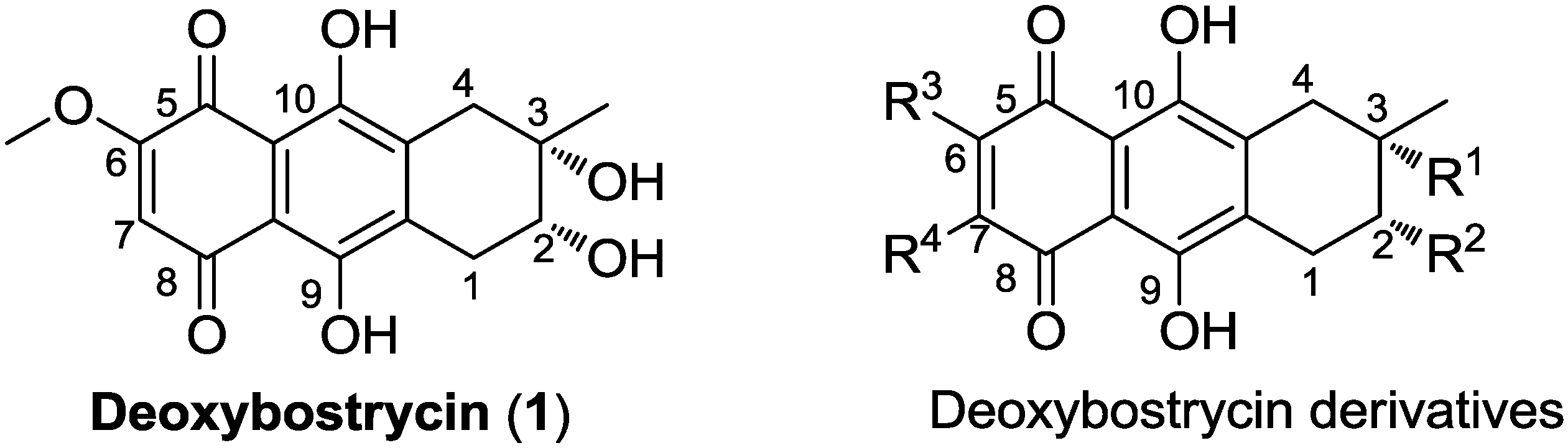
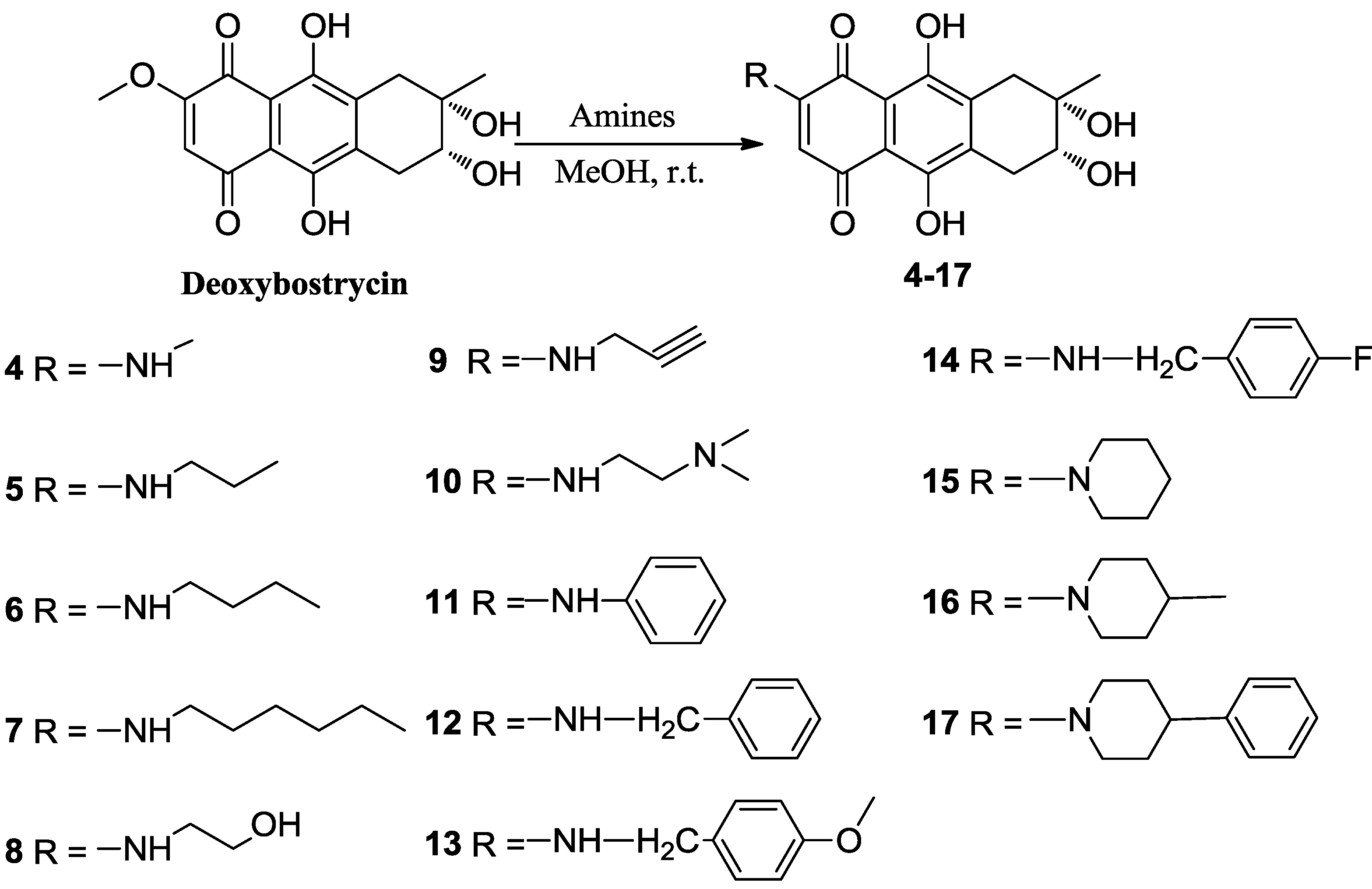
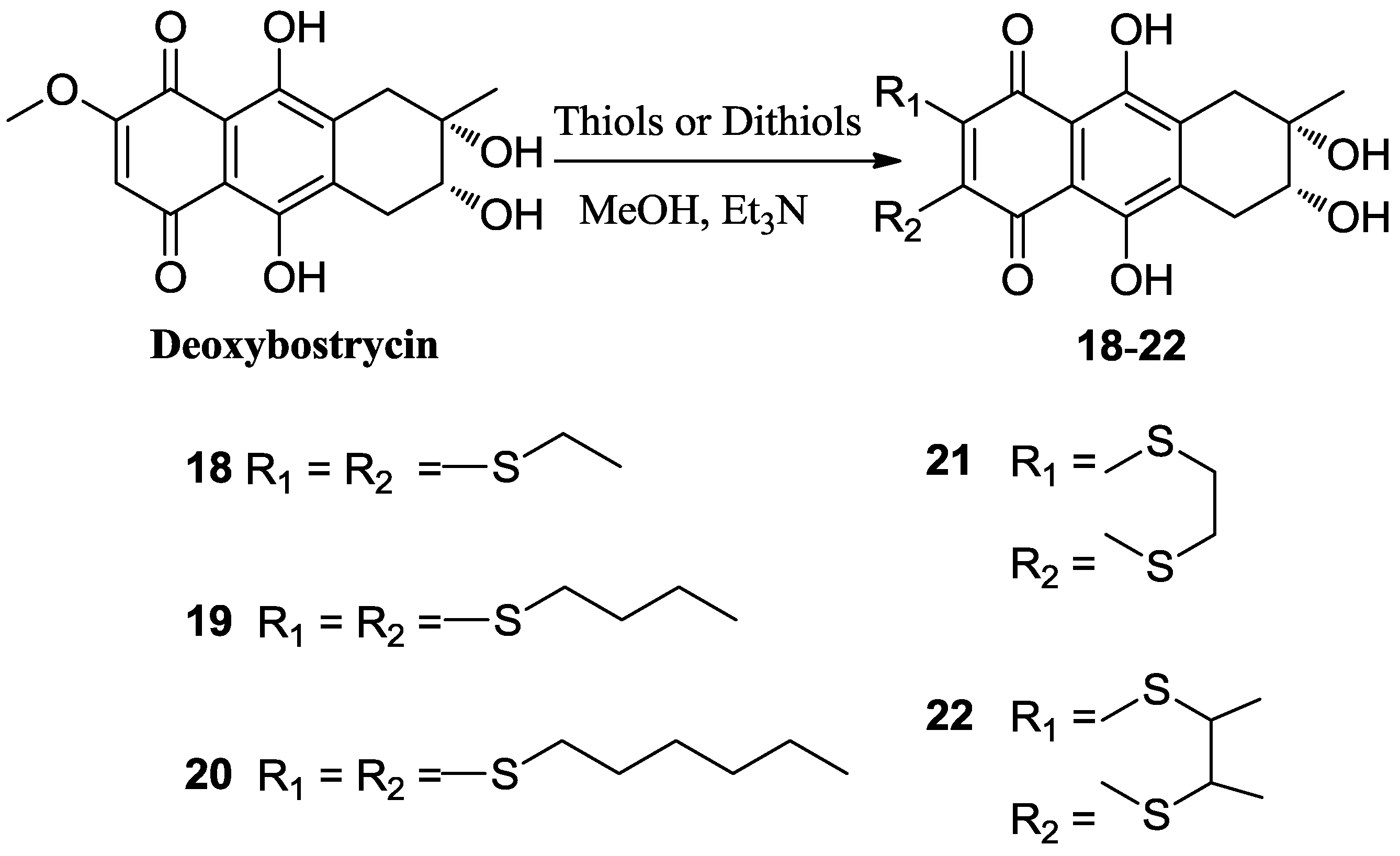
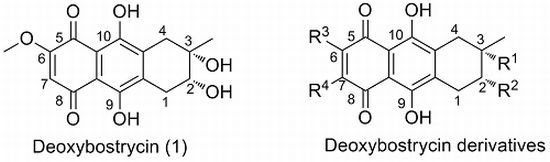
3.4. General Procedure for Preparation of Compounds (4–17)
To a solution of 1 (50 mg, 0.156 mmol) in 10 mL of methanol was added the corresponding amine (0.78 mmol). The reaction mixture was stirred at room temperature until the starting material disappeared (for aniline, the reaction mixture was stirred at 50 °C). The solvent was removed under reduced pressure. The resulting residue was subsequently purified using first silica gel chromatography with dichloromethane-methanol as eluent, then C18 reversed phase silica gel chromatography with methanol-water as eluent.
3.4.1. 6-(Methylamino) 1-Deoxy-6-demethoxybostrycin (4)
A red solid (MeOH) in a 40% yield; mp: 221–223 °C; IR (KBr): νmax = 3375, 3338, 2929, 2901, 2853, 2814, 1582, 1514, 1452, 1418 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 14.23 (s, 1H), 12.36 (s, 1H), 7.95 (q, 1H, J = 5.0 Hz), 5.55 (s, 1H), 4.75 (d, 1H, J = 5.1 Hz), 4.41 (s, 1H), 3.63 (dt, 1H, J = 7.3, 5.1 Hz), 2.83 (d, 3H, J = 5.0 Hz), 2.85 (dd, 1H, J = 18.8, 5.1 Hz), 2.77 (d, 1H, J = 17.9 Hz), 2.68 (dd, 1H, J = 18.8, 7.3 Hz), 2.57 (d, 1H, J = 17.9 Hz), 1.20 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 186.31, 183.47, 156.34, 154.56, 151.05, 139.24, 132.89, 109.17, 107.89, 98.98, 70.74, 69.38, 35.99, 30.70, 29.62, 25.93; ESI-MS m/z: 318.1 [M − H]−; HRMS (EI) calcd for C16H17NO6, 319.1056; found, 319.1059.
3.4.2. 6-(n-Propylamino) 1-Deoxy-6-demethoxybostrycin (5)
A red solid (MeOH) in a 44% yield; mp: 215–216 °C; IR (KBr): νmax = 3385, 3285, 3085, 2966, 2934, 2876, 1578, 1508, 1445, 1412 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 14.23 (s, 1H), 12.37 (s, 1H), 7.86 (t, 1H, J = 5.7 Hz), 5.63 (s, 1H), 4.74 (d, 1H, J = 5.1 Hz), 4.40 (s, 1H), 3.63 (dt, 1H, J = 7.3, 5.1 Hz), 3.18 (dt, 2H, J = 5.7, 7.2 Hz), 2.85 (1H, dd, J = 18.7, 5.1 Hz), 2.79 (d, 1H, J = 17.9 Hz), 2.68 (dd, 1H, J = 18.7, 7.3 Hz), 2.57 (d, 1H, J = 17.9 Hz), 1.61 (sextet, 2H, J = 7.2 Hz), 1.20 (s, 3H), 0.91 (t, 3H, J = 7.4 Hz); 13C NMR (100 MHz, DMSO-d6): δ 186.34, 183.54, 156.35, 154.50, 150.14, 139.28, 132.84, 109.18, 107.79, 98.96, 70.74, 69.37, 44.26, 36.00, 30.69, 25.93, 21.22, 11.82; ESI-MS m/z: 346.2 [M − H]−; HRMS (EI) calcd for C18H21NO6, 347.1369; found, 347.1370.
3.4.3. 6-(n-Butylamino) 1-Deoxy-6-demethoxybostrycin (6)
A red solid (MeOH) in a 40% yield; mp: 215–217 °C; IR (KBr): νmax = 3386, 3287, 3085, 2960, 2934, 2872, 1580, 1509, 1445, 1412 cm–1; 1H NMR (400 MHz, DMSO-d6): δ 14.22 (s, 1H), 12.36 (s, 1H), 7.85 (t, 1H, J = 6.0 Hz), 5.61 (s, 1H), 4.74 (d, 1H, J = 5.1 Hz), 4.40 (s, 1H), 3.63 (dt, 1H, J = 7.4, 5.2 Hz), 3.21 (dt, 2H, J = 6.0, 7.1 Hz), 2.85 (dd, 1H, J = 18.7, 5.2 Hz), 2.79 (d, 1H, J = 17.9 Hz), 2.68 (dd, 1H, J = 18.7, 7.4 Hz), 2.57 (d, 1H, J = 17.9 Hz), 1.57 (pentet, 2H, J = 7.1 Hz), 1.35 (sextet, 2H, J = 7.3 Hz), 1.20 (s, 3H), 0.91 (t, 3H, J = 7.3 Hz); 13C NMR (100 MHz, DMSO-d6): δ 186.30, 183.53, 156.35, 154.50, 150.09, 139.29, 132.83, 109.18, 107.79, 98.91, 70.74, 69.37, 42.33, 36.00, 30.68, 29.90, 25.94, 20.16, 14.13; ESI-MS m/z: 360.2 [M − H]−; HRMS (EI) calcd for C19H23NO6, 361.1525; found, 361.1522.
3.4.4. 6-(n-Hexylamino) 1-Deoxy-6-demethoxybostrycin (7)
A red solid (MeOH) in a 52% yield; mp: 214–215 °C; IR (KBr): νmax = 3384, 3287, 3086, 2955, 2932, 2870, 2858, 1576, 1509, 1448, 1410 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 14.25 (s, 1H), 12.38 (s, 1H), 7.88 (t, 1H, J = 6.1 Hz), 5.63 (s, 1H), 4.75 (d, 1H, J = 5.1 Hz), 4.41 (s, 1H), 3.64 (dt, 1H, J = 7.2, 5.2 Hz), 3.21 (dt, 2H, J = 6.1, 7.0 Hz), 2.86 (dd, 1H, J = 18.7, 5.2 Hz), 2.80 (d, 1H, J = 18.0 Hz), 2.69 (dd, 1H, J = 18.7, 7.2 Hz), 2.58 (d, 1H, J = 18.0 Hz), 1.57 (m, 2H), 1.37–1.25 (m, 6H), 1.19 (s, 3H), 0.87 (t, 3H, J = 6.8 Hz); 13C NMR (100 MHz, DMSO-d6): δ 186.35, 183.58, 156.36, 154.51, 150.13, 139.28, 132.88, 109.21, 107.82, 98.90, 70.73, 69.38, 42.61, 35.97, 31.39, 30.71, 27.75, 26.59, 25.91, 22.49, 14.35; ESI-MS m/z: 388.1 [M − H]−; HRMS (EI) calcd for C21H27NO6, 389.1838; found, 389.1834.
3.4.5. 6-(2′-Hydroxyethylamino) 1-Deoxy-6-demethoxybostrycin (8)
A red solid (MeOH) in a 30% yield; mp: 232–234 °C; IR (KBr): νmax = 3441, 3389, 3340, 3298, 3062, 2966, 2945, 2926, 2875, 1571, 1528, 1445, 1419 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 14.21 (s, 1H), 12.36 (s, 1H), 7.63 (t, 1H, J = 5.9 Hz), 5.70 (s, 1H), 4.89 (br t, 1H), 4.75 (d, 1H, J = 4.2 Hz), 4.41 (s, 1H), 3.66–3.59 (m, 3H), 3.28 (q, 2H, J = 5.9 Hz), 2.86 (dd, 1H, J = 18.7, 5.0 Hz), 2.80 (d, 1H, J = 18.0 Hz), 2.69 (dd, 1H, J = 18.7, 7.4 Hz), 2.58 (d, 1H, J = 18.0 Hz), 1.20 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 186.46, 183.39, 156.43, 154.57, 150.35, 139.33, 132.99, 109.12, 107.78, 99.37, 70.73, 69.38, 58.95, 45.34, 36.01, 30.70, 25.92; ESI-MS m/z: 348.1 [M − H]−; HRMS (EI) calcd for C17H19NO7, 349.1162; found, 349.1158.
3.4.6. 6-(Prop-2′-yn-1′-ylamino) 1-Deoxy-6-demethoxybostrycin (9)
A red solid (MeOH) in a 56% yield; mp: 214–216 °C; IR (KBr): νmax = 3390, 3242, 3242, 3070, 2968, 2932, 2912, 2851, 1586, 1503, 1447, 1387 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 14.03 (s, 1H), 12.36 (s, 1H), 8.08 (t, 1H, J = 5.8 Hz), 5.74 (s, 1H), 4.76 (d, 1H, J = 5.1 Hz), 4.42 (s, 1H), 4.08 (dd, 2H, J = 5.8, 2.3 Hz), 3.64 (dt, 1H, J = 7.3, 5.2 Hz), 3.27 (t, 1H, J = 2.3 Hz), 2.86 (dd, 1H, J = 18.8, 5.1 Hz), 2.80 (d, 1H, J = 18.0 Hz), 2.69 (dd, 1H, J = 18.8, 7.3 Hz), 2.59 (d, 1H, J = 18.0 Hz), 1.20 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 186.54, 183.10, 156.66, 154.94, 149.46, 139.31, 133.53, 109.10, 107.64, 101.23, 79.13, 75.25, 70.71, 69.36, 36.05, 31.83, 30.67, 25.92; ESI-MS m/z: 342.1 [M − H]−; HRMS (EI) calcd for C18H17NO6, 343.1056; found, 343.1052.
3.4.7. 6-[(2′-(Dimethylamino)ethyl)amino]-1-deoxy-6-demethoxybostrycin (10)
A red solid (MeOH) in a 60% yield; mp: 215–217 °C; IR (KBr): νmax = 3287, 3087, 2974, 2944, 2863, 2818, 1575, 1512, 1444, 1411 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 14.19 (s, 1H), 12.29 (s, 1H), 7.47 (t, 1H, J = 5.4 Hz), 5.67 (s, 1H), 4.75 (d, 1H, J = 4.6 Hz), 4.41 (s, 1H), 3.63 (dt, 1H, J = 7.4, 5.3 Hz), 3.37–3.26 (m, 4H), 2.86 (dd, 1H, J = 18.7, 5.2 Hz), 2.79 (d, 1H, J = 18.1 Hz), 2.68 (dd, 1H, J = 18.7, 7.4 Hz), 2.59 (d, 1H, J = 18.1 Hz), 2.25 (s, 6H), 1.20 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 186.44, 183.21, 156.45, 154.65, 149.81, 139.40, 133.07, 109.10, 107.78, 99.47, 70.73, 69.37, 56.39, 45.28, 36.01, 30.70, 25.92; ESI-MS m/z: 375.1 [M − H]−; HRMS (EI) calcd for C19H24N2O6, 376.1634; found, 376.1628.
3.4.8. 6-(Phenylamino) 1-Deoxy-6-demethoxybostrycin (11)
A red solid (MeOH) in a 38% yield; mp: 220–222 °C; IR (KBr): νmax = 3359, 3283, 3060, 2980, 2940, 2857, 2821, 1584, 1543, 1444, 1382 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 13.92 (s, 1H), 12.49 (s, 1H), 9.46 (s, 1H), 7.48–7.24 (m, 5H), 6.00 (s, 1H), 4.76 (br s, 1H), 4.43 (s, 1H), 3.64 (br t, 1H), 2.86 (dd, 1H, J = 18.6, 5.0 Hz), 2.82 (d, 1H, J = 18.2 Hz), 2.69 (dd, 1H, J = 18.8, 7.4 Hz), 2.60 (d, 1H, J = 18.2 Hz), 1.20 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 186.93, 183.08, 156.96, 154.94, 147.89, 139.26, 138.24, 133.89, 129.83,126.18, 124.48,109.21, 107.80, 101.70, 70.72, 69.38, 36.14, 30.66, 25.93; ESI-MS m/z: 380.1 [M − H]−; HRMS (EI) calcd for C21H19NO6, 381.1212; found, 381.1204.
3.4.9. 6-(Benzylamino) 1-Deoxy-6-demethoxybostrycin (12)
A red solid (MeOH) in 29% yield; mp: 220–222 °C; IR (KBr): νmax = 3382, 3264, 3085, 2973, 2934, 2900, 2870, 1574, 1511, 1444, 1411 cm−1; 1H NMR (300 MHz, DMSO-d6): δ 12.37 (s, 1H), 8.49 (t, 1H, J = 6.4 Hz), 7.38–7.18 (m, 5H), 5.53 (s, 1H), 4.75 (d, 1H, J = 5.3 Hz), 4.46 (d, 2H, J = 6.4 Hz), 4.42 (s, 1H), 3.61 (dt, 1H, J = 7.5, 5.3 Hz), 2.82 (dd,1H, J = 18.9, 5.3 Hz), 2.77 (d, 1H, J = 17.4 Hz), 2.66 (dd, 1H, J = 18.9, 7.5 Hz), 2.57 (d, 1H, J = 17.4 Hz,), 1.17 (s, 3H); ESI-MS m/z: 394.1 [M − H]−; HRMS (EI) calcd for C22H21NO6, 395.1369; found, 395.1361.
3.4.10. 6-(p-Methoxybenzylamino) 1-Deoxy-6-demethoxybostrycin (13)
A red solid (MeOH) in a 44% yield; mp: 223–225 °C; [α]20D = −250.0° (c = 1.00, CH3OH); IR (KBr): νmax = 3373, 3069, 2979, 2938, 2861, 2834, 1583, 1507, 1445, 1387 cm–1; 1H NMR (400 MHz, DMSO-d6): δ 14.11 (s, 1H), 12.39 (s, 1H), 8.40 (t, 1H, J = 6.5 Hz), 7.33–6.87 (m, 4H), 5.57 (s, 1H), 4.74 (d, 1H, J = 5.1 Hz), 4.40 (br s, 2H), 4.39 (s, 1H), 3.73 (s, 3H), 3.63 (dt, 1H, J = 7.2, 5.2 Hz), 2.84 (dd, 1H, J = 18.8, 5.2 Hz), 2.80 (d, 1H, J = 18.1 Hz), 2.68 (dd, 1H, J = 18.8, 7.2 Hz), 2.58 (d, 1H, J = 18.1 Hz), 1.19 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 186.33, 183.51, 158.95, 156.45, 154.65, 149.93, 139.26, 133.12, 129.42, 129.04, 129.04, 114.42, 114.42, 109.18, 107.72, 100.10, 70.71, 69.36, 55.52, 45.25, 35.99, 30.68, 25.91; ESI-MS m/z: 424.1 [M − H] −; HRMS (EI) calcd for C23H23NO7, 425.1475; found, 425.1464.
3.4.11. 6-(p-Fluorobenzylamino) 1-Deoxy-6-demethoxybostrycin (14)
A red solid (MeOH) in a 47% yield; mp: 234–236 °C; IR (KBr): νmax = 3272, 3085, 2976, 2936, 2873, 2856, 1577, 1510, 1443, 1411 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 14.07 (s, 1H), 12.40 (s, 1H), 8.46 (t, 1H, J = 6.5 Hz), 7.44–7.14 (m, 4H), 5.57 (s, 1H), 4.74 (d, 1H, J = 5.1 Hz), 4.46 (d, 2H, J = 6.5 Hz), 4.41 (s, 1H), 3.63 (dt, 1H, J = 7.3, 5.2 Hz), 2.85 (dd, 1H, J = 18.8, 5.2 Hz), 2.80 (d, 1H, J = 18.1 Hz), 2.68 (dd, 1H, J = 18.8, 7.2 Hz), 2.58 (d, 1H, J = 18.1 Hz), 1.19 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 186.43, 183.44, 160.63, 156.47, 154.68, 149.98, 139.24, 133.80, 133.20, 129.77, 129.69, 115.74, 109.19, 107.72, 100.21, 70.71, 69.36, 45.00, 35.99, 30.68, 25.90; ESI-MS m/z: 412.1 [M − H]−; HRMS (EI) calcd for C22H20FNO6, 413.1275; found, 413.1267.
3.4.12. 6-(Piperidin-1-yl) 1-Deoxy-6-demethoxybostrycin (15)
A red solid (MeOH) in a 30% yield; mp: 188–190 °C; IR (KBr): νmax = 3369, 3065, 2935, 2856, 1593, 1551, 1448, 1409 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 13.86 (s, 1H), 12.66 (s, 1H), 5.98 (s, 1H), 4.74 (d, 1H, J = 5.1 Hz), 4.39 (s, 1H), 3.63 (dt, 1H, J = 7.3, 5.2 Hz), 3.55 (br s, 4H), 2.83 (dd, 1H, J = 18.7, 5.2 Hz), 2.79 (d, 1H, J = 18.2 Hz), 2.67 (dd, 1H, J = 18.7, 7.3 Hz), 2.57 (d, 1H, J = 18.2 Hz), 1.65 (br s, 6H), 1.19 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 185.37, 184.10, 157.65, 155.43, 154.35, 138.14, 134.29, 110.61, 109.80, 107.87, 70.72, 69.40, 50.89,36.22, 30.49, 25.93, 24.15; ESI-MS m/z: 372.1 [M − H]−; HRMS (EI) calcd for C20H23NO6, 373.1525; found, 373.1522.
3.4.13. 6-(4′-Methylpiperidin-1-yl) 1-Deoxy-6-demethoxybostrycin (16)
A red solid (MeOH) in a 32% yield; mp: 198–200 °C; [α]20D = −44.8° (c = 1.00, CH3OH); IR (KBr): νmax = 3407, 3087, 2948, 2923, 2872, 1593, 1573, 1543, 1455 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 13.85 (s, 1H), 12.66 (s, 1H), 5.98 (s, 1H), 4.74 (d, 1H, J = 5.1 Hz), 4.39 (s, 1H), 4.09 (br d, 2H), 3.63 (dt, 1H, J = 7.3, 5.2 Hz), 3.01 (br t, 2H), 2.81 (dd, 1H, J = 18.7, 5.2 Hz), 2.79 (d, 1H, J = 18.2 Hz), 2.67 (dd, 1H, J = 18.7, 7.3 Hz), 2.56 (d, 1H, J = 18.2 Hz), 1.75 (m, 2H), 1.67 (m, 1H), 1.31 (m, 2H), 1.19 (s, 3H), 0.94 (d, 3H, J = 6.3 Hz); 13C NMR (100 MHz, DMSO-d6): δ 185.35, 184.07, 157.69, 155.47, 154.27, 138.16, 134.30, 110.59, 110.00, 107.86, 70.72, 69.40, 50.14, 36.22, 34.08, 30.50, 25.94; ESI-MS m/z: 386.1 [M − H]−; HRMS (EI) calcd for C21H25NO6, 387.1682; found, 387.1678.
3.4.14. 6-(4′-Phenylpiperidin-1-yl) 1-Deoxy-6-demethoxybostrycin (17)
A red solid (MeOH) in a 28% yield; mp: 227–228 °C; IR (KBr): νmax = 3332, 3084, 2939, 2927, 2876, 2847, 1593, 1567, 1537, 1454, 1407 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 13.85 (s, 1H), 12.70 (s, 1H), 7.36–7.17 (m, 5H), 6.07 (s, 1H), 4.75 (d, 1H, J = 5.1 Hz), 4.41 (s, 1H), 4.27 (br d, 2H), 3.64 (dt, 1H, J = 7.3, 5.2 Hz), 3.14 (br t, 2H), 2.87 (m, 2H), 2.79 (d, 1H, J = 18.2 Hz,), 2.69 (dd, 1H, J = 18.7, 7.2 Hz), 2.58 (d, 1H, J = 18.2 Hz), 1.81 (m, 4H), 1.20 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 185.38, 183.96, 157.85, 155.65, 154.29, 145.87, 138.20, 134.46, 128.90,127.19, 127.19,126.71, 110.63, 110.37, 107.91, 70.72, 69.41, 50.50,41.85, 36.24, 33.23,30.50, 25.94; ESI-MS m/z: 448.2 [M − H]−; HRMS (EI) calcd for C26H27NO6, 449.1838; found, 449.1837.
3.5. General Procedure for Preparation of Compounds (18–22)
To a solution of 1 (50 mg, 0.156 mmol) and triethylamine (8 equivalents) in 10 mL of methanol was added the corresponding thiol (0.624 mmol, the butane-2,3-dithiol was racemate). The reaction mixture was stirred at 0–5 °C until the starting material disappeared. The solvent was removed under reduced pressure. The resulting residue was subsequently purified using first silica gel chromatography with dichloromethane-methanol as eluent, and then C18 reversed phase silica gel column with methanol-water as eluent to obtain the corresponding products.
3.5.1. 6,7-Bis(ethylthio) 1-Deoxy-6-demethoxybostrycin (18)
A red solid (MeOH) in a 35% yield; mp: 186–188 °C; IR (KBr): νmax = 3378, 2976, 2959, 2925, 2855, 1611, 1574, 1489, 1427, 1408 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 13.11 (s, 2H), 4.79 (br s, 1H), 4.46 (s, 1H), 3.63 (br t, 1H), 3.26 (q, 4H, J = 7.4 Hz), 2.83 (dd, 1H, J = 18.1, 5.4 Hz), 2.79 (d, 1H, J = 18.7 Hz), 2.66 (dd, 1H, J = 19.0, 7.4 Hz), 2.58 (d, 1H, J = 18.7 Hz), 1.21 (m, 9H); 13C NMR (100 MHz, DMSO-d6): δ 175.21, 165.23, 165.19, 145.47, 138.77, 138.58, 109.40, 70.60, 69.34, 36.27, 30.32, 29.26,25.81, 15.63; ESI-MS m/z: 409.0 [M − H]−; HRMS (EI) calcd for C19H22O6S2, 410.0858; found, 410.0855.
3.5.2. 6,7-Bis(n-butylthio) 1-Deoxy-6-demethoxybostrycin (19)
A red solid (MeOH) in a 39% yield; mp: 178–180 °C; [α]20D = −17.4° (c = 1.00, CH3OH); IR (KBr): νmax = 3317, 2958, 2929, 2870, 1599, 1438, 1422, 1408 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 13.12 (s, 2H), 4.80 (br s, 1H), 4.46 (s, 1H), 3.63 (br t, 1H), 3.25 (t, 4H, J = 7.2 Hz), 2.83 (dd, 1H, J = 19.0, 5.6 Hz), 2.78 (d, 1H, J = 18.7 Hz), 2.66 (dd, 1H, J = 19.0, 7.4 Hz), 2.57 (d, 1H, J = 18.7 Hz), 1.56–1.47 (m, 4H), 1.38 (sextet, 4H, J = 7.3 Hz), 1.20 (s, 3H), 0.86 (t, 6H, J = 7.3 Hz); 13C NMR (100 MHz, DMSO-d6): δ 175.10, 165.35, 165.31, 145.78, 138.79, 138.62, 109.41, 70.60, 69.34, 36.28, 34.64, 32.33, 30.31, 25.81, 21.62,13.89,; ESI-MS m/z: 465.2 [M − H]−; HRMS (EI) calcd for C23H30O6S2, 466.1484; found, 466.1483.
3.5.3. 6,7-Bis(n-hexylthio) 1-Deoxy-6-demethoxybostrycin (20)
A red solid (MeOH) in a 55% yield; mp: 170–172 °C; IR (KBr): νmax = 3322, 2956, 2925, 2853, 1599, 1439, 1422, 1408 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 13.11 (s, 2H), 4.79 (br s, 1H), 4.45 (s, 1H), 3.62 (br t, 1H), 3.23 (t, 4H, J = 7.2 Hz), 2.82 (dd, 1H, J = 19.2, 5.4 Hz), 2.77 (d, 1H, J = 18.7 Hz), 2.65 (dd, 1H, J = 19.2, 7.3 Hz), 2.56 (d, 1H, J = 18.7 Hz), 1.57–1.47 (m, 4H), 1.40–1.33 (m, 4H), 1.27–1.21 (m, 8H), 1.20 (s, 3H), 0.87–0.80 (m, 6H); 13C NMR (100 MHz, DMSO-d6): δ 175.22, 165.19, 165.14, 145.83, 138.74, 138.59, 109.36,70.60, 69.32, 36.29, 34.97,31.19, 30.29, 30.20, 28.12, 25.81, 22.43, 14.28; ESI-MS m/z: 521.1 [M − H]−; HRMS (EI) calcd for C27H38O6S2, 522.2110; found, 522.2107.
3.5.4. 6,7-(Ethan-1′,2′-yl-dithio) 1-Deoxy-6-demethoxybostrycin (21)
A red solid (MeOH) in a 47% yield; mp: 226–227 °C; IR (KBr): νmax = 3529, 3479, 2974, 2922, 2849, 2824, 1588, 1515, 1449, 1415 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 12.64 (s, 2H), 4.80 (d, 1H, J = 5.2 Hz), 4.45 (s, 1H), 3.64 (dt, 1H, J = 7.5, 5.2 Hz), 3.34 (s, 4H), 2.85 (dd, 1H, J = 18.9, 5.4 Hz), 2.80 (d, 1H, J = 18.4 Hz), 2.66 (dd, 1H, J = 18.9, 7.7 Hz), 2.59 (d, 1H, J = 18.4Hz), 1.21 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 180.71, 156.93, 156.86, 140.79, 137.10, 136.93, 107.63, 107.60, 70.63, 69.29, 36.48, 30.33, 26.68, 25.89; ESI-MS m/z: 379.1 [M − H]−; HRMS (EI) calcd for C17H16O6S2, 380.0388; found, 380.0380.
3.5.5. 6,7-(Butan-2′,3′-yl-dithio) 1-Deoxy-6-demethoxybostrycin (22)
A red solid (MeOH) in a 78% yield; mp: 225–227 °C; [α]20D = −188.7° (c = 1.00, CH3OH); IR (KBr): νmax = 3484, 3406, 2969, 2930, 2873, 2818, 1582, 1515, 1443, 1413 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 12.68 (s, 1H), 12.66 (s, 1H), 4.79 (d, 1H, J = 5.2 Hz), 4.44 (s, 1H), 3.68–3.44 (m, 3H), 2.84 (dd, 1H, J = 18.6, 5.2 Hz), 2.80 (d, 1H, J =18.5 Hz), 2.66 (dd, 1H, J = 18.6, 7.7 Hz), 2.58 (d, 1H, J = 18.5 Hz), 1.32 and 1.30 (each d, 3H, J = 6.0 Hz), 1.21 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 180.96, 180.86, 156.73, 156.65, 139.83, 139.29, 136.96, 136.84, 107.75, 70.59, 69.26, 36.48, 30.30, 25.90, 23.33, 18.08; ESI-MS m/z: 407.1 [M − H]−; HRMS (EI) calcd for C19H20O6S2, 408.0698; found, 408.0701.