Nutritional Supplements for Skin Health—A Review of What Should Be Chosen and Why
Abstract
:1. Introduction
2. Aim of the Review and Search Strategy
3. Vitamin A
4. Vitamin C (L-Ascorbic Acid)
5. Vitamin E
6. Vitamin D
7. Curcumin
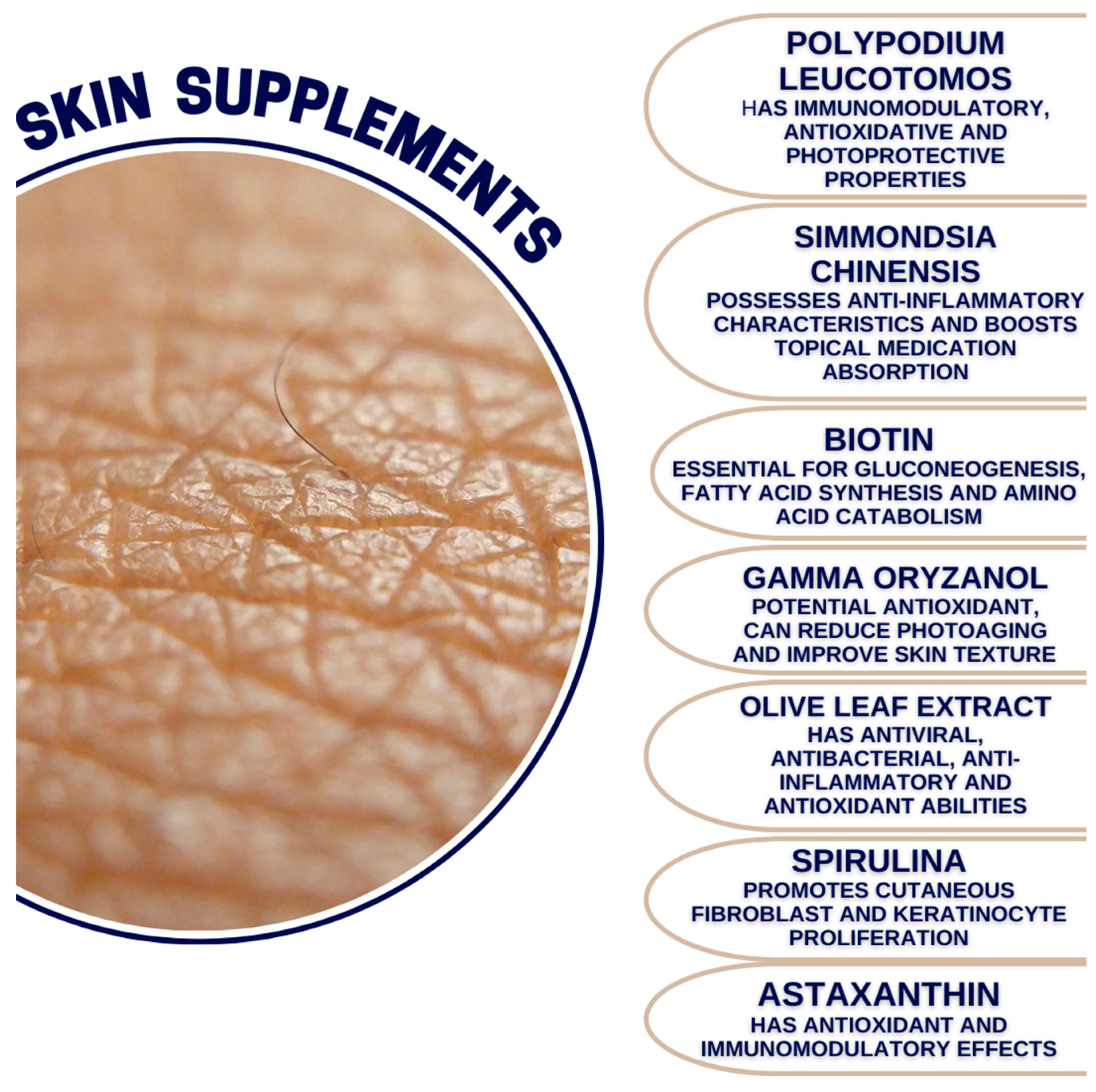
8. Polypodium leucotomos
9. Simmondsia chinensis
10. Biotin
11. Gamma Oryzanol
12. Olive Leaf Extract
13. Spirulina
14. Chlorella
15. Omega-3
16. Astaxanthin
17. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations:
| AD | atopic dermatitis |
| AMPs | antimicrobial proteins |
| ASX | astaxanthin |
| CDP | Chlorella-derived peptide |
| Cv-pdg | Chlorella virus pyrimidine dimer glycosylase |
| DM | dermatomyositis |
| EGF | epidermal growth factor |
| NEs | nanoethosomes |
| OLE | olive leaf extract |
| URI | upper respiratory illness |
| PL | Polypodium leucotomos |
| RELMα | Resistin-like molecule α |
| SFN | sulforaphane |
| SLE | systemic lupus erythematosus |
| SOD | superoxide dismutase |
| SPF | sunscreen protection factor |
| THC | tetrahydrocurcumin |
| UV | ultraviolet |
References
- Bouwstra, J.A.; Pilgram, G.S.K.; Ponex, M. Structure of the skin barrier. In Skin Barrier; Elias, P.M., Feingold, K.R., Eds.; Taylor & Francis: New York, NY, USA, 2006; pp. 65–96. [Google Scholar]
- Park, K. Role of micronutrients in skin health and function. Biomol. Ther. 2015, 23, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Boelsma, E.; Hendriks, H.F.; Roza, L. Nutritional skin care: Health effects of micronutrients and fatty acids. Am. J. Clin. Nutr. 2001, 73, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Dattola, A.; Silvestri, M.; Bennardo, L.; Passante, M.; Scali, E.; Patruno, C.; Nisticò, S.P. Role of Vitamins in Skin Health: A Systematic Review. Curr. Nutr. Rep. 2020, 9, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Harris-Tryon, T.A.; Grice, E.A. Microbiota and maintenance of skin barrier function. Science 2022, 376, 940–945. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Roche, F.C.; Harris-Tryon, T.A. Illuminating the Role of Vitamin A in Skin Innate Immunity and the Skin Microbiome: A Narrative Review. Nutrients 2021, 13, 302. [Google Scholar] [CrossRef]
- VanBuren, C.A.; Everts, H.B. Vitamin A in Skin and Hair: An Update. Nutrients 2022, 14, 2952. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, S.; Zhu, W.; Wu, L.; Chen, X. Retinoids as an Immunity-modulator in Dermatology Disorders. Arch. Immunol. Ther. Exp. 2019, 67, 355–365. [Google Scholar] [CrossRef]
- Dąbrowska, A.K.; Spano, F.; Derler, S.; Adlhart, C.; Spencer, N.D.; Rossi, R.M. The relationship between skin function, barrier properties, and body-dependent factors. Skin Res. Technol. 2018, 24, 165–174. [Google Scholar] [CrossRef]
- Qiang, L.; Yang, S.; Cui, Y.H.; He, Y.Y. Keratinocyte autophagy enables the activation of keratinocytes and fibroblastsand facilitates wound healing. Autophagy 2021, 17, 2128–2143. [Google Scholar] [CrossRef]
- Totté, J.E.; van der Feltz, W.T.; Hennekam, M.; van Belkum, A.; van Zuuren, E.J.; Pasmans, S.G.M. A Prevalence and odds of Staphylococcus aureus carriage in atopic dermatitis: A systematic review meta-analysis. Br. J. Dermatol. 2016, 175, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Paller, A.S.; Kong, H.H.; Seed, P.; Naik, S.; Scharschmidt, T.C.; Gallo, R.L.; Luger, T.; Irvine, A.D. The microbiome in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019, 143, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.A.; Gattu, S.; Propheter, D.C.; Kuang, Z.; Bel, S.; Ruhn, K.A.; Chara, A.L.; Edwards, M.; Zhang, C.; Jo, J.H.; et al. Resistin-like molecule α provides Vitamin-A-dependent antimicrobial protection in the skin. Cell Host Microbe 2019, 25, 777–788.e8. [Google Scholar] [CrossRef] [PubMed]
- Kotori, M.G. Low-Dose Vitamin “A” Tablets-Treatment of Acne Vulgaris. Med. Arch. 2015, 69, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Lietz, G.; Lange, J.; Rimbach, G. Molecular and dietary regulation of beta,beta-carotene 15,15′-monooxygenase 1 (BCMO1). Arch. Biochem. Biophys. 2010, 502, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Grune, T.; Lietz, G.; Palou, A.; Ross, A.C.; Stahl, W.; Tang, G.; Thurnham, D.; Yin, S.A.; Biesalski, H.K. Beta-carotene is an important vitamin A source for humans. J. Nutr. 2010, 140, 2268S–2285S. [Google Scholar] [CrossRef] [PubMed]
- Paiva, S.A.; Russell, R.M. Beta-carotene and other carotenoids as antioxidants. J. Am. Coll. Nutr. 1999, 18, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Bourhim, T.; Villareal, M.O.; Couderc, F.; Hafidi, A.; Isoda, H.; Gadhi, C. Melanogenesis Promoting Effect, Antioxidant Activity, and UPLC-ESI-HRMS Characterization of Phenolic Compounds of Argan Leaves Extract. Molecules 2021, 26, 371. [Google Scholar] [CrossRef]
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The Roles of Vitamin C in Skin Health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krcmova, L.K.; Javorska, L.; Pourova, J.; Mercolini, L.; Remiao, F.; Novakova, L.; Mladenka, P.; et al. Vitamin C-Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, H.; Li, W.; Qiang, M.; Dong, T.; Li, H. Role of Vitamin C in Skin Diseases. Front. Physiol. 2018, 9, 819. [Google Scholar] [CrossRef] [PubMed]
- Ravetti, S.; Clemente, C.; Brignone, S.; Hergert, L.; Allemandi, D.; Palma, S. Ascorbic acid in skin health. Cosmetics 2019, 6, 58–69. [Google Scholar] [CrossRef]
- Bechara, N.; Flood, V.M.; Gunton, J.E. A Systematic Review on the Role of Vitamin C in Tissue Healing. Antioxidants 2022, 11, 1605. [Google Scholar] [CrossRef]
- Meydani, S.N.; Lewis, E.D.; Wu, D. Perspective: Should vitamin E recommendations for older adults be increased? Adv. Nutr. 2018, 9, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Berardesca, E.; Cameli, N. Vitamin E supplementation in inflammatory skin diseases. Dermatol. Ther. 2021, 34, e15160. [Google Scholar] [CrossRef] [PubMed]
- Medovic, M.V.; Jakovljevic, V.L.; Zivkovic, V.I.; Jeremic, N.S.; Jeremic, J.N.; Bolevich, S.B.; Ravic Nikolic, A.B.; Milicic, V.M.; Srejovic, I.M. Psoriasis between Autoimmunity and Oxidative Stress: Changes Induced by Different Therapeutic Approaches. Oxid. Med. Cell. Longev. 2022, 2022, 2249834. [Google Scholar] [CrossRef] [PubMed]
- Javanbakht, M.H.; Keshavarz, S.A.; Djalali, M.; Siassi, S.; Eshraghian, M.R.; Firooz, A.; Seirafi, H.; Eshani, A.H.; Chamari, M.; Mirshafiey, A. Randomized controlled trial using vitamins E and D supplementation in atopic dermatitis. J. Dermatol. Treat. 2011, 22, 144–150. [Google Scholar] [CrossRef]
- Thiele, J.J.; Ekanayake-Mudiyanselage, S. Vitamin E in human skin: Organ-specific physiology and considerations for its use in dermatology. Mol. Asp. Med. 2007, 28, 646–667. [Google Scholar] [CrossRef]
- Ramdas, P.; Radhakrishnan, A.K.; Abdu Sani, A.A.; Kumari, M.; Rao, J.S.A.; Abdul-Rahman, P.S. Advancing the Role of Gamma-Tocotrienol as Proteasomes Inhibitor: A Quantitative Proteomic Analysis of MDA-MB-231 Human Breast Cancer Cells. BioMolecules 2019, 10, 19. [Google Scholar] [CrossRef]
- Kechichian, E.; Ezzedine, K. Vitamin D and the Skin: An Update for Dermatologists. Am. J. Clin. Dermatol. 2018, 19, 223–235. [Google Scholar] [CrossRef]
- Hahn, J.; Cook, N.R.; Alexander, E.K.; Friedman, S.; Walter, J.; Bubes, V.; Kotler, G.; Lee, I.M.; Manson, J.E.; Costenbader, K.H. Vitamin D and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial. BMJ 2022, 376, e066452. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, T.; Hung, T.; Soung, J. The role of vitamin D in psoriasis: A review. Int. J. Dermatol. 2015, 54, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Muzumdar, S.; Ferenczi, K. Nutrition and Youthful Skin. Clin. Dermatol. 2021, 39, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.M.; Raman, C.; Elmets, C.; Jetten, A.M.; Slominski, A.T.; Tuckey, R.C. The significance of CYP11A1 expression in skin physiology and pathology. Mol. Cell. Endocrinol. 2021, 530, 111238. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Chaiprasongsuk, A.; Janjetovic, Z.; Kim, T.K.; Stefan, J.; Slominski, R.M.; Hanumanthu, V.S.; Raman, C.; Qayyum, S.; Song, Y.; et al. Photoprotective Properties of Vitamin D and Lumisterol Hydroxyderivatives. Cell Biochem. Biophys. 2020, 78, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.K.; Slominski, R.M.; Song, Y.; Janjetovic, Z.; Podgorska, E.; Reddy, S.B.; Song, Y.; Raman, C.; Tang, E.K.Y.; et al. Metabolic activation of tachysterol3 to biologically active hydroxyderivatives that act on VDR, AhR, LXRs, and PPARγ receptors. FASEB J. 2022, 36, e22451. [Google Scholar] [CrossRef]
- Slominski, A.T.; Hardeland, R.; Zmijewski, M.A.; Slominski, R.M.; Reiter, R.J.; Paus, R. Melatonin: A Cutaneous Perspective on its Production, Metabolism, and Functions. J. Investig. Dermatol. 2018, 138, 490–499. [Google Scholar] [CrossRef]
- Bocheva, G.; Slominski, R.M.; Janjetovic, Z.; Kim, T.-K.; Böhm, M.; Steinbrink, K.; Reiter, R.J.; Kleszczyński, K.; Slominski, A.T. Protective Role of Melatonin and Its Metabolites in Skin Aging. Int. J. Mol. Sci. 2022, 23, 1238. [Google Scholar] [CrossRef]
- Jurenka, J.S. Anti-Inflammatory Properties of Curcumin, a Major Constituent of Curcuma Longa: A Review of Preclinical and Clinical Research. Altern. Med. Rev. 2009, 14, 141–153. [Google Scholar]
- Panahi, Y.; Hosseini, M.S.; Khalili, N.; Naimi, E.; Simental-Mendía, L.E.; Majeed, M.; Sahebkar, A. Effects of Curcumin on Serum Cytokine Concentrations in Subjects with Metabolic Syndrome: A Post-Hoc Analysis of a Randomized Controlled Trial. Biomed. Pharmacother. 2016, 82, 578–582. [Google Scholar] [CrossRef]
- Zia, A.; Farkhondeh, T.; Pourbagher-Shahri, A.M.; Samarghandian, S. The role of curcumin in aging and senescence: Molecular mechanisms. Biomed. Pharmacother. 2021, 134, 111119. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Curcumin, a Natural Antimicrobial Agent with Strain-Specific Activity. Pharmaceuticals 2020, 13, 153. [Google Scholar] [CrossRef] [PubMed]
- Baldissera, M.D.; Souza, C.F.; Zeppenfeld, C.C.; Descovi, S.; Machado, V.S.; Santos, R.C.V.; Baldisserott, B. Efficacy of Dietary Curcumin Supplementation as Bactericidal for Silver Catfish against Streptococcus Agalactiae. Microb. Pathog. 2018, 116, 237–240. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, H.R.; Kim, J.C.; Lee, E.S.; Chung, C.H.; Lee, E.Y.; Chung, B.Y. Tetrahydrocurcumin Ameliorates Skin Inflammation by Modulating Autophagy in High-Fat Diet-Induced Obese Mice. BioMed Res. Int. 2021, 2021, 6621027. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yin, X.; Gan, W.; Pan, F.; Li, S.; Xiang, Z.; Han, X.; Li, D. PRCC-TFE3 Fusion-Mediated PRKN/Parkin-Dependent Mitophagy Promotes Cell Survival and Proliferation in PRCC-TFE3 Translocation Renal Cell Carcinoma. Autophagy 2020, 17, 2475–2493. [Google Scholar] [CrossRef] [PubMed]
- Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Ramirez-Tortosa, M. Curcumin and Health. Molecules 2016, 21, 264. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.; Chiou, S.; Weng, J.; Lin, P. Curcumin and Tetrahydrocurcumin Induce Cell Death in Ara-C-Resistant Acute Myeloid Leukemia. Phytother. Res. 2019, 33, 1199–1207. [Google Scholar] [CrossRef]
- Chen, B.L.; Chen, Y.Q.; Ma, B.H.; Yu, S.F.; Li, L.Y.; Zeng, Q.X.; Zhou, Y.T.; Wu, Y.F.; Liu, W.L.; Wan, J.B. Tetrahydrocurcumin, a Major Metabolite of Curcumin, Ameliorates Allergic Airway Inflammation by Attenuating Th2 Response and Suppressing the IL-4Rα-Jak1-STAT6 and Jagged1/Jagged2 -Notch1/Notch2 Pathways in Asthmatic Mice. Clin. Exp. Allergy 2018, 48, 1494–1508. [Google Scholar] [CrossRef]
- da Mata, I.R.; da Mata, S.R.; Menezes, R.C.R.; Faccioli, L.S.; Bandeira, K.K.; Bosco, S.M.D. Benefits of Turmeric Supplementation for Skin Health in Chronic Diseases: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2020, 61, 3421–3435. [Google Scholar] [CrossRef]
- Jabczyk, M.; Nowak, J.; Hudzik, B.; Zubelowicz-Szkodzińska, B. Curcumin in Metabolic Health and Disease. Nutrients 2021, 13, 4440. [Google Scholar] [CrossRef] [PubMed]
- Akaberi, M.; Sahebkar, A.; Emami, S.A. Turmeric and Curcumin: From Traditional to Modern Medicine. Adv. Exp. Med. Biol. 2021, 1291, 15–39. [Google Scholar] [CrossRef]
- Vaughn, A.R.; Pourang, A.; Clark, A.K.; Burney, W.; Sivamani, R.K. Dietary Supplementation with Turmeric Polyherbal Formulation Decreases Facial Redness: A Randomized Double-Blind Controlled Pilot Study. J. Integr. Med. 2019, 17, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.D.; Donald, A. The Effect of an Anti-Inflammatory Botanical Cleanser/Night Mask Combination on Facial Redness Reduction. J. Drugs Dermatol. JDD 2018, 17, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, A.R.; Branum, A.; Sivamani, R.K. Effects of Turmeric (Curcuma longa) on Skin Health: A Systematic Review of the Clinical Evidence. Phytother. Res. 2016, 30, 1243–1264. [Google Scholar] [CrossRef]
- Perna, A.; Hay, E.; Sellitto, C.; Genio, E.D.; Falco, M.D.; Guerra, G.; Luca, A.D.; Blasiis, P.D.; Lucariello, A. Antiinflammatory Activities of Curcumin and Spirulina: Focus on Their Role against COVID-19. J. Diet. Suppl. 2023, 20, 372–389. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P.; Simental-Mendía, L.E.; Bo, S.; Sahebkar, A. Effect of Curcumin on Circulating Interleukin-6 Concentrations: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pharmacol. Res. 2016, 111, 394–404. [Google Scholar] [CrossRef]
- Ye, M.Y.; Xu, D.; Liu, J.C.; Lyu, H.P.; Xue, Y.; He, J.T.; Huang, H.Y.; Zhang, K.K.; Xie, X.L.; Wang, Q. IL-6 and IL-20 as potential markers for vitality of skin contusion. J. Forensic Leg. Med. 2018, 59, 8–12. [Google Scholar] [CrossRef]
- Pagano, E.; Romano, B.; Izzo, A.A.; Borrelli, F. The Clinical Efficacy of Curcumin-Containing Nutraceuticals: An Overview of Systematic Reviews. Pharmacol. Res. 2018, 134, 79–91. [Google Scholar] [CrossRef]
- Ghahartars, M.; Abtahi, S.; Zeinali, Z.; Fattahi, M.J.; Ghaderi, A. Investigation of TNF-α and IL-6 Levels in the Sera of Non-Melanoma Skin Cancer Patients. Iran. Biomed. J. 2021, 25, 88–92. [Google Scholar] [CrossRef]
- Baj, T.; Seth, R. Role of Curcumin in Regulation of TNF-α Mediated Brain Inflammatory Responses. Recent Pat. Inflamm. Allergy Drug Discov. 2018, 12, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials. AAPS J. 2012, 15, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Nestor, M.S.; Berman, B.; Swenson, N. Safety and Efficacy of Oral Polypodium Leucotomos Extract in Healthy Adult Subjects. J. Clin. Aesthetic Dermatol. 2015, 8, 19–23. [Google Scholar]
- Segars, K.; McCarver, V.; Miller, R.A. Dermatologic Applications of Polypodium leucotomos: A Literature Review. J. Clin. Aesthetic Dermatol. 2021, 14, 50–60. [Google Scholar]
- Berman, B.; Ellis, C.; Elmets, C. Polypodium Leucotomos—An Overview of Basic Investigative Findings. J. Drugs Dermatol. 2016, 15, 224–228. [Google Scholar] [PubMed]
- Tanew, A.; Radakovic, S.; Gonzalez, S.; Venturini, M.; Calzavara-Pinton, P. Oral Administration of a Hydrophilic Extract of Polypodium Leucotomos for the Prevention of Polymorphic Light Eruption. J. Am. Acad. Dermatol. 2012, 66, 58–62. [Google Scholar] [CrossRef]
- Parrado, C.; Mascaraque, M.; Gilaberte, Y.; Juarranz, A.; Gonzalez, S. Fernblock (Polypodium leucotomos Extract): Molecular Mechanisms and Pleiotropic Effects in Light-Related Skin Conditions, Photoaging and Skin Cancers, a Review. Int. J. Mol. Sci. 2016, 17, 1026. [Google Scholar] [CrossRef]
- Emanuele, E.; Bertona, M.; Biagi, M. Comparative effects of a fixed Polypodium leucotomos/Pomegranate combination versus Polypodium leucotomos alone on skin biophysical parameters. Neuro Endocrinol. Lett. 2017, 38, 38–42. [Google Scholar]
- Rodríguez-Luna, A.; Zamarrón, A.; Juarranz, Á.; Gonzalez, S. Clinical Applications of Polypodium leucotomos (Fernblock®): An Update. Life 2023, 13, 1513. [Google Scholar] [CrossRef]
- Shakhbazova, A.; Wu, H.; Chambers, C.J.; Sivamani, R.K. A Systematic Review of Nutrition, Supplement, and Herbal-Based Adjunctive Therapies for Vitiligo. J. Altern. Complement. Med. 2021, 27, 294–311. [Google Scholar] [CrossRef]
- Pourang, A.; Dourra, M.; Ezekwe, N.; Kohli, I.; Hamzavi, I.; Lim, H.W. The potential effect of Polypodium leucotomos extract on ultraviolet- and visible light-induced photoaging. Photochem. Photobiol. Sci. 2021, 20, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Komarova, E.A.; Krivokrysenko, V.; Wang, K.; Neznanov, N.; Chernov, M.V.; Komarov, P.G.; Brennan, M.L.; Golovkina, T.V.; Rokhlin, O.W.; Kuprash, D.V.; et al. P53 Is a Suppressor of Inflammatory Response in Mice. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2005, 19, 1030–1032. [Google Scholar] [CrossRef] [PubMed]
- Parrado, C.; Nicolas, J.; Juarranz, A.; Gonzalez, S. The role of the aqueous extract Polypodium leucotomos in photoprotection. Photochem. Photobiol. Sci. 2020, 19, 831–843. [Google Scholar] [CrossRef] [PubMed]
- Jańczyk, A.; Garcia-Lopez, M.A.; Fernandez-Peñas, P.; Alonso-Lebrero, J.L.; Benedicto, I.; López-Cabrera, M.; Gonzalez, S. A Polypodium Leucotomos Extract Inhibits Solar-Simulated Radiation-Induced TNF-? And INOS Expression, Transcriptional Activation and Apoptosis. Exp. Dermatol. 2007, 16, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.; Guarino, R.; Ottes Vasconcelos, R.; Celleno, L.; Calviello, G. The Combination of Sulforaphane and Fernblock® XP Improves Individual Beneficial Effects in Normal and Neoplastic Human Skin Cell Lines. Nutrients 2020, 12, 1608. [Google Scholar] [CrossRef] [PubMed]
- González, S.; Pathak, M.A. Inhibition of Ultraviolet-Induced Formation of Reactive Oxygen Species, Lipid Peroxidation, Erythema and Skin Photosensitization by Polypodium Leucotomos. Photodermatol. Photoimmunol. Photomed. 1996, 12, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Babbush, K.M.; Babbush, R.A.; Khachemoune, A. Treatment of melasma: A review of less commonly used antioxidants. Int. J. Dermatol. 2021, 60, 166–173. [Google Scholar] [CrossRef]
- Boozer, C.N.; Herron, A.J. Simmondsin for Weight Loss in Rats. Int. J. Obes. 2006, 30, 1143–1148. [Google Scholar] [CrossRef]
- Tietel, Z.; Melamed, S.; Eretz-Kdosha, N.; Guetta, A.; Gvirtz, R.; Ogen-Shtern, N.; Cohen, G. Anti-Herpes Simplex 1 Activity of Simmondsia chinensis (Jojoba) Wax. Molecules 2021, 26, 6059. [Google Scholar] [CrossRef]
- Shawer, R.; El-Shazly, M.M.; Khider, A.M.; Baeshen, R.S.; Hikal, W.M.; Kordy, A.M. Botanical Oils Isolated from Simmondsia chinensis and Rosmarinus officinalis Cultivated in Northern Egypt: Chemical Composition and Insecticidal Activity against Sitophilus oryzae (L.) and Tribolium castaneum (Herbst). Molecules 2022, 27, 4383. [Google Scholar] [CrossRef]
- Harry-O’kuru, R.E.; Biresaw, G.; Gordon, S.; Xu, J. Physical Characteristics of Tetrahydroxy and Acylated Derivatives of Jojoba Liquid Wax in Lubricant Applications. J. Anal. Methods Chem. 2018, 2018, 7548327. [Google Scholar] [CrossRef] [PubMed]
- Ranzato, E.; Martinotti, S.; Burlando, B. Wound Healing Properties of Jojoba Liquid Wax: An in Vitro Study. J. Ethnopharmacol. 2011, 134, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Gruber, J.V.; Terpak, N.; Massard, S.; Schwartz, A.; Bojanowski, K. Passive Enhancement of Retinol Skin Penetration by Jojoba Oil Measured Using the Skin Parallel Artificial Membrane Permeation Assay (Skin-PAMPA): A Pilot Study. Clin. Cosmet. Investig. Dermatol. 2023, 16, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Orchard, A.; Kamatou, G.; Viljoen, A.M.; Patel, N.; Mawela, P.; van Vuuren, S.F. The Influence of Carrier Oils on the Antimicrobial Activity and Cytotoxicity of Essential Oils. Evid.-Based Complement. Altern. Med. 2019, 2019, 6981305. [Google Scholar] [CrossRef]
- De Prijck, K.; Peeters, E.; Nelis, H.J. Comparison of Solid-Phase Cytometry and the Plate Count Method for the Evaluation of the Survival of Bacteria in Pharmaceutical Oils. Lett. Appl. Microbiol. 2008, 47, 571–573. [Google Scholar] [CrossRef]
- Ellaithy, H.M.; El-Shaboury, K.M.F. The Development of Cutina Lipogels and Gel Microemulsion for Topical Administration of Fluconazole. AAPS PharmSciTech 2002, 3, 77–85. [Google Scholar] [CrossRef]
- Realdon, N.; Ragazzi, E.; Ragazzi, E. Effect of Gelling Conditions and Mechanical Treatment on Drug Availability from a Lipogel. Drug Dev. Ind. Pharm. 2001, 27, 165–170. [Google Scholar] [CrossRef]
- Assaf, S.; Maaroof, K.; Altaani, B.; Ghareeb, M.M.; Alhayyal, A.A.A. Jojoba Oil-Based Microemulsion for Transdermal Drug Delivery. Res. Pharm. Sci. 2021, 16, 326. [Google Scholar] [CrossRef]
- Chacko, A.; Newton, A.M.J. Synthesis and Characterization of Valacyclovir HCl Hybrid Solid Lipid Nanoparticles by Using Natural Oils. Recent Pat. Drug Deliv. Formul. 2019, 13, 46–61. [Google Scholar] [CrossRef]
- Basto, R.; Andrade, R.; Nunes, C.; Lima, S.A.C.; Reis, S. Topical Delivery of Niacinamide to Skin Using Hybrid Nanogels Enhances Photoprotection Effect. Pharmaceutics 2021, 13, 1968. [Google Scholar] [CrossRef]
- Starr, P. Oral Nicotinamide Prevents Common Skin Cancers in High-Risk Patients, Reduces Costs. Am. Health Drug Benefits 2015, 8, 13–14. [Google Scholar] [PubMed]
- Chen, A.C.; Martin, A.J.; Choy, B.; Fernandez-Penas, P.; Dalziell, R.A.; McKenzie, C.A.; Scolyer, R.A.; Dhillon, H.M.; Vardy, J.L.; Kricker, A.; et al. A Phase 3 Randomized Trial of Nicotinamide for Skin-Cancer Chemoprevention. N. Engl. J. Med. 2015, 373, 1618–1626. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M.; Abdel-Hamid, S.; Moftah, N.H.; Fadel, M.; Alyoussef, A.A. Jojoba Oil Soft Colloidal Nanocarrier of a Synthetic Retinoid: Preparation, Characterization and Clinical Efficacy in Psoriatic Patients. Curr. Drug Deliv. 2017, 14, 426–432. [Google Scholar] [CrossRef]
- Meier, L.; Stange, R.; Michalsen, A.; Uehleke, B. Clay Jojoba Oil Facial Mask for Lesioned Skin and Mild Acne—Results of a Prospective, Observational Pilot Study. Forsch. Komplementärmedizin/Res. Complement. Med. 2012, 19, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Belostozky, A.; Bretler, S.; Kolitz-Domb, M.; Grinberg, I.; Margel, S. Solidification of oil liquids by encapsulation within porous hollow silica microspheres of narrow size distribution for pharmaceutical and cosmetic applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Zempleni, J.; Kuroishi, T. Biotin. Adv. Nutr. 2012, 3, 213–214. [Google Scholar] [CrossRef]
- Lipner, S. Update on Biotin Therapy in Dermatology: Time for a Change. J. Drugs Dermatol. 2020, 19, 1264–1265. [Google Scholar] [CrossRef] [PubMed]
- Saleem, F.; Soos, M.P. Biotin Deficiency. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Mock, D.M. Biotin: From Nutrition to Therapeutics. J. Nutr. 2017, 147, 1487–1492. [Google Scholar] [CrossRef]
- Perez-Sanchez, A.C.; Burns, E.K.; Perez, V.M.; Tantry, E.K.; Prabhu, S.; Katta, R. Safety Concerns of Skin, Hair and Nail Supplements in Retail Stores. Cureus 2020, 12, e9477. [Google Scholar] [CrossRef]
- Waqas, B.; Wu, A.; Yim, E.; Lipner, S.R. A Survey-Based Study of Physician Practices Regarding Biotin Supplementation. J. Dermatol. Treat. 2020, 33, 573–574. [Google Scholar] [CrossRef]
- Rosner, I.; Rogers, E.; Maddrey, A.; Goldberg, D.M. Clinically Significant Lab Errors due to Vitamin B7 (Biotin) Supplementation: A Case Report Following a Recent FDA Warning. Cureus 2019, 11, e5470. [Google Scholar] [CrossRef] [PubMed]
- Waqas, B.; Lipner, S.R. Biotin Interference in Routine Laboratory Tests: A Bibliometric Analysis. J. Am. Acad. Dermatol. 2020, 83, 1834–1838. [Google Scholar] [CrossRef] [PubMed]
- Gunsolus, I.L.; Matias, M.; Prostko, J.; Mohr, P.; Sokoll, L.J. Prevalence of Detectable Biotin in Five US Emergency Department Patient Cohorts. Clin. Biochem. 2021, 93, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Lundin, M.S.; Alratroot, A.; Abu Rous, F.; Aldasouqi, S. When Thyroid Labs Do Not Add Up, Physicians Should Ask Patients about Biotin Supplements. BMJ Case Rep. 2020, 13, e231337. [Google Scholar] [CrossRef]
- Patel, D.P.; Swink, S.M.; Castelo-Soccio, L. A Review of the Use of Biotin for Hair Loss. Skin Appendage Disord. 2017, 3, 166–169. [Google Scholar] [CrossRef] [PubMed]
- DiBaise, M.; Tarleton, S.M. Hair, Nails, and Skin: Differentiating Cutaneous Manifestations of Micronutrient Deficiency. Nutr. Clin. Pract. 2019, 34, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Radelfahr, F.; Riedhammer, K.M.; Keidel, L.F.; Gramer, G.; Meitinger, T.; Klopstock, T.; Wagner, M. Biotinidase deficiency: A treatable cause of hereditary spastic paraparesis. Neurol. Genet. 2020, 6, e525. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, J.Y.; Chen, X.J. Biotinidase deficiency characterized by skin and hair findings. Clin. Dermatol. 2020, 38, 477–483. [Google Scholar] [CrossRef]
- Lipner, S.R. Rethinking biotin therapy for hair, nail, and skin disorders. J. Am. Acad. Dermatol. 2018, 78, 1236–1238. [Google Scholar] [CrossRef]
- Angelis, A.; Urbain, A.; Halabalaki, M.; Aligiannis, N.; Skaltsounis, A.L. One-Step Isolation of γ-Oryzanol from Rice Bran Oil by Non-Aqueous Hydrostatic Countercurrent Chromatography. J. Sep. Sci. 2011, 34, 2528–2537. [Google Scholar] [CrossRef]
- Ramazani, E.; Akaberi, M.; Emami, S.A.; Tayarani-Najaran, Z. Biological and Pharmacological Effects of Gamma-oryzanol: An Updated Review of the Molecular Mechanisms. Curr. Pharm. Des. 2021, 27, 2299–2316. [Google Scholar] [CrossRef] [PubMed]
- Minatel, I.O.; Francisqueti, F.V.; Corrêa, C.R.; Lima, G.P.P. Antioxidant Activity of γ-Oryzanol: A Complex Network of Interactions. Int. J. Mol. Sci. 2016, 17, 1107. [Google Scholar] [CrossRef] [PubMed]
- Masuzaki, H.; Kozuka, C.; Okamoto, S.; Yonamine, M.; Tanaka, H.; Shimabukuro, M. Brown rice-specific γ-oryzanol as a promising prophylactic avenue to protect against diabetes mellitus and obesity in humans. J. Diabetes Investig. 2019, 10, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Zeinali, M.; Abbaspour-Ravasjani, S.; Soltanfam, T.; Paiva-Santos, A.C.; Babaei, H.; Veiga, F.; Hamishehkar, H. Prevention of UV-Induced Skin Cancer in Mice by Gamma Oryzanol-Loaded Nanoethosomes. Life Sci. 2021, 283, 119759. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Chakraborty, S.; Roy, C.; Rajabalaya, R.; Mohaimin, A.W.; Khanam, J.; Nanda, A.; David, S.R. Ethosomes as Novel Vesicular Carrier: An Overview of the Principle, Preparation and Its Applications. Curr. Drug Deliv. 2018, 15, 795–817. [Google Scholar] [CrossRef]
- Toorani, M.R.; Golmakani, M.-T.; Hashemi Gahruie, H. Antioxidant Activity and Inhibitory Mechanism of γ-Oryzanol as Influenced by the Unsaturation Degree of Lipid Systems. LWT 2020, 133, 109930. [Google Scholar] [CrossRef]
- Heydari, S.; Ghanbarzadeh, S.; Anoush, B.; Ranjkesh, M.; Javadzadeh, Y.; Kouhsoltani, M.; Hamishehkar, H. Nanoethosomal Formulation of Gammaoryzanol for Skin-Aging Protection and Wrinkle Improvement: A Histopathological Study. Drug Dev. Ind. Pharm. 2017, 43, 1154–1162. [Google Scholar] [CrossRef]
- Manosroi, A.; Chutoprapat, R.; Abe, M.; Manosroi, W.; Manosroi, J. Anti-aging efficacy of topical formulations containing niosomes entrapped with rice bran bioactive compounds. Pharm. Biol. 2012, 50, 208–224. [Google Scholar] [CrossRef]
- Shin, S.Y.; Kim, H.W.; Jang, H.H.; Hwang, Y.J.; Choe, J.S.; Lim, Y.; Lee, Y.H. γ-Oryzanol-Rich Black Rice Bran Extract Enhances the Innate Immune Response. J. Med. Food 2017, 20, 855–863. [Google Scholar] [CrossRef]
- Badalkhani, O.; Pires, P.C.; Mohammadi, M.; Babaie, S.; Paiva-Santos, A.C.; Hamishehkar, H. Nanogel Containing Gamma-Oryzanol-Loaded Nanostructured Lipid Carriers and TiO2/MBBT: A Synergistic Nanotechnological Approach of Potent Natural Antioxidants and Nanosized UV Filters for Skin Protection. Pharmaceuticals 2023, 16, 670. [Google Scholar] [CrossRef]
- Wanitphakdeedecha, R.; Ng, J.N.C.; Junsuwan, N.; Phaitoonwattanakij, S.; Phothong, W.; Eimpunth, S.; Manuskiatti, W. Efficacy of Olive Leaf Extract-Containing Cream for Facial Rejuvenation: A Pilot Study. J. Cosmet. Dermatol. 2020, 19, 1662–1666. [Google Scholar] [CrossRef] [PubMed]
- Somerville, V.; Moore, R.; Braakhuis, A. The Effect of Olive Leaf Extract on Upper Respiratory Illness in High School Athletes: A Randomised Control Trial. Nutrients 2019, 11, 358. [Google Scholar] [CrossRef] [PubMed]
- Allegretta, C.; Difonzo, G.; Caponio, F.; Tamma, G.; Laselva, O. Olive Leaf Extract (OLE) as a Novel Antioxidant That Ameliorates the Inflammatory Response in Cystic Fibrosis. Cells 2023, 12, 1764. [Google Scholar] [CrossRef] [PubMed]
- Grubić Kezele, T.; Ćurko-Cofek, B. Neuroprotective Panel of Olive Polyphenols: Mechanisms of Action, Anti-Demyelination, and Anti-Stroke Properties. Nutrients 2022, 14, 4533. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S.; Simon, S.R. A Novel Pro-Melanogenic Effect of Standardized Dry Olive Leaf Extract on Primary Human Melanocytes from Lightly Pigmented and Moderately Pigmented Skin. Pharmaceuticals 2021, 14, 252. [Google Scholar] [CrossRef] [PubMed]
- Khayyal, M.; El-Ghazaly, M.; Abdallah, D.; Nassar, N.; Okpanyi, S.; Kreuter, M.-H. Blood Pressure Lowering Effect of an Olive Leaf Extract {Olea europaed) in L-NAME Induced Hypertension in Rats. Arzneimittelforschung 2011, 52, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Karanovic, D.; Mihailovic-Stanojevic, N.; Miloradovic, Z.; Ivanov, M.; Vajic, U.-J.; Grujic-Milanovic, J.; Markovic-Lipkovski, J.; Dekanski, D.; Jovovic, D. Olive Leaf Extract Attenuates Adriamycin-Induced Focal Segmental Glomerulosclerosis in Spontaneously Hypertensive Rats via Suppression of Oxidative Stress, Hyperlipidemia, and Fibrosis. Phytother. Res. PTR 2021, 35, 1534–1545. [Google Scholar] [CrossRef] [PubMed]
- Dekanski, D.; Selaković, V.; Piperski, V.; Radulović, Z.; Korenić, A.; Radenović, L. Protective Effect of Olive Leaf Extract on Hippocampal Injury Induced by Transient Global Cerebral Ischemia and Reperfusion in Mongolian Gerbils. Phytomedicine Int. J. Phytother. Phytopharm. 2011, 18, 1137–1143. [Google Scholar] [CrossRef]
- Hayes, J.E.; Allen, P.; Brunton, N.; O’Grady, M.N.; Kerry, J.P. Phenolic Composition and in Vitro Antioxidant Capacity of Four Commercial Phytochemical Products: Olive Leaf Extract (Olea europaea L.), Lutein, Sesamol and Ellagic Acid. Food Chem. 2011, 126, 948–955. [Google Scholar] [CrossRef]
- da Silva, A.C.P.; Paiva, J.P.; Diniz, R.R.; Dos Anjos, V.M.; Silva, A.B.S.M.; Pinto, A.V.; Dos Santos, E.P.; Leitão, A.C.; Cabral, L.M.; Rodrigues, C.R.; et al. Photoprotection Assessment of Olive (Olea europaea L.) Leaves Extract Standardized to Oleuropein: In Vitro and In Silico Approach for Improved Sunscreens. J. Photochem. Photobiol. B Biol. 2019, 193, 162–171. [Google Scholar] [CrossRef]
- De Cicco, P.; Ercolano, G.; Tenore, G.C.; Ianaro, A. Olive leaf extract inhibits metastatic melanoma spread through suppression of epithelial to mesenchymal transition. Phytother. Res. 2022, 36, 4002–4013. [Google Scholar] [CrossRef]
- Soengas, M.S.; Lowe, S.W. Apoptosis and Melanoma Chemoresistance. Oncogene 2003, 22, 3138–3151. [Google Scholar] [CrossRef] [PubMed]
- Ruzzolini, J.; Peppicelli, S.; Andreucci, E.; Bianchini, F.; Scardigli, A.; Romani, A.; Marca, G.I.; Nediani, C.; Calorini, L. Oleuropein, the Main Polyphenol of Olea europaea Leaf Extract, Has an Anti-Cancer Effect on Human BRAF Melanoma Cells and Potentiates the Cytotoxicity of Current Chemotherapies. Nutrients 2018, 10, 1950. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Sumiyoshi, M. Olive Leaf Extract and Its Main Component Oleuropein Prevent Chronic Ultraviolet B Radiation-Induced Skin Damage and Carcinogenesis in Hairless Mice. J. Nutr. 2009, 139, 2079–2086. [Google Scholar] [CrossRef] [PubMed]
- Inomata, S.; Matsunaga, Y.; Amano, S.; Takada, K.; Kobayashi, K.; Tsunenaga, M.; Nishiyama, T.; Kohno, Y.; Fukuda, M. Possible Involvement of Gelatinases in Basement Membrane Damage and Wrinkle Formation in Chronically Ultraviolet B-Exposed Hairless Mouse. J. Investig. Dermatol. 2003, 120, 128–134. [Google Scholar] [CrossRef]
- Kaliski, A.; Maggiorella, L.; Cengel, K.A.; Mathe, D.; Rouffiac, V.; Opolon, P.; Lassau, N.; Bourhis, J.; Deutsch, E. Angiogenesis and Tumor Growth Inhibition by a Matrix Metalloproteinase Inhibitor Targeting Radiation-Induced Invasion. Mol. Cancer Ther. 2005, 4, 1717–1728. [Google Scholar] [CrossRef]
- Ikeda, I.K.; Sydney, E.B.; Sydney, A.C.N. Potential Application of Spirulinain Dermatology. J. Cosmet. Dermatol. 2022, 21, 4205–4214. [Google Scholar] [CrossRef]
- Burke, K.E. Mechanisms of Aging and Development—A New Understanding of Environmental Damage to the Skin and Prevention with Topical Antioxidants. Mech. Ageing Dev. 2018, 172, 123–130. [Google Scholar] [CrossRef]
- Pina-Pérez, M.C.; Úbeda-Manzanaro, M.; Beyrer, M.; Martinez, A.; Rodrigo, D. In Vivo Assessment of Cold Atmospheric Pressure Plasma Technology on the Bioactivity of Spirulina. Front. Microbiol. 2022, 12, 781871. [Google Scholar] [CrossRef]
- Liu, P.; Lee, M.; Choi, J.; Choi, Y.; Nam, T. Crude Protein from Spirulina Increases the Viability of CCD-986sk Cells via the EGFR/MAPK Signaling Pathway. Int. J. Mol. Med. 2018, 43, 771–778. [Google Scholar] [CrossRef]
- Gunes, S.; Tamburaci, S.; Dalay, M.C.; Gurhan, I.D. In Vitro Evaluation of Spirulina Platensis Extract Incorporated Skin Cream with Its Wound Healing and Antioxidant Activities. Pharm. Biol. 2017, 55, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Nihal, B.; Gupta, N.V.; Gowda, D.V.; Manohar, M. Formulation and Development of Topical Anti Acne Formulation of Spirulina Extract. Int. J. Appl. Pharm. 2018, 10, 229. [Google Scholar] [CrossRef]
- Józsa, L.; Ujhelyi, Z.; Vasvári, G.; Sinka, D.; Nemes, D.; Fenyvesi, F.; Varadi, J.; Vecsernyes, M.; Szabo, J.; Kallo, G.; et al. Formulation of Creams Containing Spirulina Platensis Powder with Different Nonionic Surfactants for the Treatment of Acne Vulgaris. Molecules 2020, 25, 4856. [Google Scholar] [CrossRef] [PubMed]
- Bax, C.E.; Chakka, S.; Concha, J.S.S.; Zeidi, M.; Werth, V.P. The Effects of Immunostimulatory Herbal Supplements on Autoimmune Skin Diseases. J. Am. Acad. Dermatol. 2021, 84, 1051–1058. [Google Scholar] [CrossRef]
- Bax, C.E.; Maddukuri, S.; Ravishankar, A.; Pappas-Taffer, L.; Werth, V.P. Environmental triggers of dermatomyositis: A narrative review. Ann. Transl. Med. 2021, 9, 434. [Google Scholar] [CrossRef] [PubMed]
- Zeidi, M.; Chansky, P.B.; Werth, V.P. Acute Onset/Flares of Dermatomyositis Following Ingestion of IsaLean Herbal Supplement: Clinical and Immunostimulatory Findings. J. Am. Acad. Dermatol. 2019, 80, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Konno, T.; Umeda, Y.; Umeda, M.; Kawachi, I.; Oyake, M.; Fujita, N. A Case of Inflammatory Myopathy with Widely Skin Rash Following Use of Supplements Containing Spirulina. Rinsho Shinkeigaku 2011, 51, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Kraigher, O.; Wohl, Y.; Gat, A.; Brenner, S. A Mixed Immunoblistering Disorder Exhibiting Features of Bullous Pemphigoid and Pemphigus Foliaceus Associated with Spirulina Algae Intake. Int. J. Dermatol. 2007, 47, 61–63. [Google Scholar] [CrossRef]
- Prete, P.E. The Mechanism of Action of L-Canavanine in Inducing Autoimmune Phenomena. Arthritis Rheum. 1985, 28, 1198–1200. [Google Scholar] [CrossRef]
- Juszkiewicz, A.; Basta, P.; Petriczko, E.; Machaliński, B.; Trzeciak, J.; Łuczkowska, K.; Skarpańska-Stejnborn, A. An attempt to induce an immunomodulatory effect in rowers with spirulina extract. J. Int. Soc. Sports Nutr. 2018, 15, 9. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, H.S. Monocyte chemoattractant protein-1 polymorphism interaction with spirulina immunomodulatory effects in healthy Korean elderly: A 16 week, double-blind randomized clinical trial. Nutr. Res. Pract. 2017, 11, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, H.; Tominaga, A.; Fukuoka, S.; Taguchi, T.; Kusumoto, Y.; Ono, S. Spirulina Lipopolysaccharides Inhibit Tumor Growth in a Toll-like Receptor 4-Dependent Manner by Altering the Cytokine Milieu from Interleukin-17/Interleukin-23 to Interferon-γ. Oncol. Rep. 2017, 37, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Trushina, E.N.; Gladkikh, O.; Gadzhieva, Z.M.; Mustafina, O.K.; Pozdniakov, A.L. The Influence of Spirulina and Selen-Spirulina on Some Indexes of Rat’s Immune Status. Vopr. Pitan. 2007, 76, 21–25. [Google Scholar] [PubMed]
- Finamore, A.; Palmery, M.; Bensehaila, S.; Peluso, I. Antioxidant, Immunomodulating, and Microbial-Modulating Activities of the Sustainable and Ecofriendly Spirulina. Oxidative Med. Cell. Longev. 2017, 2017, 3247528. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, Z.; Lin, Q.; Xia, X.; Lin, Y.; Yan, J.; Huang, M.; Huang, R. Anti-colon cancer effects of Spirulina polysaccharide and its mechanism based on 3D models. Int. J. Biol. Macromol. 2023, 228, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Kawashima, H.; Osada, H.; Toda, E.; Homma, K.; Nagai, N.; Imai, Y.; Tsubota, K.; Ozawa, Y. Dietary Spirulina Supplementation Protects Visual Function From Photostress by Suppressing Retinal Neurodegeneration in Mice. Transl. Vis. Sci. Technol. 2019, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, P.; Pugh, N.D.; Ma, G.; Pasco, D.S. Toll-like Receptor 2-Dependent Activation of Monocytes by Spirulina Polysaccharide and Its Immune Enhancing Action in Mice. Int. Immunopharmacol. 2006, 6, 1808–1814. [Google Scholar] [CrossRef] [PubMed]
- Sibiya, T.; Ghazi, T.; Chuturgoon, A. The Potential of Spirulina platensis to Ameliorate the Adverse Effects of Highly Active Antiretroviral Therapy (HAART). Nutrients 2022, 14, 3076. [Google Scholar] [CrossRef]
- Sibiya, T.; Ghazi, T.; Mohan, J.; Nagiah, S.; Chuturgoon, A.A. Spirulina platensis Mitigates the Inhibition of Selected miRNAs that Promote Inflammation in HAART-Treated HepG2 Cells. Plants 2022, 12, 119. [Google Scholar] [CrossRef]
- Chen, W.; Chen, Y.H.; Liao, Y.C.; Huang, X.W.; Lu, T.J.; Shih, S.R. Effect of hot water extracts of Arthrospira maxima (spirulina) against respiratory syncytial virus. Phytomedicine 2023, 110, 154611. [Google Scholar] [CrossRef]
- Ngo-Matip, M.-E.; Pieme, C.A.; Azabji-Kenfack, M.; Moukette, B.M.; Korosky, E.; Stefanini, P.; Ngogang, J.Y.; Mbofung, C.M. Impact of Daily Supplementation of Spirulina Platensis on the Immune System of Naïve HIV-1 Patients in Cameroon: A 12-Months Single Blind, Randomized, Multicenter Trial. Nutr. J. 2015, 14, 70. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Choi, J.; Lee, M.; Choi, Y.; Nam, T. Spirulina Protein Promotes Skin Wound Repair in a Mouse Model of Full-Thickness Dermal Excisional Wound. Int. J. Mol. Med. 2020, 46, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Choi, J.-W.; Lee, M.-K.; Choi, Y.-K.; Nam, T.-J. Wound Healing Potential of Spirulina Protein on CCD-986sk Cells. Mar. Drugs 2019, 17, 130. [Google Scholar] [CrossRef] [PubMed]
- Pappas, A.; Tsiokanos, A.; Fatouros, I.G.; Poulios, A.; Kouretas, D.; Goutzourelas, N.; Giakas, G.; Jamurtas, A.Z. The Effects of Spirulina Supplementation on Redox Status and Performance Following a Muscle Damaging Protocol. Int. J. Mol. Sci. 2021, 22, 3559. [Google Scholar] [CrossRef] [PubMed]
- Plikus, M.V.; Guerrero-Juarez, C.F.; Ito, M.; Li, Y.R.; Dedhia, P.H.; Zheng, Y.; Shao, M.; Gay, D.L.; Ramos, R.; Hsi, T.-C.; et al. Regeneration of Fat Cells from Myofibroblasts during Wound Healing. Science 2017, 355, 748–752. [Google Scholar] [CrossRef]
- Souza, C.; Campos, P.M.B.G.M. Development and Photoprotective Effect of a Sunscreen Containing the Antioxidants Spirulina and Dimethylmethoxy Chromanol on Sun-Induced Skin Damage. Eur. J. Pharm. Sci. 2017, 104, 52–64. [Google Scholar] [CrossRef]
- Byeon, S.Y.; Cho, M.K.; Shim, K.H.; Kim, H.J.; Song, H.G.; Shin, H.S. Development of a Spirulina Extract/Alginate-Imbedded PCL Nanofibrous Cosmetic Patch. J. Microbiol. Biotechnol. 2017, 27, 1657–1663. [Google Scholar] [CrossRef]
- Bito, T.; Okumura, E.; Fujishima, M.; Watanabe, F. Potential of Chlorella as a Dietary Supplement to Promote Human Health. Nutrients 2020, 12, 2524. [Google Scholar] [CrossRef]
- Woortman, D.V.; Fuchs, T.; Striegel, L.; Fuchs, M.; Weber, N.; Bruck, T.B.; Rychlik, M. Microalgae a Superior Source of Folates: Quantification of Folates in Halophile Microalgae by Stable Isotope Dilution Assay. Front. Bioeng. Biotechnol. 2019, 7, 481. [Google Scholar] [CrossRef]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for High-Value Products Towards Human Health and Nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef]
- Wilson, L.R.; Tripkovic, L.; Hart, K.H.; Lanhma-New, S.A. Vitamin D Deficiency as a Public Health Issue: Using Vitamin D2or Vitamin D3in Future Fortification Strategies. Proc. Nutr. Soc. 2017, 76, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Li, H.; Wei, Z.; Lv, K.; Gao, C.; Liu, Y.; Zhao, L. Isolation, structures and biological activities of polysaccharides from Chlorella: A review. Int. J. Biol. Macromol. 2020, 163, 2199–2209. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, D.H.; Jo, S.; Cho, M.J.; Cho, Y.R.; Lee, Y.J.; Byun, S. Immunomodulatory functional foods and their molecular mechanisms. Exp. Mol. Med. 2022, 54, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.K.; Park, S.B.; Kim, C.H. Effects of dietary supplementation with a chlorella by-product on the growth performance, immune response, intestinal microflora and intestinal mucosal morphology in broiler chickens. J. Anim. Physiol. Anim. Nutr. 2017, 101, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Hart, A.N.; Zaske, L.A.; Patterson, K.M.; Drapeau, C.; Jensen, G.S. Natural Killer Cell Activation and Modulation of Chemokine Receptor Profile in Vitro by an Extract from the Cyanophyta Aphanizomenon Flos-Aquae. J. Med. Food 2007, 10, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.S.; Resende, D.I.S.P.; Lobo, J.M.S.; Sousa, E.; Almeida, I.F. Marine Ingredients for Sensitive Skin: Market Overview. Mar. Drugs 2021, 19, 464. [Google Scholar] [CrossRef] [PubMed]
- De Melo, R.G.; de Andrade, A.F.; Bezerra, R.P.; Marques, D.A.V.; da Silva Jr, V.A.; Paz, S.T.; Filho, J.L.L.; Porto, A.L.F. Hydrogel-Based Chlorella vulgaris Extracts: A New Topical Formulation for Wound Healing Treatment. J. Appl. Phycol. 2019, 31, 3653–3663. [Google Scholar] [CrossRef]
- Machmud, E.; Ruslin, M.; Waris, R.; Asse, R.A.; Qadafi, A.M.; Achmad, H. Effect of the Application of Chlorella vulgaris Ointment to the Number of Fibroblast Cells as an Indicator of Wound Healing in the Soft Tissue of Pig Ears. Pesqui. Bras. Odontopediatria Clínica Integr. 2020, 20. [Google Scholar] [CrossRef]
- Kang, H.; Lee, C.; Kim, J.; Kwon, J.; Seo, S.; Han, J.; Kim, B.; Kim, J.; Lee, K. Chlorella vulgaris Attenuates Dermatophagoides Farinae-Induced Atopic Dermatitis-like Symptoms in NC/Nga Mice. Int. J. Mol. Sci. 2015, 16, 21021–21034. [Google Scholar] [CrossRef]
- Singh, A.; Singh, S.P.; Bamezai, R. Inhibitory Potential of Chlorella vulgaris (E-25) on Mouse Skin Papillomagenesis and Xenobiotic Detoxication System. Anticancer Res. 1999, 19, 1887–1891. [Google Scholar]
- Utsunomiya, N.; Oyama, N.; Chino, T.; Utsunomiya, A.; Hida, Y.; Hasegawa, M. Dietary Supplement Product Composed of Natural Ingredients as a Suspected Cause of Erythema Multiforme: A Case Report and Identification for the Confident False Positivity of Lymphocyte Transformation Test. J. Dermatol. 2019, 46, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Shih, M.F.; Cherng, J.Y. Protective Effects of Chlorella-Derived Peptide against UVC-Induced Cytotoxicity through Inhibition of Caspase-3 Activity and Reduction of the Expression of Phosphorylated FADD and Cleaved PARP-1 in Skin Fibroblasts. Molecules 2012, 17, 9116–9128. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ye, T.; Li, C.; Li, X.; Chen, L.; Wang, G. Cell damage repair mechanism in a desert green algae Chlorella sp. against UV-B radiation. Ecotoxicol. Environ. Saf. 2022, 242, 113916. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Zhang, F.; Zhou, K.; Zhao, Q.; Sun, H.; Wang, S.; Zhao, Y.; Fu, J. Breeding of high protein Chlorella sorokiniana using protoplast fusion. Bioresour. Technol. 2020, 313, 123624. [Google Scholar] [CrossRef]
- Johnson, J.L.; Lowell, B.C.; Ryabinina, O.P.; Stephen Lloyd, R.; McCullough, A.K. TAT-Mediated Delivery of a DNA Repair Enzyme to Skin Cells Rapidly Initiates Repair of UV-Induced DNA Damage. J. Investig. Dermatol. 2011, 131, 753–761. [Google Scholar] [CrossRef]
- Hacker, E.; Muller, H.K.; Hayward, N.; Fahey, P.; Walker, G. Enhancement of DNA Repair Using Topical T4 Endonuclease v Does Not Inhibit Melanoma Formation in Cdk4(R24C/R24C)/Tyr-Nras(Q61K) Mice Following Neonatal UVR. Pigment. Cell Melanoma Res. 2010, 23, 121–128. [Google Scholar] [CrossRef]
- Tiberg, E.; Dreborg, S.; Bjorksten, B. Allergy to Green Algae (Chlorella) among Children. J. Allergy Clin. Immunol. 1995, 96, 257–259. [Google Scholar] [CrossRef]
- Balić, A.; Vlašić, D.; Žužul, K.; Marinovic, B.; Mokos, Z.B. Omega-3 Versus Omega-6 Polyunsaturated Fatty Acids in the Prevention and Treatment of Inflammatory Skin Diseases. Int. J. Mol. Sci. 2020, 21, 741. [Google Scholar] [CrossRef]
- Thomsen, B.J.; Chow, E.Y.; Sapijaszko, M.J. The Potential Uses of Omega-3 Fatty Acids in Dermatology: A Review. J. Cutan. Med. Surg. 2020, 24, 481–494. [Google Scholar] [CrossRef]
- Elagizi, A.; Lavie, C.J.; O’Keefe, E.; Marshall, K.; O’Keefe, J.H.; Milani, R.V. An Update on Omega-3 Polyunsaturated Fatty Acids and Cardiovascular Health. Nutrients 2021, 13, 204. [Google Scholar] [CrossRef]
- Sawada, Y.; Saito-Sasaki, N.; Nakamura, M. Omega 3 Fatty Acid and Skin Diseases. Front. Immunol. 2021, 11, 623052. [Google Scholar] [CrossRef] [PubMed]
- Garbicz, J.; Całyniuk, B.; Górski, M.; Buczkowska, M.; Piecuch, M.; Kulik, A.; Rozentryt, P. Nutritional Therapy in Persons Suffering from Psoriasis. Nutrients 2021, 14, 119. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, W.H.; Schön, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Dogra, S.; Mehta, H. Biological treatment for erythrodermic psoriasis. Expert. Opin. Biol. Ther. 2022, 22, 1531–1543. [Google Scholar] [CrossRef]
- Clark, C.C.T.; Taghizadeh, M.; Nahavandi, M.; Jafarnejad, S. Efficacy of ω-3 supplementation in patients with psoriasis: A meta-analysis of randomized controlled trials. Clin. Rheumatol. 2019, 38, 977–988. [Google Scholar] [CrossRef]
- Williams, H.C.; Chalmers, J. Prevention of Atopic Dermatitis. Acta Derm. Venereol. 2020, 100, adv00166. [Google Scholar] [CrossRef]
- Best, K.P.; Gold, M.; Kennedy, D.; Martin, J.; Markides, M. Omega-3 long-chain PUFA intake during pregnancy and allergic disease outcomes in the offspring: A systematic review and meta-analysis of observational studies and randomized controlled trials. Am. J. Clin. Nutr. 2016, 103, 128–143. [Google Scholar] [CrossRef]
- Trikamjee, T.; Comberiati, P.; D’Auria, E.; Peroni, D.; Zuccotti, G.V. Nutritional Factors in the Prevention of Atopic Dermatitis in Children. Front. Pediatr. 2021, 8, 577413. [Google Scholar] [CrossRef]
- Freitas, R.D.S.; Campos, M.M. Protective Effects of Omega-3 Fatty Acids in Cancer-Related Complications. Nutrients 2019, 11, 945. [Google Scholar] [CrossRef]
- Braha, A.; Albai, A.; Timar, B.; Negru, S.; Sorin, S.; Roman, D.; Popovici, D. Nutritional Interventions to Improve Cachexia Outcomes in Cancer-A Systematic Review. Medicina 2022, 58, 966. [Google Scholar] [CrossRef]
- Liu, X.; Peng, Y.; Tao, R.; Meng, L.; Li, X. Mendelian Randomization Study of Causal Relationship between Omega-3 Fatty Acids and Risk of Lung Cancer. Biomed. Res. Int. 2022, 2022, 2786567. [Google Scholar] [CrossRef] [PubMed]
- Vega, O.M.; Abkenari, S.; Tong, Z.; Tedman, A.; Huerta-Yepez, S. Omega-3 Polyunsaturated Fatty Acids and Lung Cancer: Nutrition or Pharmacology? Nutr. Cancer 2021, 73, 541–561. [Google Scholar] [CrossRef] [PubMed]
- Innes, J.K.; Calder, P.C. Marine Omega-3 (N-3) Fatty Acids for Cardiovascular Health: An Update for 2020. Int. J. Mol. Sci. 2020, 21, 1362. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.S.; Brown, T.J.; Brainard, J.S.; Biswas, P.; Thorpe, G.C.; Moore, H.J.; Deane, K.H.; Alabdulghafoor, F.K.; Summerbell, C.D.; Worthington, H.V.; et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2018, 7, CD003177, Erratum in Cochrane Database Syst. Rev. 2018, 11, CD003177. [Google Scholar] [CrossRef] [PubMed]
- PubChem Astaxanthin. Available online: https://pubchem.ncbi.nlm.nih.gov/substance/347827776 (accessed on 30 July 2022).
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in Skin Health, Repair, and Disease: A Comprehensive Review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef] [PubMed]
- Stachowiak, B.; Szulc, P. Astaxanthin for the Food Industry. Molecules 2021, 26, 2666. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-Producing Green Microalga Haematococcus Pluvialis: From Single Cell to High Value Commercial Products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.N.; Patil, S.; Barkate, H. Protective effects of astaxanthin on skin: Recent scientific evidence, possible mechanisms, and potential indications. J. Cosmet. Dermatol. 2020, 19, 22–27. [Google Scholar] [CrossRef]
- Ito, N.; Seki, S.; Ueda, F. The Protective Role of Astaxanthin for UV-Induced Skin Deterioration in Healthy People-A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2018, 10, 817. [Google Scholar] [CrossRef]
- Davinelli, S.; Bertoglio, J.C.; Polimeni, A.; Scapagnini, G. Cytoprotective Polyphenols against Chronological Skin Aging and Cutaneous Photodamage. Curr. Pharm. Des. 2018, 24, 99–105. [Google Scholar] [CrossRef]
- Yuan, L.; Qu, Y.; Li, Q.; An, T.; Chen, Z.; Chen, Y.; Deng, X.; Bai, D. Protective effect of astaxanthin against La2O3 nanoparticles induced neurotoxicity by activating PI3K/AKT/Nrf-2 signaling in mice. Food Chem. Toxicol. 2020, 144, 111582. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Yeo, I.J.; Han, J.H.; Suh, J.W.; Lee, H.P.; Hong, J.T. Anti-Inflammatory Effect of Astaxanthin in Phthalic Anhydride-Induced Atopic Dermatitis Animal Model. Exp. Dermatol. 2018, 27, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.E.; Shin, C.Y.; Han, S.H.; Kwon, K.J. Astaxanthin Suppresses PM2.5-Induced Neuroinflammation by Regulating Akt Phosphorylation in BV-2 Microglial Cells. Int. J. Mol. Sci. 2020, 21, 7227. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sahu, D.S.; Chandra, G.; Yadav, S.P.; Kumar, R.; Ali, N.; Roy, D.; Maurya, P.S. Effect of Astaxanthin and Copper Supplementation on Growth, Immunity, Antioxidant, and Blood Biochemical Status of Growing Murrah Buffalo Heifers. Biol. Trace Elem. Res. 2022, 200, 5052–5063. [Google Scholar] [CrossRef] [PubMed]
- Chew, B.P.; Mathison, B.D.; Hayek, M.G.; Massimino, S.; Reinhart, G.A.; Park, J.S. Dietary Astaxanthin Enhances Immune Response in Dogs. Vet. Immunol. Immunopathol. 2011, 140, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Mathison, B.D.; Hayek, M.G.; Massimino, S.; Reinhart, G.A.; Chew, B.P. Astaxanthin Stimulates Cell-Mediated and Humoral Immune Responses in Cats. Vet. Immunol. Immunopathol. 2011, 144, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Kageyama, H.; Zhang, Y.; Hibino, T.; Goto, M. Oral Supplementation with Z-Isomer-Rich Astaxanthin Inhibits Ultraviolet Light-Induced Skin Damage in Guinea Pigs. Mar. Drugs 2022, 20, 414. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.Y.; Park, S.H.; Yun, S.Y.; Yu, D.S.; Lee, Y.B. Astaxanthin Protects Ultraviolet B-Induced Oxidative Stress and Apoptosis in Human Keratinocytes via Intrinsic Apoptotic Pathway. Ann. Dermatol. 2022, 34, 125. [Google Scholar] [CrossRef]
- Heo, Y.; Shin, S.-W.; Kim, D.-S.; Lee, S.; Park, S.-Y.; Baek, S.-W.; Lee, J.-K.; Kim, J.H.; Han, D.K. Bioactive PCL Microspheres with Enhanced Biocompatibility and Collagen Production for Functional Hyaluronic Acid Dermal Fillers. Biomater. Sci. 2022, 10, 947–959. [Google Scholar] [CrossRef]
- Baby, A.R.; Morocho-Jácome, A.L. Dermocosmetic Applications of Microalgal Pigments. Adv. Appl. Microbiol. 2021, 117, 63–93. [Google Scholar] [CrossRef]
- Zhou, X.; Cao, Q.; Orfila, C.; Zhao, J.; Zhang, L. Systematic Review and Meta-Analysis on the Effects of Astaxanthin on Human Skin Ageing. Nutrients 2021, 13, 2917. [Google Scholar] [CrossRef] [PubMed]
- Lima, S.G.M.; Freire, M.C.L.C.; Oliveira, V.d.S.; Soliso, C.; Converti, A.; de Lima, A.A.N. Astaxanthin Delivery Systems for Skin Application: A Review. Mar. Drugs 2021, 19, 511. [Google Scholar] [CrossRef] [PubMed]
- Ponto, T.; Latter, G.; Luna, G.; Leite-Silva, R.; Wright, A.; Benson, H.A.E. Novel Self-Nano-Emulsifying Drug Delivery Systems Containing Astaxanthin for Topical Skin Delivery. Pharmaceutics 2021, 13, 649. [Google Scholar] [CrossRef] [PubMed]
- Geng, Q.; Zhao, Y.; Wang, L.; Xu, L.; Chen, X.; Han, J. Development and Evaluation of Astaxanthin as Nanostructure Lipid Carriers in Topical Delivery. AAPS PharmSciTech 2020, 21, 318. [Google Scholar] [CrossRef] [PubMed]
- Gürsoy, K.; Teymur, H.; Koca, G.; Isikci, O.T.; Demircan, F.B.G.; Kankaya, Y.; Kocer, U. The Effect of Astaxanthin on Random Pattern Skin Flaps. Ann. Plast. Surg. 2019, 84, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.A. Benefits and liabilities of vitamin A and carotenoids. J. Nutr. 1996, 126 (Suppl. S4), 1208S–1212S. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.M.; Ameer, M.A.; Goyal, A. Vitamin A Toxicity. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Abdullah, M.; Jamil, R.T.; Attia, F.N. Vitamin C (Ascorbic Acid). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Telang, P. Vitamin C in dermatology. Indian Dermatol. Online J. 2013, 4, 143. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Manousaki, D.; Rosen, C.; Trajanoska, K.; Rivadeneira, F.; Richards, J.B. The health effects of vitamin D supplementation: Evidence from human studies. Nat. Rev. Endocrinol. 2022, 18, 96–110. [Google Scholar] [CrossRef]
- Asif, A.; Farooq, N. Vitamin D Toxicity. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Turmeric. In Drugs and Lactation Database (LactMed®) [Internet]; National Institute of Child Health and Human Development: Bethesda, MD, USA, 2022.
- Winkelmann, R.R.; Del Rosso, J.; Rigel, D.S. Polypodium leucotomos extract: A status report on clinical efficacy and safety. J. Drugs Dermatol. 2015, 14, 254–261. [Google Scholar]
- Pazyar, N.; Yaghoobi, R.; Ghassemi, M.R.; Kazerouni, A.; Rafeie, E.; Jamshydian, N. Jojoba in dermatology: A succinct review. G. Ital. Dermatol. Venereol. 2013, 148, 687–691. [Google Scholar] [PubMed]
- Gad, H.A.; Roberts, A.; Hamzi, S.H.; Gad, H.A.; Touiss, I.; Altyar, A.E.; Kensara, O.A.; Ashour, M.L. Jojoba Oil: An Updated Comprehensive Review on Chemistry, Pharmaceutical Uses, and Toxicity. Polymers 2021, 13, 1711. [Google Scholar] [CrossRef] [PubMed]
- Bistas, K.G.; Tadi, P. Biotin. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Cheng, H.H.; Ma, C.-Y.; Chou, T.-W.; Chen, Y.-Y.; Lai, M.-H. Gamma-oryzanol Ameliorates Insulin Resistance and Hyperlipidemia in Rats with Streptozotocin/nicotinamide-induced Type 2 Diabetes. Int. J. Vitam. Nutr. Res. 2010, 80, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Vogel, P.; Kasper Machado, I.; Garavaglia, J.; Zani, V.T.; de Souza, D.; Morelo Dal Bosco, S. Polyphenols benefits of olive leaf (Olea europaea L.) to human health. Nutr. Hosp. 2014, 31, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Karkos, P.D.; Leong, S.C.; Karkos, C.D.; Sivaji, N.; Assimakopoulos, D.A. Spirulina in clinical practice: Evidence-based human applications. Evid.-Based Complement. Altern. Med. 2011, 2011, 531053. [Google Scholar] [CrossRef] [PubMed]
- Marles, R.J.; Barrett, M.L.; Barnes, J.; Chavez, M.L.; Gardiner, P.; Ko, R.; Mahady, G.B.; Dog, T.L.; Sarma, N.D.; Giancaspro, G.I.; et al. United States Pharmacopeia Safety Evaluation of Spirulina. Crit. Rev. Food Sci. Nutr. 2011, 51, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Nakano, S.; Takekoshi, H.; Nakano, M. Supplementation Decreases Dioxin and Increases Immunoglobulin A Concentrations in Breast Milk. J. Med. Food 2007, 10, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Ruxton, C. Health benefits of omega-3 fatty acids. Nurs. Stand. 2004, 18, 38–42. [Google Scholar] [CrossRef]
- Gammone, M.; Riccioni, G.; Parrinello, G.; D’Orazio, N. Omega-3 Polyunsaturated Fatty Acids: Benefits and Endpoints in Sport. Nutrients 2018, 11, 46. [Google Scholar] [CrossRef]
- Higuera-Ciapara, I.; Félix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef]
- Ng, Q.X.; de Deyn, M.L.Z.Q.; Loke, W.; Foo, N.X.; Chan, H.W.; Yeo, W.S. Effects of Astaxanthin Supplementation on Skin Health: A Systematic Review of Clinical Studies. J. Diet. Suppl. 2021, 18, 169–182. [Google Scholar] [CrossRef] [PubMed]

| Name of Compound | Benefits | Side Effects |
|---|---|---|
| Vitamin A | Modulates the immune response Maintains homeostasis of epithelial tissues and mucosa through retinoic acid Anti-inflammatory mechanisms [229] | Skin irritation Erythema [230] |
| Vitamin C | Assists in antioxidant defense against UV-induced photodamage Induces collagen biosynthesis as a key promoter in formation Squalene vitamin C increases epidermal thickness Accelerates wound healing Helps in burn wound healing [231] | Skin pigmentation Hypopigmented hair Stinging Erythema Dryness [232] |
| Vitamin D | Anti-inflammatory and antiproliferative effects Inhibits the production of psoriasin and koebnerisin [233] | Weakness Fatigue Loss of skin turgor Dry mucous membrane [234] |
| Curcumin | Anti-inflammatory and antioxidant properties [235] | Nausea Diarrhea Allergic reactions Interactions with warfarin [236] |
| Polypodium leucotomos | Immunomodulatory properties Antioxidant defense Photoprotection [66] | Gastrointestinal symptoms Pruritus [237] |
| Simmondsia chinesis | Anti-inflammatory properties Boosts topical medication absorption Functions in the restoration of injured skin Speeds wound closure Stimulates collagen I production Enhanced antibacterial action Photoprotection [238] | Allergic reactions [239] |
| Biotin | Promotes the grow of nails and hair [240] | Possible interactions with, e.g., ciprofloxacin, azithromycin, and clarithromycin (biotin presents interactions with more than 70 different drugs) Teratogenicity hazards Interferes with thyroid and troponin tests [240] |
| Gamma Oryzanol | Antioxidant and skin anti-aging properties Prevents sola UV-related skin cancer [113] | Ameliorates insulin resistance and hyperlipidemia in rats [241] |
| Olive leaf extract | Antiviral, antibacterial, anti-inflammatory, and antioxidant activities Stimulates repigmentation in vitiligo [242] | Allergic reactions [124] |
| Spirulina | Antioxidant and anti-inflammatory activity Emphasizes anti-aging, photoprotection, and wound-healing [243] | Diarrhea Bloating Flatulence Edema Headache Muscle pain Skin redness Sweating [244] |
| Chlorella | Immunomodulatory, antioxidant, antidiabetic, antihypertensive, and antihyperlipidemic effects Increases collagen production in skin [170] | Nausea Diarrhea Abdominal cramping Flatulence Photosensitivity Interactions with warfarin [245] |
| Omega-3 | Anti-inflammatory benefits Prevents from UV radiation Antihypertensive and antihyperlipidemic effects [246] | Diarrhea Nausea Abdominal pain Constipation Vomiting Fatigue [247] |
| Astaxanthin | Antioxidant and anti-inflammatory properties Cytoprotective effects [248] | Nausea Diarrhea [249] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Januszewski, J.; Forma, A.; Zembala, J.; Flieger, M.; Tyczyńska, M.; Dring, J.C.; Dudek, I.; Świątek, K.; Baj, J. Nutritional Supplements for Skin Health—A Review of What Should Be Chosen and Why. Medicina 2024, 60, 68. https://doi.org/10.3390/medicina60010068
Januszewski J, Forma A, Zembala J, Flieger M, Tyczyńska M, Dring JC, Dudek I, Świątek K, Baj J. Nutritional Supplements for Skin Health—A Review of What Should Be Chosen and Why. Medicina. 2024; 60(1):68. https://doi.org/10.3390/medicina60010068
Chicago/Turabian StyleJanuszewski, Jacek, Alicja Forma, Julita Zembala, Michał Flieger, Magdalena Tyczyńska, James Curtis Dring, Iga Dudek, Kamila Świątek, and Jacek Baj. 2024. "Nutritional Supplements for Skin Health—A Review of What Should Be Chosen and Why" Medicina 60, no. 1: 68. https://doi.org/10.3390/medicina60010068





