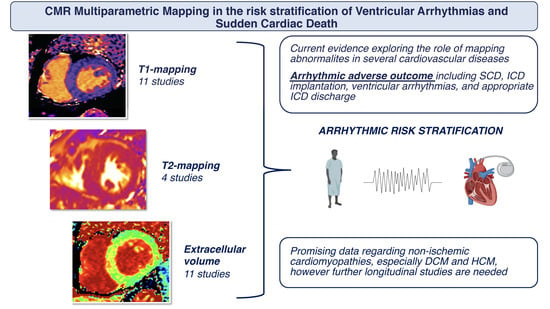
Multiparametric Mapping via Cardiovascular Magnetic Resonance in the Risk Stratification of Ventricular Arrhythmias and Sudden Cardiac Death
Abstract
:1. Introduction
2. Association of CMR Mapping Alterations and Ventricular Arrhythmias in Cardiovascular Diseases
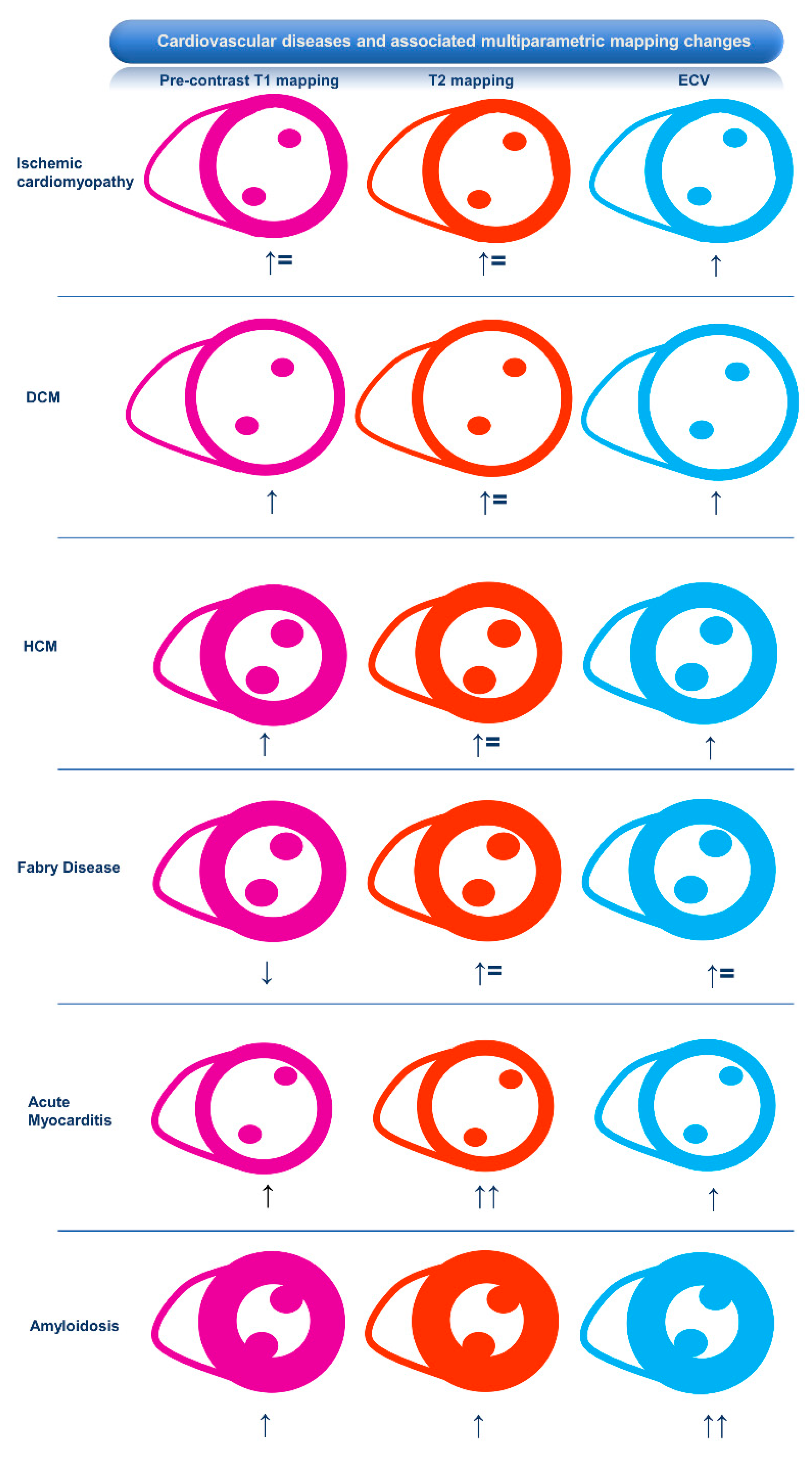
2.1. Ischemic Heart Disease
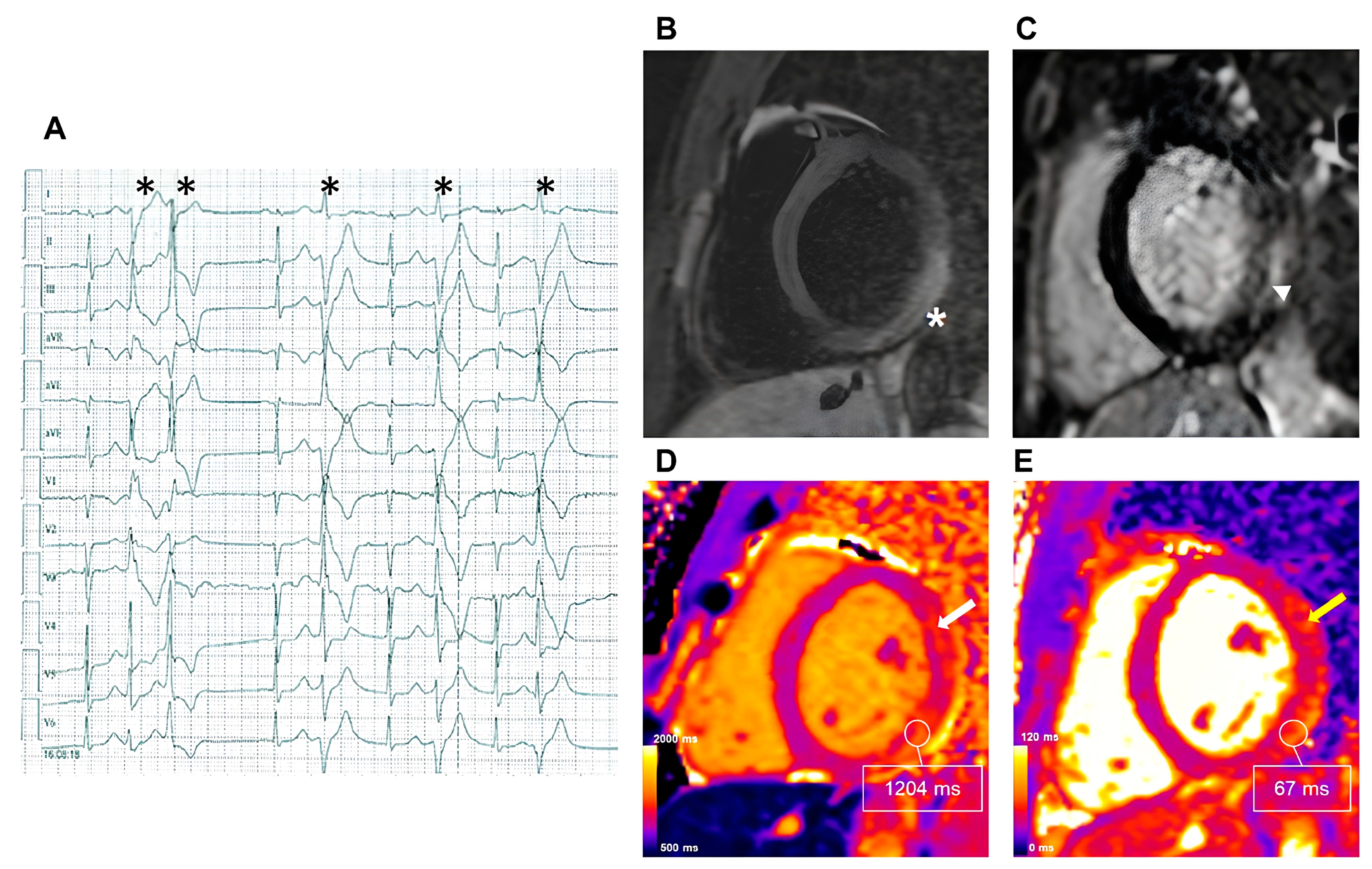
2.2. Inflammatory Cardiomyopathy
2.2.1. Myocarditis
2.2.2. Sarcoidosis
2.2.3. Connective Tissue Disorders
2.2.4. Chagas Disease
2.2.5. Takotsubo Cardiomyopathy
2.3. Hypertrophic Cardiomyopathy and Phenocopies
2.3.1. Hypertrophic Cardiomyopathy
2.3.2. Fabry Disease
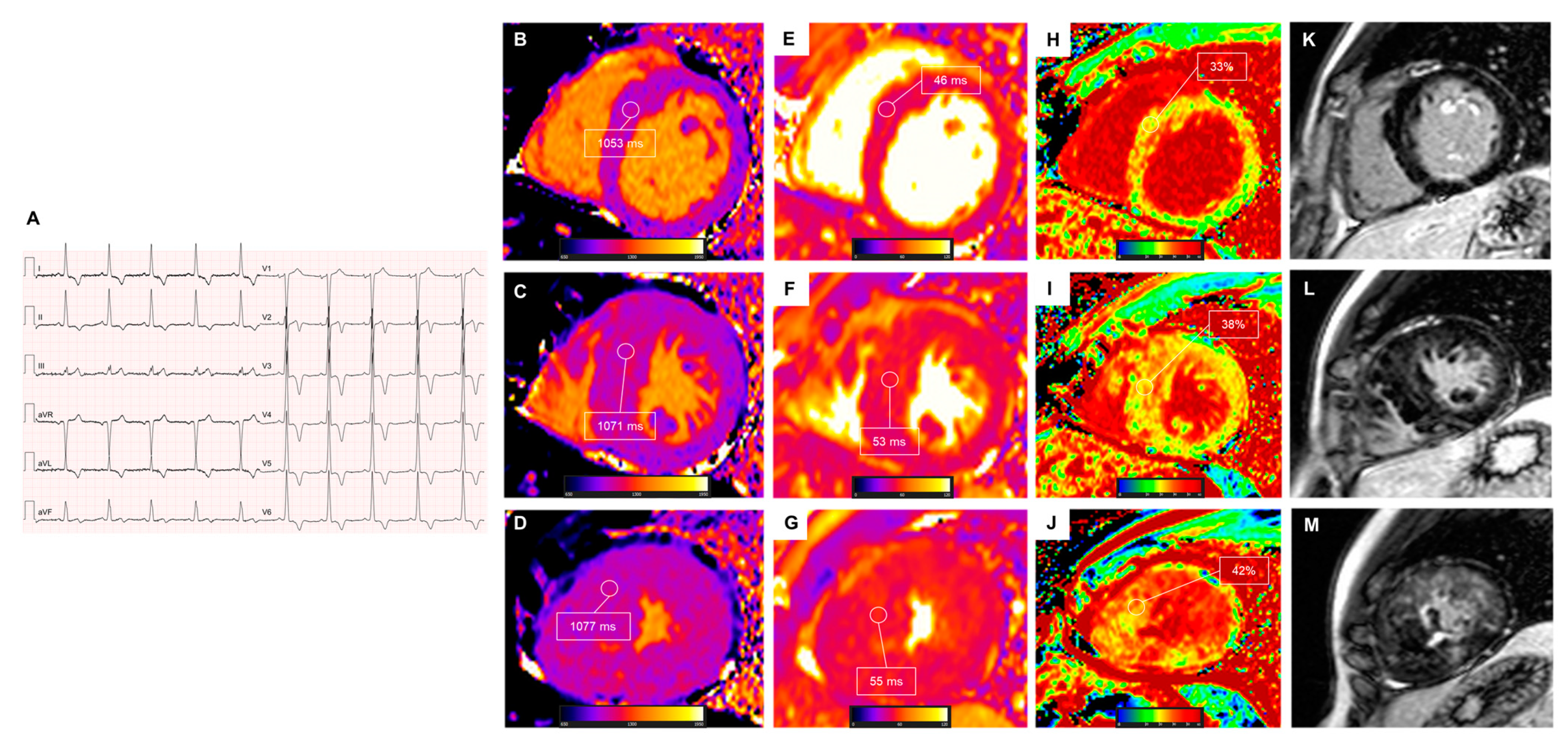
2.3.3. Amyloidosis
2.4. Dilated Cardiomyopathy
2.5. Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy
2.6. Mitral Valve Prolapse
3. Future Perspectives for CMR Mapping in Clinical Practice
4. Conclusions
Funding
Conflicts of Interest
Abbreviations
| DCM | dilated cardiomyopathy |
| ECV | extracellular volume |
| FD | Fabry disease |
| HCM | hypertrophic cardiomyopathy |
| ICD | implantable cardioverter-defibrillator |
| ICI | immune check-point inhibitor |
| LGE | late gadolinium enhancement |
| LV | left ventricular |
| MVP | mitral valve prolapse |
| SCD | sudden cardiac death |
References
- Tamene, A.; Tholakanahalli, V.N.; Chandrashekhar, Y. Cardiac imaging in evaluating patients prone to sudden death. Indian Heart J. 2014, 66 (Suppl. S1), S61–S70. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Sohal, M.; Voigt, T.; Sammut, E.; Tobon-Gomez, C.; Child, N.; Jackson, T.; Shetty, A.; Bostock, J.; Cooklin, M.; et al. Myocardial tissue characterization by cardiac magnetic resonance imaging using T1 mapping predicts ventricular arrhythmia in ischemic and non-ischemic cardiomyopathy patients with implantable cardioverter-defibrillators. Heart Rhythm. 2015, 12, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.C.; Messroghli, D.R.; Kellman, P.; Piechnik, S.K.; Robson, M.D.; Ugander, M.; Gatehouse, P.D.; Arai, A.E.; Friedrich, M.G.; Neubauer, S.; et al. Myocardial T1 mapping and extracellular volume quantification: A Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J. Cardiovasc. Magn. Reson. 2013, 15, 92. [Google Scholar] [CrossRef] [PubMed]
- Keil, L.; Chevalier, C.; Kirchhof, P.; Blankenberg, S.; Lund, G.; Müllerleile, K.; Magnussen, C. CMR-Based Risk Stratification of Sudden Cardiac Death and Use of Implantable Cardioverter-Defibrillator in Non-Ischemic Cardiomyopathy. Int. J. Mol. Sci. 2021, 22, 7115. [Google Scholar] [CrossRef] [PubMed]
- Figliozzi, S.; Georgiopoulos, G.; Lopes, P.M.; Bauer, K.B.; Moura-Ferreira, S.; Tondi, L.; Mushtaq, S.; Censi, S.; Pavon, A.G.; Bassi, I.; et al. Myocardial Fibrosis at Cardiac MRI Helps Predict Adverse Clinical Outcome in Patients with Mitral Valve Prolapse. Radiology 2023, 306, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Georgiopoulos, G.; Figliozzi, S.; Pateras, K.; Nicoli, F.; Bampatsias, D.; Beltrami, M.; Finocchiaro, G.; Chiribiri, A.; Masci, P.G.; Olivotto, I. Comparison of Demographic, Clinical, Biochemical, and Imaging Findings in Hypertrophic Cardiomyopathy Prognosis: A Network Meta-Analysis. JACC Heart Fail. 2023, 11, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Georgiopoulos, G.; Figliozzi, S.; Sanguineti, F.; Aquaro, G.D.; di Bella, G.; Stamatelopoulos, K.; Chiribiri, A.; Garot, J.; Masci, P.G.; Ismail, T.F. Prognostic Impact of Late Gadolinium Enhancement by Cardiovascular Magnetic Resonance in Myocarditis: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Imaging 2021, 14, e011492. [Google Scholar] [CrossRef] [PubMed]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [PubMed]
- Carrabba, N.; Amico, M.A.; Guaricci, A.I.; Carella, M.C.; Maestrini, V.; Monosilio, S.; Pedrotti, P.; Ricci, F.; Monti, L.; Figliozzi, S.; et al. CMR Mapping: The 4th-Era Revolution in Cardiac Imaging. J. Clin. Med. 2024, 13, 337. [Google Scholar] [CrossRef]
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P.; Mascherbauer, J.; Nezafat, R.; Salerno, M.; Schelbert, E.B.; et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J. Cardiovasc. Magn. Reson. 2017, 19, 75. [Google Scholar]
- Jugdutt, B.I. Ventricular remodeling after infarction and the extracellular collagen matrix: When is enough enough? Circulation 2003, 108, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Antzelevitch, C.; Burashnikov, A. Overview of Basic Mechanisms of Cardiac Arrhythmia. Card. Electrophysiol. Clin. 2011, 3, 23–45. [Google Scholar] [CrossRef] [PubMed]
- Olausson, E.; Wertz, J.; Fridman, Y.; Bering, P.; Maanja, M.; Niklasson, L.; Wong, T.C.; Fukui, M.; Cavalcante, J.L.; Cater, G.; et al. Diffuse myocardial fibrosis associates with incident ventricular arrhythmia in implantable cardioverter defibrillator recipients. medRxiv 2023. [Google Scholar] [CrossRef] [PubMed]
- Gräni, C.; Bière, L.; Eichhorn, C.; Kaneko, K.; Agarwal, V.; Aghayev, A.; Steigner, M.; Blankstein, R.; Jerosch-Herold, M.; Kwong, R.Y. Incremental value of extracellular volume assessment by cardiovascular magnetic resonance imaging in risk stratifying patients with suspected myocarditis. Int. J. Cardiovasc. Imaging 2019, 35, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Thavendiranathan, P.; Zhang, L.; Zafar, A.; Drobni, Z.D.; Mahmood, S.S.; Cabral, M.; Awadalla, M.; Nohria, A.; Zlotoff, D.A.; Thuny, F.; et al. Myocardial T1 and T2 Mapping by Magnetic Resonance in Patients with Immune Checkpoint Inhibitor-Associated Myocarditis. J. Am. Coll. Cardiol. 2021, 77, 1503–1516. [Google Scholar] [CrossRef] [PubMed]
- Crouser, E.D.; Ono, C.; Tran, T.; He, X.; Raman, S.V. Improved detection of cardiac sarcoidosis using magnetic resonance with myocardial T2 mapping. Am. J. Respir. Crit. Care Med. 2014, 189, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Crouser, E.D.; Ruden, E.; Julian, M.W.; Raman, S.V. Resolution of abnormal cardiac MRI T2 signal following immune suppression for cardiac sarcoidosis. J. Investig. Med. 2016, 64, 1148–1150. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, M.V.T.; Moll-Bernardes, R.J.; Camargo, G.C.; Siqueira, F.P.; de Azevedo, C.F.; de Holanda, M.T.; Mendes, F.d.S.N.S.; Sangenis, L.H.C.; Mediano, M.F.F.; de Sousa, A.S. Associations between Cardiac Magnetic Resonance T1 Mapping Parameters and Ventricular Arrhythmia in Patients with Chagas Disease. Am. J. Trop. Med. Hyg. 2020, 103, 745–751. [Google Scholar] [CrossRef]
- Melo, R.J.L.; Assunção, A.N., Jr.; Morais, T.C.; Nomura, C.H.; Scanavacca, M.I.; Martinelli-Filho, M.; Ramires, F.J.A.; Fernandes, F.; Ianni, B.M.; Mady, C.; et al. Detection of Early Diffuse Myocardial Fibrosis and Inflammation in Chagas Cardiomyopathy with T1 Mapping and Extracellular Volume. Radiol. Cardiothorac. Imaging 2023, 5, e220112. [Google Scholar] [CrossRef]
- Qin, L.; Min, J.; Chen, C.; Zhu, L.; Gu, S.; Zhou, M.; Yang, W.; Yan, F. Incremental values of T1 mapping in the prediction of sudden cardiac death risk in hypertrophic cardiomyopathy: A comparison with two guidelines. Front. Cardiovasc. Med. 2021, 8, 661673. [Google Scholar] [CrossRef]
- Avanesov, M.; Münch, J.; Weinrich, J.; Well, L.; Säring, D.; Stehning, C.; Tahir, E.; Bohnen, S.; Radunski, U.K.; Muellerleile, K.; et al. Prediction of the estimated 5-year risk of sudden cardiac death and syncope or non-sustained ventricular tachycardia in patients with hypertrophic cardiomyopathy using late gadolinium enhancement and extracellular volume CMR. Eur. Radiol. 2017, 27, 5136–5145. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Cai, Z.; Yang, Z.; Lin, W.; Su, Y.; Li, J.; Xie, S.; Shen, J. The Value of Myocardial Fibrosis Parameters Derived from Cardiac Magnetic Resonance Imaging in Risk Stratification for Patients with Hypertrophic Cardiomyopathy. Acad. Radiol. 2023, 30, 1962–1978. [Google Scholar] [CrossRef] [PubMed]
- McLELLAN, A.J.A.; Ellims, A.H.; Prabhu, S.; Voskoboinik, A.; Iles, L.M.; Hare, J.L.; Kaye, D.M.; Macciocca; Mariani, J.A.; Kalman, J.M.; et al. Diffuse ventricular fibrosis on cardiac magnetic resonance imaging associates with ventricular tachycardia in patients with hypertrophic cardiomyopathy. J. Cardiovasc. Electrophysiol. 2016, 27, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, J.; Cheng, W.; Wan, K.; Li, W.; Pu, L.; Xu, Y.; Sun, J.; Han, Y.; Chen, Y. Incremental significance of myocardial oedema for prognosis in hypertrophic cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Orsborne, C.; Bradley, J.; Bonnett, L.J.; Pleva, L.A.; Naish, J.H.; Clark, D.G.; Abidin, N.; Woolfson, P.; Nucifora, G.; Schmitt, M.; et al. Validated model for prediction of adverse cardiac outcome in patients with fabry disease. J. Am. Coll. Cardiol. 2022, 80, 982–994. [Google Scholar] [CrossRef] [PubMed]
- Nakamori, S.; Bui, A.H.; Jang, J.; El-Rewaidy, H.A.; Kato, S.; Ngo, L.H.; Josephson, M.E.; Manning, W.J.; Nezafat, R. Increased myocardial native T1 relaxation time in patients with nonischemic dilated cardiomyopathy with complex ventricular arrhythmia. J. Magn. Reson. Imaging 2018, 47, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Barison, A.; Del Torto, A.; Chiappino, S.; Aquaro, G.D.; Todiere, G.; Vergaro, G.; Passino, C.; Lombardi, M.; Emdin, M.; Masci, P.G. Prognostic significance of myocardial extracellular volume fraction in nonischaemic dilated cardiomyopathy. J. Cardiovasc. Med. 2015, 16, 681. [Google Scholar] [CrossRef] [PubMed]
- Cadour, F.; Quemeneur, M.; Biere, L.; Donal, E.; Bentatou, Z.; Eicher, J.-C.; Roubille, F.; Lalande, A.; Giorgi, R.; Rapacchi, S.; et al. Prognostic value of cardiovascular magnetic resonance T1 mapping and extracellular volume fraction in nonischemic dilated cardiomyopathy. J. Cardiovasc. Magn. Reson. 2023, 25, 7. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhou, D.; Sirajuddin, A.; He, J.; Xu, J.; Zhuang, B.; Huang, J.; Yin, G.; Fan, X.; Wu, W.; et al. T1 mapping and extracellular volume fraction in dilated cardiomyopathy: A prognosis study. JACC Cardiovasc. Imaging 2022, 15, 578–590. [Google Scholar] [CrossRef]
- Rubiś, P.P.; Dziewięcka, E.M.; Banyś, P.; Urbańczyk-Zawadzka, M.; Krupiński, M.; Mielnik, M.; Łach, J.; Ząbek, A.; Wiśniowska-Śmiałek, S.; Podolec, P.; et al. Extracellular volume is an independent predictor of arrhythmic burden in dilated cardiomyopathy. Sci. Rep. 2021, 11, 24000. [Google Scholar] [CrossRef]
- Chun, K.-H.; Oh, J.; Hong, Y.J.; Yu, H.T.; Lee, C.J.; Kim, T.; Joung, B.; Pak, H.; Lee, M.; Kim, Y.J.; et al. Prognostic cardiac magnetic resonance markers of left ventricular involvement in arrhythmogenic cardiomyopathy for predicting heart failure outcomes. J. Am. Heart Assoc. 2022, 11, e023167. [Google Scholar] [CrossRef] [PubMed]
- Pavon, A.G.; Arangalage, D.; Pascale, P.; Hugelshofer, S.; Rutz, T.; Porretta, A.P.; Le Bloa, M.; Muller, O.; Pruvot, E.; Schwitter, J.; et al. Myocardial extracellular volume by T1 mapping: A new marker of arrhythmia in mitral valve prolapse. J. Cardiovasc. Magn. Reson. 2021, 23, 102. [Google Scholar] [CrossRef] [PubMed]
- Bui, A.H.; Roujol, S.; Foppa, M.; Kissinger, K.V.; Goddu, B.; Hauser, T.H.; Zimetbaum, P.J.; Ngo, L.H.; Manning, W.J.; Nezafat, R.; et al. Diffuse myocardial fibrosis in patients with mitral valve prolapse and ventricular arrhythmia. Heart 2017, 103, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Amoni, M.; Dries, E.; Ingelaere, S.; Vermoortele, D.; Roderick, H.L.; Claus, P.; Willems, R.; Sipido, K.R. Ventricular Arrhythmias in Ischemic Cardiomyopathy-New Avenues for Mechanism-Guided Treatment. Cells 2021, 10, 2629. [Google Scholar] [CrossRef]
- Haaf, P.; Garg, P.; Messroghli, D.R.; Broadbent, D.A.; Greenwood, J.P.; Plein, S. Cardiac T1 Mapping and Extracellular Volume (ECV) in clinical practice: A comprehensive review. J. Cardiovasc. Magn. Reson. 2016, 18, 89. [Google Scholar] [CrossRef] [PubMed]
- de Bakker, J.M.; van Capelle, F.J.; Janse, M.J.; Wilde, A.A.; Coronel, R.; Becker, A.E.; Dingemans, K.P.; van Hemel, N.M.; Hauer, R.N. Reentry as a cause of ventricular tachycardia in patients with chronic ischemic heart disease: Electrophysiologic and anatomic correlation. Circulation 1988, 77, 589–606. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.M.; Piechnik, S.K.; Dall’Armellina, E.; Karamitsos, T.D.; Francis, J.M.; Choudhury, R.P.; Friedrich, M.G.; Robson, M.D.; Neubauer, S. Non-contrast T1-mapping detects acute myocardial edema with high diagnostic accuracy: A comparison to T2-weighted cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2012, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Jiménez, R.; Sánchez-González, J.; Aguero, J.; del Trigo, M.; Galán-Arriola, C.; Fuster, V.; Ibáñez, B. Fast T2 gradient-spin-echo (T2-GraSE) mapping for myocardial edema quantification: First in vivo validation in a porcine model of ischemia/reperfusion. J. Cardiovasc. Magn. Reson. 2015, 17, 92. [Google Scholar] [CrossRef] [PubMed]
- Harmon, K.G.; Asif, I.M.; Maleszewski, J.J.; Owens, D.S.; Prutkin, J.M.; Salerno, J.C.; Zigman, M.L.; Ellenbogen, R.; Rao, A.L.; Ackerman, M.J.; et al. Incidence and etiology of sudden cardiac arrest and death in high school athletes in the united states. Mayo Clin. Proc. 2016, 91, 1493–1502. [Google Scholar] [CrossRef]
- Maron, B.J.; Udelson, J.E.; Bonow, R.O.; Owens, D.S.; Prutkin, J.M.; Salerno, J.C.; Zigman, M.L.; Ellenbogen, R.; Rao, A.L.; Ackerman, M.J.; et al. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task force 3: Hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy and other cardiomyopathies, and myocarditis: A scientific statement from the american heart association and american college of cardiology. J. Am. Coll. Cardiol. 2015, 66, 2362–2371. [Google Scholar]
- Cooper, L.T. Ventricular arrhythmias and sudden cardiac death in lymphocytic myocarditis. J. Am. Coll. Cardiol. 2020, 75, 1058–1060. [Google Scholar] [CrossRef] [PubMed]
- Palaskas, N.; Lopez-Mattei, J.; Durand, J.B.; Iliescu, C.; Deswal, A. Immune Checkpoint Inhibitor Myocarditis: Pathophysiological Characteristics, Diagnosis, and Treatment. J. Am. Heart Assoc. 2020, 9, e013757. [Google Scholar] [CrossRef] [PubMed]
- Nordenswan, H.-K.; Pöyhönen, P.; Lehtonen, J.; Ekström, K.; Uusitalo, V.; Niemelä, M.; Vihinen, T.; Kaikkonen, K.; Haataja, P.; Kerola, T.; et al. Incidence of Sudden Cardiac Death and Life-Threatening Arrhythmias in Clinically Manifest Cardiac Sarcoidosis with and without Current Indications for an Implantable Cardioverter Defibrillator. Circulation 2022, 146, 964–975. [Google Scholar] [CrossRef] [PubMed]
- Mayr, A.; Kitterer, D.; Latus, J.; Steubing, H.; Henes, J.; Vecchio, F.; Kaesemann, P.; Patrascu, A.; Greiser, A.; Groeninger, S.; et al. Evaluation of myocardial involvement in patients with connective tissue disorders: A multi-parametric cardiovascular magnetic resonance study. J. Cardiovasc. Magn. Reson. 2016, 18, 67. [Google Scholar] [CrossRef] [PubMed]
- Ross, L.; Costello, B.; Brown, Z.; Hansen, D.; Lindqvist, A.; Stevens, W.; Burns, A.; Prior, D.; Nikpour, M.; La Gerche, A. Myocardial fibrosis and arrhythmic burden in systemic sclerosis. Rheumatology 2022, 61, 4497–4502. [Google Scholar] [CrossRef] [PubMed]
- Duran-Crane, A.; Rojas, C.A.; Cooper, L.T.; Medina, H.M. Cardiac magnetic resonance imaging in Chagas’ disease: A parallel with electrophysiologic studies. Int. J. Cardiovasc. Imaging 2020, 36, 2209–2219. [Google Scholar] [CrossRef] [PubMed]
- Manolis, A.A.; Manolis, T.A.; Melita, H.; Manolis, A.S. Takotsubo syndrome and sudden cardiac death. Angiology 2023, 74, 105–128. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.; Voskoboinik, A.; Neil, C. Arrhythmias and their electrophysiological mechanisms in takotsubo syndrome: A narrative review. Heart Lung Circ. 2022, 31, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Citro, R.; Okura, H.; Ghadri, J.R.; Izumi, C.; Meimoun, P.; Izumo, M.; Dawson, D.; Kaji, S.; Eitel, I.; Kagiyama, N.; et al. Multimodality imaging in takotsubo syndrome: A joint consensus document of the European Association of Cardiovascular Imaging (EACVI) and the Japanese Society of Echocardiography (JSE). Eur. Heart J. Cardiovasc. Imaging 2020, 21, 1184–1207. [Google Scholar] [CrossRef]
- Scally, C.; Rudd, A.; Mezincescu, A.; Wilson, H.; Srivanasan, J.; Horgan, G.; Broadhurst, P.; Newby, D.E.; Henning, A.; Dawson, D.K. Persistent Long-Term Structural, Functional, and Metabolic Changes after Stress-Induced (Takotsubo) Cardiomyopathy. Circulation 2018, 137, 1039–1048. [Google Scholar] [CrossRef]
- Schwarz, K.; Ahearn, T.; Srinivasan, J.; Neil, C.J.; Scally, C.; Rudd, A.; Jagpal, B.; Frenneaux, M.P.; Pislaru, C.; Horowitz, J.D.; et al. Alterations in Cardiac Deformation, Timing of Contraction and Relaxation, and Early Myocardial Fibrosis Accompany the Apparent Recovery of Acute Stress-Induced (Takotsubo) Cardiomyopathy: An End to the Concept of Transience. J. Am. Soc. Echocardiogr. 2017, 30, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Mathai, S.; Sehmi, J.; Auger, D.; Heureux, C.L.; Keenan, N.G. Role of T1 mapping in identification of convalescent Takotsubo cardiomyopathy. Eur. Heart J.-Cardiovasc. Imaging 2021, 22 (Suppl. S1), jeaa356.313. [Google Scholar] [CrossRef]
- Cau, R.; Pisu, F.; Porcu, M.; Cademartiri, F.; Montisci, R.; Bassareo, P.; Muscogiuri, G.; Amadu, A.; Sironi, S.; Esposito, A.; et al. Machine learning approach in diagnosing Takotsubo cardiomyopathy: The role of the combined evaluation of atrial and ventricular strain, and parametric mapping. Int. J. Cardiol. 2023, 373, 124–133. [Google Scholar] [CrossRef]
- Gil, K.E.; Truong, V.T.; Zareba, K.M.; Varghese, J.; Simonetti, O.P.; Rajpal, S. Parametric mapping by cardiovascular magnetic resonance imaging in sudden cardiac arrest survivors. Int. J. Cardiovasc. Imaging 2023, 39, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.H.; Maron, B.J.; Olivotto, I.; Pencina, M.J.; Assenza, G.E.; Haas, T.; Lesser, J.R.; Gruner, C.; Crean, A.M.; Rakowski, H.; et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation 2014, 130, 484–495. [Google Scholar] [CrossRef]
- Weng, Z.; Yao, J.; Chan, R.H.; He, J.; Yang, X.; Zhou, Y.; He, Y. Prognostic Value of LGE-CMR in HCM: A Meta-Analysis. JACC Cardiovasc. Imaging 2016, 9, 1392–1402. [Google Scholar] [CrossRef] [PubMed]
- Stankowski, K.; Figliozzi, S.; Lisi, C.; Catapano, F.; Panico, C.; Cannata, F.; Mantovani, R.; Frontera, A.; Bragato, R.M.; Stefanini, G.; et al. Solving the Riddle of Sudden Cardiac Death in Hypertrophic Cardiomyopathy: The Added Role of Cardiac Magnetic Resonance. J. Cardiovasc. Dev. Dis. 2023, 10, 226. [Google Scholar] [CrossRef]
- Huang, L.; Ran, L.; Zhao, P.; Tang, D.; Han, R.; Ai, T.; Xia, L.; Tao, Q. MRI native T1 and T2 mapping of myocardial segments in hypertrophic cardiomyopathy: Tissue remodeling manifested prior to structure changes. Br. J. Radiol. 2019, 92, 20190634. [Google Scholar] [CrossRef] [PubMed]
- Camporeale, A.; Pieroni, M.; Pieruzzi, F.; Lusardi, P.; Pica, S.; Spada, M.; Mignani, R.; Burlina, A.; Bandera, F.; Guazzi, M.; et al. Predictors of clinical evolution in prehypertrophic fabry disease. Circ. Cardiovasc. Imaging 2019, 12, e008424. [Google Scholar] [CrossRef]
- Nordin, S.; Kozor, R.; Medina-Menacho, K.; Abdel-Gadir, A.; Baig, S.; Sado, D.M.; Lobascio, I.; Murphy, E.; Lachmann, R.H.; Mehta, A.; et al. Proposed stages of myocardial phenotype development in fabry disease. JACC Cardiovasc. Imaging 2019, 12 Pt 2, 1673–1683. [Google Scholar] [CrossRef]
- Figliozzi, S.; Camporeale, A.; Boveri, S.; Pieruzzi, F.; Pieroni, M.; Lusardi, P.; Spada, M.; Mignani, R.; Burlina, A.; Graziani, F.; et al. ECG-based score estimates the probability to detect Fabry Disease cardiac involvement. Int. J. Cardiol. 2021, 339, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Augusto, J.B.; Nordin, S.; Vijapurapu, R.; Baig, S.; Bulluck, H.; Castelletti, S.; Alfarih, M.; Knott, K.; Captur, G.; Kotecha, T.; et al. Myocardial edema, myocyte injury, and disease severity in fabry disease. Circ. Cardiovasc. Imaging 2020, 13, e00171. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.; Lo, P.; Cho, K.; Subbiah, R. Ventricular arrhythmias in cardiac amyloidosis: A review of current literature. Clin. Med. Insights Cardiol. 2020, 14, 1179546820963055. [Google Scholar] [CrossRef] [PubMed]
- Banypersad, S.M.; Fontana, M.; Maestrini, V.; Sado, D.M.; Captur, G.; Petrie, A.; Piechnik, S.K.; Whelan, C.J.; Herrey, A.S.; Gillmore, J.D.; et al. T1 mapping and survival in systemic light-chain amyloidosis. Eur. Heart J. 2015, 36, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Naharro, A.; Kotecha, T.; Norrington, K.; Boldrini, M.; Rezk, T.; Quarta, C.; Treibel, T.A.; Whelan, C.J.; Knight, D.S.; Kellman, P.; et al. Native T1 and Extracellular Volume in Transthyretin Amyloidosis. JACC Cardiovasc. Imaging 2019, 12, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Nelson, T.; Garg, P.; Clayton, R.H.; Lee, J. The Role of Cardiac MRI in the Management of Ventricular Arrhythmias in Ischaemic and Non-Ischaemic Dilated Cardiomyopathy. Arrhythm. Electrophysiol. Rev. 2019, 8, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Porcari, A.; De Luca, A.; Grigoratos, C.; Biondi, F.; Faganello, G.; Vitrella, G.; Nucifora, G.; Aquaro, G.D.; Merlo, M.; Sinagra, G. Arrhythmic risk stratification by cardiac magnetic resonance tissue characterization: Disclosing the arrhythmic substrate within the heart muscle. Heart Fail. Rev. 2022, 27, 49–69. [Google Scholar] [CrossRef] [PubMed]
- Agbaedeng, T.A.; Roberts, K.A.; Colley, L.; Noubiap, J.J.; Oxborough, D. Incidence and predictors of sudden cardiac death in arrhythmogenic right ventricular cardiomyopathy: A pooled analysis. Europace 2022, 24, 1665–1674. [Google Scholar] [CrossRef]
- Dowd, R.; Dhanjal, T.; Schmucki, M.; Kanagala, P.; Khan, J.N. Unique role of cardiovascular magnetic resonance imaging parametric mapping in the diagnosis of arrhythmogenic left ventricular cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2021, 22, e96. [Google Scholar] [CrossRef]
- Noseworthy, P.A.; Asirvatham, S.J. The knot that binds mitral valve prolapse and sudden cardiac death. Circulation 2015, 132, 551–552. [Google Scholar] [CrossRef]
- Guglielmo, M.; Fusini, L.; Muscogiuri, G.; Baessato, F.; Loffreno, A.; Cavaliere, A.; Rizzon, G.; Baggiano, A.; Rabbat, M.G.; Muratori, M.; et al. T1 mapping and cardiac magnetic resonance feature tracking in mitral valve prolapse. Eur. Radiol. 2021, 31, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Guglielmo, M.; Arangalage, D.; Bonino, M.A.; Angelini, G.; Bonanni, M.; Pontone, G.; Pascale, P.; Leo, L.A.; Faletra, F.; Schwitter, J.; et al. Additional value of cardiac magnetic resonance feature tracking parameters for the evaluation of the arrhythmic risk in patients with mitral valve prolapse. J. Cardiovasc. Magn. Reson. 2023, 25, 32. [Google Scholar] [CrossRef] [PubMed]
- Pradella, S.; Grazzini, G.; Brandani, M.; Calistri, L.; Nardi, C.; Mori, F.; Miele, V.; Colagrande, S. Cardiac magnetic resonance in patients with mitral valve prolapse: Focus on late gadolinium enhancement and T1 mapping. Eur. Radiol. 2019, 29, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Argentiero, A.; Carella, M.C.; Mandunzio, D.; Greco, G.; Mushtaq, S.; Baggiano, A.; Fazzari, F.; Fusini, L.; Muscogiuri, G.; Basile, P.; et al. Cardiac Magnetic Resonance as Risk Stratification Tool in Non-Ischemic Dilated Cardiomyopathy Referred for Implantable Cardioverter Defibrillator Therapy—State of Art and Perspectives. J. Clin. Med. 2023, 12, 7752. [Google Scholar] [CrossRef] [PubMed]
- Køber, L.; Thune, J.J.; Nielsen, J.C.; Haarbo, J.; Videbæk, L.; Korup, E.; Jensen, G.; Hildebrandt, P.; Steffensen, F.H.; Bruun, N.E.; et al. Defibrillator Implantation in Patients with Nonischemic Systolic Heart Failure. N. Engl. J. Med. 2016, 375, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Rabbat, M.G.; Kwong, R.Y.; Heitner, J.F.; Young, A.A.; Shanbhag, S.M.; Petersen, S.E.; Selvanayagam, J.B.; Berry, C.; Nagel, E.; Heydari, B.; et al. The Future of Cardiac Magnetic Resonance Clinical Trials. JACC Cardiovasc. Imaging 2022, 15, 2127–2138. [Google Scholar] [CrossRef] [PubMed]
- Nakou, E.; Patel, R.K.; Fontana, M.; Bucciarelli-Ducci, C. Cardiovascular Magnetic Resonance Parametric Mapping Techniques: Clinical Applications and Limitations. Curr. Cardiol. Rep. 2021, 23, 185. [Google Scholar] [CrossRef] [PubMed]
- Tampakis, K.; Pastromas, S.; Sykiotis, A.; Kampanarou, S.; Kourgiannidis, G.; Pyrpiri, C.; Bousoula, M.; Rozakis, D.; Andrikopoulos, G. Real-time cardiovascular magnetic resonance-guided radiofrequency ablation: A comprehensive review. World J. Cardiol. 2023, 15, 415–426. [Google Scholar] [CrossRef] [PubMed]
- De Zan, G.; Calò, L.; Borrelli, A.; Guglielmo, M.; De Ruvo, E.; Rier, S.; van Driel, V.; Ramanna, H.; Patti, G.; Rebecchi, M.; et al. Cardiac magnetic resonance-guided cardiac ablation: A case series of an early experience. Eur. Heart J. Suppl. 2023, 25 (Suppl. C), C265–C270. [Google Scholar] [CrossRef] [PubMed]
- Guglielmo, M.; Rier, S.; Zan, G.; Krafft, A.J.; Schmidt, M.; Kunze, K.P.; Botnar, R.M.; Prieto, C.; van der Heijden, J.; Van Driel, V.; et al. Cardiac magnetic resonance for early atrial lesion visualization post atrial fibrillation radiofrequency catheter ablation. J. Cardiovasc. Electrophysiol. 2024, 35, 258–266. [Google Scholar] [CrossRef]
| Study First Author, Year | Type of Cardiomyopathy | Number of Patients | Type of Study | Mapping Parameter | Study Endpoint | Association of Mapping Parameter with the Study Endpoint |
|---|---|---|---|---|---|---|
| Chen, 2015 [2] | Ischemic cardiomyopathy | 130 | Prospective | 10 ms increase of native T1 mapping | Appropriate ICD therapy or documented sustained VA | HR 1.1 (95% CI 1.0–1.2) |
| Olausson, 2023 [13] | Ischemic cardiomyopathy | 215 | Retrospective | 5% increase in ECV | Time from ICD implantation to appropriate shock or anti-tachycardia pacing | HR 2.2 (95% CI 1.2–4.0) |
| Gräni, 2019 [14] | Myocarditis | 179 | Retrospective | ECV ≥ 35% | MACE (all-cause death, HF hospitalization, heart transplantation, documented sustained VA, and recurrent myocarditis) | HR 3.3 (95% CI 1.4–8.0) |
| Thavendiranathan, 2021 [15] | Myocarditis | 136 | Retrospective | Every 1-unit increase in T1 mapping z-score | MACE (cardiovascular death, cardiogenic shock, cardiac arrest, and complete heart block) | HR 1.4 (95% CI 1.1–1.8) |
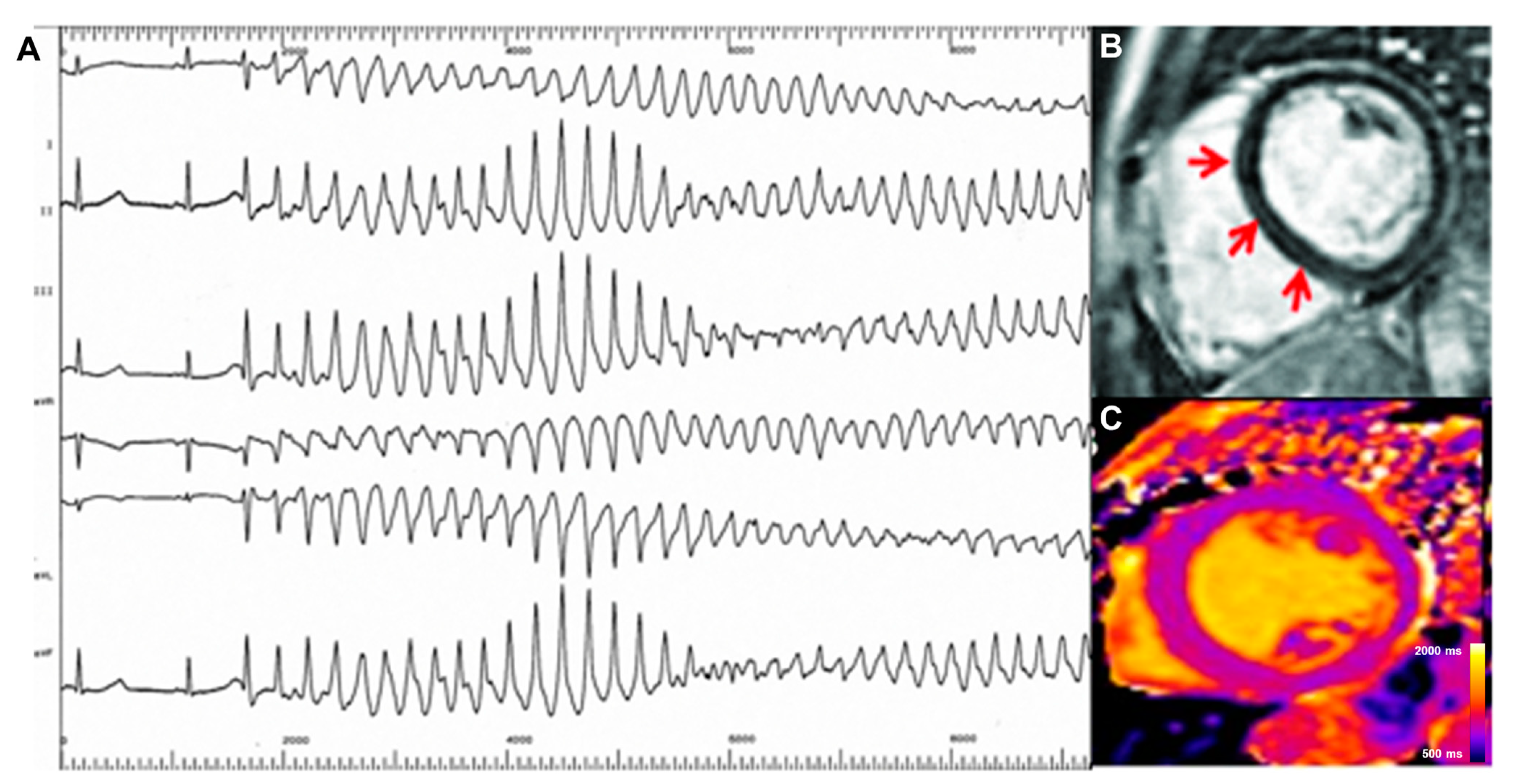
| Crouser, 2014 [16] | Sarcoidosis | 50 | Retrospective | T2 mapping | Conduction system disease and cardiac arrhythmias (atrial arrhythmia, ventricular arrhythmia, atrioventricular block, or QRS complex duration > 120 ms) | T2 mapping significantly higher in patients with the study endpoint |
| Crouser, 2016 [17] | Sarcoidosis | 8 | Retrospective | T2 mapping > 70 ms | Reversible cardiac arrhythmias (atrial arrhythmia, ventricular arrhythmia, atrioventricular block, or QRS complex duration > 120 ms) after immune suppression therapy | T2 mapping significantly higher in patients with the study endpoint |
| Pinheiro, 2020 [18] | Chagas | 62 | Cross-sectional | T1 mapping > 1200 ms, ECV > 25% | NSVT | AUC 0.81 (95% CI 0.65–0.97) and 0.85 (95% CI 0.71–0.99) |
| Melo, 2023 [19] | Chagas | 90 | Prospective | Remote native T1 value > 1100 ms | ICD implantation, heart transplant, or death | HR 12 (95% CI 4.1–34.2) |
| Qin, 2021 [20] | HCM | 203 | Prospective | Native T1 mapping > 1300 ms | MACE (cardiac death, transplantation, HF admission, and ICD implantation) | HR 1.45 (95% CI 1.26–1.77) |
| Avanesov, 2017 [21] | HCM | 73 | Retrospective | Global ECV ≥ 35% | SCD, syncope, and NSVT | AUC 0.83 (95% CI 0.73–0.91) |
| Yu, 2023 [22] | HCM | 108 | Retrospective | Global ECV ≥ 35% | SCD | HR 1.27 (95% CI 1.10–1.47) |
| McLellan, 2016 [23] | HCM | 100 | Prospective | Post-contrast T1 mapping (median value: 422 ± 54 ms) | NSVT | Post-contrast T1 (p = 0.004) |
| Xu, 2023 [24] | HCM | 674 | Prospective | 2 ms increase in T2 mapping | Cardiovascular death and appropriate ICD discharge | HR 1.43 (95% CI 1.18–1.72) |
| Orsborne, 2022 [25] | Fabry disease | 200 | Prospective | T1 dispersion | Adverse cardiac outcome (first hospitalization for HF, MI, coronary revascularization, VT sustained or nonsustained, new AF, bradyarrhythmia necessitating PM implantation, aborted SCD, appropriate ICD therapy, or cardiovascular death) | HR 1.012 (95% CI 1.002–1.021) |
| Nakamori, 2018 [26] | DCM | 107 | Retrospective | 10 ms increase in T1 mapping | Complex VA | OR 1.14 (95% CI 1.03–1.25) |
| Barison, 2015 [27] | DCM | 89 | Retrospective | ECV > 29% | Cardiovascular death, hospitalization for HF and appropriate ICD intervention | p < 0.05 |
| Cadour, 2023 [28] | DCM | 225 | Prospective | T1 mapping Z-score > 4.2, ECV > 30.5% | MACE (HF-related events and arrhythmia-related events) | HR 2.86 (95% CI 1.06–7.68) and HR 2.72 (95% CI 1.01–7.36) |
| Li, 2022 [29] | DCM | 659 | Retrospective | T1 mapping > 1000 ms, ECV > 30.5% | Cardiac-related death, heart transplantation, hospitalization for HF, VA, and ICD or CRT implantation | HR 1.13 (95% CI 1.10–1.36) and HR 1.32; (95% CI 1.12–1.53) |
| Rubiś, 2021 [30] | DCM | 102 | Prospective | ECV | Arrhythmic burden (ventricular tachycardia or a high burden of PVCs) | HR 1.12 (95% CI 1.00–1.25) |
| Chun, 2022 [31] | ARVD/C | 60 | Retrospective | T1 mapping, T2 mapping, and ECV | HF-related events (hospitalization, heart transplantation, and cardiac death) and ventricular tachycardia events | More HF-related events: higher native T1 (log-rank p = 0.002), T2 (log-rank p = 0.002), and ECV (log-rank p = 0.002) |
| Pavon, 2021 [32] | MVP | 30 | Retrospective | Synthetic ECV > 27% | Ventricular arrhythmic events (recent history of unexplained resuscitated OHCA and complex PVC) | AUC 0.83 |
| Bui, 2017 [33] | MVP | 41 | Retrospective | Post-contrast T1 mapping | Complex VA | Reduced post-contrast T1 mapping in patients with complex VA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo Monaco, M.; Stankowski, K.; Figliozzi, S.; Nicoli, F.; Scialò, V.; Gad, A.; Lisi, C.; Marchini, F.; Dellino, C.M.; Mollace, R.; et al. Multiparametric Mapping via Cardiovascular Magnetic Resonance in the Risk Stratification of Ventricular Arrhythmias and Sudden Cardiac Death. Medicina 2024, 60, 691. https://doi.org/10.3390/medicina60050691
Lo Monaco M, Stankowski K, Figliozzi S, Nicoli F, Scialò V, Gad A, Lisi C, Marchini F, Dellino CM, Mollace R, et al. Multiparametric Mapping via Cardiovascular Magnetic Resonance in the Risk Stratification of Ventricular Arrhythmias and Sudden Cardiac Death. Medicina. 2024; 60(5):691. https://doi.org/10.3390/medicina60050691
Chicago/Turabian StyleLo Monaco, Maria, Kamil Stankowski, Stefano Figliozzi, Flavia Nicoli, Vincenzo Scialò, Alessandro Gad, Costanza Lisi, Federico Marchini, Carlo Maria Dellino, Rocco Mollace, and et al. 2024. "Multiparametric Mapping via Cardiovascular Magnetic Resonance in the Risk Stratification of Ventricular Arrhythmias and Sudden Cardiac Death" Medicina 60, no. 5: 691. https://doi.org/10.3390/medicina60050691