Antegrade Posterior Column Acetabulum Fracture Screw Fixation via Posterior Approach: A Biomechanical Comparative Study
Abstract
:1. Introduction
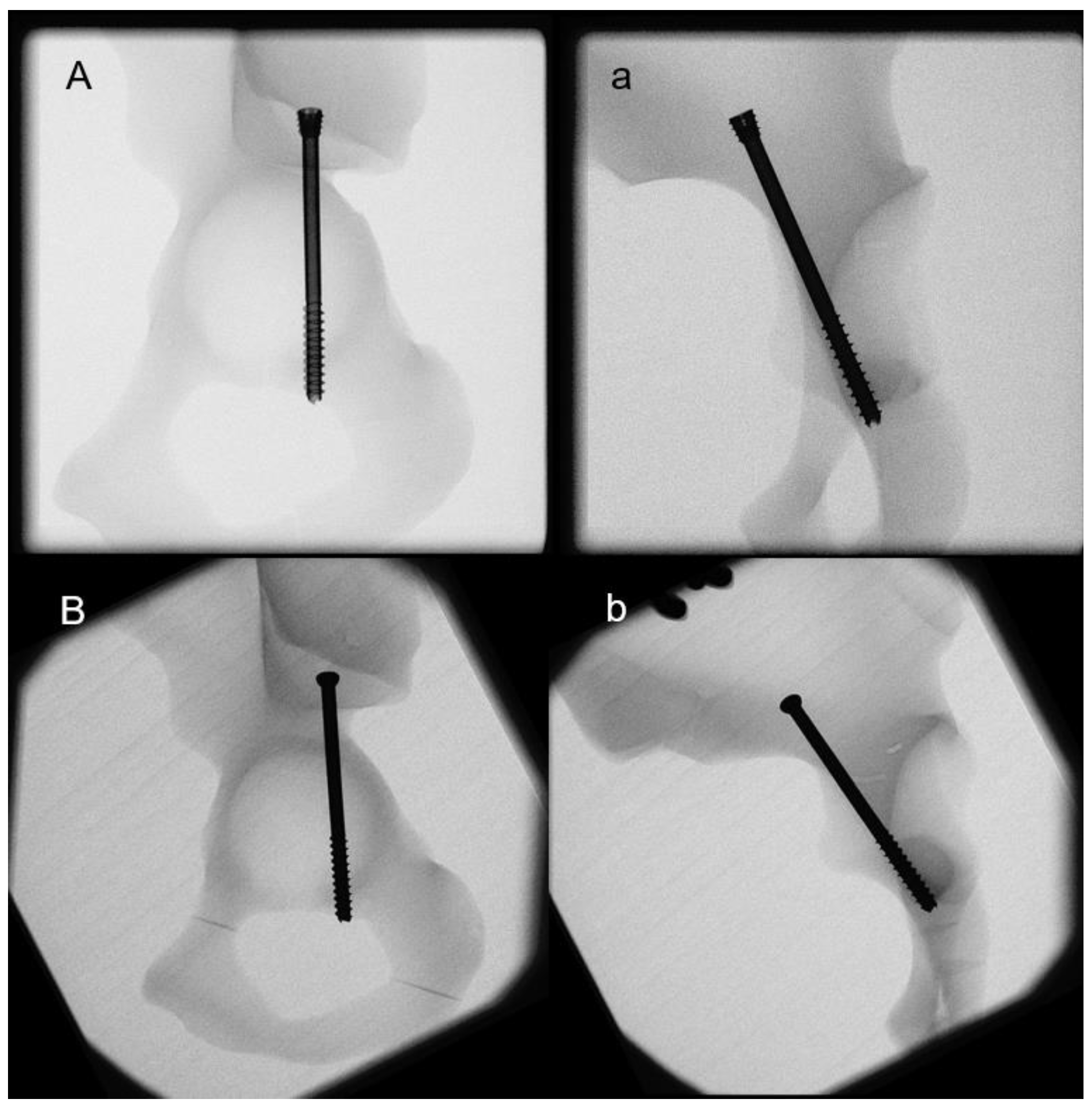
2. Materials and Methods
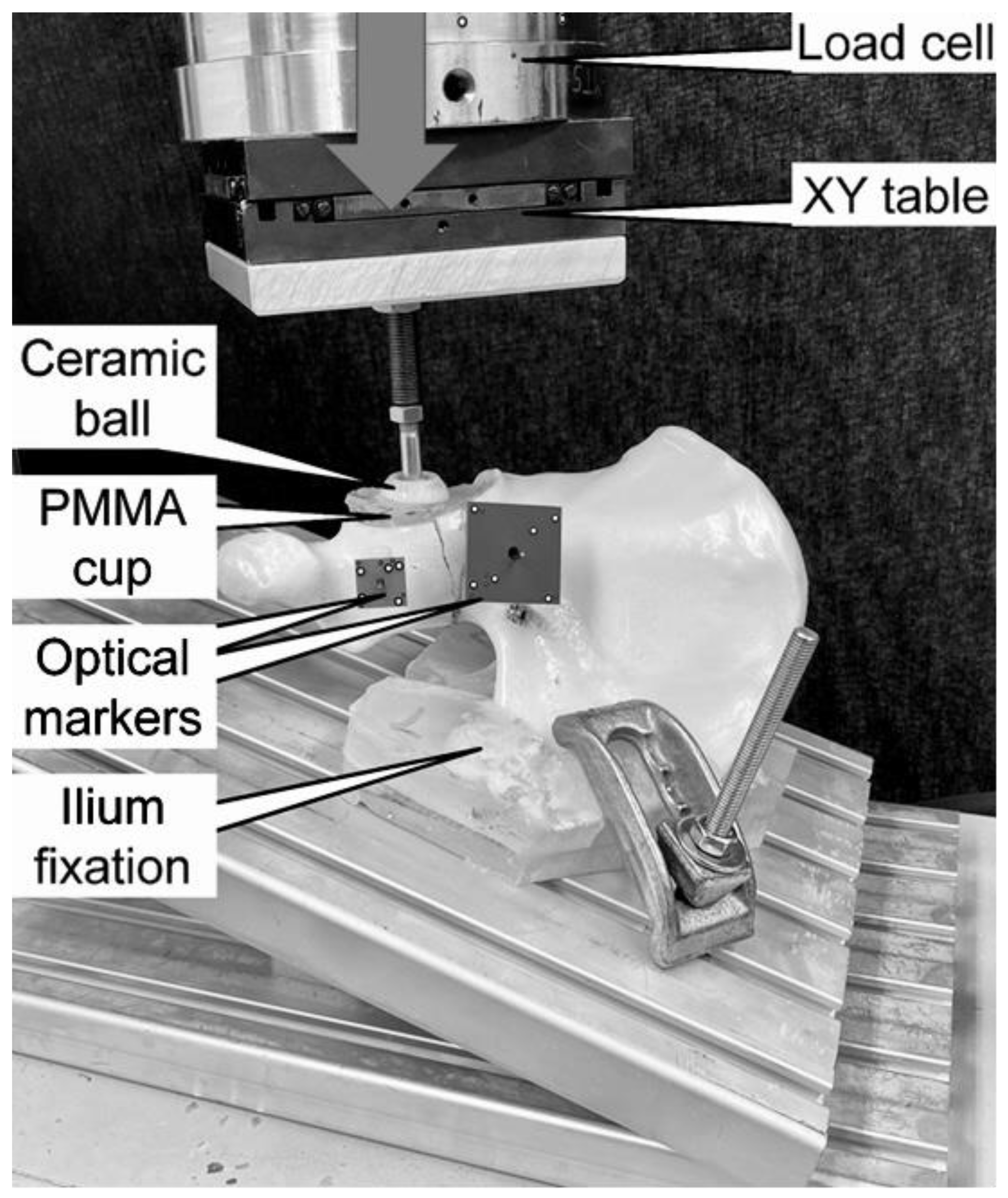
2.1. Biomechanical Testing
2.2. Data Collection and Analysis
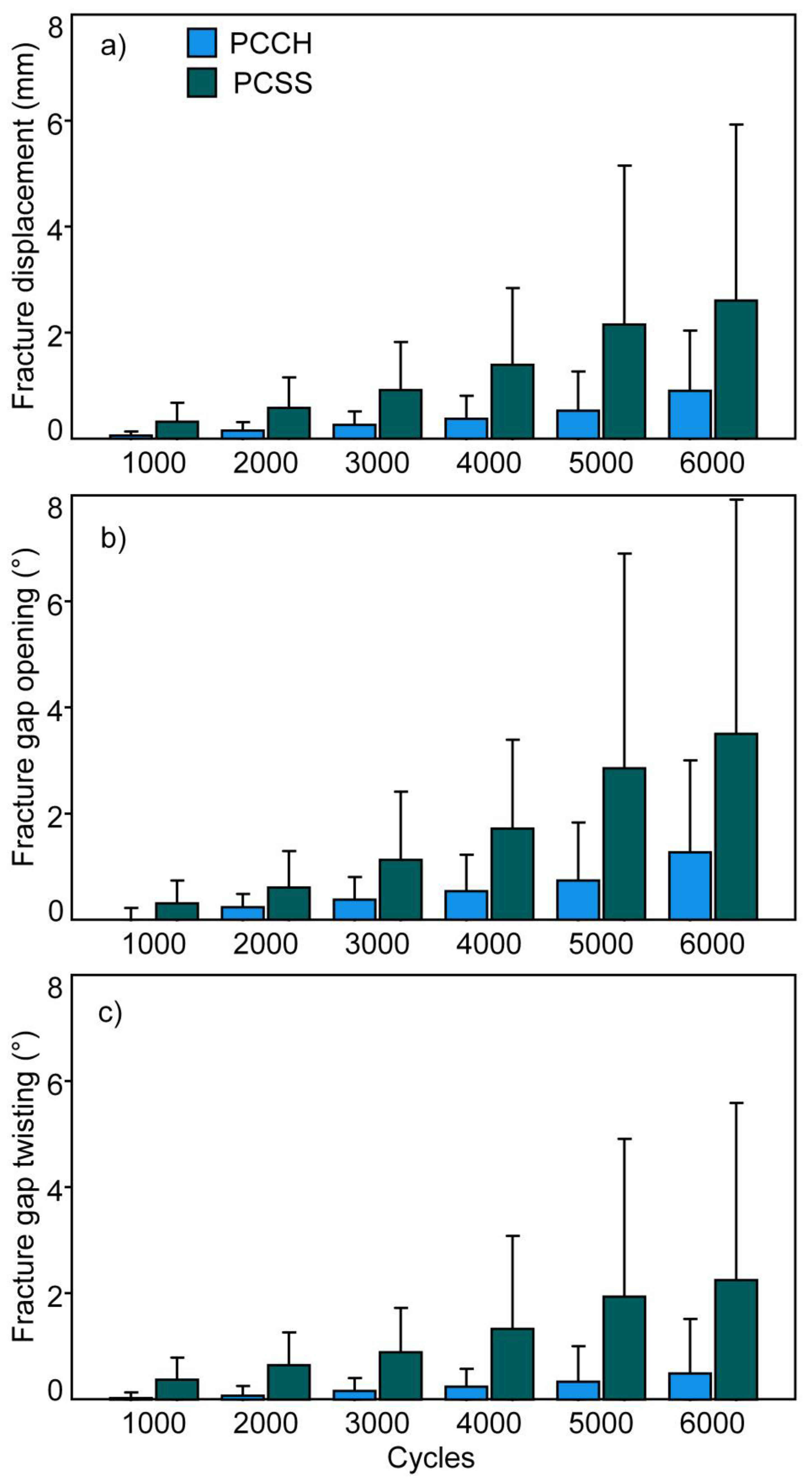
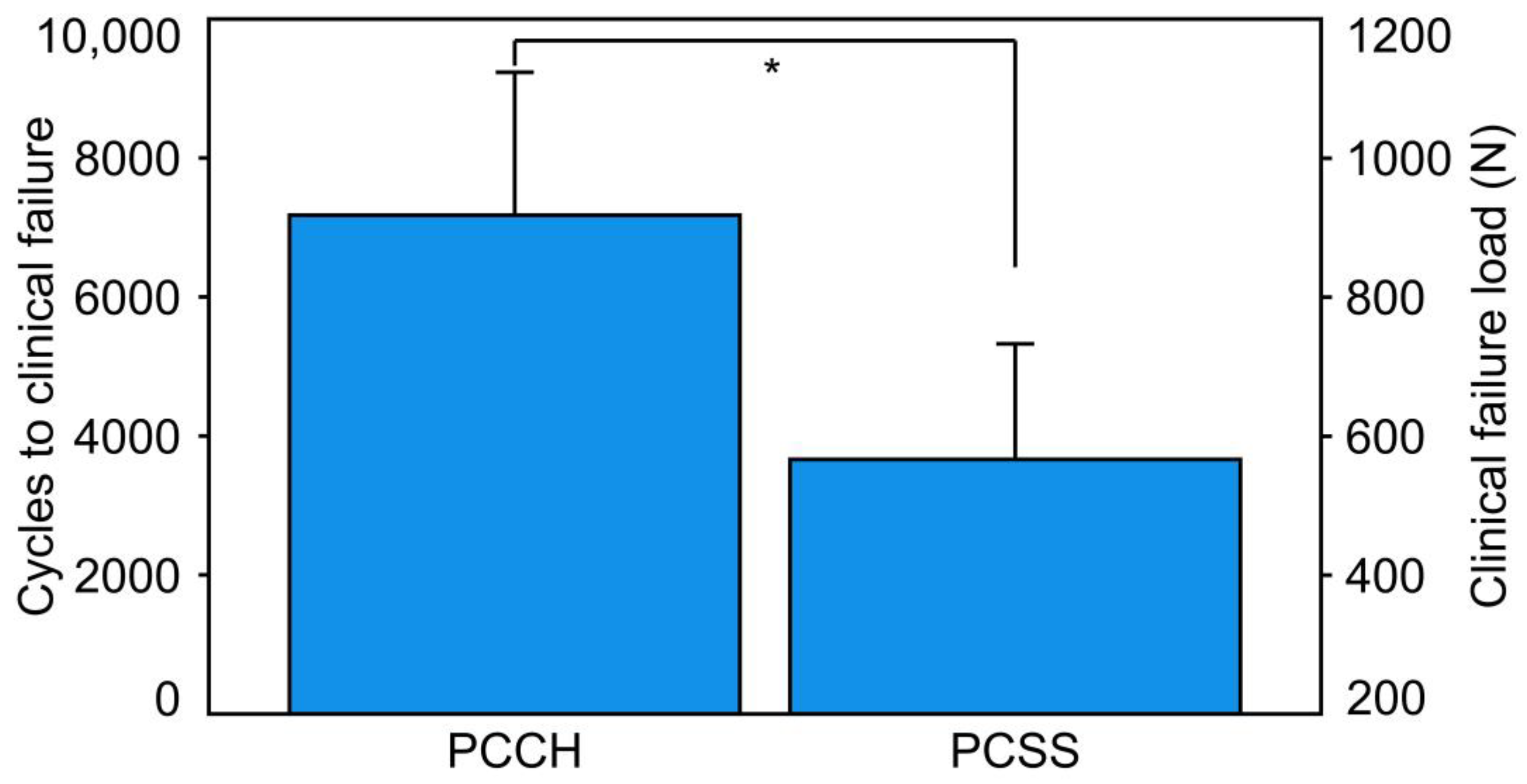
3. Results
4. Discussion
- PCCH was associated with significantly higher construct stiffness versus PCSS.
- PCCH demonstrated significantly fewer interfragmentary movements during the first 6000 test cycles compared to PCSS.
- Cycles to clinical failure and clinical failure load were significantly higher for PCCH versus PCSS.
Strength and Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, W.; Zhang, Z.; Lu, Y.; Li, J.; Zhang, Y.; Shen, Y. Fluoroscopic views for safe insertion of lag screws into the posterior column of the acetabulum. BMC Musculoskelet. Disord. 2014, 15, 303. [Google Scholar]
- Yu, K.; Zhou, R.; Gao, S.; Liang, A.; Yang, M.; Yang, H. The placement of percutaneous retrograde acetabular posterior column screw based on imaging anatomical study of acetabular posterior column corridor. J. Orthop. Surg. Res. 2022, 17, 492. [Google Scholar]
- Jung, G.-H.; Lee, Y.; Kim, J.-W.; Kim, J.-W. Computational analysis of the safe zone for the antegrade lag screw in posterior column fixation with the anterior approach in acetabular fracture: A cadaveric study. Injury 2017, 48, 608–614. [Google Scholar]
- Feng, X.; Zhang, S.; Luo, Q.; Fang, J.; Lin, C.; Leung, F.; Chen, B. Definition of a safe zone for antegrade lag screw fixation of fracture of posterior column of the acetabulum by 3D technology. Injury 2016, 47, 702–706. [Google Scholar]
- Stöckle, U.; Hoffmann, R.; Nittinger, M.; Südkamp, N.; Haas, N. Screw fixation of acetabular fractures. Int. Orthop. 2000, 24, 143–147. [Google Scholar]
- Starr, A.J.; Reinert, C.M.; Jones, A.L. Percutaneous fixation of the columns of the acetabulum: A new technique. J. Orthop. Trauma 1998, 12, 51–58. [Google Scholar] [CrossRef]
- Hong, G.; Cong-Feng, L.; Cheng-Fang, H.; Chang-Qing, Z.; Bing-Fang, Z. Percutaneous screw fixation of acetabular fractures with 2D fluoroscopy-based computerized navigation. Arch. Orthop. Trauma Surg. 2010, 130, 1177–1183. [Google Scholar]
- Mu, W.-D.; Wang, X.-Q.; Jia, T.-H.; Zhou, D.-S.; Cheng, A.-X. Quantitative anatomic basis of antegrade lag screw placement in posterior column of acetabulum. Arch. Orthop. Trauma Surg. 2009, 129, 1531–1537. [Google Scholar]
- Shiramizu, K.; Naito, M.; Yatsunami, M. Quantitative anatomic characterisation of the pelvic brim to facilitate internal fixation through an anterior approach. J. Orthop. Surg. 2003, 11, 137–140. [Google Scholar]
- Starr, A.J.; Jones, A.L.; Reinert, C.M.; Borer, D.S. Preliminary results and complications following limited open reduction and percutaneous screw fixation of displaced fractures of the acetabulum. Inj. Int. J. Care Inj. 2001, 32, 45–50. [Google Scholar] [CrossRef]
- Azzam, K.; Siebler, J.; Bergmann, K.; Daccarett, M.; Mormino, M. Percutaneous retrograde posterior column acetabular fixation: Is the sciatic nerve safe? A cadaveric study. J. Orthop. Trauma 2014, 28, 37–40. [Google Scholar]
- Mouhsine, E.; Garofalo, R.; Borens, O.; Wettstein, M.; Blanc, C.-H.; Fischer, J.-F.; Moretti, B.; Leyvraz, P.-F. Percutaneous retrograde screwing for stabilisation of acetabular fractures. Injury 2005, 36, 1330–1336. [Google Scholar]
- Ochs, B.G.; Gonser, C.; Shiozawa, T.; Badke, A.; Weise, K.; Rolauffs, B.; Stuby, F.M. Computer-assisted periacetabular screw placement: Comparison of different fluoroscopy-based navigation procedures with conventional technique. Injury 2010, 41, 1297–1305. [Google Scholar]
- Letournel, E. Acetabulum fractures: Classification and management. Orthop. Trauma Dir. 2007, 5, 27–33. [Google Scholar]
- Wenzel, L.; Sandriesser, S.; Glowalla, C.; Gueorguiev, B.; Perl, M.; Stuby, F.M.; Augat, P.; Hungerer, S. Biomechanical comparison of acetabular fracture fixation with stand-alone THA or in combination with plating. Eur. J. Trauma Emerg. Surg. 2022, 48, 3185–3192. [Google Scholar]
- Morosato, F.; Traina, F.; Cristofolini, L. Standardization of hemipelvis alignment for in vitro biomechanical testing. J. Orthop. Res. 2018, 36, 1645–1652. [Google Scholar]
- Bergmann, G.; Deuretzbacher, G.; Heller, M.; Graichen, F.; Rohlmann, A.; Strauss, J.; Duda, G.N. Hip contact forces and gait patterns from routine activities. J. Biomech. 2001, 34, 859–871. [Google Scholar] [CrossRef]
- Gueorguiev, B.; Ockert, B.; Schwieger, K.; Wähnert, D.; Lawson-Smith, M.; Windolf, M.; Stoffel, K. Angular stability potentially permits fewer locking screws compared with conventional locking in intramedullary nailed distal tibia fractures: A biomechanical study. J. Orthop. Trauma 2011, 25, 340–346. [Google Scholar]
- Windolf, M.; Muths, R.; Braunstein, V.; Gueorguiev, B.; Hänni, M.; Schwieger, K. Quantification of cancellous bone-compaction due to DHS® Blade insertion and influence upon cut-out resistance. Clin. Biomech. 2009, 24, 53–58. [Google Scholar]
- Meys, G.; Kalmet, P.H.; Sanduleanu, S.; Van Horn, Y.Y.; Maas, G.J.; Poeze, M.; Brink, P.R.; Seelen, H.A. A protocol for permissive weight-bearing during allied health therapy in surgically treated fractures of the pelvis and lower extremities. J. Rehabil. Med. 2019, 51, 290–297. [Google Scholar]
- Yoshida, H.; Faust, A.; Wilckens, J.; Kitagawa, M.; Fetto, J.; Chao, E.Y.-S. Three-dimensional dynamic hip contact area and pressure distribution during activities of daily living. J. Biomech. 2006, 39, 1996–2004. [Google Scholar]
- Hayes, J.; Richards, R. The use of titanium and stainless steel in fracture fixation. Expert Rev. Med. Devices 2010, 7, 843–853. [Google Scholar]
- Beumer, A.; Campo, M.M.; Niesing, R.; Day, J.; Kleinrensink, G.-J.; Swierstra, B.A. Screw fixation of the syndesmosis: A cadaver model comparing stainless steel and titanium screws and three and four cortical fixation. Injury 2005, 36, 60–64. [Google Scholar]
- Christensen, F.B.; Dalstra, M.; Sejling, F.; Overgaard, S.; Bünger, C. Titanium-alloy enhances bone-pedicle screw fixation: Mechanical and histomorphometrical results of titanium-alloy versus stainless steel. Eur. Spine J. 2000, 9, 97–103. [Google Scholar]
- Attias, N.; Lindsey, R.W.; Starr, A.J.; Borer, D.; Bridges, K.; Hipp, J.A. The use of a virtual three-dimensional model to evaluate the intraosseous space available for percutaneous screw fixation of acetabular fractures. J. Bone Jt. Surg. Br. Vol. 2005, 87B, 1520–1523. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.-K.; Gill, S.S.; Zura, R.D.; Krause, W.R.; Wang, G.-J. Comparative strength of three methods of fixation of transverse acetabular fractures. Clin. Orthop. Relat. Res. 2001, 392, 433–441. [Google Scholar]
- Nambiar, M.; West, L.R.; Bingham, R. AO Surgery Reference: A comprehensive guide for management of fractures. Br. J. Sport. Med. 2017, 51, 545–546. [Google Scholar]
- Gardner, M.P.; Chong, A.; Pollock, A.G.; Wooley, P.H. Mechanical evaluation of large-size fourth-generation composite femur and tibia models. Ann. Biomed. Eng. 2010, 38, 613–620. [Google Scholar]
- Heiner, A.D. Structural properties of fourth-generation composite femurs and tibias. J. Biomech. 2008, 41, 3282–3284. [Google Scholar]
- Zdero, R.; Olsen, M.; Bougherara, H.; Schemitsch, E. Cancellous bone screw purchase: A comparison of synthetic femurs, human femurs, and finite element analysis. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2008, 222, 1175–1183. [Google Scholar]
- Elfar, J.; Stanbury, S.; Menorca, R.M.G.; Reed, J.D. Composite bone models in orthopaedic surgery research and education. J. Am. Acad. Orthop. Surg. 2014, 22, 111. [Google Scholar]
- Camino Willhuber, G.; Zderic, I.; Gras, F.; Wahl, D.; Sancineto, C.; Barla, J.; Windolf, M.; Richards, R.G.; Gueorguiev, B. Analysis of sacro-iliac joint screw fixation: Does quality of reduction and screw orientation influence joint stability? A biomechanical study. Int. Orthop. 2016, 40, 1537–1543. [Google Scholar]
- Gardner, M.J.; Kendoff, D.; Ostermeier, S.; Citak, M.; Hüfner, T.; Krettek, C.; Nork, S.E. Sacroiliac joint compression using an anterior pelvic compressor: A mechanical study in synthetic bone. J. Orthop. Trauma 2007, 21, 435–441. [Google Scholar]
- Yinger, K.; Scalise, J.; Olson, S.A.; Bay, B.K.; Finkemeier, C.G. Biomechanical comparison of posterior pelvic ring fixation. J. Orthop. Trauma 2003, 17, 481–487. [Google Scholar] [CrossRef]
- Sahin, O.; Demirors, H.; Akgun, R.; Tuncay, I. Internal fixation of bilateral sacroiliac dislocation with transiliac locked plate: A biomechanical study on pelvic models. Acta Orthop. Traumatol. Turc. 2013, 47, 411–416. [Google Scholar]
- Sagi, H.; Ordway, N.; DiPasquale, T. Biomechanical analysis of fixation for vertically unstable sacroiliac dislocations with iliosacral screws and symphyseal plating. J. Orthop. Trauma 2004, 18, 138–143. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berk, T.; Zderic, I.; Schwarzenberg, P.; Drenchev, L.; Skulev, H.K.; Pfeifer, R.; Pastor, T.; Richards, G.; Gueorguiev, B.; Pape, H.-C. Antegrade Posterior Column Acetabulum Fracture Screw Fixation via Posterior Approach: A Biomechanical Comparative Study. Medicina 2023, 59, 1214. https://doi.org/10.3390/medicina59071214
Berk T, Zderic I, Schwarzenberg P, Drenchev L, Skulev HK, Pfeifer R, Pastor T, Richards G, Gueorguiev B, Pape H-C. Antegrade Posterior Column Acetabulum Fracture Screw Fixation via Posterior Approach: A Biomechanical Comparative Study. Medicina. 2023; 59(7):1214. https://doi.org/10.3390/medicina59071214
Chicago/Turabian StyleBerk, Till, Ivan Zderic, Peter Schwarzenberg, Ludmil Drenchev, Hristo Kostov Skulev, Roman Pfeifer, Tatjana Pastor, Geoff Richards, Boyko Gueorguiev, and Hans-Christoph Pape. 2023. "Antegrade Posterior Column Acetabulum Fracture Screw Fixation via Posterior Approach: A Biomechanical Comparative Study" Medicina 59, no. 7: 1214. https://doi.org/10.3390/medicina59071214





