Montelukast Influence on Lung in Experimental Diabetes
Abstract
:1. Introduction
2. Material and Methods
2.1. Histopathological Analysis
2.2. Biochemical Analysis
2.3. Statistical Interpretation of the Data
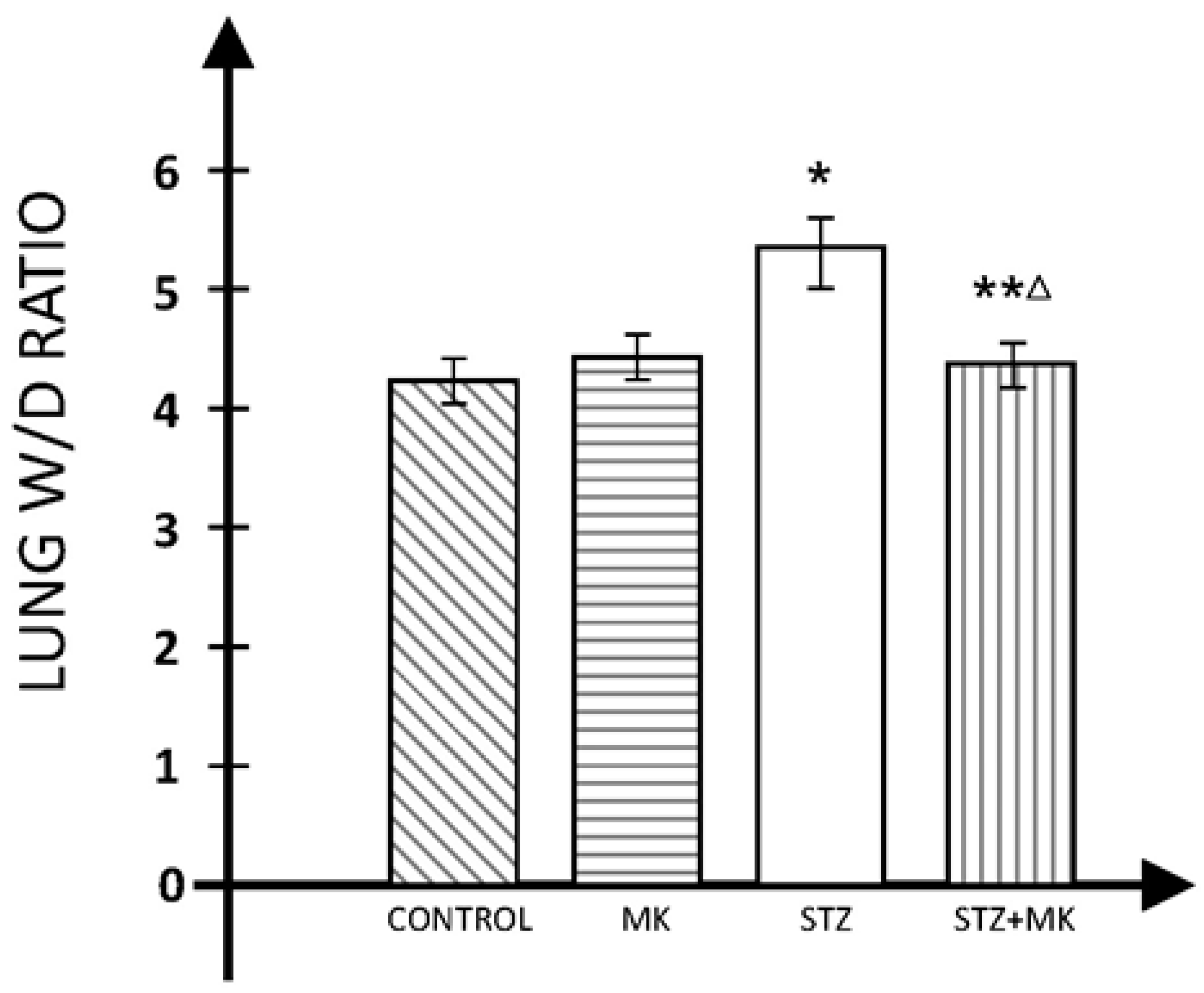
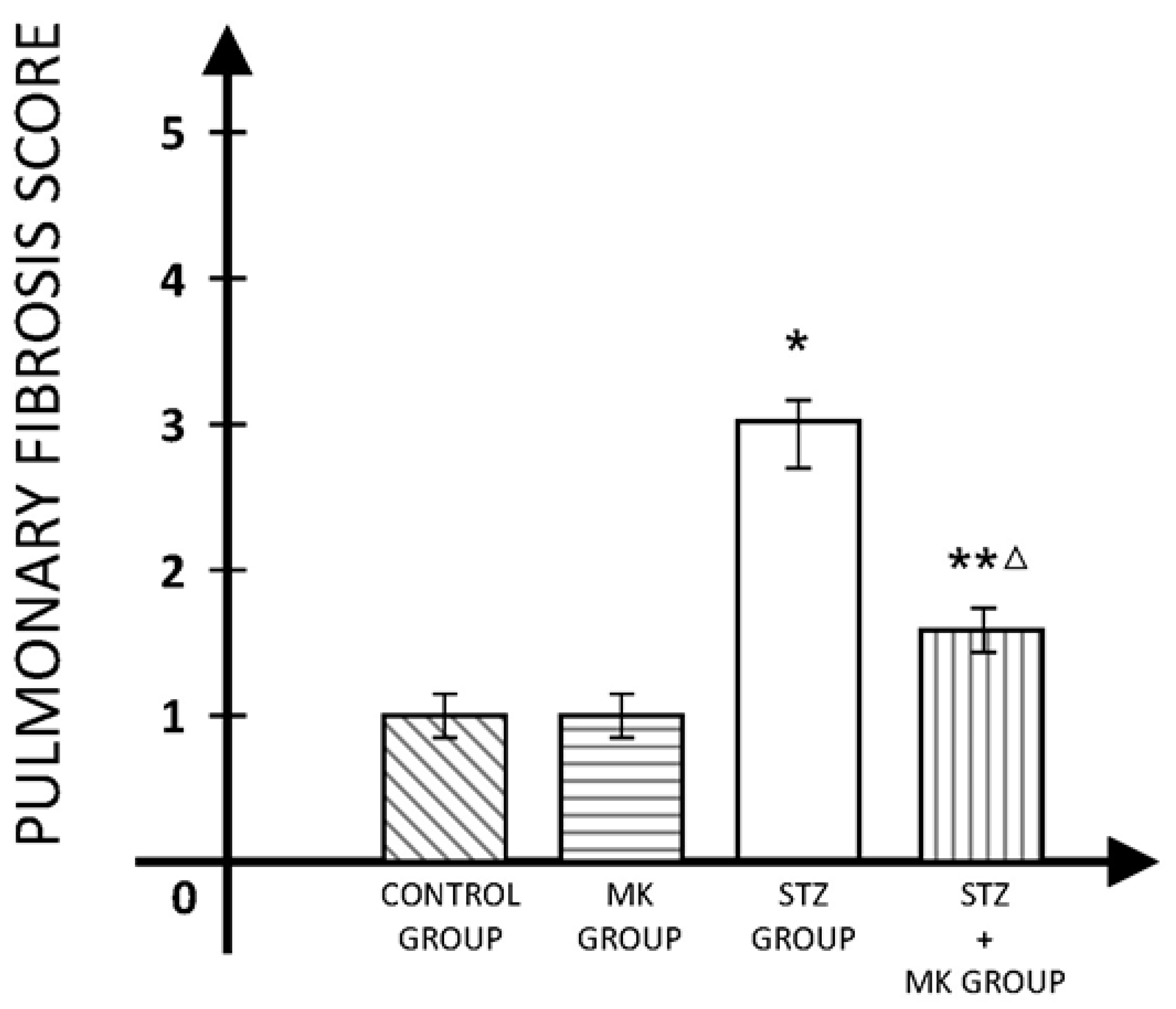
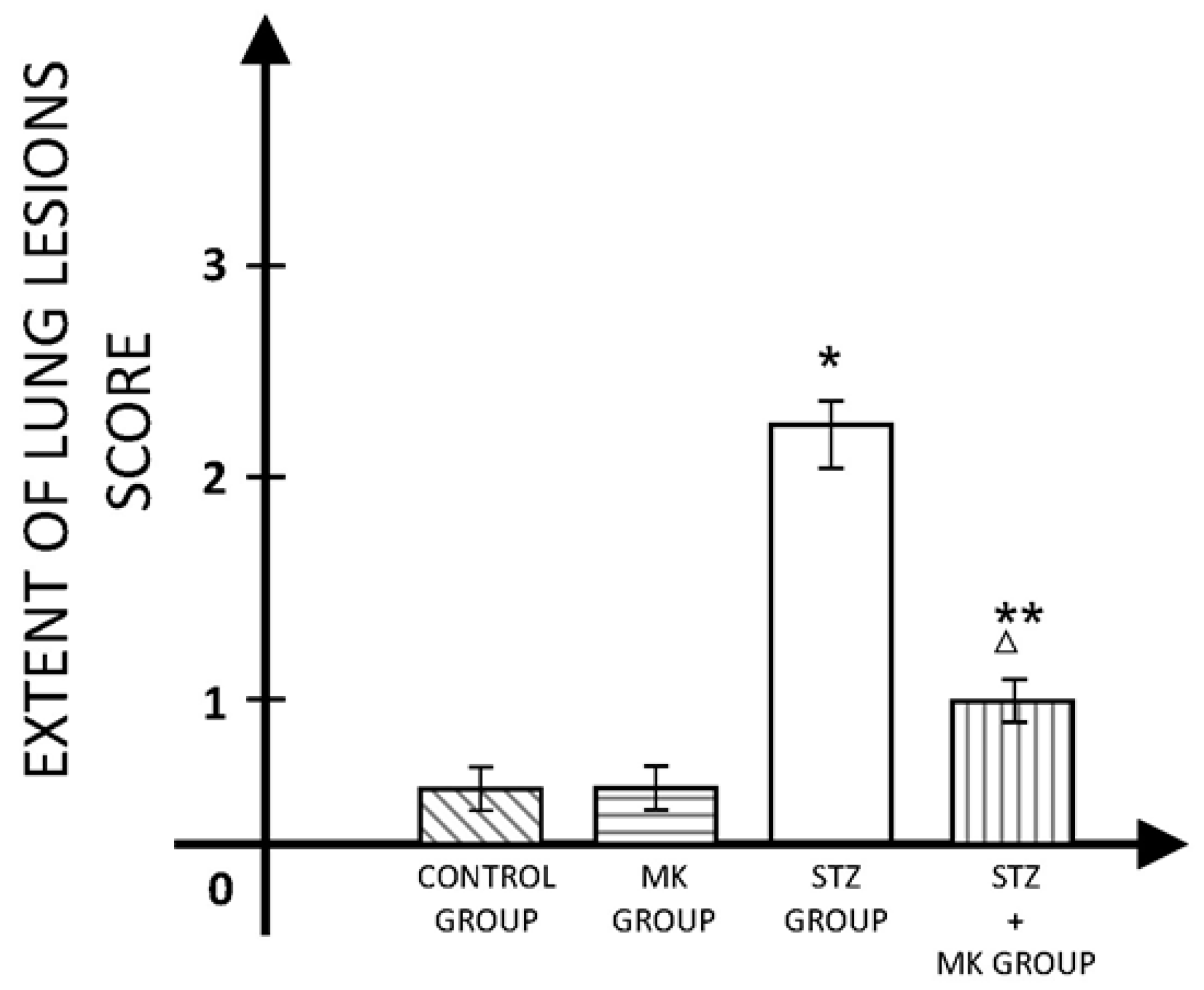
3. Results
4. Discussions
5. Conclusions
Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lovic, D.; Piperidou, A.; Zografou, I.; Grassos, H.A.; Pittaras, A.; Manolis, A. The Growing Epidemic of Diabetes Mellitus. Curr. Vasc. Pharmacol. 2020, 18, 104–109. [Google Scholar] [CrossRef]
- Guazzi, M.; Brambilla, R.; Pontone, G.; Agostoni, P.; Guazzi, M.D. Effects of non-insulin dependent diabetes mellitus on pulmonary function and exercise tolerance in chronic congestive heart failure. Am. J. Cardiol. 2002, 89, 191–197. [Google Scholar] [CrossRef]
- Boulbou, M.S.; Gourgoulianis, K.I.; Klisiaris, V.K.; Tsikrikas, T.S.; Stathakis, N.E.; Molyvdas, P.A. Diabetes mellitus and lung function. Princ. Pract. Med. 2003, 12, 87–91. [Google Scholar] [CrossRef]
- Kaparianos, A.; Argyropoulou, E.; Sampsonas, F.; Karkoulias, K.; Tsiamita, M.; Spiropoulos, K. Pulmonary complications in diabetes mellitus. Chronic Respir. Dis. 2008, 5, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Fontaine-Delaruelle, C.; Viart-Ferber, C.; Luyton, C.; Couraud, S. Lung function in patients with diabetes mellitus. Rev. Pneumol. Clin. 2016, 72, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Ardigo, D.; Valtuena, S.; Zavaroni, I.; Baroni, M.C.; Delsignore, R. Pulmonary complications of diabetes mellitus: The role of glycemic control. Curr. Drug Targets Inflamm. Allergy 2004, 3, 455–458. [Google Scholar] [CrossRef]
- Peters-Golden, M.; Henderson, W.R. Leukotrienes. N. Engl. J. Med. 2007, 357, 1841–1854. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.J.; Chang, W.A.; Chuang, C.H.; Wu, K.L.; Cheng, C.H.; Sheu, C.C.; Hsu, Y.-L.; Hung, J.-Y. Cysteinyl leukotriene pathway and cancer. Int. J. Mol. Sci. 2021, 23, 120. [Google Scholar] [CrossRef]
- Austen, K.F.; Maekawa, A.; Kanaoka, Y.; Boyce, J.A. The leukotriene E4 puzzle: Finding the missing pieces and revealing the pathobiologic implications. J. Allergy Clin. Immunol. 2009, 124, 406–414. [Google Scholar] [CrossRef]
- Singh, R.K.; Gupta, S.; Dastidar, S.; Ray, A. Cysteinyl leukotrienes and their receptors: Molecular and functional characteristics. Pharmacology 2010, 85, 336–349. [Google Scholar] [CrossRef]
- Jo-Watanabe, A.; Okuno, T.; Yokomizo, T. The Role of Leukotrienes as Potential Therapeutic Targets in Allergic Disorders. Int. J. Mol. Sci. 2019, 20, 3580. [Google Scholar] [CrossRef]
- Gelfand, E.W. Importance of the leukotriene B4-BLT1 and LTB4-BLT2 pathways in asthma. Semin. Immunol. 2017, 33, 44–51. [Google Scholar] [CrossRef]
- Scott, J.P.; Peters-Golden, M. Antileukotriene agents for the treatment of lung disease. Am. J. Respir. Crit. Care Med. 2013, 188, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Molehin, O.R.; Oloyede, O.I.; Adefegha, S.A. Streptozotocin-induced diabetes in rats: Effects of White Butterfly (Clerodendrum volubile) leaves on blood glucose levels, lipid profile and antioxidant status. Toxicol. Mech. Methods 2018, 28, 573–586. [Google Scholar] [CrossRef]
- Hübner, R.H.; Gitter, W.; El Mokhtari, N.E.; Mathiak, M.; Both, M.; Bolte, H.; Freitag-Wolf, S.; Bewig, B. Standardized quantification of pulmonary fibrosis in histological samples. Biotechniques 2008, 44, 507–511. [Google Scholar] [CrossRef]
- Ashcroft, T.; Simpson, J.M.; Timbrell, V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J. Clin. Pathol. 1988, 41, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Jeong, S.H.; Kim, J.; Kang, J.Y.; Nam, J.N.; Togloom, A.; Cha, J.; Lee, K.Y.; Lee, C.H.; Park, E.K.; et al. Evaluation of the effect of filtered ultrafine particulate matter on bleomycin-induced lung fibrosis in a rat model using computed tomography, histopathologic analysis, and RNA sequencing. Sci. Rep. 2021, 11, 22672. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, Y.; Du, D.; Zhao, H.; Xu, M.; Xu, M.; Liu, Z. The effects of the combination of sodium ferulate and oxymatrine on lipopolysaccharide-induced acute lung injury in mice. Inflammation 2012, 35, 1161–1168. [Google Scholar] [CrossRef]
- Jin, Z.; Li, M.Y.; Tang, L.; Zou, Y.; Chen, K. Protective effect of Ulinastatin on acute lung injury in diabetic sepsis rats. Int. Immunopharmacol. 2022, 108, 108908. [Google Scholar] [CrossRef]
- Schiffrin, E.L. Oxidative stress, nitric oxide synthase, and superoxide dismutase: A matter of imbalance underlies endothelial dysfunction in the human coronary circulation. Hypertension 2008, 51, 31–32. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- McLauchlan, W.R.; Sanderson, J.; Quinlan, M.; Williamson, G. Measurement of the Total Antioxidant Activity of Human Aqueous Humor. Clin. Chem. 1998, 44, 888–889. [Google Scholar] [CrossRef] [PubMed]
- Forgiarini, L.A., Jr.; Kretzmann, N.A.; Porawski, M.; Dias, A.S.; Marroni, N.A.P. Experimental diabetes mellitus: Oxidative stress and changes in lung structure. J. Bras. Pneumol. 2009, 35, 788–791. [Google Scholar] [CrossRef]
- Welliver, R.C.; Hintz, K.H.; Glori, M.; Welliver, R.C., Sr. Zileuton reduces respiratory illness and lung inflammation, during respiratory syncytial virus infection, in mice. J. Infect. Dis. 2003, 187, 1773–1779. [Google Scholar] [CrossRef] [PubMed]
- Eum, S.Y.; Maghni, K.; Hamid, Q.; Campbell, H.; Eidelman, D.H.; Martin, J.G. Involvement of the cysteinyl-leukotrienes in allergen-induced airway eosinophilia and hyperresponsiveness in the mouse. Am. J. Respir. Cell Mol. Biol. 2003, 28, 25–32. [Google Scholar] [CrossRef]
- Cernak, I.; Savic, J.; Malicevic, Z.; Zunic, G.; Radosevic, P.; Ivanovic, I. Leukotrienes in the pathogenesis of pulmonary blast injury. J. Trauma 1996, 40 (Suppl. 3), S148–S151. [Google Scholar] [CrossRef]
- Kordjazy, N.; Amini, S. A review of the therapeutic potential of the cysteinyl leukotriene antagonist Montelukast in the treatment of bronchiolitis obliterans syndrome following lung and hematopoietic-stem cell transplantation and its possible mechanisms. Ther. Adv. Respir. Dis. 2024, 18, 17534666241232284. [Google Scholar] [CrossRef]
- Prost, N.; El-Karakm, C.; Avila, M.; Ichinose, F.; Vidal Melo, M.F. Changes in cysteinyl leukotrienes during and after cardiac surgery with cardiopulmonary bypass in patients with and without chronic obstructive pulmonary disease. J. Thorac. Cardiovasc. Surg. 2011, 141, 1496–1502.e3. [Google Scholar] [CrossRef]
- Kanaoka, Y.; Boyce, J.A. Cysteinyl leukotrienes and their receptors; emerging concepts. Allergy Asthma Immunol. Res. 2014, 6, 288–295. [Google Scholar] [CrossRef]
- Das, U.N. Essential Fatty Acids and Their Metabolites in the Pathobiology of Inflammation and Its Resolution. Biomolecules 2021, 11, 1873. [Google Scholar] [CrossRef]
- Xiong, X.Q.; Wang, W.T.; Wang, L.R.; Jin, L.D.; Lin, L.N. Diabetes increases inflammation and lung injury associated with protective ventilation strategy in mice. Int. Immunopharmacol. 2012, 13, 280–283. [Google Scholar] [CrossRef]
- Kato, J.; Kohyama, T.; Okazaki, H.; Desaki, M.; Nagase, T.; Rennard, S.I.; Takizawa, H. Leukotriene D4 potentiates fibronectin-induced migration of human lung fibroblasts. Clin. Immunol. 2005, 117, 177–181. [Google Scholar] [CrossRef]
- Ochkur, S.I.; Protheroe CALi, W.; Colbert, D.C.; Zellner, K.R.; Shen, H.H.; Luster, A.D.; Irvin, C.G.; Lee, J.J.; Lee, N.A. Cys-leukotrienes promote fibrosis in a mouse model of eosinophil-mediated respiratory inflammation. Am. J. Respir. Cell Mol. Biol. 2013, 49, 1074–1084. [Google Scholar] [CrossRef]
- Wiley, C.D.; Brumwell, A.N.; Davis, S.S.; Jackson, J.R.; Valdovinos, A.; Calhoun, C.; Alimirah, F.; Carlos, A.; Castellanos, C.A.; Ruan, R.; et al. Secretion of leukotrienes by senescent lung fibroblasts promotes pulmonary fibrosis. JCI Insight 2019, 4, e130056. [Google Scholar] [CrossRef]
- Beller, T.C.; Friend, D.S.; Maekawa, A.; Lam, B.K.; Austen, K.F.; Kanaoka, Y. Cysteinyl leukotriene 1 receptor controls the severity of chronic pulmonary inflammation and fibrosis. Proc. Natl. Acad. Sci. USA 2004, 101, 3047–3052. [Google Scholar] [CrossRef]
- Bao, W.A.; Wang, Y.Z.; Zhu, X.; Lin, J.; Fan, J.F.; Yang, Y.; Zhou, X. Baicalin Ameliorates Radiation-Induced Lung Injury by Inhibiting the CysLTs/CysLT1 Signaling Pathway. Evid.-Based Complement. Altern. Med. 2022, 2022, 2765354. [Google Scholar] [CrossRef] [PubMed]
- Shimbori, C.; Shiota, N.; Okunishi, H. Involvement of leukotrienes in the pathogenesis of silica-induced pulmonary fibrosis in mice. Exp. Lung Res. 2010, 36, 292–301. [Google Scholar] [CrossRef]
- Matsubara, T.; Hara, F. The pulmonary function and histopathological studies of the lung in diabetes mellitus. Nihon Ika Daigaku Igakkai Zasshi 1991, 58, 528–536. [Google Scholar] [CrossRef]
- Wang, L.M.; Zhong, N.Z.; Liu, S.J.; Zhu, X.Y.; Liu, Y.J. Hypoxia-induced acute lung injury is aggravated in streptozotocin diabetic mice. Exp. Lung Res. 2015, 41, 146–154. [Google Scholar] [CrossRef]
- Talakatta, G.; Sarikhani, M.; Muhamed, J.; Dhanya, K.; Somashekar, B.S.; Mahesh, P.A.; Sundaresan, N.; Ravindra, P.V. Diabetes induces fibrotic changes in the lung through the activation of TGF-β signaling pathways. Sci. Rep. 2018, 8, 11920. [Google Scholar] [CrossRef]
- Thompson-Souza, G.A.; Gropillo, I.; Neves, J.S. Cysteinyl Leukotrienes in Eosinophil Biology: Functional Roles and Therapeutic Perspectives in Eosinophilic Disorders. Front. Med. (Lausanne) 2017, 4, 106. [Google Scholar] [CrossRef]
- Ahmed, A.E. Protective effect of montelukast on paraquat-induced lung toxicity in rats. BioScience Trends 2009, 3, 63–72. [Google Scholar] [PubMed]
- Elnoury, H.A.; Elgendy, S.A.; Baloza, S.H.; Ghamry, H.I.; Soliman, M.; Abdel-Aziz, E.A.M. Synergistic impacts of Montelukast and Klotho against doxorubicin-induced cardiac toxicity in Rats. Toxicol. Res. 2022, 11, 592–604. [Google Scholar] [CrossRef]
- Alnfakh, Z.A.; Al-Mudhafar, D.H.; Al-Nafakh, R.T.; Jasim, A.E.; Hadi, N.R. The anti-inflammatory and antioxidant effects of Montelukast on lung sepsis in adult mice. J. Med. Life 2022, 15, 819–827. [Google Scholar] [CrossRef]
- Erşahin, M.; Çevik, O.; Akakın, D.; Şener, A.; Özbay, L.; Yegen, B.C.; Şener, G. Montelukast inhibits caspase-3 activity and ameliorates oxidative damage in the spinal cord and urinary bladder of rats with spinal cord injury. Prostaglandins Other Lipid Mediat. 2012, 99, 131–139. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Z.; Wang, Y.; Zhou, D.; Zhang, J.; Chen, X.; Li, X.; Shao, Q. Impacts of lipopolysaccharide on fetal lung developmental maturity and surfactant protein B and surfactant protein C protein expression in gestational diabetes mellitus rats. Bioengineered 2022, 13, 834–843. [Google Scholar] [CrossRef]
- Yuksel, H.; Ozbilgin, K.; Coskun, S.; Tuglu, I. Protective effect of leukotriene receptor antagonist montelukast against smoking induced lung injury in Wister rats. Acta Medica Okayama 2003, 57, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Schmitt-Grohé, S.; Eickmeier, O.; Schubert, R.; Bez, C.; Zielen, S. Anti-inflammatory effects of montelukast in mild cystic fibrosis. Ann. Allergy Asthma Immunol. 2002, 89, 599–605. [Google Scholar] [CrossRef]
- Sener, G.; Sehirli, O.; Velioğlu-Oğünç, A.; Cetinel, S.; Gedik, N.; Caner, M.; Sakarcan, A.; Yeğen, B.C. Montelukast protects against renal ischemia/reperfusion injury in rats. Pharmacol. Res. 2006, 54, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Avogaro, A.; Albiero, M.; Menegazzo, L.; de Kreutzenberg, S.; Fadini, G.P. Endothelial dysfunction in diabetes: The role of reparatory mechanisms. Diabetes Care 2011, 34, S285–S290. [Google Scholar] [CrossRef]
- Stehouwer, C.D.; Lambert, J.; Donker, A.J.; van Hinsbergh, V.W. Endothelial dysfunction and pathogenesis of diabetic angiopathy. Cardiovasc. Res. 1997, 34, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Mauser, P.J.; House, A.; Jones, H.; Correll, C.; Boyce, C.; Chapman, R.W. Pharmacological characterization of the late phase reduction in lung functions and correlations with microvascular leakage and lung edema in allergen-challenged Brown Norway rats. Pulm. Pharmacol. Ther. 2013, 26, 677–684. [Google Scholar] [CrossRef]
- Fujii, Y.; Abe, T.; Ikegami, K. Diabetic Pathophysiology Enhances Inflammation during Extracorporeal Membrane Oxygenation in a Rat Model. Membranes 2021, 11, 283. [Google Scholar] [CrossRef] [PubMed]
- Nechifor, M.; Cuciureanu, M.; Chelarescu, D.; Ciubotariu, D.; Pascu, M. Magnesium and other bivalent cations influence upon sodium montelukast effect in experimental-induced thermoalgesia. Magnes. Res. 2008, 21, 38–42. [Google Scholar]
- El-Baz, A.M.; Shata, A.; Hassan, H.M.; El-Sokkary, M.M.A.; Khodir, A.E. The therapeutic role of lactobacillus and montelukast in combination with metformin in diabetes mellitus complications through modulation of gut microbiota and suppression of oxidative stress. Int. Immunopharmacol. 2021, 96, 107757. [Google Scholar] [CrossRef]
- Tuğtepe, H.; Sener, G.; Cetinel, S.; Velioğlu-Oğünç, A.; Yeğen, B.C. Oxidative renal damage in pyelonephritic rats is ameliorated by montelukast, a selective leukotriene CysLT1 receptor antagonist. Eur. J. Pharmacol. 2007, 557, 69–75. [Google Scholar] [CrossRef]
- Cuciureanu, M.; Căruntu, I.D.; Nechifor, M. The influence of leukotriene receptors’ antagonists on experimentally induced ulcer in rats. Rev. Med. Chir. Soc. Medici Si Nat. Din Iasi 2008, 112, 750–756. [Google Scholar]
- Teslariu, O.; Nechifor, M.; Plămădeală, P.; Miron, I.C. Influence of montelukast on cisplatin-induced experimental acute renal failure. Rev. Med. Chir. Soc. Medici Si Nat. Din Iasi 2014, 118, 612–617. [Google Scholar]
- Rizk, F.H.; Ibrahim, M.A.A.; Abd-Elsalam, M.M.; Soliman, N.A.; Abd-Elsalam, S.M. Gastroprotective effects of montelukast and Nigella sativa oil against corticosteroid-induced gastric damage: They are much more than antiasthmatic drugs. Can. J. Physiol. Pharmacol. 2017, 95, 714–720. [Google Scholar] [CrossRef]
- Pleasants, R.; Tighe, R.M. Management of idiopathic pulmonary fibrosis. Ann. Pharmacother. 2019, 53, 1238–1248. [Google Scholar] [CrossRef] [PubMed]
- Glassberg, M.K. Overview of idiopathic pulmonary fibrosis, evidence-based guidelines, and recent developments in the treatment landscape. Am. J. Manag. Care 2019, 25, S195–S203. [Google Scholar] [PubMed]
- Finnerty, J.P.; Ponnuswamy, A.; Dutta, P.; Abdelaziz, A.; Kamil, H. Efficacy of antifibrotic drugs, nintedanib and pirfenidone, in treatment of progressive pulmonary fibrosis in both idiopathic pulmonary fibrosis (IPF) and non-IPF: A systematic review and meta-analysis. BMC Pulm. Med. 2021, 21, 411. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zhang, D.; Zhang, Y.; Peng, Y.; Li, M.; Song, H.; Chen, H.; Li, W.; Li, X. Vitamin D3 alleviates lung fibrosis of type 2 diabetic rats via SIRT3 mediated suppression of pyroptosis. Apoptosis 2023, 28, 1618–1627. [Google Scholar] [CrossRef]
- D’Alessandro-Gabazza, C.N.; Yasuma, T.; Kobayashi, T.; Toda, M.; Abdel-Hamid, A.M.; Fujimoto, H.; Hataji, O.; Nakahara, H.; Takeshita, A.; Nishihama, K.; et al. Inhibition of lung microbiota-derived proapoptotic peptides ameliorates acute exacerbation of pulmonary fibrosis. Nat. Commun. 2022, 13, 1558. [Google Scholar] [CrossRef] [PubMed]
| Group | Initial | p | After 72 h | p | p* | After 8 Weeks | p | p* |
|---|---|---|---|---|---|---|---|---|
| Control | 41.85 ± 2.71 | NS | 40.18 ± 1.46 | <0.01 | NS | 40.08 ± 2.11 | <0.01 | NS |
| MK | 39.55 ± 1.23 | NS | 37.65 ± 2.32 | <0.01 | NS | 38.76 ± 2.41 | <0.01 | NS |
| STZ | 39.89 ± 1.03 | 23.42 ± 2.03 | <0.05 | 25.52 ± 2.09 | <0.01 | |||
| STZ+MK | 40.44 ± 2.09 | NS | 27.45 ± 1.24 | <0.05 | <0.05 | 33.29 ± 1.64 | <0.01 | <0.05 |
| Group | Initial | p | After 72 h | p | p* | After 8 Weeks | p | p* |
|---|---|---|---|---|---|---|---|---|
| Control | 129.5 ±18.3 | NS | 133.2 ± 15.7 | <0.01 | NS | 128.4 ± 13.8 | <0.01 | NS |
| MK | 135.1 ± 16.8 | NS | 129.6 ± 16.5 | <0.01 | NS | 132 ± 10.5 | <0.01 | NS |
| STZ | 134.4 ± 19.5 | 76.1 ± 14.3 | <0.01 | 77.2 ± 11.9 | <0.01 | |||
| STZ+MK | 130.7 ± 17.7 | NS | 91.3 ± 12.9 | <0.05 | <0.05 | 95.7 ± 13.3 | <0.05 | <0.05 |
| Group | Initial | p | After 72 h | p | p* | After 8 Weeks | p | p* |
|---|---|---|---|---|---|---|---|---|
| Control | 1.37 ± 0.13 | NS | 1.39 ± 0.14 | <0.01 | NS | 1.43 ± 0.10 | <0.01 | NS |
| MK | 1.41 ± 0.16 | NS | 1.42 ± 0.09 | <0.01 | NS | 1.44 ± 0.13 | <0.01 | NS |
| STZ | 1.36 ± 0.12 | 2.67 ± 0.2 | <0.01 | 2.72 ± 0.22 | <0.01 | |||
| STZ+MK | 1.42 ±0.11 | NS | 2.11 ± 0.17 | <0.05 | <0.05 | 2.07 ± 0.15 | <0.0.5 | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gales, C.; Stoica, B.; Rusu-Zota, G.; Nechifor, M. Montelukast Influence on Lung in Experimental Diabetes. Medicina 2024, 60, 749. https://doi.org/10.3390/medicina60050749
Gales C, Stoica B, Rusu-Zota G, Nechifor M. Montelukast Influence on Lung in Experimental Diabetes. Medicina. 2024; 60(5):749. https://doi.org/10.3390/medicina60050749
Chicago/Turabian StyleGales, Cristina, Bogdan Stoica, Gabriela Rusu-Zota, and Mihai Nechifor. 2024. "Montelukast Influence on Lung in Experimental Diabetes" Medicina 60, no. 5: 749. https://doi.org/10.3390/medicina60050749





