Uterine Cesarean Scar Tissue—An Immunohistochemical Study
Abstract
:1. Introduction
1.1. The Wound Healing Process
1.2. The Cluster of Differentiation 31 Antigen
1.3. Structural Proteins in the Repair and Healing Process
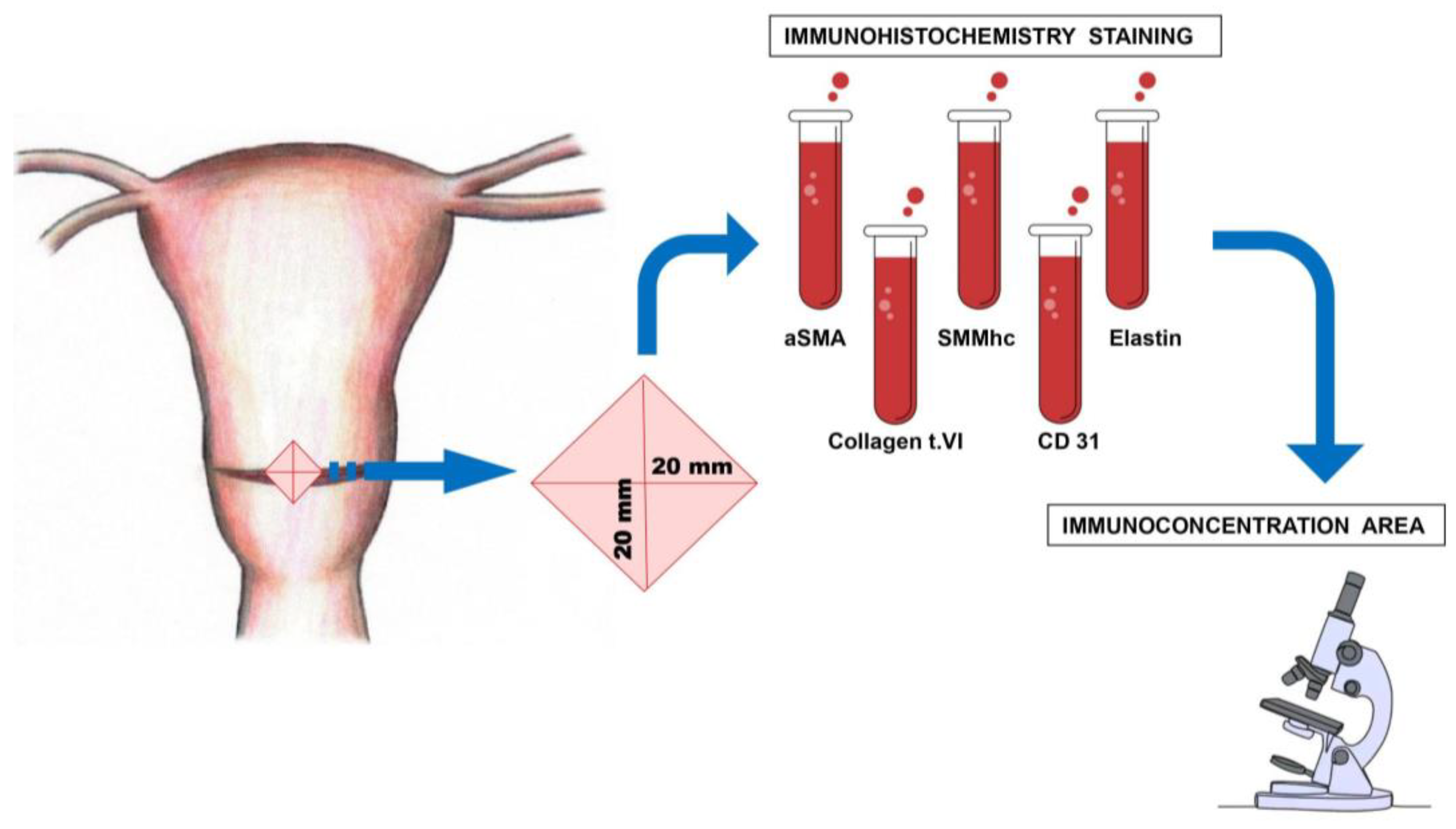
2. Material and Methods
- Group 1 consisted of 52 primipara participants.
- Group 2 consisted of 15 participants, 13–23 months.
- Group 3 consisted of 17 participants, 24–30 months.
- Group 4 consisted of 21 participants, 31–36 months.
- Group 5 consisted of 16 participants, 37–42 months.
- Group 6 consisted of 29 participants, 43–60 months.
- Group 7 consisted of 27 participants, more than 60 months.
2.1. Reagents Used for Immunohistochemical Determinations
2.2. Surgical Procedures
2.3. Morphological Study
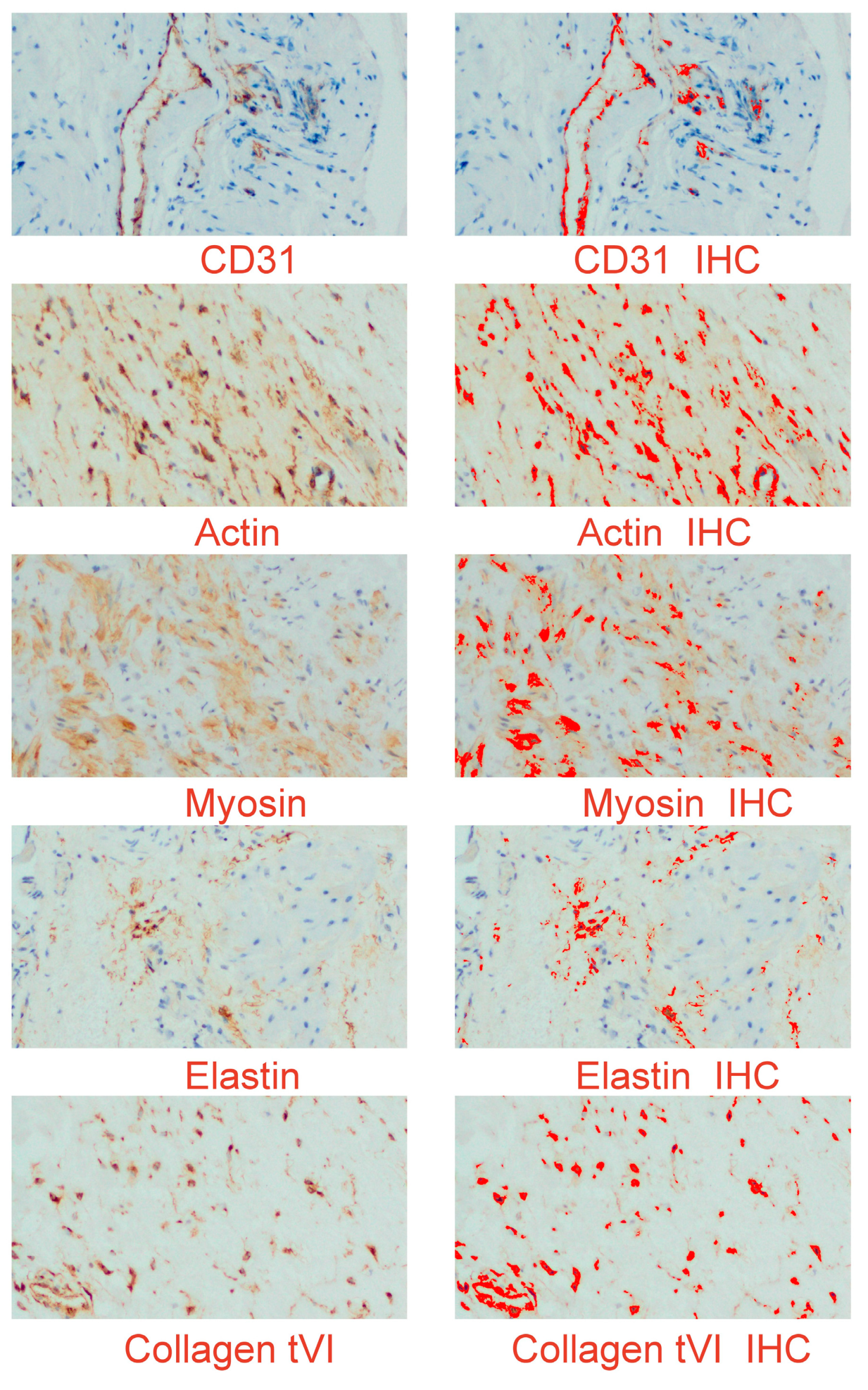
2.4. Immunohistochemistry
2.5. Digital Image Analysis (Digital Computer-Assisted Analysis Technique)
2.6. Statistical Analysis
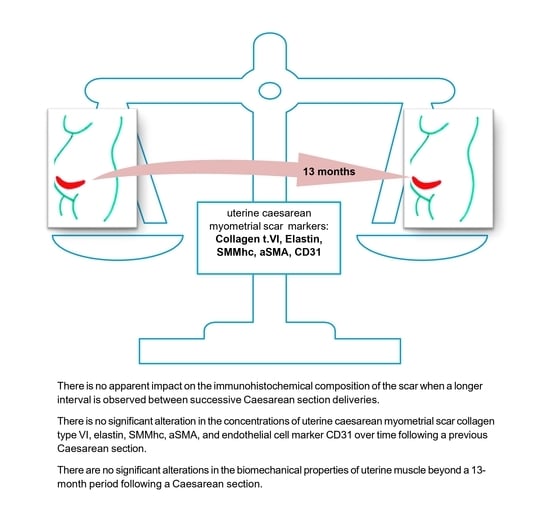
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| aSMA | Alpha-smooth muscle actin |
| BM | basement membranes |
| CD31 | cluster of differentiation 31 |
| CS | cesarean section |
| ECM | extracellular matrix |
| IHC | immunohistochemistry |
| SMC | smooth muscle cells |
| SMMhc | smooth muscle myosin heavy chain |
References
- Hamilton, B.E.; Martin, J.A.; Osterman, M.J.; Curtin, S.C.; Matthews, T.J. Birth: Final Data for 2014. Natl. Vital. Stat. Rep. 2015, 64, 1–64. [Google Scholar] [PubMed]
- Melo-Cerda, I. Cesarean scar defect. Ginecol. Obstet. Mex. 2014, 82, 530–534. [Google Scholar] [PubMed]
- Bishop, A. Role of oxygen in wound healing. J. Wound Care 2008, 17, 399–402. [Google Scholar] [CrossRef]
- Bauer, S.M.; Bauer, R.J.; Velazquez, O.C. Angiogenesis, vasculogenesis, and induction of healing in chronic wounds. Vasc. Endovascular Surg. 2005, 39, 293–306. [Google Scholar] [CrossRef]
- Takada, S.; Hojo, M.; Takebe, N.; Tanigaki, K.; Miyamoto, S. Role of Endothelial-to-Mesenchymal Transition in the Pathogenesis of Central Nervous System Hemangioblastomas. World Neurosurg. 2018, 117, e187–e193. [Google Scholar] [CrossRef]
- Lertkiatmongkol, P.; Liao, D.; Mei, H.; Hu, Y.; Newman, P.J. Endothelial functions of platelet/endothelial cell adhesion molecule-1 (CD31). Curr. Opin. Hematol. 2016, 23, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Levillain, A.; Orhant, M.; Turquier, F.; Hoc, T. Contribution of collagen and elastin fibers to the mechanical behavior of an abdominal connective tissue. J. Mech. Behav. Biomed. Mater. 2016, 61, 308–317. [Google Scholar] [CrossRef]
- Nallasamy, S.; Yoshida, K.; Akins, M.; Myers, K.; Iozzo, R.; Mahendroo, M. Steroid Hormones Are Key Modulators of Tissue Mechanical Function via Regulation of Collagenand Elastic Fibers. Endocrinology 2017, 158, 950–962. [Google Scholar] [CrossRef]
- Varma, S.; Stéphenne, X.; Komuta, M.; Bouzin, C.; Ambroise, J.; Smets, F.; Reding, R.; Sokal, E.M. The histological quantification of alpha-smooth muscle actin predicts future graft fibrosis in pediatric liver transplant recipients. Pediatr. Transplant. 2017, 21, e12834. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques, 5th ed.; Churchil Livingstone: London, UK, 2002. [Google Scholar]
- Broughton, G., 2nd; Janis, J.E.; Attinger, C.E. The basic science of wound healing. Plast. Reconstr. Surg. 2006, 117, 12S–34S. [Google Scholar] [CrossRef]
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, J.H. Fibroblasts and myofibroblasts in wound healing: Force generation and measurement. J. Tissue Viability 2011, 20, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Roeder, H.A.; Cramer, S.F.; Leppert, P.C. A look at uterine wound healing through a histopathological study of uterine scars. Reprod. Sci. 2012, 19, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Borel, J. Uterine collagens. General. Review. Rev. Fr. Gynecol. Obstet. 1991, 86, 715–722. [Google Scholar] [PubMed]
- Luther, D.J.; Thodeti, C.K.; Shamhart, P.E.; Adapala, R.K.; Hodnichak, C.; Weihrauch, D.; Bonaldo, P.; Chilian, W.M.; Meszaros, J.G. Absence of type VI collagen paradoxically improves cardiac function, structure, and remodeling after myocardial infarction. Circ. Res. 2012, 110, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Buhimschi, C.S.; Buhimschi, I.A.; Yu, C.; Wang, H.; Sharer, D.J.; Diamond, M.P.; Petkova, A.P.; Garfield, R.E.; Saade, G.R.; Weiner, C.P. The effect of dystocia and previous cesarean uterine scar on the tensile properties of the lower uterine segment. Am. J. Obstet. Gynecol. 2006, 194, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Pollio, F.; Staibano, S.; Mascolo, M.; Salvatore, G.; Persico, F.; De Falco, M.; Di Lieto, A. Uterine dehiscence in term pregnant patients with one previous cesarean delivery: Growth factor immunoexpression and collagen content in the scarred lower uterine segment. Am. J. Obstet. Gynecol. 2006, 194, 527–534. [Google Scholar] [CrossRef]
- House, M.; Kaplan, D.L.; Socrate, S. Relationships between mechanical properties and extracellular matrix constituents of the cervical stroma during pregnancy. Semin. Perinatol. 2009, 33, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Leppert, P.C.; Jayes, F.L.; Segars, J.H. The extracellular matrix contributes to mechanotransduction in uterine fibroids. Obstet. Gynecol. Int. 2014, 2014, 783289. [Google Scholar] [CrossRef]
- Woessner, J.F.; Brewer, T.H. Formation and breakdown of collagen and elastin in the human uterus during pregnancy and post-partum involution. Biochem. J. 1963, 89, 75–82. [Google Scholar] [CrossRef]
- Dawning, K.T.; Billah, M.; Raparia, E.; Shah, A.; Silverstein, M.C.; Ahmad, A.; Boutis, G.S. The role of mode of delivery on elastic fiber architecture and vaginal vault elasticity: A rodent model study. J. Mech. Behav. Biomed. Mater. 2014, 29, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Dhital, B.; Gul-E-Noor, F.; Downing, K.T.; Hirsch, S.; Boutis, G.S. Pregnancy-Induced Dynamical and Structural Changes of Reproductive Tract Collagen. Biophys. J. 2016, 111, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Morrione, T.G.; Seifter, S. Alteration in the collagen content of the human uterus during pregnancy and post partum involution. J. Exp. Med. 1962, 115, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Stone, P.J.; Franzblau, C. Increase in urinary desmosine and pyridinoline during postpartum involution of the uterus in humans. Proc. Soc. Exp. Biol. Med. 1995, 210, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Word, R.A.; Pathi, S.; Schaffer, J.I. Pathophysiology of pelvic organ prolapse. Obstet. Gynecol. Clin. North. Am. 2009, 36, 521–539. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Gabbiani, G. Cell-matrix and cell-cell contacts of myofibroblasts: Role in connective tissue remodeling. Thromb. Haemost. 2003, 90, 993–1002. [Google Scholar] [PubMed]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Roman, C.D.; Choy, H.; Nanney, L.; Riordan, C.; Parman, K.; Johnson, D.; Beauchamp, R.D. Vascular endothelial growth factor-mediated angiogenesis inhibition and postoperative wound healing in rats. J. Surg. Res. 2002, 105, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, D. Neovascularisation in wound healing. J. Wound Care 2008, 17, 298–300, 302. [Google Scholar] [CrossRef]
- Wilgus, T.A.; Ferreira, A.M.; Oberyszyn, T.M. Regulation of scar formation by vascular endothelial growth factor. Lab. Investig. 2008, 88, 579–590. [Google Scholar] [CrossRef]
- Wietecha, M.S.; DiPietro, L.A. Therapeutic Approaches to the Regulation of Wound Angiogenesis. Adv. Wound Care 2013, 2, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Nissen, N.N.; Polverini, P.J.; Koch, A.E.; Volin, M.V.; Gamelli, R.L.; DiPietro, L.A. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am. J. Pathol. 1998, 152, 1445–1452. [Google Scholar] [PubMed]
- Bangal, V.B.; Giri, P.A.; Shinde, K.K. Vaginal birth after cesarean section. N. Am. J. Med. Sci. 2013, 5, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Guise, J.M.; McDonagh, M.S.; Osterweil, P.; Nygren, P.; Chan, B.K.S.; Helfand, M. Systematic review of the incidence and consequences of uterine rupture in women with previous caesarean section. BMJ 2004, 329, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Ehsan, A.; Arif, S.; Murtaza, J.; Hanif, A. Evaluating trial of scar in patients with a history of caesarean section. N. Am. J. Med. Sci. 2011, 3, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Landon, M.B.; Leindecker, S.; Spong, C.Y.; Hauth, J.C.; Bloom, S.; Varner, M.W.; Moawad, A.H.; Caritis, S.N.; Harper, M.; Wapner, R.J.; et al. The MFMU Cesarean Registry: Factors affecting the success of trial of labor after previous cesarean delivery. Am. J. Obstet. Gynecol. 2005, 193 Pt 2, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Landon, M.B.; Spong, C.Y.; Thom, E.; Hauth, J.C.; Bloom, S.L.; Varner, M.W.; Moawad, A.H.; Caritis, S.N.; Harper, M.; Wapner, R.J.; et al. Risk of uterine rupture with a trial of labor in women with multiple and single prior cesarean delivery. Obstet. Gynecol. 2006, 108, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Macones, G.A.; Cahill, A.G.; Stamilio, D.M.; Odibo, A.; Peipert, J.; Stevens, E.J. Can uterine rupture in patients attempting vaginal birth after cesarean delivery be predicted? Am. J. Obstet. Gynecol. 2006, 195, 1148–1152. [Google Scholar] [CrossRef]
- Conde-Agudelo, A.; Rosas-Bermudez, A.; Kafury-Goeta, A.C. Birth spacing and risk of adverse perinatal outcomes: A metaanalysis. JAMA 2006, 295, 1809–1823. [Google Scholar] [CrossRef]
- Conde-Agudelo, A.; Rosas-Bermudez, A.; Kafury-Goeta, A.C. Effects of birth spacing on maternal health: A systematic review. Am. J. Obstet. Gynecol. 2007, 196, 297–308. [Google Scholar] [CrossRef]
- Esposito, M.A.; Menihan, C.A.; Malee, M.P. Association of interpregnancy interval with uterine scar failure in labor: A case–control study. Am. J. Obstet. Gynecol. 2000, 183, 1180–1183. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.M.; Zelop, C.M. Vaginal birth after cesarean: Assessing maternal and perinatal risks- -contemporary management. Clin. Obstet. Gynecol. 2006, 49, 147–153. [Google Scholar] [CrossRef]
- Birth after Previous Caesarean Birth. Green-Top Guideline Number 45; Royal College of Obstetricians and Gynaecologists: London, UK, 2015. [Google Scholar]
- Bujold, E.; Gauthier, R.J. Risk of uterine rupture associated with an interdelivery interval between 18 and 24 months. Obstet. Gynecol. 2010, 115, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Bujold, E.; Mehta, S.H.; Bujold, C.; Gauthier, R.J. Interdelivery interval and uterine rupture. Am. J. Obstet. Gynecol. 2002, 187, 1199–1202. [Google Scholar] [CrossRef] [PubMed]
- Cahill, A.G.; Macones, G.A. Vaginal birth after cesarean delivery: Evidence-based practice. Clin. Obstet. Gynecol. 2007, 50, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Shipp, T.D.; Zelop, C.M.; Repke, J.T.; Cohen, A.; Lieberman, E. Interdelivery interval and risk of symptomatic uterine rupture. Obstet. Gynecol. 2001, 97, 175–177. [Google Scholar]
- Stamilio, D.M.; DeFranco, E.; Pare, E.; Odibo, A.O.; Peipert, J.F.; Allsworth, J.E.; Stevens, E.; Macones, G.A. Short interpregnancy interval risk of uterine rupture and complications of vaginal birth after cesarean delivery. Obstet. Gynecol. 2007, 110, 1075–1082. [Google Scholar] [CrossRef]
| Interpregnancy Interval (Months) | Maternal Age (Years) * | Maternal Weight (kg) * | Gestational Age (Weeks) * | Birth Weight (kg) * | |
|---|---|---|---|---|---|
| Group I | - | 28.05 ± 3.71 | 75.04 ± 8.09 | 38.6 ± 1.2 | 3.256 ± 0.556 |
| Group II | 20.07 ± 3.03 | 32.5 ± 4.46 | 82.45 ± 18.61 | 38.67 ± 0.98 | 3.354 ± 0.279 |
| Group III | 26.35 ± 2.45 | 31.27 ± 4.13 | 78.63 ± 12.62 | 38.42 ± 1.52 | 3.318 ± 0.674 |
| Group IV | 34.75 ± 1.29 | 34.81 ± 5.0 | 81.21 ± 14.57 | 37.95 ± 2.85 | 3.281 ± 0.683 |
| Group V | 44.25 ± 3.39 | 33.71 ± 7.12 | 81.39 ± 15.92 | 38.06 ± 2.14 | 3.215 ± 0.568 |
| Group VI | 54.69 ± 5.11 | 33.36 ± 4.56 | 80.61 ± 12.36 | 38.14 ± 1.15 | 3.334 ± 0.328 |
| Group VII | 92.61 ± 33.10 | 32.82 ± 4.83 | 81.96 ± 11.63 | 37.93 ± 2.1 | 3.231 ± 0.656 |
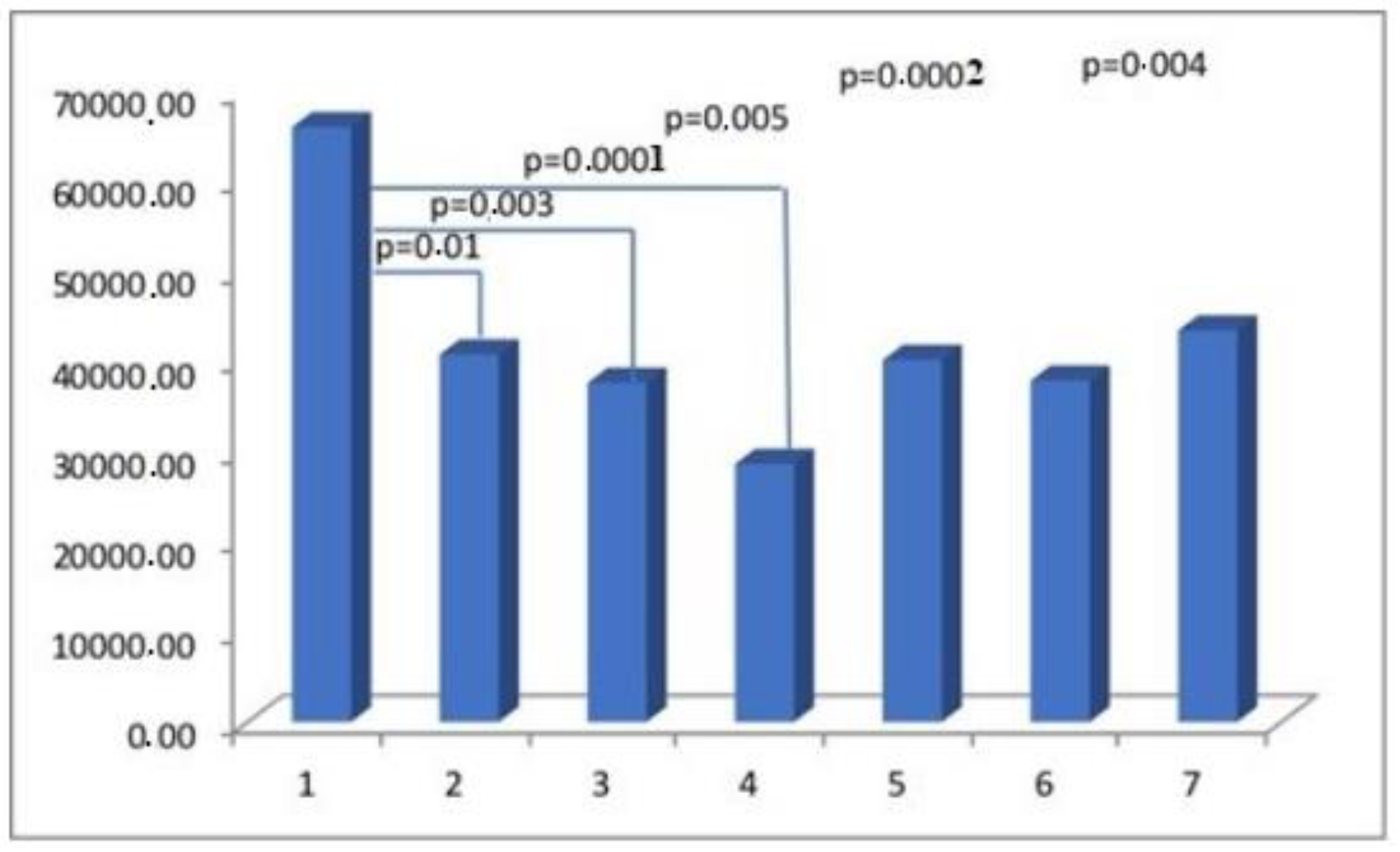
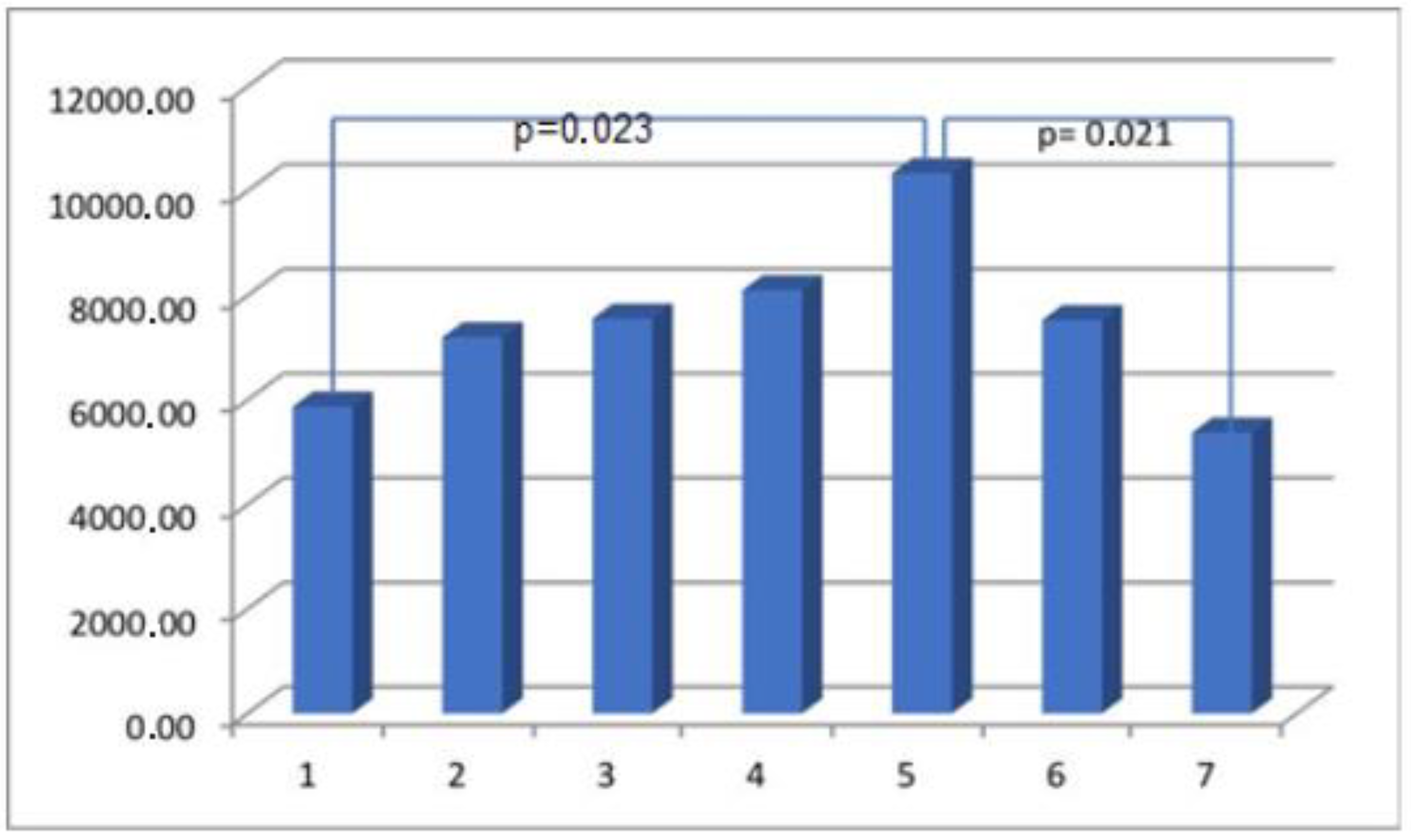
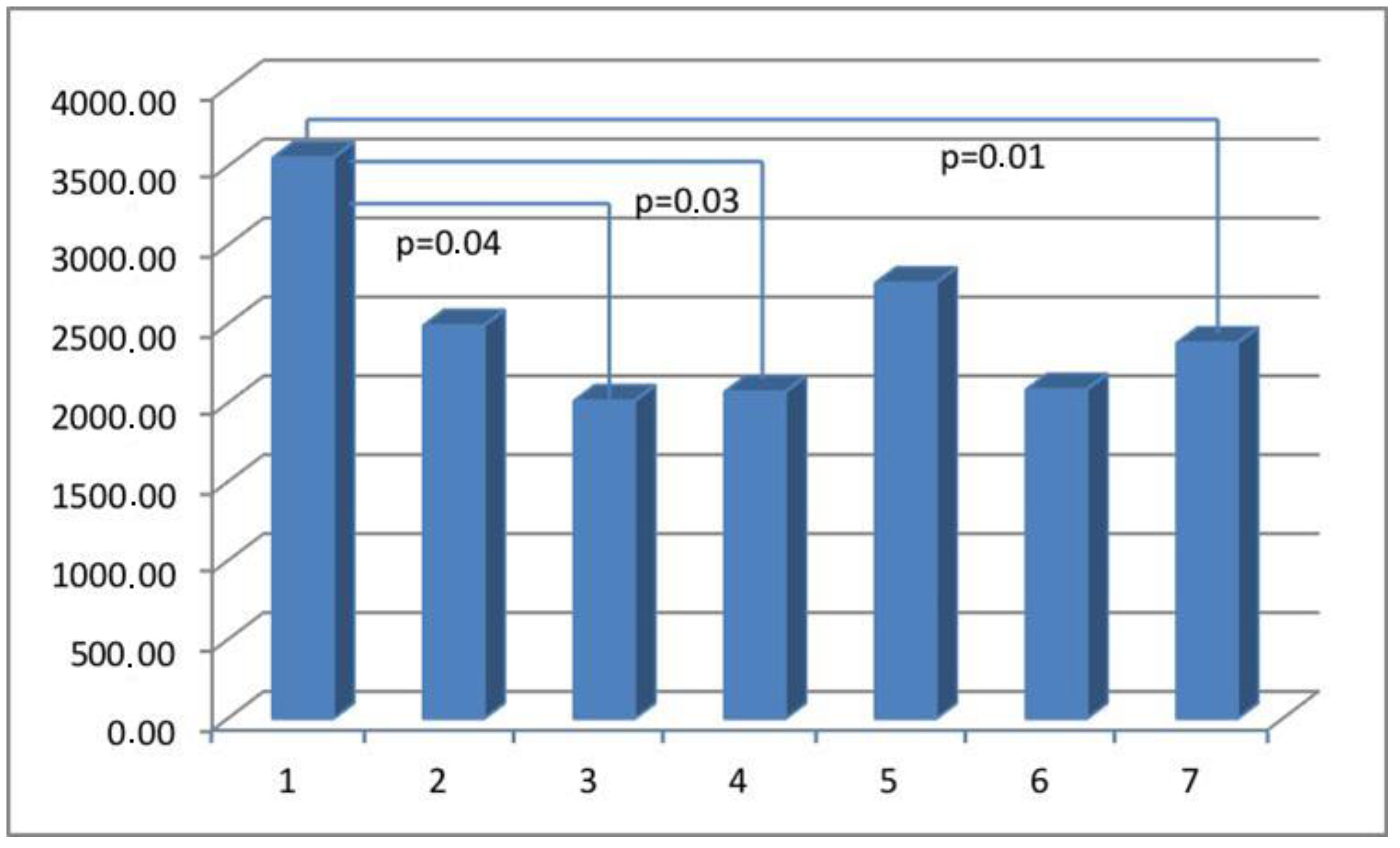
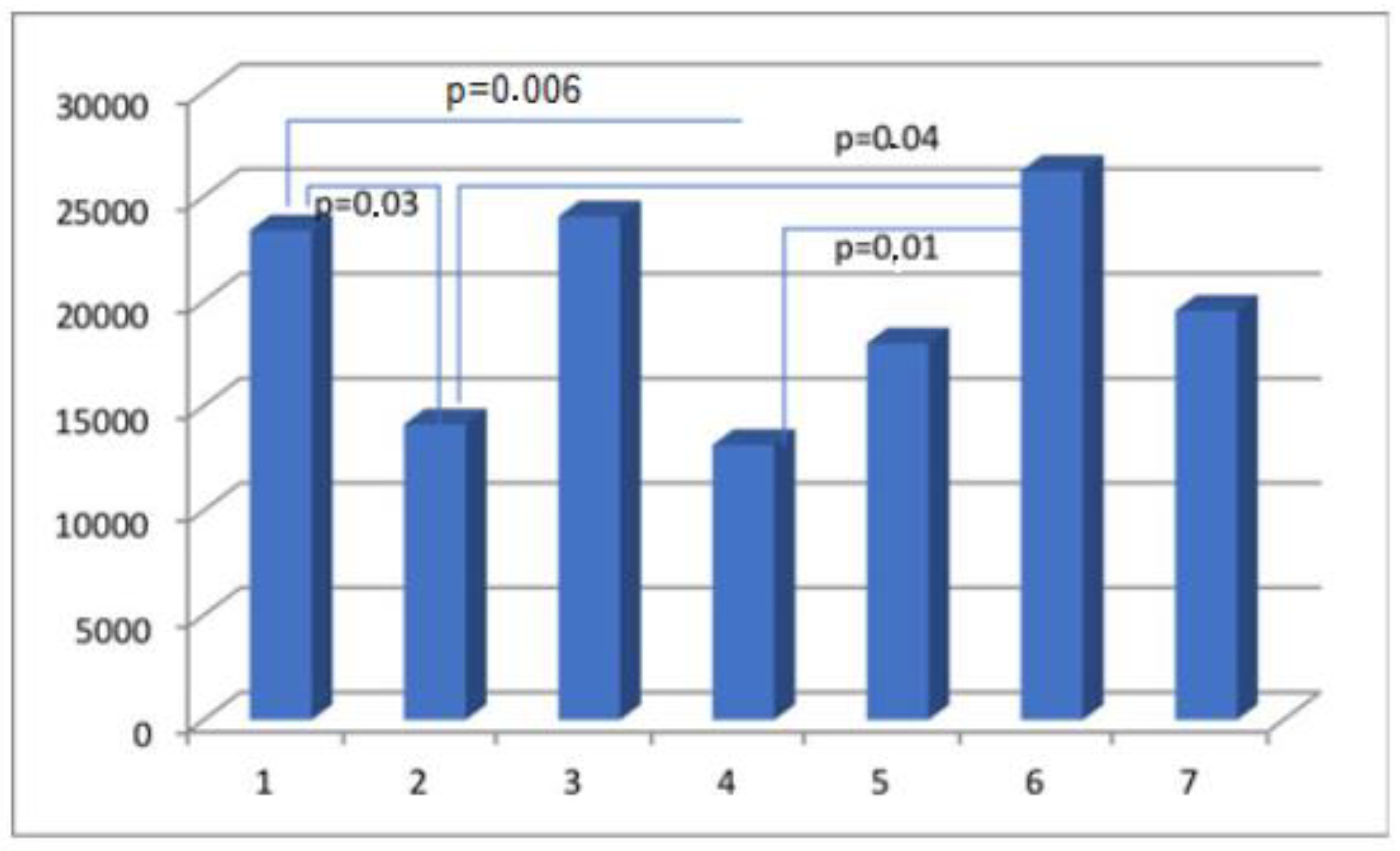
| Parameter | Actin Area (aSMA) | Myosin Area (SMMhc) | Elastin Area | Collagen Area (Collagen t.VI) | CD 31 Area (PECAM-1) |
|---|---|---|---|---|---|
| Group I | 65,933.65 ± 33,522.77 | 5870.08 ± 5939.30 | 5002.57 ± 3823.45 | 23,379.68 ± 14,945.39 | 3553.13 ± 2932.26 |
| Group II | 40,730.74 ± 27,791.32 | 7198.77 ± 6749.24 | 5522.69 ± 6390.30 | 14,122.49 ± 11,119.03 | 2490.21 ± 1523.59 |
| Group III | 37,578.51 ± 33,305.28 | 7554.65 ± 8615.21 | 4972.82 ± 4437.13 | 24,045.47 ± 21,182.52 | 2013.35 ± 1587.92 |
| Group IV | 28,598.35 ± 23,383.7 | 8097.26 ± 8845.94 | 3805.66 ± 4234.11 | 13,136.13 ± 11,771.61 | 2070.71 ± 1417.69 |
| Group V | 40,118.16 ± 21,442.87 | 10,338.99 ± 9192.84 | 7909.23 ± 9559.51 | 17,991.79 ± 13,068.37 | 2760.05 ± 2155.23 |
| Group VI | 37,833.75 ± 26,360.83 | 7569.92 ± 6656.56 | 9693.78 ± 17235.27 | 26,225.81 ± 20,777.57 | 2084.36 ± 1282.20 |
| Group VII | 43,394.51 ± 30,965.22 | 4837.22 ± 6096.49 | 5604.20 ± 5449.66 | 19,540.48 ± 15,068.75 | 2380.13 ± 1564.63 |
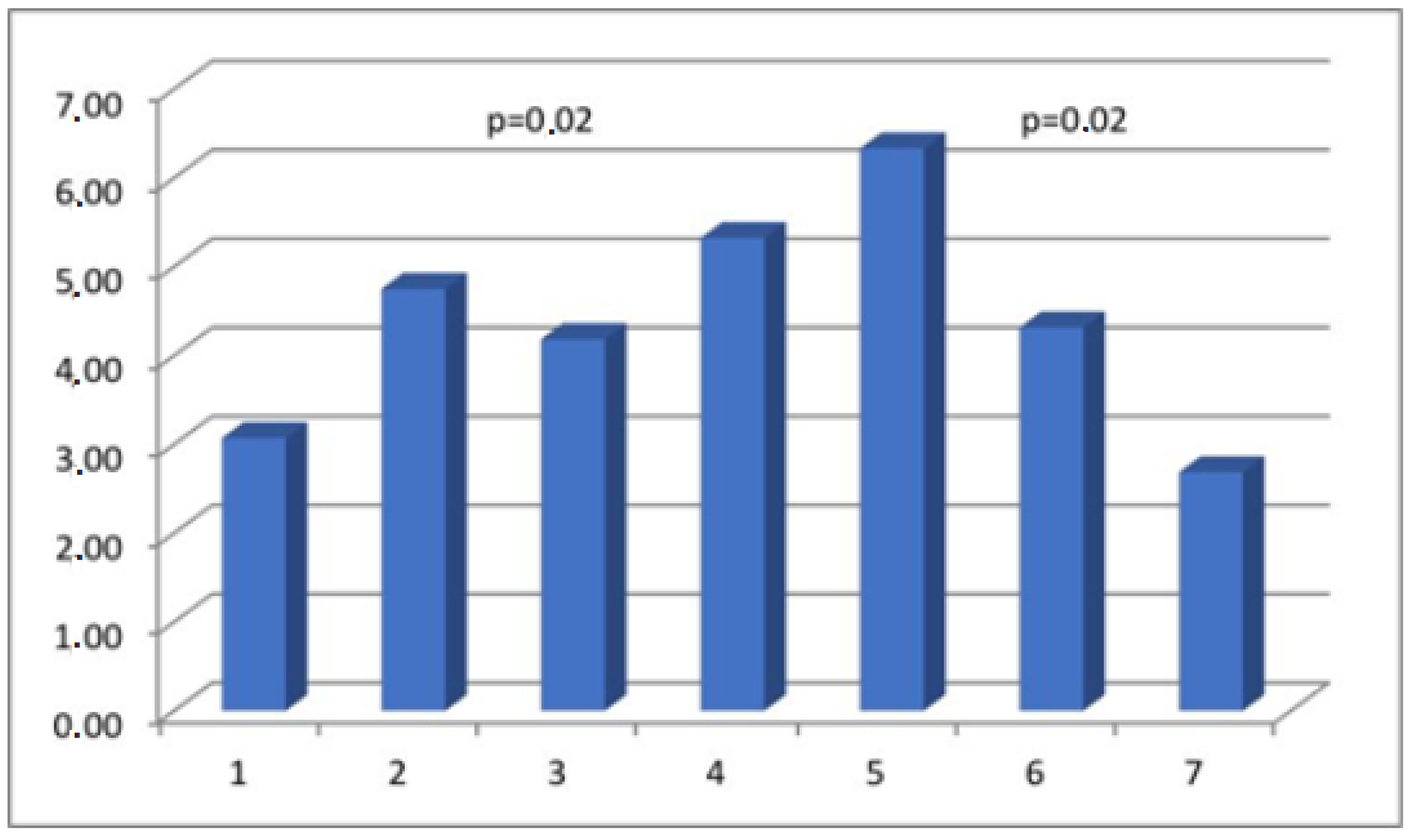
| Parameter | Actin % (aSMA) | Myosin % (SMMhc) | Elastin % | Collagen % (Collagen t.VI) | CD 31% (PECAM-1) |
|---|---|---|---|---|---|
| Group I | 41.33 ± 14.68 | 3.07 ± 4.3 | 2.11 ± 3.19 | 14.65 ± 10.91 | 2.06 ± 1.21 |
| Group II | 29.92 ± 19.23 | 4.75 ± 4.93 | 3.59 ± 4.64 | 9.94 ± 7.83 | 1.54 ± 0.83 |
| Group III | 21.72 ± 17.8 | 4.18 ± 5.16 | 3.19 ± 3.31 | 12.63 ± 8.14 | 1.10 ± 0.67 |
| Group IV | 19.28 ± 16.38 | 5.32 ± 5.64 | 2.27 ± 3.17 | 9.05 ± 8.43 | 1.34 ± 1.0 |
| Group V | 24.88 ± 15.83 | 6.33 ± 6.16 | 2.74 ± 3.64 | 12.12 ± 9.62 | 1.45 ± 0.79 |
| Group VI | 23.77 ± 17.43 | 4.32 ± 3.06 | 4.39 ± 4.78 | 13.76 ± 9.30 | 1.24 ± 0.8 |
| Group VII | 30.16 ± 22.2 | 2.69 ± 3.9 | 3.43 ± 4.07 | 12.49 ± 10.78 | 1.43 ± 1.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziętek, M.; Świątkowska-Feund, M.; Ciećwież, S.; Machałowski, T.; Szczuko, M. Uterine Cesarean Scar Tissue—An Immunohistochemical Study. Medicina 2024, 60, 651. https://doi.org/10.3390/medicina60040651
Ziętek M, Świątkowska-Feund M, Ciećwież S, Machałowski T, Szczuko M. Uterine Cesarean Scar Tissue—An Immunohistochemical Study. Medicina. 2024; 60(4):651. https://doi.org/10.3390/medicina60040651
Chicago/Turabian StyleZiętek, Maciej, Małgorzata Świątkowska-Feund, Sylwester Ciećwież, Tomasz Machałowski, and Małgorzata Szczuko. 2024. "Uterine Cesarean Scar Tissue—An Immunohistochemical Study" Medicina 60, no. 4: 651. https://doi.org/10.3390/medicina60040651