Colorectal Cancer: From Risk Factors to Oncogenesis
Abstract
:1. Introduction
2. Oncogenesis in Colorectal Cancer
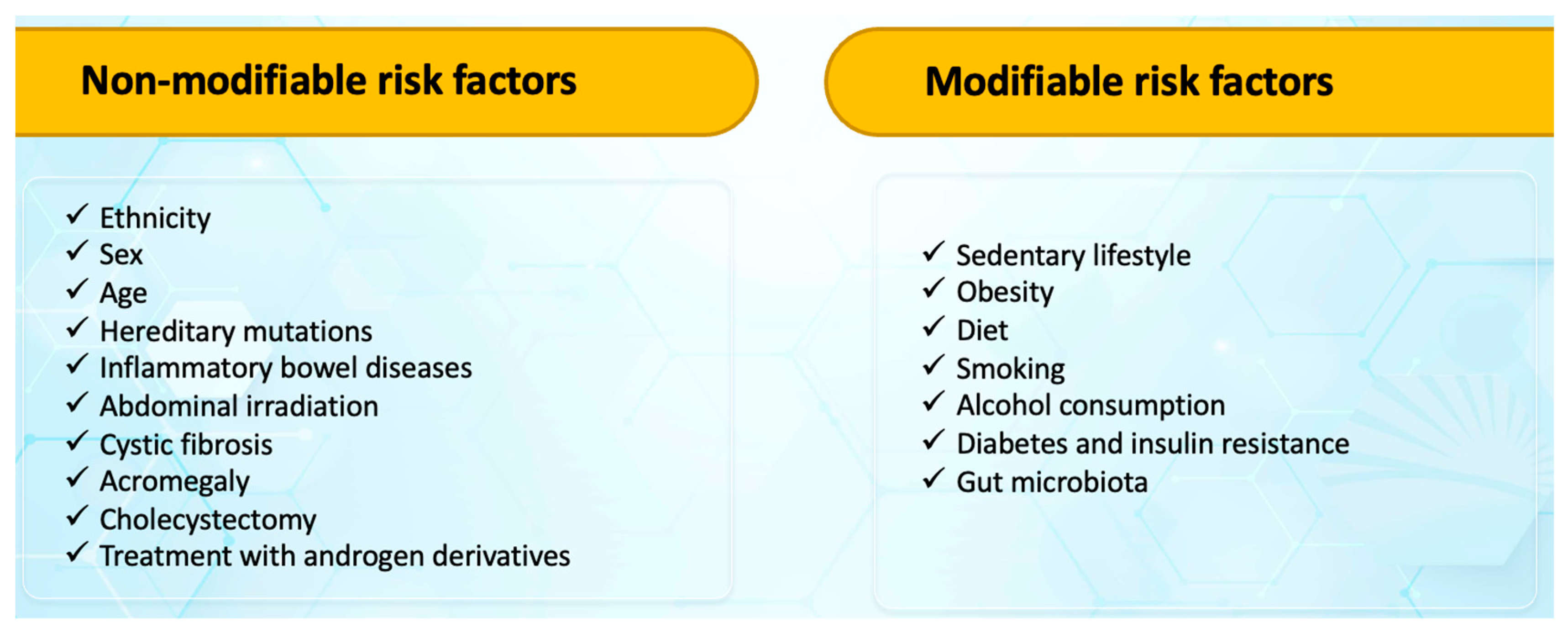
3. Risk Factors for Colorectal Cancers
- pro-inflammatory diets are associated with an increased risk of colorectal cancer (carbohydrates, proteins, trans fats, cholesterol, saturated fatty acids, iron, etc.).
- foods with anti-inflammatory potential—associated with a lower risk of colorectal cancer (fibers, monounsaturated fatty acids, polyunsaturated fatty acids, omega 3, omega 6, niacin, thiamine, vitamin B6, vitamin B12, selenium, zinc, magnesium, fat-soluble vitamins (A, D, E, K), beta-carotene, folic acid, caffeine, tea, etc.) [48,49,50].
4. Future Research Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Colorectal Cancer. Globocan. 2020. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/10_8_9-Colorectum-fact-sheet.pdf (accessed on 2 July 2023).
- International Agency for Research on Cancer. Available online: https://www.iarc.who.int/news-events/global-burden-of-colorectal-cancer-in-2020-and-2040-incidence-and-mortality-estimates-from-globocan/ (accessed on 2 July 2023).
- Romania Globocan. 2020. Available online: https://gco.iarc.fr/today/data/factsheets/populations/642-romania-fact-sheets.pdf (accessed on 2 July 2023).
- Poursheikhani, A.; Abbaszadegan, M.R.; Nokhandani, N.; Kerachian, M.A. Integration analysis of long non-coding RNA (lncRNA) role in tumorigenesis of colon adenocarcinoma. BMC Med. Genom. 2020, 13, 108. [Google Scholar] [CrossRef] [PubMed]
- Barresi, V. Colorectal Cancer: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines 2021, 9, 1858. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Q.; Zhou, Y.C.; Ge, Z.Y.J.; Qin, G.; Yin, T.F.; Zhao, D.Y.; Tan, C.; Yao, S.K. Comprehensive proteomic signature and identification of CDKN2A as a promising prognostic biomarker and therapeutic target of colorectal cancer. World J. Clin. Cases 2022, 10, 7686–7697. [Google Scholar] [CrossRef]
- Sameer, A.S. Colorectal Cancer: Molecular Mutations and Polymorphisms. Front. Oncol. 2013, 3, 114. [Google Scholar] [CrossRef] [PubMed]
- La Vecchia, S.; Sebastian, C. Metabolic pathways regulating colorectal cancer initiation and progression. Semin. Cell Dev. Biol. 2020, 98, 63–70. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Przegląd Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef]
- Zhao, H.; Ming, T.; Tang, S.; Ren, S.; Yang, H.; Liu, M.; Tao, Q.; Xu, H. Wnt signaling in colorectal cancer: Pathogenic role and therapeutic target. Mol. Cancer 2022, 21, 144. [Google Scholar] [CrossRef]
- Pabla, B.; Bissonnette, M.; Konda, V.J. Colon cancer and the epidermal growth factor receptor: Current treatment paradigms, the importance of diet, and the role of chemoprevention. World J. Clin. Oncol. 2015, 6, 133–141. [Google Scholar] [CrossRef]
- Yamamoto, D.; Oshima, H.; Wang, D.; Takeda, H.; Kita, K.; Lei, X.; Nakayama, M.; Murakami, K.; Ohama, T.; Takemura, H.; et al. Characterization of RNF43 frameshift mutations that drive Wnt ligand- and R-spondin-dependent colon cancer. J. Pathol. 2022, 257, 39–52. [Google Scholar] [CrossRef]
- Zhang, L.; Shay, J.W. Multiple Roles of APC and its Therapeutic Implications in Colorectal Cancer. J. Natl. Cancer Inst. 2017, 109, djw332. [Google Scholar] [CrossRef]
- Valle, L.; de Voer, R.M.; Goldberg, Y.; Sjursen, W.; Forsti, A.; Ruiz-Ponte, C.; Caldes, T.; Garre, P.; Olsen, M.F.; Nordling, M.; et al. Update on genetic predisposition to colorectal cancer and polyposis. Mol. Asp. Med. 2019, 69, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Henrikson, N.B.; Webber, E.M.; Goddars, K.A.; Scrol, A.; Piper, M.; Williams, M.S.; Zallen, D.T.; Calonge, N.; Ganiats, T.G.; Janssens, A.C.J.W.; et al. Family history and the natural history of colorectal cancer: Systematic review. Genet. Med. 2015, 17, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Marx, O.; Mankarious, M.; Ychum, G. Molecular genetics of early-onset colorectal cancer. World J. Biol. Chem. 2023, 14, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Deng, X.; Luo, J.; Lei, Y.; Jin, X.; Zhu, J.; Lv, G. Metabolomic Comparison of Patients with Colorectal Cancer at Different Anticancer Treatment Stages. Front. Oncol. 2021, 11, 574318. [Google Scholar] [CrossRef]
- Gold, A.; Choueiry, F.; Jin, N.; Mo, X.; Zhu, J. The Application of Metabolomics in Recent Colorectal Cancer Studies: A State-of-the-Art Review. Cancers 2022, 14, 725. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Sauer, G.A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef]
- Saraiva, M.R.; Rosa, I.; Claro, I. Early-onset colorectal cancer: A review of current knowledge. World J. Gastroenterol. 2023, 29, 1289–1303. [Google Scholar] [CrossRef]
- Boardman, L.A.; Vilar, E.; You, Y.N.; Samadder, J. AGA Clinical Practice Update on Young Adult-Onset Colorectal Cancer Diagnosis and Management: Expert Review. Clin. Gastroenterol. Hepatol. 2020, 18, 2415–2424. [Google Scholar] [CrossRef]
- Mauri, G.; Sartore-Bianchi, A.; Russo, A.G.; Marsoni, S.; Bardelli, A.; Siena, S. Early-onset colorectal cancer in young individuals. Mol. Oncol. 2019, 13, 109–131. [Google Scholar] [CrossRef]
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef]
- Cho, Y.A.; Lee, J.; Oh, J.H.; Chang, H.J.; Sohn, D.K.; Shin, A.; Kim, J. Genetic Risk Score, Combined Lifestyle Factors and Risk of Colorectal Cancer. Cancer Res. Treat. 2019, 51, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Chen, Y.; Gu, J. Risk factors for advanced colorectal neoplasm in young adults: A meta-analysis. Future Oncol. 2023, 19, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Meester, R.G.; Doubeni, C.A.; Lansdorp-Vogelaar, I.; Goede, S.L.; Levin, T.R.; Quinn, V.P.; Ballegooijen, M.V.; Corley, D.A.; Zauber, A.G. Colorectal cancer deaths attributable to nonuse of screening in the United States. Ann. Epidemiol. 2015, 25, 208–213.e1. [Google Scholar] [CrossRef]
- O’Sullivan, D.E.; Sutherland, R.L.; Town, S.; Chow, K.; Fan, J.; Forbes, N.; Heitman, S.J.; Hilsden, R.J.; Brenner, D.R. Risk Factors for Early-Onset Colorectal Cancer: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2022, 20, 1229–1240.e5. [Google Scholar] [CrossRef] [PubMed]
- Olén, O.; Erichsen, R.; Sachs, M.C.; Pedersen, L.; Halfvarson, J.; Askling, J.; Ekbom, A.; Sørensen, H.T.; Ludvigsson, J.F. Colorectal cancer in ulcerative colitis: A Scandinavian population-based cohort study. Lancet 2020, 395, 123. [Google Scholar] [CrossRef] [PubMed]
- Yashiro, M. Ulcerative colitis-associated colorectal cancer. World J. Gastroenterol. 2014, 20, 16389–16397. [Google Scholar] [CrossRef]
- Li, W.; Zhao, T.; Wu, D.; Li, J.; Wang, M.; Sun, Y.; Hou, S. Colorectal Cancer in Ulcerative Colitis: Mechanisms Surveillance and Chemoprevention. Curr. Oncol. 2022, 29, 6091–6114. [Google Scholar] [CrossRef]
- Lakatos, P.L.; Lakatos, L. Risk for colorectal cancer in ulcerative colitis: Changes, causes and management strategies. World J. Gastroenterol. 2008, 14, 3937–3947. [Google Scholar] [CrossRef]
- Olén, O.; Erichsen, R.; Sachs, M.C.; Pedersen, L.; Halfvarson, J.; Asklin, J.; Ekbom, A.; Sørensen, H.T.; Ludvigsson, J.F. Colorectal cancer in Crohn’s disease: A Scandinavian population-based cohort study. Lancet Gastroenterol. Hepatol. 2020, 5, 475–484. [Google Scholar] [CrossRef]
- Freeman, H.J. Colorectal cancer risk in Crohn’s disease. World J. Gastroenterol. 2008, 14, 1810–1811. [Google Scholar] [CrossRef]
- Shah, S.C.; Itzkowitz, S.H. Colorectal cancer in inflammatory bowel disease: Mechanisms and management. Gastroenterology 2022, 162, 715–730. [Google Scholar] [CrossRef] [PubMed]
- Keller, D.S.; Windsor, A.; Cohen, R.; Chand, M. Colorectal cancer in inflammatory bowel disease: Review of the evidence. Tech. Coloproctol. 2019, 23, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Arif, A.A.; Chahal, D.; Ladua, G.K.; Bhang, E.; Salh, B.; Rosenfeld, G.; Loree, J.M.; Donnellan, F. Hereditary and inflammatory bowel disease-related only onset colorectal cancer have unique characteristics and clinical course compared with sporadic disease. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, H.; Misumi, M.; Brenner, A.; Grant, E.J.; Sakata, R.; Sadakane, A.; Utada, M.; Preston, D.L.; Mabuchi, K.; Ozasa, K. Radiation risk of incident colorectal cancer by anatomical site among atomic bomb survivors:1958–2009. Int. J. Cancer 2020, 146, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Rombouts, A.J.M.; Hugen, N.; Elferink, M.A.G.; Poortmans, P.M.P.; Nagtegaal, I.D.; de Wilt, J.H.W. Increased risk for second primary rectal cancer after pelvic radiation therapy. Eur. J. Cancer 2020, 124, 142. [Google Scholar] [CrossRef]
- Ho, C.H.; Cheng, K.C.; Chao, C.M.; Lai, C.C.; Chiang, S.R.; Chen, C.M.; Liao, K.M.; Wang, J.J.; Lee, P.H.; Hung, C.M.; et al. Does radiotherapy increase the risk of colorectal cancer among prostate cancer patients? A large population-based study. J. Cancer 2020, 11, 6204–6212. [Google Scholar] [CrossRef]
- Children’s Oncology Group Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers, Version 5.0 (October 2018). Available online: http://www.survivorshipguidelines.org/ (accessed on 2 July 2023).
- Desautels, D.; Czaykowski, P.; Nugent, Z.; Demers, A.A.; Mahmud, S.M.; Singh, H. Risk of colorectal cancer after the diagnosis of prostate cancer: A population-based study. Cancer 2016, 122, 1254–1260. [Google Scholar] [CrossRef]
- Yamada, A.; Komaki, Y.; Komaki, F.; Micic, D.; Zullow, S.; Sakuraba, A. Risk of gastrointestinal cancers in patients with cystic fibrosis: A systematic review and meta-analysis. Lancet Oncol. 2018, 19, 758–767. [Google Scholar] [CrossRef]
- Scott, P.; Anderson, K.; Singhania, M.; Cormier, R. Cystic Fibrosis, CFTR, and Colorectal Cancer. Int. J. Mol. Sci. 2020, 21, 2891. [Google Scholar] [CrossRef]
- Ingravalle, F.; Casella, G.; Ingravalle, A.; Monti, C.; De Salvatore, F.; Stillitano, D.; Villanaci, V. Surveillance of Colorectal cancer (CCR) in Cystic Fibrosis (CF) Patients. Gastrointest. Disord. 2021, 3, 84–95. [Google Scholar] [CrossRef]
- Birch, R.J.; Peckham, D.; Wood, H.M.; Quirke, P.; Konstant-Hambling, R.; Brownlee, K.; Cosgriff, R.; Genomocs england Research Consortium; Burr, N.; Downing, A. The risk of colorectal cancer in individuals with mutations of the cystic fibrosis transmembrane conductance regulator (CFTR) gene: An English population-based study. J. Cyst. Fibros. 2023, 22, 499–504. [Google Scholar] [CrossRef]
- Than, B.L.; Linnekamp, J.F.; Starr, T.K.; Largaespada, D.A.; Rod, A.; Zhang, Y.; Bruner, V.; Abrahante, J.; Schumann, A.; Luczak, T.; et al. CFTR is a tumor suppressor gene in murine and human intestinal cancer. Oncogene 2016, 35, 4179–4187. [Google Scholar] [CrossRef] [PubMed]
- Lagergren, J.; Ye, W.; Ekbom, A. Intestinal cancer after cholecystectomy: Is bile involved in carcinogenesis? Gastroenterology 2001, 121, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Godos, J.; Hebert, J.R.; Wirth, M.D.; Piuri, G.; Speciani, A.F.; Grosso, G. Dietary Inflammatory Index and Colorectal Cancer Risk-A Meta-Analysis. Nutrients 2017, 9, 1043. [Google Scholar] [CrossRef] [PubMed]
- Marx, W.; Veronese, N.; Kelly, J.T.; Smith, L.; Hockey, M.; Collins, S.; Trakman, G.L.; Hoare, E.; Teasdale, S.B.; Wade, A.; et al. The Dietary Inflammatory Index and Human Health: An Umbrella Review of Meta-Analyses of Observational Studies. Adv. Nutr. 2021, 12, 1681–1690. [Google Scholar] [CrossRef]
- Liang, Z.; Feng, Y.; Shivappa, N.; Hebert, J.R.; Xu, X. Dietary Inflammatory Index and Mortality from All Causes, Cardiovascular Disease, and Cancer: A Prospective Study. Cancers 2022, 14, 4609. [Google Scholar] [CrossRef]
- Ye, P.; Xi, Y.; Huang, Z.; Xu, P. Linking obesity with colorectal cancer: Epidemiology and Mechanistic Insights. Cancers 2020, 12, 1408. [Google Scholar] [CrossRef]
- Socol, C.T.; Chira, A.; Martinez-Sanchez, M.A.; Nunez-Sanchez, M.A.; Maerescu, C.M.; Mierlita, D.; Rusu, A.V.; Ruiz-Alcaraz, A.J.; Trif, M.; Ramos-Molina, B. Leptin Signaling in Obesity and Colorectal cancer. Int. J. Mol. Sci. 2022, 23, 4713. [Google Scholar] [CrossRef]
- Soltani, G.; Poursheikhani, A.; Yassi, M.; Hayatbakhsh, A.; Kerachian, M.; Kerachian, M.A. Obesity, diabetes and the risk of colorectal adenoma and cancer. BMC Endocr. Disord. 2019, 19, 113. [Google Scholar] [CrossRef]
- Bull, C.J.; Bell, J.A.; Murphy, N.; Sanderson, E.; Smith, G.D.; Timpson, N.J.; Bunbury, B.L.; Albanes, D.; Berndt, S.I.; Bezieau, S.; et al. Adiposity, metabolites, and colorectal cancer risk: Mendelian randomization study. BMC Med. 2020, 18, 396. [Google Scholar] [CrossRef]
- Fletcher, R.; Wang, Y.J.; Schoen, R.E.; Finn, O.J.; Yu, J.; Zhang, L. Colorectal cancer prevention: Immune modulation taking the stage. Biochim. Biophys. Acta Rev. Cancer 2018, 1869, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Ajouz, H.; Mukherji, D.; Shamseddine, A. Secondary bile acids: An underrecognized cause of colon cancer. World J. Surg. Oncol. 2014, 12, 164. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Ung, T.T.; Kim, N.H.; Jung, Y.D. Role of bile acids in colon carcinogenesis. World J. Clin. Cases 2018, 6, 577–588. [Google Scholar] [CrossRef]
- Thanikachalam, K.; Khan, G. Colorectal cancer and nutrition. Nutrients 2019, 11, 164. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, L.; Xiao, J.; Sun, J.; Yu, L.; Zhang, H.; Meng, X.; Yuan, S.; Timofeeva, M.; Law, P.J.; et al. Alcohol consumption, DNA methylation and colorectal cancer risk: Results from pooled cohort studies and Mendelian randomization analysis. Int. J. Cancer 2022, 151, 83–94. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, J.; Wong, M.C.S. Associations of alcohol and coffee with colorectal cancer risk in East Asian populations: A Mendelian randomization study. Eur. J. Nutr. 2023, 62, 749–756. [Google Scholar] [CrossRef]
- Cai, S.; Li, Y.; Ding, Y.; Chen, K.; Jin, M. Alcohol drinking and the risk of colorectal cancer death: A meta-analysis. Eur. J. Cancer Prev. 2014, 23, 532–539. [Google Scholar] [CrossRef]
- Gram, I.T.; Park, S.Y.; Wilkens, L.R.; Haiman, C.A.; Le Marchand, L. Smoking-related risks of colorectal cancer by anatomical subsite and sex. Am. J. Epidemiol. 2020, 189, 543–553. [Google Scholar] [CrossRef]
- Bai, X.; Wei, H.; Liu, W.; Coker, O.O.; Gou, H.; Liu, C.; Zhao, L.; Li, C.; Zhou, Y.; Wang, G.; et al. Cigarette smoke promotes colorectal cancer through modulation of gut microbiota and related metabolite. Gut 2022, 71, 2439–2450. [Google Scholar] [CrossRef]
- Botteri, E.; Borroni, E.; Sloan, E.K.; Bagnardi, V.; Bosetti, C.; Peveri, G.; Santucci, C.; Specchia, C.; van den Brandt, P.; Gallus, S.; et al. Smoking and Colorectal Cancer Risk, Overall and by Molecular Subtypes: A Meta-Analysis. Am. J. Gastroenterol. 2020, 115, 1940–1949. [Google Scholar] [CrossRef]
- Cheng, J.; Chen, Y.; Wang, X.; Yan, Z.; Gong, G.; Li, G.; Li, C. Meta-analysis of prospective cohort studies of cigarette smoking and the incidence of colon and rectal cancers. Eur. J. Cancer Prev. 2015, 24, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Limsui, D.; Vierkant, R.A.; Tillmans, L.S.; Wang, A.H.; Weisenberg, D.J.; Laird, P.W.; Lynch, C.F.; Anderson, K.E.; French, A.J.; Haile, R.W.; et al. Cigarette smoking and colorectal cancer risk by molecularly defined subtypes. J. Natl. Cancer Inst. 2010, 102, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Winkels, R.M.; Botma, A.; Van Duijnhoven, F.J.B.; Nagengast, F.M.; Kleibeuker, J.H.; Vasen, F.A.; Kampaman, E. Smoking Increases the risk for colorectal adenomas in patients with Lynch syndrome. Gastroenterology 2012, 142, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Yuhara, H.; Steinmaus, C.; Cohen, S.E.; Corley, D.A.; Tei, Y.; Buffler, P.A. Is Diabetes Mellitus an Independent Risk Factor for Colon Cancer and Rectal Cancer? Am. J. Gastroenterol. 2011, 106, 1911–1921. [Google Scholar] [CrossRef]
- Kim, D.S.; Scherer, P.E. Obesity, Diabetes, and Increased Cancer Progression. Diabetes Metabol. J. 2021, 45, 799–812. [Google Scholar] [CrossRef]
- Goto, A.; Yamaji, T.; Sawada, N.; Momozawa, Y.; Kamatani, Y.; Kubo, M.; Shimazu, T.; Inoue, M.; Noda, M.; Tsugane, S.; et al. Diabetes and cancer risk: A Mendelian randomization study. Int. J. Cancer 2020, 146, 712–719. [Google Scholar] [CrossRef]
- Murphy, N.; Song, M.; Papadimitriou, N.; Carreras-Torres, R.; Langenberg, C.; Martin, R.M.; Tsilidis, K.K.; Barroso, I.; Chen, J.; Frayling, T.M.; et al. Association between Glycemic Traits and Colorectal cancer: A Mendelian Randomization Analysis. J. Natl. Cancer Inst. 2022, 114, 740–751. [Google Scholar] [CrossRef]
- Xiao, W.; Huang, J.; Zhao, C.; Ding, L.; Wang, X.; Wu, B. Diabetes and risks of right-sided and left-sided colon cancer: A meta-analysis of prospective cohorts. Front. Oncol. 2022, 12, 737330. [Google Scholar] [CrossRef]
- Dehal, A.N.; Newton, C.C.; Jacobs, E.J.; Patel, A.V.; Gapstur, S.M.; Campbell, P.T. Impact of diabetes mellitus and insulin use on survival after colorectal cancer diagnosis: The Cancer Prevention Study-II Nutrition Cohort. J. Clin. Oncol. 2012, 30, 53. [Google Scholar] [CrossRef]
- Ta, H.D.K.; Nguyen, N.N.; Ho, D.K.N.; Nguyen, H.D.; Ni, Y.C.; Yee, K.X.; Pan, S.R.; Nguyen, H.S.; Phuoc, T.T.H.; Chen, M.J.; et al. Association of diabetes mellitus with early-onset colorectal cancer: A systematic review and meta-analysis of 19 studies including 10 million individuals and 30,000 events. Diabetes Metab. Syndr. 2023, 17, 102828. [Google Scholar]
- Wong, S.H.; Yu, J. Gut microbiota in colorectal cancer: Mechanisms of action and clinical applications. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 690–704. [Google Scholar] [CrossRef] [PubMed]
- Rebersek, M. Gut Microbiome and its role in colorectal cancer. BMC Cancer 2021, 21, 1325. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Ling, Z.; Li, L. The intestinal microbiota and colorectal cancer. Front. Immunol. 2020, 11, 615056. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Coker, O.O.; Nakatsu, G.; Wu, W.K.K.; Zhao, L.; Chen, Z.; Chan, F.K.L.; Kristiansen, K.; Sung, J.J.Y.; Wong, H.S.; et al. Multi-cohort analysis of colorectal cancer metagenome identified altered bacteria across populations and universal bacterial markers. Microbiome 2018, 6, 70. [Google Scholar] [CrossRef]
- Debelius, J.W.; Engstrand, L.; Matussek, A.; Brusselaers, N.; Morton, J.T.; Stenmarker, M.; Olsen, R.S. The Local Tumor Microbiome Is Associated with Survival in Late-Stage Colorectal Cancer Patients. Microbiol. Spectr. 2023, 11, e05066-22. [Google Scholar] [CrossRef]
- Wirbel, J.; Pyl, P.T.; Kartal, E.; Zych, K.; Kashani, A.; Milanese, A.; Fleck, J.S.; Voigt, A.Y.; Palleja, A.; Ponnudurai, R.; et al. Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer. Nat. Med. 2019, 25, 679–689. [Google Scholar] [CrossRef]
- Wang, T.; Cai, G.; Qiu, Y.; Fei, N.; Zhang, M.; Pang, X.; Jia, W.; Cai, S.; Zhao, L. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J. 2012, 6, 320–329. [Google Scholar] [CrossRef]
- Zackular, J.P.; Rogers, M.A.; Ruffin, M.T.T.; Schloss, P.D. The human gut microbiome as a screening tool for colorectal cancer. Cancer Prev. Res. 2014, 7, 1112–1121. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The controversial role of human gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef]
- Hajjar, R.; Richard, C.S.; Santos, M.M. The role of butyrate in surgical and oncological outcomes in colorectal cancer. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G601–G608. [Google Scholar] [CrossRef]
- Young, C.; Wood, H.M.; Seshadri, R.A.; Van Nang, P.; Vaccaro, C.; Melendez, L.C.; Bose, M.; Van Doi, M.; Piñero, T.A.; Valladares, C.T.; et al. The colorectal cancer-associated faecal microbiome of developing countries resembles that of developed countries. Genome Med. 2021, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Cao, S.; Liu, S.; Yao, Z.; Sun, T.; Li, Y.; Li, J.; Zhang, D.; Zhou, Y. Could gut microbiota serve as prognostic biomarker associated with colorectal cancer patients’ survival? A pilot study on relevant mechanism. Oncotarget 2016, 7, 46158–46172. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Du, L.; Shi, D.; Kong, C.; Liu, J.; Liu, G.; Li, X.; Ma, Y. Dysbiosis of human gut microbiome in young-onset colorectal cancer. Nat. Commun. 2021, 12, 6757. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, H.; Li, Y.; Xie, Y.; Huang, C.; Zhao, H.; Miyagishi, M.; Kasim, V. Transcription factor YY1 promotes cell proliferation by directly activating the pentose phosphate pathway. Cancer Res. 2018, 78, 4549–4562. [Google Scholar] [CrossRef]
- Jain, S.; Maque, J.; Galoosian, A.; Osuna-Garcia, A.; May, F.P. Optimal Strategies for Colorectal Cancer Screening. Curr. Treat. Options Oncol. 2022, 23, 474–493. [Google Scholar] [CrossRef]
- Shaukat, A.; Kahi, C.; Carol, B.; Rabeneck, L.; Sauer, B.; Rex, D. ACG Clinical Guidelines: Colorectal Cancer Screening 2021. Am. J. Gastroenterol. 2021, 116, 458–479. [Google Scholar] [CrossRef]
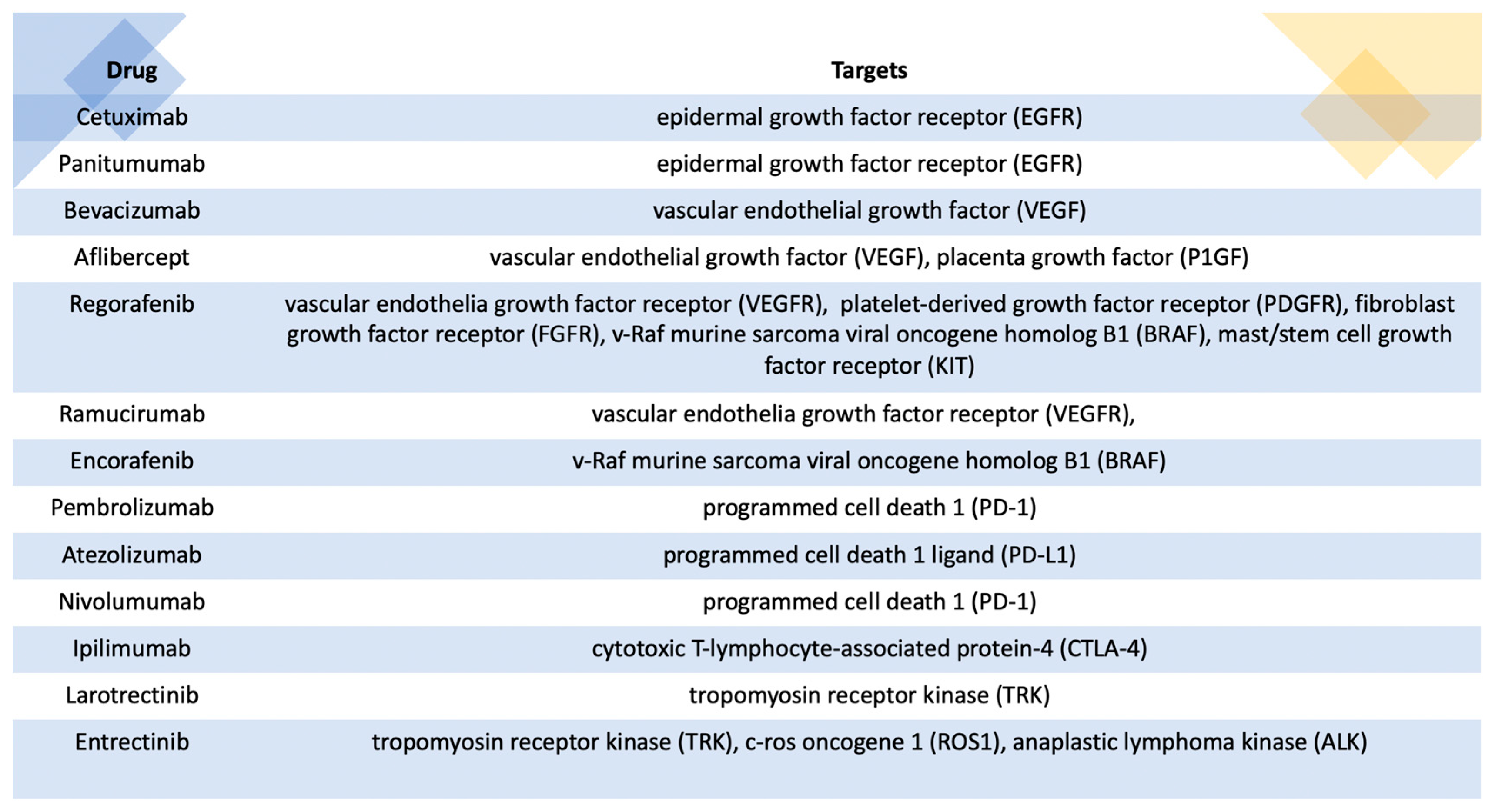
- Ohishi, T.; Kaneko, M.K.; Yoshida, Y.; Takashima, A.; Kato, Y.; Kawada, M. Current Targeted therapy for Metastatic Colorectal Cancer. Int. J. Mol. Sci. 2023, 24, 1702. [Google Scholar] [CrossRef]
- Rico, G.T.; Price, T.J. Atezolizumab for the treatment of colorectal cancer: The latest evidence and clinical potential. Expert. Opin. Biol. Ther. 2018, 18, 449–457. [Google Scholar] [CrossRef]
| Classification | Description |
|---|---|
| CMS 1 | associated with activation of the JAK-STAT signaling pathway, micro-satellite instability, and hypermutated tumor DNA |
| CMS 2 | associated with activation of the Wnt/MYC signaling pathway |
| CMS 3 | associated with metabolic changes |
| CMS 4 | associated with epithelial–mesenchymal transition and immunosuppression |
| Consensus molecular classification (CMS); Janus kinase/signal transduction and transcription activation (JAK-STAT); Wingless-related integration site/myelocytomatosis oncogene (Wnt/MYC). | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ionescu, V.A.; Gheorghe, G.; Bacalbasa, N.; Chiotoroiu, A.L.; Diaconu, C. Colorectal Cancer: From Risk Factors to Oncogenesis. Medicina 2023, 59, 1646. https://doi.org/10.3390/medicina59091646
Ionescu VA, Gheorghe G, Bacalbasa N, Chiotoroiu AL, Diaconu C. Colorectal Cancer: From Risk Factors to Oncogenesis. Medicina. 2023; 59(9):1646. https://doi.org/10.3390/medicina59091646
Chicago/Turabian StyleIonescu, Vlad Alexandru, Gina Gheorghe, Nicolae Bacalbasa, Alexandru Laurentiu Chiotoroiu, and Camelia Diaconu. 2023. "Colorectal Cancer: From Risk Factors to Oncogenesis" Medicina 59, no. 9: 1646. https://doi.org/10.3390/medicina59091646







