG) | MMP-9 | 0.57 ± 0.27 | 1.01 ± 0.23 | 0.65 ± 0.26 | 1.37 ± 0.24 | 3 |
| TIMP-1 | 0.39 ± 0.27 | 0.77 ± 0.27 | 0.98 ± 0.24 | 0.96 ± 0.43 | 3 | |
| TNF-a | 0.44 ± 0.19 | 0.42 ± 0.23 | 0.57 ± 0.31 | 0.87 ± 0.66 | 3 | |
| NF-kB | 1.30 ± 0.24 | 1.07 ± 0.20 | 1.08 ± 0.31 | 1.10 ± 0.01 | 3 | |
| Experimental Groups | Granulation Tissue Formation (%) | ||||
|---|---|---|---|---|---|
| Period of Treatment | |||||
| Day 4 | Day 7 | Day 10 | Day 14 | N | |
| Normal control (0 µg/m3) | 1.38 ± 0.38 | 0.74 ± 0.42 | 0.70 ± 0.26 | 1.01 ± 0.26 | 3 |
| Group 1 (80 µg/m3) | 1.20 ± 0.83 | 0.88 ± 0.45 | 1.31 ± 0.45 | 0.38 ± 0.23 | 3 |
| Group 2 (80 µg/m3,LGG + KRG) | 0.95 ± 0.48 | 0.93 ± 0.42 | 0.95 ± 0.63 | 1.68 ± 0.62 | 3 |
| Experimental Groups | Inflammatory Cells Infiltration (%) | ||||
|---|---|---|---|---|---|
| Period of Treatment | |||||
| Day 4 | Day 7 | Day 10 | Day 14 | N | |
| Normal control (0 µg/m3) | 1.27 ± 0.19 | 2.41 ± 0.15 | 1.61 ± 0.25 | 1.10 ± 0.18 | 3 |
| Group 1 (80 µg/m3) | 2.09 ± 0.38 | 2.63 ± 0.14 | 1.83 ± 0.36 | 1.19 ± 0.09 | 3 |
| Group 2 (80 µg/m3, LGG + KRG) | 1.16 ± 0.14 | 1.74 ± 0.47 | 1.91 ± 0.46 | 0.98 ± 0.34 | 3 |
| Experimental Groups | Score of IHC Staining(%) | |||||
|---|---|---|---|---|---|---|
| Period of Treatment | ||||||
| Gene | Day 4 | Day 7 | Day 10 | Day 14 | N | |
| Normal control (0 µg/m3) | MMP-9 | 3.29 ± 1.88 | 3.56 ± 0.51 | 4.21 ± 1.70 | 4.42 ± 1.86 | 3 |
| TIMP-1 | 3.60 ± 1.41+ | 5.78 ± 1.06 | 4.83 ± 1.82 | 4.44 ± 0.98 | 3 | |
| TNF-a | 2.46 ± 0.64 | 3.07 ± 0.29 | 3.24 ± 0.91 | 2.62 ± 0.91 | 3 | |
| NF-kB | 3.08 ± 0.52 | 6.00 ± 0.00 | 2.54 ± 0.36 | 5.77 ± 2.35 | 3 | |
| Group 1 (80 µg/m3) | MMP-9 | 2.48 ± 0.58 | 3.05 ± 0.20 | 2.67 ± 0.47 | 4.59 ± 3.19 | 3 |
| TIMP-1 | 4.83 ± 2.89 | 4.79 ± 1.39 | 4.55 ± 1.26 | 4.08 ± 1.21 | 3 | |
| TNF-a | 3.96 ± 0.73 | 3.21 ± 0.59 | 3.23 ± 0.91 | 3.79 ± 1.61 | 3 | |
| NF-kB | 3.83 ± 0.38 | 3.96 ± 0.94 | 3.19 ± 0.05 | 2.96 ± 0.44 | 3 | |
| Group 2 (80 µg/m3,LGG + KRG) | MMP-9 | 6.08 ± 3.17 | 6.66 ± 2.23 | 3.04 ± 1.06 | 4.50 ± 1.44 | 3 |
| TIMP-1 | 5.79 ± 1.58 | 6.08 ± 1.61 | 4.59 ± 0.95 | 6.92 ± 2.06 | 3 | |
| TNF-a | 3.54 ± 0.92 | 4.63 ± 1.20 | 3.00 ± 0.25 | 3.67 ± 0.88 | 3 | |
| NF-kB | 4.58 ± 0.38 | 5.19 ± 2.19 | 4.41 ± 0.96 | 4.95 ± 0.88 | 3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, H.M.; Choi, Y.S.; Bae, S.K.; Lee, Y.K. Effect of the Combination of Probiotics and Korean Red Ginseng on Diabetic Wound Healing Exposed to Diesel Exhaust Particles(DEPs). Medicina 2023, 59, 1155. https://doi.org/10.3390/medicina59061155
An HM, Choi YS, Bae SK, Lee YK. Effect of the Combination of Probiotics and Korean Red Ginseng on Diabetic Wound Healing Exposed to Diesel Exhaust Particles(DEPs). Medicina. 2023; 59(6):1155. https://doi.org/10.3390/medicina59061155
Chicago/Turabian StyleAn, Hye Min, Young Suk Choi, Sung Kyoung Bae, and Young Koo Lee. 2023. "Effect of the Combination of Probiotics and Korean Red Ginseng on Diabetic Wound Healing Exposed to Diesel Exhaust Particles(DEPs)" Medicina 59, no. 6: 1155. https://doi.org/10.3390/medicina59061155




