Enhancing Chronic Non-Cancer Pain Management: A Systematic Review of Mindfulness Therapies and Guided Imagery Interventions
Abstract
:1. Introduction
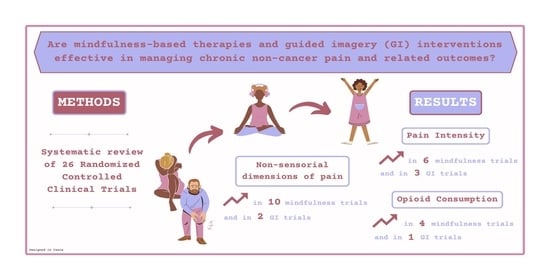
2. Materials and Methods
3. Results
4. Discussion
4.1. General Findings
4.2. Effects on Pain Intensity
4.3. Effects on Non-Sensorial Dimensions of Pain
4.4. Opioid Consumption
4.5. Cancer Pain
4.6. Limitations
4.7. Strengths
4.8. Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mäntyselkä, P.; Kumpusalo, E.; Ahonen, R.; Kumpusalo, A.; Kauhanen, J.; Viinamäki, H.; Halonen, P.; Takala, J. Pain as a reason to visit the doctor: A study in Finnish primary health care. Pain 2001, 89, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef]
- Nicholas, M.; Vlaeyen, J.W.S.; Rief, W.; Barke, A.; Aziz, Q.; Benoliel, R.; Cohen, M.; Evers, S.; Giamberardino, M.A.; Goebel, A.; et al. The IASP classification of chronic pain for ICD-11: Chronic primary pain. Pain 2019, 160, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Treede, R.D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic pain as a symptom or a disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019, 160, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Engel, G.L. The need for a new medical model: A challenge for biomedicine. Science 1977, 196, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Hylands-White, N.; Duarte, R.V.; Raphael, J.H. An overview of treatment approaches for chronic pain management. Rheumatol. Int. 2017, 37, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic pain: An update on burden, best practices, and new advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef] [PubMed]
- Dahlhamer, J.; Lucas, J.; Zelaya, C.; Nahin, R.; Mackey, S.; DeBar, L.; Kerns, R.; Von Korff, M.; Porter, L.; Helmick, C. Prevalence of Chronic Pain and High-Impact Chronic Pain among Adults—United States, 2016. MMWR. Morb. Mortal. Wkly. Rep. 2018, 67, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Johannes, C.B.; Le, T.K.; Zhou, X.; Johnston, J.A.; Dworkin, R.H. The prevalence of chronic pain in United States adults: Results of an Internet-based survey. J. Pain 2010, 11, 1230–1239. [Google Scholar] [CrossRef]
- Nahin, R.L. Estimates of pain prevalence and severity in adults: United States, 2012. J Pain 2015, 16, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Steglitz, J.; Buscemi, J.; Ferguson, M.J. The future of pain research, education, and treatment: A summary of the IOM report “Relieving pain in America: A blueprint for transforming prevention, care, education, and research”. Transl. Behav. Med. 2012, 2, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Rice, A.S.C.; Smith, B.H.; Blyth, F.M. Pain and the global burden of disease. Pain 2016, 157, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Gatchel, R.J.; McGeary, D.D.; McGeary, C.A.; Lippe, B. Interdisciplinary chronic pain management: Past, present, and future. Am. Psychol. 2014, 69, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.P.; Turk, D.C. Contributions of psychology to the understanding and treatment of people with chronic pain: Why it matters to ALL psychologists. Am. Psychol. 2014, 69, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Mackey, S. Outcomes in pain medicine: A brief review. Pain Ther. 2012, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Meints, S.M.; Edwards, R.R. Evaluating psychosocial contributions to chronic pain outcomes. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 87, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, R.; Bourbonnais, D.; Higgins, J.; Mireault, M.; Harris, P.G.; Danino, M.A. Pain interference may be an important link between pain severity, impairment, and self-reported disability in participants with wrist/hand pain. J. Hand Ther. 2020, 33, 562–570.e561. [Google Scholar] [CrossRef] [PubMed]
- Clauw, D.J.; Essex, M.N.; Pitman, V.; Jones, K.D. Reframing chronic pain as a disease, not a symptom: Rationale and implications for pain management. Postgrad. Med. 2019, 131, 185–198. [Google Scholar] [CrossRef]
- Loeser. The Role of Chronic Pain Clinics in Managing Back Pain; Raven Press: New York, NY, USA, 1991; pp. 221–229. [Google Scholar]
- Voon, P.; Karamouzian, M.; Kerr, T. Chronic pain and opioid misuse: A review of reviews. Subst. Abus. Treat. Prev. Policy 2017, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Atcheson, R. Opioid and chronic non-cancer pain. J. Anaesthesiol. Clin. Pharmacol. 2013, 29, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Kabat-Zinn, J. Wherever You Go There You Are: Mindfulness Meditation in Everyday Life; Hyperion: New York, NY, USA, 1994. [Google Scholar]
- Creswell, J.D. Mindfulness Interventions. Annu. Rev. Psychol. 2017, 68, 491–516. [Google Scholar] [CrossRef] [PubMed]
- Kabat-Zinn, J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: Theoretical considerations and preliminary results. Gen. Hosp. Psychiatry 1982, 4, 33–47. [Google Scholar] [CrossRef]
- Majeed, M.H.; Ali, A.A.; Sudak, D.M. Mindfulness-based interventions for chronic pain: Evidence and applications. Asian J. Psychiatr. 2018, 32, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Chaskalson, M.; Hadley, S.G. Mindfulness in Organizations. Foundations, Research and Applications; Reb, J., Atkins, P.W.B., Eds.; Cambridge University Press: Cambridge, UK, 2015; Volume 3. [Google Scholar]
- Janssen, M.; Heerkens, Y.; Kuijer, W.; van der Heijden, B.; Engels, J. Effects of Mindfulness-Based Stress Reduction on employees’ mental health: A systematic review. PLoS ONE 2018, 13, e0191332. [Google Scholar] [CrossRef] [PubMed]
- Kabat-Zinn, J. Full Catastrophe Living: Using the Wisdom of Your Body and Mind to Face Stress, Pain and Illness; Delacorte: New York, NY, USA, 1990. [Google Scholar]
- Khoury, B.; Sharma, M.; Rush, S.E.; Fournier, C. Mindfulness-based stress reduction for healthy individuals: A meta-analysis. J. Psychosom. Res. 2015, 78, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, S.; Greeson, J.M.; Reibel, D.K.; Green, J.S.; Jasser, S.A.; Beasley, D. Mindfulness-based stress reduction for chronic pain conditions: Variation in treatment outcomes and role of home meditation practice. J. Psychosom. Res. 2010, 68, 29–36. [Google Scholar] [CrossRef]
- Segal, Z.; Williams, J.; Teasdale, J. Mindfulness-Based Cognitive Therapy for Depression: A New Approach to Preventing Relapse; The Guildford Press: New York, NY, USA, 2002. [Google Scholar]
- Sipe, W.E.; Eisendrath, S.J. Mindfulness-based cognitive therapy: Theory and practice. Can. J. Psychiatry 2012, 57, 63–69. [Google Scholar] [CrossRef]
- Kuyken, W.; Warren, F.C.; Taylor, R.S.; Whalley, B.; Crane, C.; Bondolfi, G.; Hayes, R.; Huijbers, M.; Ma, H.; Schweizer, S.; et al. Efficacy of Mindfulness-Based Cognitive Therapy in Prevention of Depressive Relapse: An Individual Patient Data Meta-analysis From Randomized Trials. JAMA Psychiatry 2016, 73, 565–574. [Google Scholar] [CrossRef]
- Piet, J.; Hougaard, E. The effect of mindfulness-based cognitive therapy for prevention of relapse in recurrent major depressive disorder: A systematic review and meta-analysis. Clin. Psychol. Rev. 2011, 31, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Delaney, M.C. Caring for the caregivers: Evaluation of the effect of an eight-week pilot mindful self-compassion (MSC) training program on nurses’ compassion fatigue and resilience. PLoS ONE 2018, 13, e0207261. [Google Scholar] [CrossRef] [PubMed]
- Neff, K.D. Compassion and Wisdom in Psychotherapy; Guilford Press: New York, NY, USA, 2012. [Google Scholar]
- Neff, K.D. Self-Compassion: Theory, Method, Research, and Intervention. Annu. Rev. Psychol. 2023, 74, 193–218. [Google Scholar] [CrossRef] [PubMed]
- Torrijos-Zarcero, M.; Mediavilla, R.; Rodriguez-Vega, B.; Del Rio-Dieguez, M.; Lopez-Alvarez, I.; Rocamora-Gonzalez, C.; Palao-Tarrero, A. Mindful Self-Compassion program for chronic pain patients: A randomized controlled trial. Eur. J. Pain 2021, 25, 930–944. [Google Scholar] [CrossRef] [PubMed]
- Bowen, S.; Witkiewitz, K.; Clifasefi, S.L.; Grow, J.; Chawla, N.; Hsu, S.H.; Carroll, H.A.; Harrop, E.; Collins, S.E.; Lustyk, M.K.; et al. Relative efficacy of mindfulness-based relapse prevention, standard relapse prevention, and treatment as usual for substance use disorders: A randomized clinical trial. JAMA Psychiatry 2014, 71, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Daley, D.; Marlatt, G. Overcoming Your Alcohol or Drug Problem: Effective Recovery Strategies: Therapist Guide, 2nd ed.; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Marlatt, G.; Gordon, J. Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors; Guilford Press: New York, NY, USA, 1985. [Google Scholar]
- Garland, E.L. Mindfulness-Oriented Recovery Enhancement for Addiction, Stress, and Pain; NASW Press: Washington, DC, USA, 2013. [Google Scholar]
- Garland, E.L. Mindful Positive Emotion Regulation as a Treatment for Addiction: From Hedonic Pleasure to Self-Transcendent Meaning. Curr. Opin. Behav. Sci. 2021, 39, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Garland, E.L.; Fredrickson, B.L. Positive psychological states in the arc from mindfulness to self-transcendence: Extensions of the Mindfulness-to-Meaning Theory and applications to addiction and chronic pain treatment. Curr. Opin. Psychol. 2019, 28, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Hanley, A.W.; Nakamura, Y.; Garland, E.L. The Nondual Awareness Dimensional Assessment (NADA): New tools to assess nondual traits and states of consciousness occurring within and beyond the context of meditation. Psychol. Assess. 2018, 30, 1625–1639. [Google Scholar] [CrossRef] [PubMed]
- Parisi, A.; Roberts, R.L.; Hanley, A.W.; Garland, E.L. Mindfulness-Oriented Recovery Enhancement for Addictive Behavior, Psychiatric Distress, and Chronic Pain: A Multilevel Meta-Analysis of Randomized Controlled Trials. Mindfulness 2022, 13, 2396–2412. [Google Scholar] [CrossRef] [PubMed]
- Feuille, M.; Pargament, K. Pain, mindfulness, and spirituality: A randomized controlled trial comparing effects of mindfulness and relaxation on pain-related outcomes in migraineurs. J. Health Psychol. 2015, 20, 1090–1106. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.M.; Mickes, L.; Stolarz-Fantino, S.; Evrard, M.; Fantino, E. Increased False-Memory Susceptibility After Mindfulness Meditation. Psychol. Sci. 2015, 26, 1567–1573. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E.A.; Dowsey, M.M.; Knowles, S.R.; Castle, D.J.; Salzberg, M.R.; Monshat, K.; Dunin, A.J.; Choong, P.F. Systematic review of the efficacy of pre-surgical mind-body based therapies on post-operative outcome measures. Complement. Ther. Med. 2013, 21, 697–711. [Google Scholar] [CrossRef] [PubMed]
- Posadzki, P.; Lewandowski, W.; Terry, R.; Ernst, E.; Stearns, A. Guided imagery for non-musculoskeletal pain: A systematic review of randomized clinical trials. J. Pain Symptom Manag. 2012, 44, 95–104. [Google Scholar] [CrossRef]
- Luberto, C.M.; Hall, D.L.; Park, E.R.; Haramati, A.; Cotton, S. A Perspective on the Similarities and Differences Between Mindfulness and Relaxation. Glob. Adv. Health Med. 2020, 9, 2164956120905597. [Google Scholar] [CrossRef] [PubMed]
- Morton, M.L. A Systematic Review of Mindfulness Interventions on Psychophysiological Responses to Acute Stress. Mindfulness 2020, 11, 2039–2054. [Google Scholar] [CrossRef]
- Kaplun, A.; Alperovitch-Najenson, D.; Kalichman, L. Effect of Guided Imagery on Pain and Health-Related Quality of Life in Musculoskeletal Medicine: A Comprehensive Narrative Review. Curr. Pain Headache Rep. 2021, 25, 76. [Google Scholar] [CrossRef] [PubMed]
- Felix, M.; Ferreira, M.B.G.; da Cruz, L.F.; Barbosa, M.H. Relaxation Therapy with Guided Imagery for Postoperative Pain Management: An Integrative Review. Pain Manag. Nurs. 2019, 20, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Kosslyn, S.M.; Ganis, G.; Thompson, W.L. Neural foundations of imagery. Nat. Rev. Neurosci. 2001, 2, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Baird, C.L.; Murawski, M.M.; Wu, J. Efficacy of guided imagery with relaxation for osteoarthritis symptoms and medication intake. Pain Manag. Nurs. 2010, 11, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Francis, A.J. Relaxation and imagery for chronic, nonmalignant pain: Effects on pain symptoms, quality of life, and mental health. Pain Manag. Nurs. 2010, 11, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, W.; Jacobson, A. Bridging the gap between mind and body: A biobehavioral model of the effects of guided imagery on pain, pain disability, and depression. Pain Manag. Nurs. 2013, 14, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, W.; Jacobson, A.; Palmieri, P.A.; Alexander, T.; Zeller, R. Biological mechanisms related to the effectiveness of guided imagery for chronic pain. Biol. Res. Nurs. 2011, 13, 364–375. [Google Scholar] [CrossRef]
- Menzies, V.; Lyon, D.E.; Elswick, R.K., Jr.; McCain, N.L.; Gray, D.P. Effects of guided imagery on biobehavioral factors in women with fibromyalgia. J. Behav. Med. 2014, 37, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.M.; Davies, A.; Griffiths, G. Facilitating comfort for hospitalized patients using non-pharmacological measures: Preliminary development of clinical practice guidelines. Int. J. Nurs. Pract. 2009, 15, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, L.F.; Costa-Pereira, A.; Mendonca, L.; Dias, C.C.; Castro-Lopes, J.M. Epidemiology of chronic pain: A population-based nationwide study on its prevalence, characteristics and associated disability in Portugal. J. Pain 2012, 13, 773–783. [Google Scholar] [CrossRef]
- Gouveia, B.; Fonseca, S.; Pozza, D.H.; Xara, D.; Sa Rodrigues, A. Relationship between Postoperative Pain and Sociocultural Level in Major Orthopedic Surgery. Adv. Orthop. 2022, 2022, 7867719. [Google Scholar] [CrossRef] [PubMed]
- Deyo, R.A.; Mirza, S.K.; Martin, B.I. Back pain prevalence and visit rates: Estimates from U.S. national surveys, 2002. Spine 2006, 31, 2724–2727. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.W.; Jensen, M.P.; Thorn, B.; Lillis, T.A.; Carmody, J.; Newman, A.K.; Keefe, F. Cognitive therapy, mindfulness-based stress reduction, and behavior therapy for the treatment of chronic pain: Randomized controlled trial. Pain 2022, 163, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Cherkin, D.C.; Sherman, K.J.; Balderson, B.H.; Cook, A.J.; Anderson, M.L.; Hawkes, R.J.; Hansen, K.E.; Turner, J.A. Effect of Mindfulness-Based Stress Reduction vs Cognitive Behavioral Therapy or Usual Care on Back Pain and Functional Limitations in Adults with Chronic Low Back Pain: A Randomized Clinical Trial. JAMA 2016, 315, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Day, M.A.; Ward, L.C.; Ehde, D.M.; Thorn, B.E.; Burns, J.; Barnier, A.; Mattingley, J.B.; Jensen, M.P. A Pilot Randomized Controlled Trial Comparing Mindfulness Meditation, Cognitive Therapy, and Mindfulness-Based Cognitive Therapy for Chronic Low Back Pain. Pain Med. 2019, 20, 2134–2148. [Google Scholar] [CrossRef] [PubMed]
- Dowd, H.; Hogan, M.J.; McGuire, B.E.; Davis, M.C.; Sarma, K.M.; Fish, R.A.; Zautra, A.J. Comparison of an Online Mindfulness-based Cognitive Therapy Intervention with Online Pain Management Psychoeducation: A Randomized Controlled Study. Clin. J. Pain 2015, 31, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Garland, E.L.; Hanley, A.W.; Nakamura, Y.; Barrett, J.W.; Baker, A.K.; Reese, S.E.; Riquino, M.R.; Froeliger, B.; Donaldson, G.W. Mindfulness-Oriented Recovery Enhancement vs Supportive Group Therapy for Co-occurring Opioid Misuse and Chronic Pain in Primary Care: A Randomized Clinical Trial. JAMA Intern. Med. 2022, 182, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Garland, E.L.; Hanley, A.W.; Riquino, M.R.; Reese, S.E.; Baker, A.K.; Salas, K.; Yack, B.P.; Bedford, C.E.; Bryan, M.A.; Atchley, R.; et al. Mindfulness-oriented recovery enhancement reduces opioid misuse risk via analgesic and positive psychological mechanisms: A randomized controlled trial. J. Consult. Clin. Psychol. 2019, 87, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Garland, E.L.; Manusov, E.G.; Froeliger, B.; Kelly, A.; Williams, J.M.; Howard, M.O. Mindfulness-oriented recovery enhancement for chronic pain and prescription opioid misuse: Results from an early-stage randomized controlled trial. J. Consult. Clin. Psychol. 2014, 82, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Hearn, J.H.; Finlay, K.A. Internet-delivered mindfulness for people with depression and chronic pain following spinal cord injury: A randomized, controlled feasibility trial. Spinal Cord 2018, 56, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Henriksson, J.; Wasara, E.; Ronnlund, M. Effects of Eight-Week-Web-Based Mindfulness Training on Pain Intensity, Pain Acceptance, and Life Satisfaction in Individuals with Chronic Pain. Psychol. Rep. 2016, 119, 586–607. [Google Scholar] [CrossRef] [PubMed]
- Howarth, A.; Riaz, M.; Perkins-Porras, L.; Smith, J.G.; Subramaniam, J.; Copland, C.; Hurley, M.; Beith, I.; Ussher, M. Pilot randomised controlled trial of a brief mindfulness-based intervention for those with persistent pain. J. Behav. Med. 2019, 42, 999–1014. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.; Said, A.S.A. Mindfulness-Based Meditation versus Progressive Relaxation Meditation: Impact on Chronic Pain in Older Female Patients with Diabetic Neuropathy. J. Evid. Based Integr. Med. 2019, 24, 2515690X19876599. [Google Scholar] [CrossRef] [PubMed]
- Ussher, M.; Spatz, A.; Copland, C.; Nicolaou, A.; Cargill, A.; Amini-Tabrizi, N.; McCracken, L.M. Immediate effects of a brief mindfulness-based body scan on patients with chronic pain. J. Behav. Med. 2014, 37, 127–134. [Google Scholar] [CrossRef]
- Williams, R.M.; Day, M.A.; Ehde, D.M.; Turner, A.P.; Ciol, M.A.; Gertz, K.J.; Patterson, D.; Hakimian, S.; Suri, P.; Jensen, M.P. Effects of hypnosis vs mindfulness meditation vs education on chronic pain intensity and secondary outcomes in veterans: A randomized clinical trial. Pain 2022, 163, 1905–1918. [Google Scholar] [CrossRef] [PubMed]
- Zgierska, A.E.; Burzinski, C.A.; Cox, J.; Kloke, J.; Stegner, A.; Cook, D.B.; Singles, J.; Mirgain, S.; Coe, C.L.; Backonja, M. Mindfulness Meditation and Cognitive Behavioral Therapy Intervention Reduces Pain Severity and Sensitivity in Opioid-Treated Chronic Low Back Pain: Pilot Findings from a Randomized Controlled Trial. Pain Med. 2016, 17, 1865–1881. [Google Scholar] [CrossRef] [PubMed]
- la Cour, P.; Petersen, M. Effects of mindfulness meditation on chronic pain: A randomized controlled trial. Pain Med. 2015, 16, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Onieva-Zafra, M.D.; Garcia, L.H.; Del Valle, M.G. Effectiveness of guided imagery relaxation on levels of pain and depression in patients diagnosed with fibromyalgia. Holist. Nurs. Pract. 2015, 29, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Polaski, A.M.; Phelps, A.L.; Smith, T.J.; Helm, E.R.; Morone, N.E.; Szucs, K.A.; Kostek, M.C.; Kolber, B.J. Integrated Meditation and Exercise Therapy: A Randomized Controlled Pilot of a Combined Nonpharmacological Intervention Focused on Reducing Disability and Pain in Patients with Chronic Low Back Pain. Pain Med. 2021, 22, 444–458. [Google Scholar] [CrossRef] [PubMed]
- Cooperman, N.A.; Hanley, A.W.; Kline, A.; Garland, E.L. A pilot randomized clinical trial of mindfulness-oriented recovery enhancement as an adjunct to methadone treatment for people with opioid use disorder and chronic pain: Impact on illicit drug use, health, and well-being. J. Subst. Abus. Treat. 2021, 127, 108468. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, W.; Good, M.; Draucker, C.B. Changes in the meaning of pain with the use of guided imagery. Pain Manag. Nurs. 2005, 6, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Baird, C.L.; Sands, L. A pilot study of the effectiveness of guided imagery with progressive muscle relaxation to reduce chronic pain and mobility difficulties of osteoarthritis. Pain Manag. Nurs. 2004, 5, 97–104. [Google Scholar] [CrossRef]
- Morone, N.E.; Greco, C.M.; Moore, C.G.; Rollman, B.L.; Lane, B.; Morrow, L.A.; Glynn, N.W.; Weiner, D.K. A Mind-Body Program for Older Adults with Chronic Low Back Pain: A Randomized Clinical Trial. JAMA Intern. Med. 2016, 176, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Cathcart, S.; Galatis, N.; Immink, M.; Proeve, M.; Petkov, J. Brief mindfulness-based therapy for chronic tension-type headache: A randomized controlled pilot study. Behav. Cogn. Psychother. 2014, 42, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.A.; Jones, A.K. Psychobiological correlates of improved mental health in patients with musculoskeletal pain after a mindfulness-based pain management program. Clin. J. Pain 2013, 29, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Lauche, R.; Schuth, M.; Schwickert, M.; Ludtke, R.; Musial, F.; Michalsen, A.; Dobos, G.; Choi, K.E. Efficacy of the Alexander Technique in treating chronic non-specific neck pain: A randomized controlled trial. Clin Rehabil. 2016, 30, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Fincham, G.W.; Mavor, K.; Dritschel, B. Effects of Mindfulness Meditation Duration and Type on Well-being: An Online Dose-Ranging Randomized Controlled Trial. Mindfulness 2023, 14, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Grover, M.P. An experimental investigation of the effects and mechanisms of mindfulness meditation versus self-hypnosis versus an attention control on cold pressor outcomes. Mindfulness 2021, 12, 923–935. [Google Scholar] [CrossRef]
- Swain, N.R. A comparison of therapist-present or therapist-free delivery of very brief mindfulness and hypnosis for acute experimental pain. J. Psychol. 2014, 43, 22–28. [Google Scholar]
- Dear, B.F.; Titov, N.; Perry, K.N.; Johnston, L.; Wootton, B.M.; Terides, M.D.; Rapee, R.M.; Hudson, J.L. The Pain Course: A randomised controlled trial of a clinician-guided Internet-delivered cognitive behaviour therapy program for managing chronic pain and emotional well-being. Pain 2013, 154, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Danon, N.; Al-Gobari, M.; Burnand, B.; Rodondi, P.Y. Are mind-body therapies effective for relieving cancer-related pain in adults? A systematic review and meta-analysis. Psychooncology 2022, 31, 345–371. [Google Scholar] [CrossRef] [PubMed]
- Garland, E.L.; Brintz, C.E.; Hanley, A.W.; Roseen, E.J.; Atchley, R.M.; Gaylord, S.A.; Faurot, K.R.; Yaffe, J.; Fiander, M.; Keefe, F.J. Mind-Body Therapies for Opioid-Treated Pain: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2020, 180, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Arola, H.M.; Nicholls, E.; Mallen, C.; Thomas, E. Self-reported pain interference and symptoms of anxiety and depression in community-dwelling older adults: Can a temporal relationship be determined? Eur. J. Pain 2010, 14, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Bair, M.J.; Robinson, R.L.; Katon, W.; Kroenke, K. Depression and pain comorbidity: A literature review. Arch. Intern. Med. 2003, 163, 2433–2445. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Wu, J.; Bair, M.J.; Krebs, E.E.; Damush, T.M.; Tu, W. Reciprocal relationship between pain and depression: A 12-month longitudinal analysis in primary care. J. Pain 2011, 12, 964–973. [Google Scholar] [CrossRef]
- Lewandowski Holley, A.; Law, E.F.; Zhou, C.; Murphy, L.; Clarke, G.; Palermo, T.M. Reciprocal longitudinal associations between pain and depressive symptoms in adolescents. Eur. J. Pain 2013, 17, 1058–1067. [Google Scholar] [CrossRef] [PubMed]


| Reference/Study Type | Study/Participant Characteristics | Pain Variables/Emotional State at Baseline | Intervention (n)/Time | Control (n)/Time | Main Results |
|---|---|---|---|---|---|
| Classical Mindfulness Interventions | |||||
| [78]/RCT |
|
| HYP (110)
| ED (110)
|
|
| [76]/RCT |
|
| MM (36)
| CM (32)
|
|
| [68]/RCT |
|
| MBCT (23)
| MM (23)
|
|
| [79]/RCT (pilot) |
|
| Meditation-CBT + TAU (21)
| TAU (14)
|
|
| [38]/RCT |
|
| MSC (62)
| CBT (61)
|
|
| [66]/RCT |
|
| BT (77)
| TAU (77)
|
|
| [74]/RCT |
|
| MBSR (55)
| Anonymous discussion forum (52)
|
|
| [86]/RCT |
|
| MBSR (140)
| Health education program (142)
|
|
| [67]/RCT |
|
| MBSR (116)
| UC (113)
|
|
| [80]/RCT |
|
| MBSR (43)
| TAU (47)
|
|
| [77]/RCT |
|
| MBSR (27)
| Reading about natural history (28)
|
|
| [82]/RCT (pilot) |
|
| MedExt (18)
| Audiobook (20)
|
|
| [73]/RCT (pilot) |
|
| Online mindfulness intervention (36)
| Internet-delivered psychoeducation (31)
|
|
| [69]/RCT |
|
| MIA (62)
| PE (62)
|
|
| [47]/RCT |
|
| STM (22)
| R (25)
|
|
| [75]/RCT (pilot) |
|
| MBI (37)
| Distraction audios (34)
|
|
| [87]/RCT (pilot) |
|
| MBT (23)
| Waiting list control (19)
|
|
| [88]/RCT |
|
| MBPM (15)
| TAU (13)
|
|
| Novel Mindfulness Interventions | |||||
| [70]/RCT |
|
| MORE (129)
| SG (121)
|
|
| [83]/RCT (pilot) |
|
| MORE + TAU (15)
| TAU (15)
|
|
| [71]/RCT |
|
| MORE (50)
| SG (45)
|
|
| [72]/RCT (pilot) |
|
| MORE (57)
| SG (58)
|
|
| Guided Imagery Interventions | |||||
| [89]/RCT |
|
| Alexander technique (24)
| Local heat (23)
|
|
| [81]/RCT |
|
| GI (30)
| Control (30)
|
|
| [84]/RCT |
|
| GI (21)
| Control (21)
|
|
| [85]/RCT (pilot) |
|
| GI + PMR (18)
| Standard care (10)
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, B.M.; Tavares, I.; Pozza, D.H. Enhancing Chronic Non-Cancer Pain Management: A Systematic Review of Mindfulness Therapies and Guided Imagery Interventions. Medicina 2024, 60, 686. https://doi.org/10.3390/medicina60050686
Pinto BM, Tavares I, Pozza DH. Enhancing Chronic Non-Cancer Pain Management: A Systematic Review of Mindfulness Therapies and Guided Imagery Interventions. Medicina. 2024; 60(5):686. https://doi.org/10.3390/medicina60050686
Chicago/Turabian StylePinto, Beatriz Manarte, Isaura Tavares, and Daniel Humberto Pozza. 2024. "Enhancing Chronic Non-Cancer Pain Management: A Systematic Review of Mindfulness Therapies and Guided Imagery Interventions" Medicina 60, no. 5: 686. https://doi.org/10.3390/medicina60050686