Exosomes and Signaling Nanovesicles from the Nanofiltration of Preconditioned Adipose Tissue with Skin-B® in Tissue Regeneration and Antiaging: A Clinical Study and Case Report
Abstract
:1. Introduction
2. Material and Methods
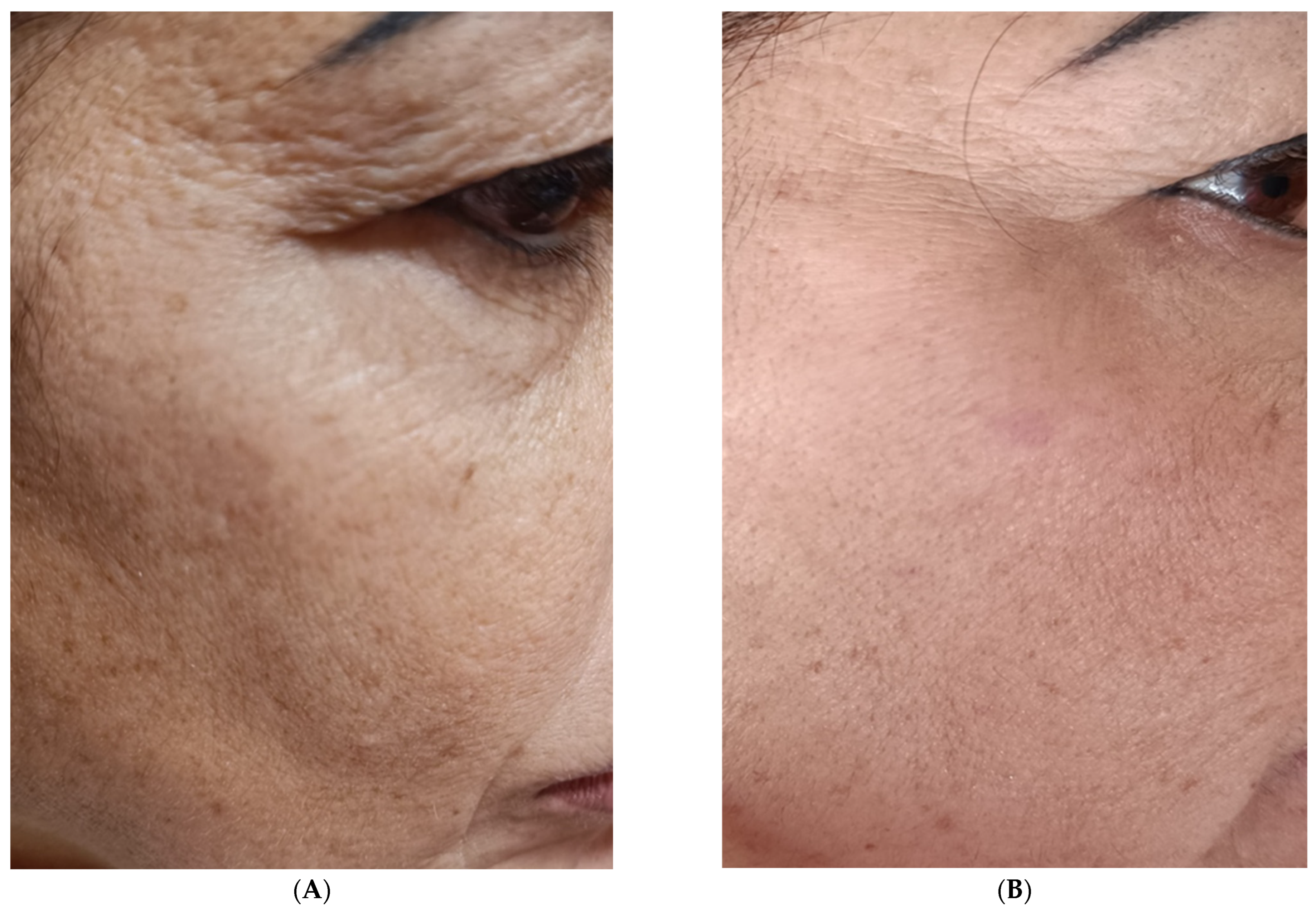
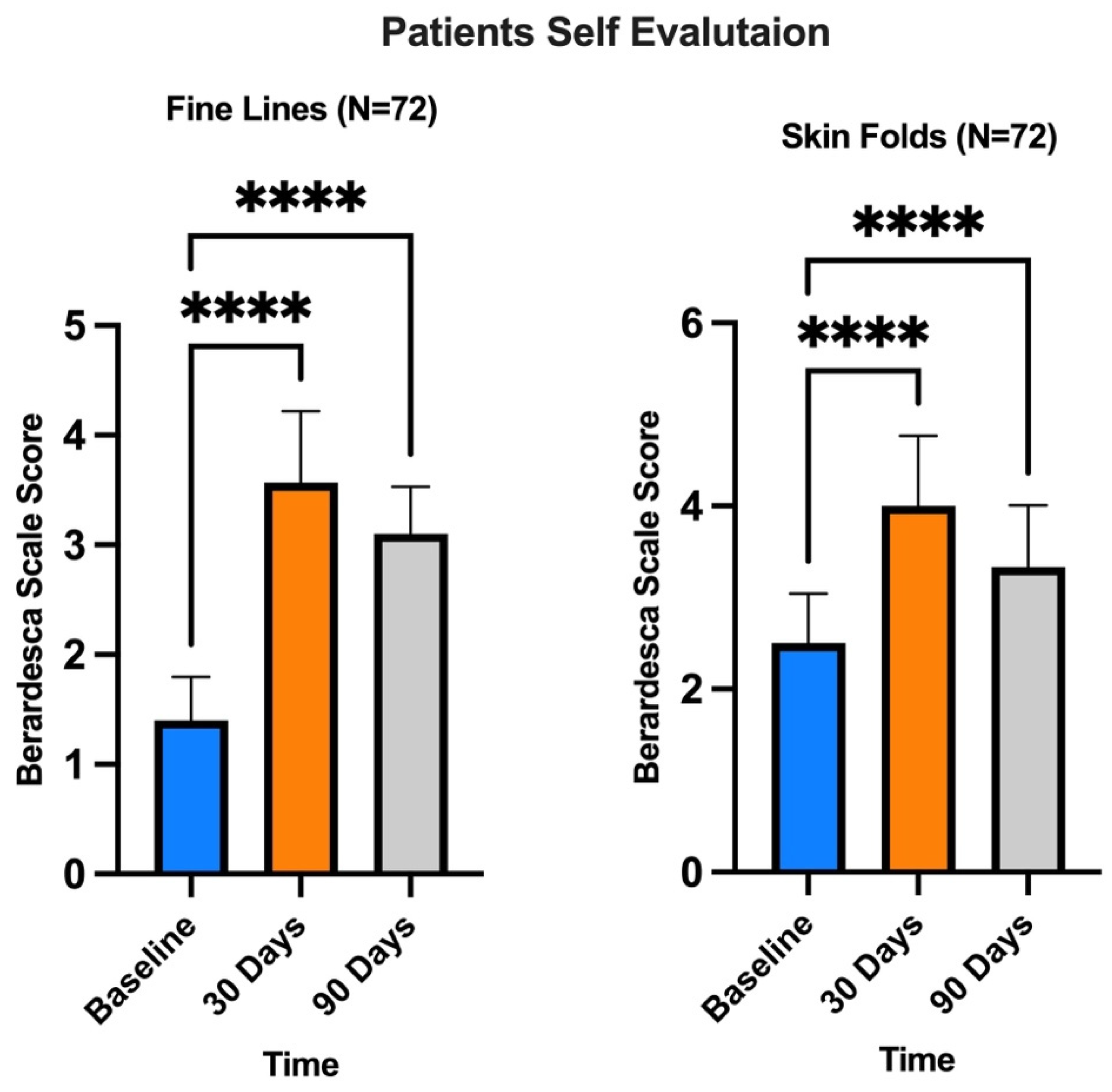
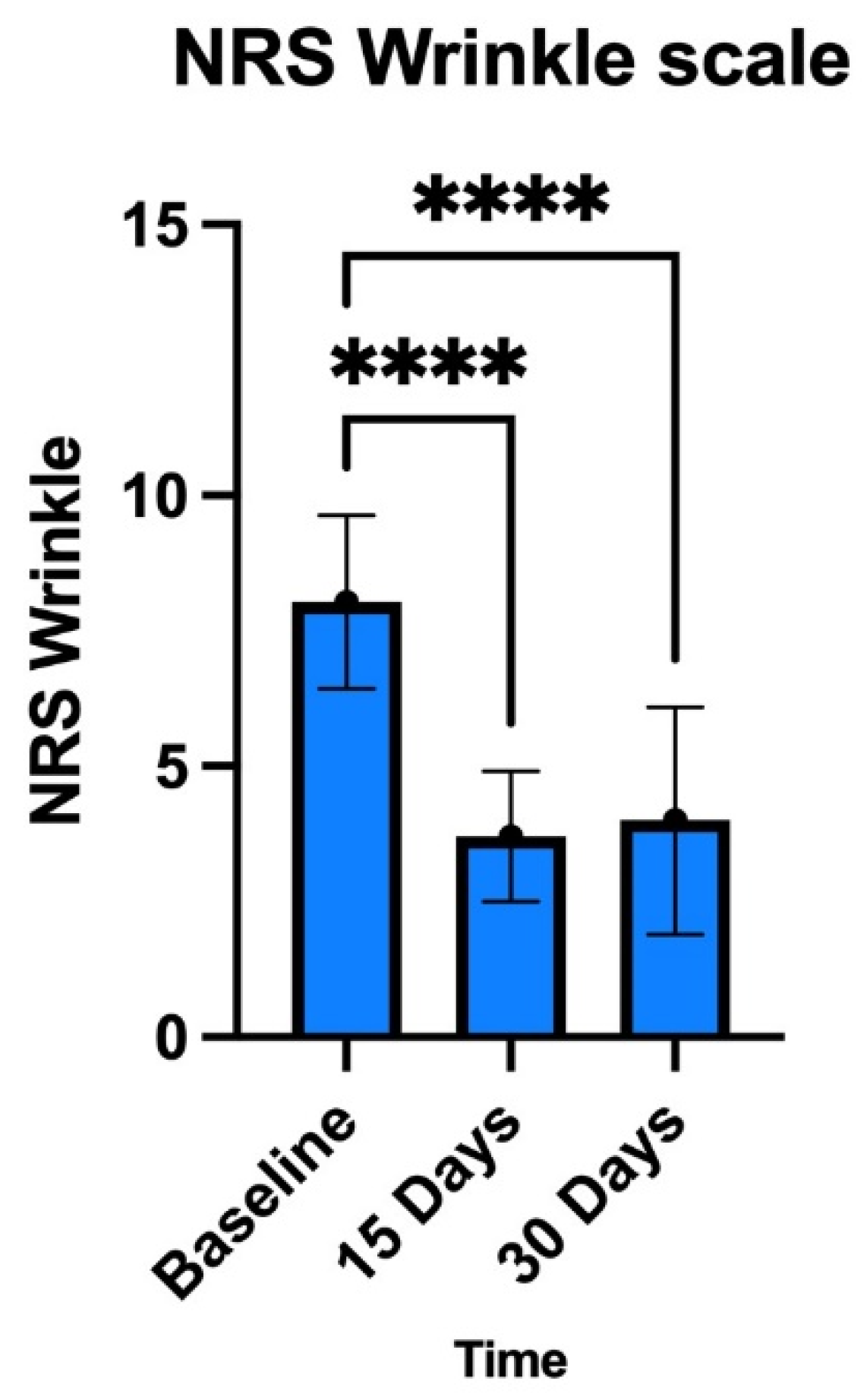
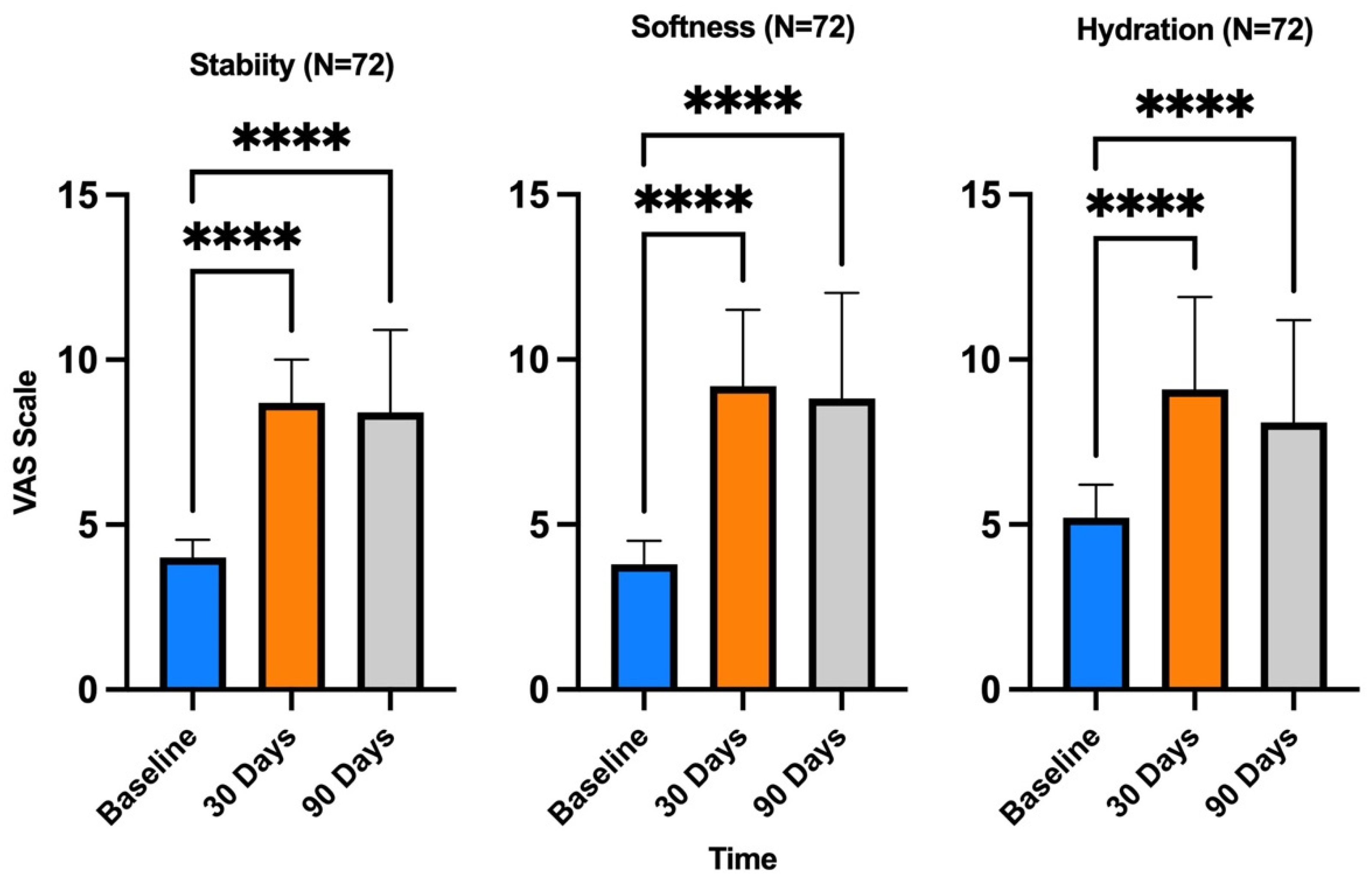
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAT | Alpha-1-antitrypsin |
| ADSCa | Adult mesenchymal stem cells (MSCa) derived from adipose tissue |
| EGF | Epidermal growth factor |
| EVs | Extracellular vesicles |
| FGF2 | Fibroblast growth factor |
| GF | Growth factor |
| IFN-γ | Interferon-γ |
| IL-1β | Interleukin-1β |
| IL-1017 | Interleukin-1017 |
| MMPs | Matrix metalloproteinases |
| NF-κB | Nuclear factor kappa B |
| NRS | Numeric Rating Scale |
| PDGFA | Platelet-derived growth factor A |
| SOD1 | Superoxide dismutase |
| TGF-β1 | Transforming growth factor β1 |
| TK | Tyrosine kinase |
| TLR4 | Toll-like receptor 4 |
| TNF-α | Tumor necrosis factor-α |
| VAS | Visual Analogue Scale |
| VEGFA | Vascular endothelial growth factor A |
References
- Svolacchia, F.; De Francesco, F.; Trovato, L.; Graziano, A.; Ferraro, G.A. An innovative regenerative treatment of scars with dermal micrografts. J. Cosmet. Dermatol. 2016, 15, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Svolacchia, F.; Svolacchia, L. Microfiltered vs only disaggregated mesenchymal stem cells from adipose tissue in regenerative medicine. Scr. Med. 2020, 51, 152–157. [Google Scholar] [CrossRef]
- Hass, R.; Kasper, C.; Böhm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 2011, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.X.F.; Johansson, H.; Wood, M.J.A.; Andaloussi, S.E. Considerations and Implications in the Purification of Extracellular Vesicles—A Cautionary Tale. Front. Neurosci. 2019, 13, 1067. [Google Scholar] [CrossRef] [PubMed]
- Tonnard, P.; Verpaele, A.; Peeters, G.; Hamdi, M.; Cornelissen, M.; Declercq, H. Nanofat grafting: Basic research and clinical applications. Plast. Reconstr. Surg. 2013, 132, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Svolacchia, F.; Svolacchia, L. A protocol of a new regenerative treatment of chrono-aging and photo-aging with progenitors cells from adipose Micrograft obtained from MilliGraft® Kit. Acta Sci. Med. Sci. 2019, 3, 30–35. [Google Scholar]
- Svolacchia, F.; Svolacchia, L. Dermal regeneration with MilliGraft® kit of nanofat: The micrograft of adipose tissue. A clinical assessment study. Scr. Med. 2019, 50, 117–121. [Google Scholar] [CrossRef]
- Golebiewska, A.; Brons, N.H.C.; Bjerkvig, R.; Niclou, S.P. Critical appraisal of the side population assay in stem cell and cancer stem cell research. Cell Stem Cell 2011, 8, 136–147. [Google Scholar] [CrossRef]
- Cittadini, E.; Brucculeri, A.M.; Quartararo, F.; Vaglica, R.; Miceli, V.; Conaldi, P.G. Stem cell therapy in the treatment of organic and dysfunctional endometrial pathology. Minerva Obstet. Gynecol. 2022, 74, 504–515. [Google Scholar] [CrossRef]
- Toh, W.S.; Lai, R.C.; Zhang, B.; Lim, S.K. MSC exosome works through a protein-based mechanism of action. Biochem. Soc. Trans. 2018, 46, 843–853. [Google Scholar] [CrossRef]
- Timothy, D.; Anthony, S.; Hector, P.; Naresh, M.; Stephen, R.; Anne, M.L.; Miguel, F.-B. Proteomic and transcriptomic analysis of human extracellular vesicles as surrogate markers of Age-related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2023, 64, 2739. [Google Scholar]
- Liu, A.; Zhang, X.; He, H.; Zhou, L.; Naito, Y.; Sugita, S.; Lee, J.W. Therapeutic potential of mesenchymal stem/stromal cell-derived secretome and vesicles for lung injury and disease. Expert Opin. Biol. Ther. 2020, 20, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhang, B.; Shi, H.; Qian, H.; Xu, W. MSC-exosome: A novel cell-free therapy for cutaneous regeneration. Cytotherapy 2018, 20, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Toh, W.S.; Lai, R.C.; Hui, J.H.P.; Lim, S.K. MSC exosome as a cell-free MSC therapy for cartilage regeneration: Implications for osteoarthritis treatment. Semin. Cell. Dev. Biol. 2017, 67, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, T.; Lei, P.; Tang, X.; Huang, P. Exosomes released by bone marrow mesenchymal stem cells attenuate lung injury induced by intestinal ischemia reperfusion via the TLR4/NF-κB pathway. Int. J. Med. Sci. 2019, 16, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.H.; Kim, H.K.; Lee, J.; Kwon, H.H.; Park, G.H.; Yang, S.H.; Jung, J.Y.; Choi, H.; Lee, J.H.; Sung, S.; et al. Mesenchymal Stem/Stromal Cell-Derived Exosomes for Immunomodulatory Therapeutics and Skin Regeneration. Cells 2020, 9, 1157. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, X.; Hu, J.; Chen, F.; Qiao, S.; Sun, X.; Gao, L.; Xie, J.; Xu, B. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc. Res. 2019, 115, 1205–1216. [Google Scholar] [CrossRef]
- Ma, Z.J.; Wang, Y.H.; Li, Z.G.; Wang, Y.; Li, B.Y.; Kang, H.Y.; Wu, X.Y. Immunosuppressive effect of exosomes from mesenchymal stromal cells in defined medium on experimental colitis. Int. J. Stem Cells 2019, 12, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Shang, Q.; Pan, Z.; Bai, Y.; Li, Z.; Zhang, H.; Zhang, Q.; Guo, C.; Zhang, L.; Wang, Q. Exosomes from Adipose-Derived Stem Cells Attenuate Adipose Inflammation and Obesity through Polarizing M2 Macrophages and Beiging in White Adipose Tissue. Diabetes 2018, 67, 235–247. [Google Scholar] [CrossRef]
- Zou, L.; Ma, X.; Lin, S.; Wu, B.; Chen, Y.; Peng, C. Bone marrow mesenchymal stem cell-derived exosomes protect against myocardial infarction by promoting autophagy. Exp. Ther. Med. 2019, 18, 2574–2582. [Google Scholar] [CrossRef]
- He, X.; Dong, Z.; Cao, Y.; Wang, H.; Liu, S.; Liao, L.; Jin, Y.; Yuan, L.; Li, B. MSC-derived exosome promotes M2 polarization and enhances cutaneous wound healing. Stem Cells Int. 2019, 2019, 7132708. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, S.; Luo, L.; Wang, J.; Zhu, Z.; Xiang, Q.; Deng, Y.; Zhao, Z. Mesenchymal Stem Cell-derived Exosomes Ameliorate Erection by Reducing Oxidative Stress Damage of Corpus Cavernosum in a Rat Model of Artery Injury. J. Cell. Mol. Med. 2019, 23, 7462–7473. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Wang, Y.; Lan, Q.; Wu, W.; Li, Z.; Ma, X.; Yu, L. Exosomes derived from mesenchymal stem cells ameliorate hypoxia/reoxygenation-injured ECs via transferring microRNA-126. Stem Cells Int. 2019, 2019, 2831756. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Yu, B.; Wang, J.; Wang, Y.; Liu, M.; Paul, C.; Millard, R.W.; Xiao, D.S.; Ashraf, M.; Xu, M. Mesenchymal stem cells release exosomes that transfer miRNAs to endothelial cells and promote angiogenesis. Oncotarget 2017, 8, 45200–45212. [Google Scholar] [CrossRef] [PubMed]
- Calabria, E.; Scambi, I.; Bonafede, R.; Schiaffino, L.; Peroni, D.; Potrich, V.; Capelli, C.; Schena, F.; Mariotti, R. ASCs-Exosomes Recover Coupling Efficiency and Mitochondrial Membrane Potential in an in vitro Model of ALS. Front. Neurosci. 2019, 13, 1070. [Google Scholar] [CrossRef] [PubMed]
- Bari, E.; Ferrarotti, I.; Di Silvestre, D.; Grisoli, P.; Barzon, V.; Balderacchi, A.; Torre, M.L.; Rossi, R.; Mauri, P.; Corsico, A.G.; et al. Adipose Mesenchymal Extracellular Vesicles as Alpha-1-Antitrypsin Physiological Delivery Systems for Lung Regeneration. Cells 2019, 8, 965. [Google Scholar] [CrossRef] [PubMed]
- Tooi, M.; Komaki, M.; Morioka, C.; Honda, I.; Iwasaki, K.; Yokoyama, N.; Ayame, H.; Izumi, Y.; Morita, I. Placenta Mesenchymal Stem Cell Derived Exosomes Confer Plasticity on Fibroblasts. J. Cell. Biochem. 2016, 117, 1658–1670. [Google Scholar] [CrossRef]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Salvia, R.; Grimaldi, A.; Girardello, R.; Scieuzo, C.; Scala, A.; Bufo, S.A.; Vogel, H.; Falabella, P. Aphidius ervi Teratocytes Release Enolase and Fatty Acid Binding Protein Through Exosomal Vesicles. Front. Physiol. 2019, 10, 715. [Google Scholar] [CrossRef]
- Yin, K.; Wang, S.; Zhao, R.C. Exosomes from mesenchymal stem/stromal cells: A new therapeutic paradigm. Biomark. Res. 2019, 7, 1–8. [Google Scholar] [CrossRef]
- Zhang, J.; Yi, Y.; Yang, S.; Zhu, Y.; Hu, X. Effects of adipose-derived stem cell released exosomes on proliferation, migration, and tube-like differentiation of human umbilical vein endothelial cells. Chin. J. Reparative Reconstr. Surg. 2018, 32, 1351–1357. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, L.; Zhan, Y.; Yu, S.; Kang, T. Adipose-derived stem cell-derived microvesicle-released miR-210 promoted proliferation, migration and invasion of endothelial cells by regulating RUNX3. Cell Cycle 2018, 17, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.; Liu, C.; Lin, Y.; Xie, Z.; Hou, C.; Lin, H. Effect of exosomes from adipose-derived stem cells on peripheral nerve regeneration. Chin. J. Reparative Reconstr. Surg. 2018, 32, 1592–1596. [Google Scholar] [CrossRef]
- Zhang, W.; Bai, X.; Zhao, B.; Li, Y.; Zhang, Y.; Li, Z.; Wang, X.; Luo, L.; Han, F.; Zhang, J.; et al. Cell-Free Therapy Based on Adipose Tissue Stem Cell-Derived Exosomes Promotes Wound Healing via the PI3K/Akt Signaling Pathway. Exp. Cell Res. 2018, 370, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Fu, B.; Yang, X.; Xiao, Y.; Pan, M. Adipose mesenchymal stem cell-derived exosomes promote cell proliferation, migration, and inhibit cell apoptosis via Wnt/β-catenin signaling in cutaneous wound healing. J. Cell. Biochem. 2019, 120, 10847–10854. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wang, J.; Zhou, X.; Xiong, Z.; Zhao, J.; Yu, R.; Huang, F.; Zhang, H.; Chen, L. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci. Rep. 2016, 6, 32993. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Chen, J.; Duscher, D.; Liu, Y.; Guo, G.; Kang, Y.; Xiong, H.; Zhan, P.; Wang, Y.; Wang, C.; et al. Microvesicles from human adipose stem cells promote wound healing by optimizing cellular functions via AKT and ERK signaling pathways. Stem Cell Res. Ther. 2019, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tao, M.; Wei, M.; Du, S.; Wang, H.; Wang, X. Mesenchymal stem cells derived exosomal miR-323-3p promotes proliferation and inhibits apoptosis of cumulus cells in polycystic ovary syndrome [PCOS]. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3804–3813. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-D.; Bai, Y.; Yan, X.-L.; Ren, J.; Zeng, Q.; Li, X.-D.; Pei, X.-T.; Han, Y. Co-transplantation of exosomes derived from hypoxia-preconditioned adipose mesenchymal stem cells promotes neovascularization and graft survival in fat grafting. Biochem. Biophys. Res. Commun. 2018, 497, 305–312. [Google Scholar] [CrossRef]
- Li, P.; Kaslan, M.; Lee, S.H.; Yao, J.; Gao, Z. Progress in Exosome Isolation Techniques. Theranostics 2017, 7, 789–804. [Google Scholar] [CrossRef]
- Svolacchia, F.; Svolacchia, L. The Exosomes of Adipose Tissue in Regenerative Medicine. The Results of our Protocol in a Clinical Study of Anti-Aging. J. Plast. Pathol. Dermatol. 2020, 16, 17–23. [Google Scholar]
- Quintana, J.F.; Makepeace, B.L.; A Babayan, S.; Ivens, A.; Pfarr, K.M.; Blaxter, M.; Debrah, A.; Wanji, S.; Ngangyung, H.F.; Bah, G.S.; et al. Extracellular Onchocerca-derived small RNAs in host nodules and blood. Parasites Vectors 2015, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-W.; Scutaru, T.T.; Ghetu, N.; Carasevici, E.; Lupascu, C.D.; Ferariu, D.; Pieptu, D.; Coman, C.-G.; Danciu, M. The Effects of Adipose-Derived Stem Cell-Differentiated Adipocytes on Skin Burn Wound Healing in Rats. J. Burn Care Res. 2017, 38, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, M.; Dai, M.; Chen, C.; Tang, Q.; Jing, W.; Wang, H.; Tian, W. miR-450a-5p within rat adipose tissue exosome-like vesicles promotes adipogenic differentiation by targeting WISP2. J. Cell Sci. 2017, 130, 1158–1168. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Chen, C.; Zhang, H.; Chen, Y.; Zhou, S. Adipose stem cell-derived exosomes in combination with hyaluronic acid accelerate wound healing through enhancing re-epithelialization and vascularization. Br. J. Dermatol. 2019, 181, 854–856. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lee, E.H.; Yang, Z. Hypoxia conditioned mesenchymal stem cells in tissue regeneration application. Tissue Eng. Part B Rev. 2022, 28, 966–977. [Google Scholar] [CrossRef] [PubMed]
- Karcioglu, O.; Topacoglu, H.; Dikme, O.; Dikme, O. A systematic review of the pain scales in adults: Which to use? Am. J. Emerg. Med. 2018, 36, 707–714. [Google Scholar] [CrossRef]
- Qiu, X.; Liu, J.; Zheng, C.; Su, Y.; Bao, L.; Zhu, B.; Liu, S.; Wang, L.; Wang, X.; Wang, Y.; et al. Exosomes released from educated mesenchymal stem cells accelerate cutaneous wound healing via promoting angiogenesis. Cell. Prolif. 2020, 53, e12830. [Google Scholar] [CrossRef]
- Hu, C.X.; Li, L.J. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. J. Cell Mol. Med. 2018, 22, 1428–1442. [Google Scholar] [CrossRef]
- Oksvold, M.P.; Neurauter, A.; Pedersen, K.W. Magnetic Bead-Based Isolation of Exosomes. Methods Mol. Biol. 2015, 1218, 465–481. [Google Scholar] [CrossRef]
- Gargett, C.E.; Schwab, K.E.; Deane, J.A. Endometrial stem/progenitor cells: The first 10 years. Hum. Reprod. Update 2016, 22, 137–163. [Google Scholar] [CrossRef] [PubMed]
- Svolacchia, L.; Prisco, C.; Giuzio, F.; Svolacchia, F. Adipose Autologous Micrograft and Its Derived Mesenchymal Stem Cells in a Bio Cross-Linked Hyaluronic Acid Scaffold for Correction Deep Wrinkles, Facial Depressions, Scars, Face Dermis and Its Regenerations: A Pilot Study and Cases Report. Medicina 2022, 58, 1692. [Google Scholar] [CrossRef] [PubMed]
- Berardesca, E.; Distante, F.; Anthoine, P.; Rabbiosi, G.; Aubert, L. Clinical and instrumental evaluation of the activity of an anti-wrinkle cosmetic product on cutaneous relief and photoaged skin. J. Appl. Cosmetol. 1997, 15, 69–75. [Google Scholar] [CrossRef]
- Mckinnon, B.T.; Avis, K.E. Membrane Filtration of Pharmaceutical Solutions. Am. J. Hosp. Pharm. 1993, 50, 1921–1936. [Google Scholar] [CrossRef] [PubMed]
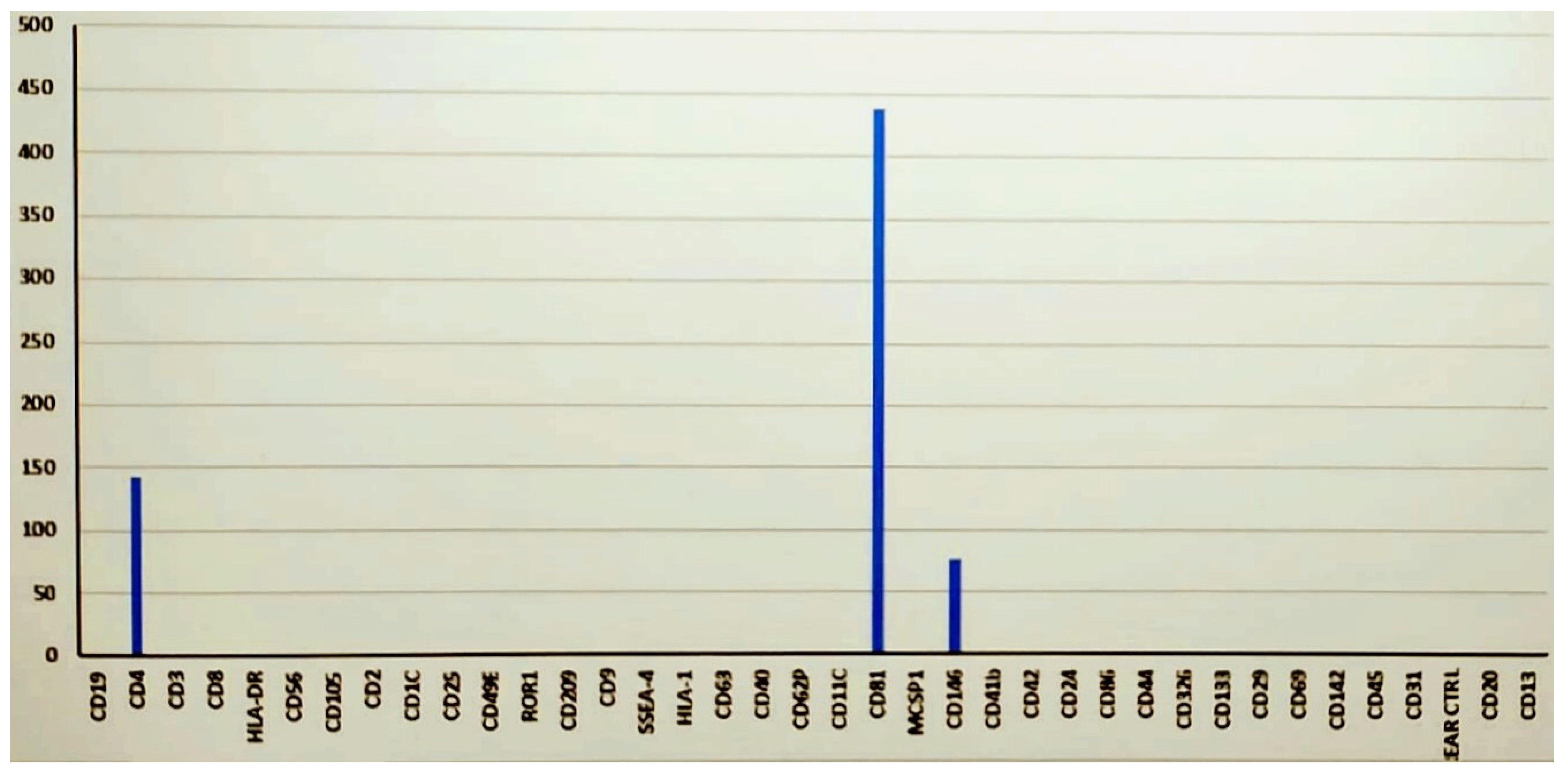
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Svolacchia, F.; Svolacchia, L.; Falabella, P.; Scieuzo, C.; Salvia, R.; Giglio, F.; Catalano, A.; Saturnino, C.; Di Lascio, P.; Guarro, G.; et al. Exosomes and Signaling Nanovesicles from the Nanofiltration of Preconditioned Adipose Tissue with Skin-B® in Tissue Regeneration and Antiaging: A Clinical Study and Case Report. Medicina 2024, 60, 670. https://doi.org/10.3390/medicina60040670
Svolacchia F, Svolacchia L, Falabella P, Scieuzo C, Salvia R, Giglio F, Catalano A, Saturnino C, Di Lascio P, Guarro G, et al. Exosomes and Signaling Nanovesicles from the Nanofiltration of Preconditioned Adipose Tissue with Skin-B® in Tissue Regeneration and Antiaging: A Clinical Study and Case Report. Medicina. 2024; 60(4):670. https://doi.org/10.3390/medicina60040670
Chicago/Turabian StyleSvolacchia, Fabiano, Lorenzo Svolacchia, Patrizia Falabella, Carmen Scieuzo, Rosanna Salvia, Fabiana Giglio, Alessia Catalano, Carmela Saturnino, Pierpaolo Di Lascio, Giuseppe Guarro, and et al. 2024. "Exosomes and Signaling Nanovesicles from the Nanofiltration of Preconditioned Adipose Tissue with Skin-B® in Tissue Regeneration and Antiaging: A Clinical Study and Case Report" Medicina 60, no. 4: 670. https://doi.org/10.3390/medicina60040670





