Lipids of Prokaryotic Origin at the Base of Marine Food Webs
Abstract
:1. Introduction
2. Lipid Production in Marine Micro-Organisms
2.1. Lipids as Protecting Agents in Marine Environments
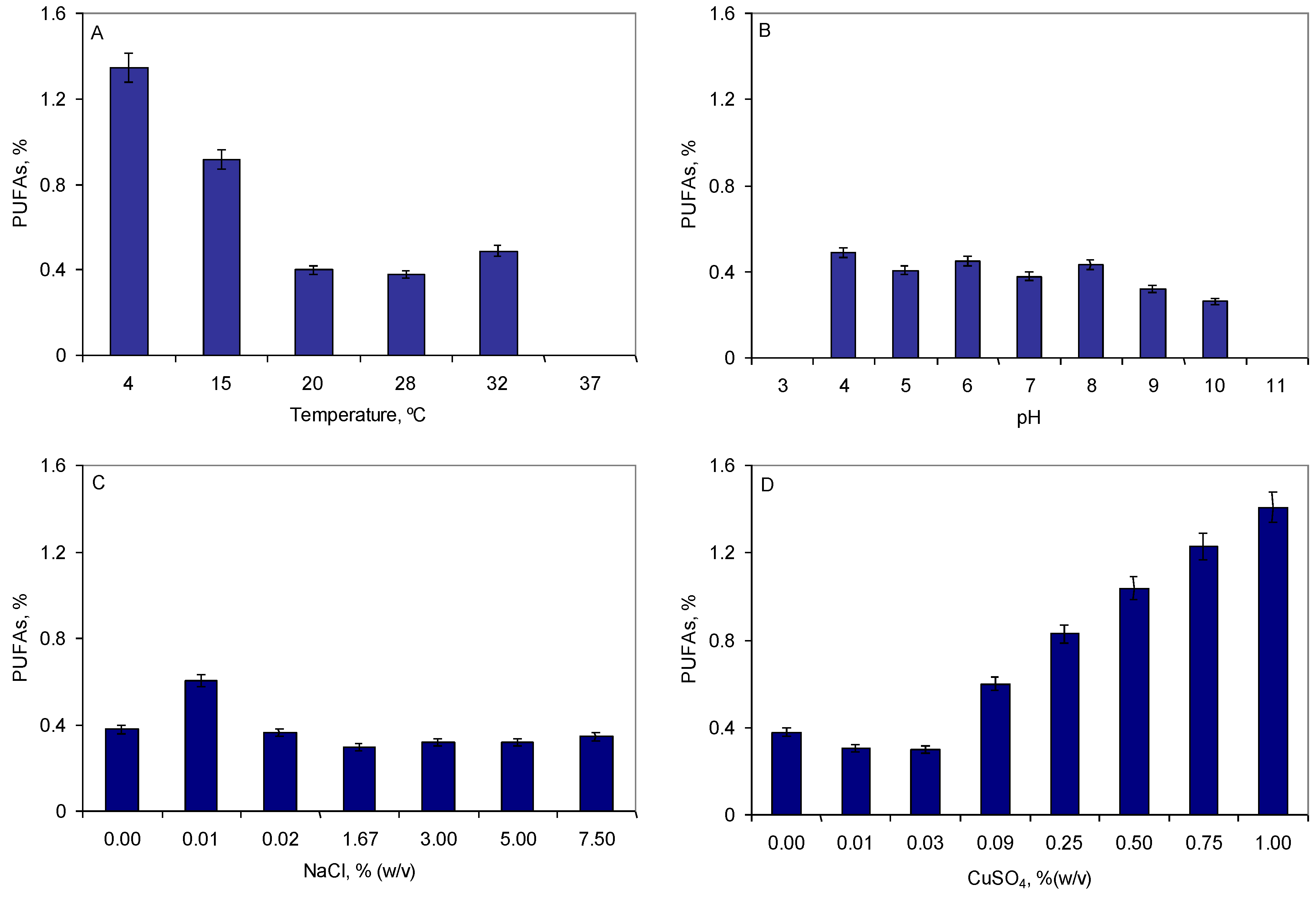
2.2. Production of Specialized Lipids
| Bacteria | |||
| Thermotoga maritima | 15,16-dimethyl-30-glyceryloxy-triacontanedioic acid | 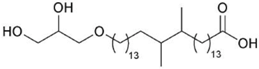 | [33] |
| Bacteria from fish microbiome | sebastenoic acid |  | [49] |
| Marine bacteria such as Shewanella putrefaciens | furan-acids | 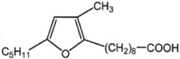 | [50,51] |
| Bacillus sp. | ω-cycloheptane fatty acids |  | [52] |
| Cyanobacteria | |||
| Lyngbya majuscula | malyngamide G | 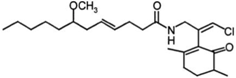 | [45] |
| Archaea | |||
| Thermoplasma acidophilum | main polar lipid | 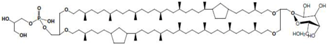 | [37] |
| Halobacterium salinarum | 2,3-diphytanyl-sn-glycerol-1-phospho- 3′-sn-glycerol-1′-methylphosphate |  | [53] |
2.3. Non-Polar Phospholipids, Non-Phosphorous Polar Lipids and Neutral Lipids
3. Transfer of Bacterial Lipids to Metazoans in Marine Foodwebs
3.1. Transfer and Transformation of Bacterial Fatty Acids to Protists
3.2. “Transfer” of Sterol
4. Conclusions
Acknowledgments
- Samples Availability: Available from the authors.
References
- De Carvalho, C.C.C.R.; Fernandes, P. Production of metabolites as bacterial responses to the marine environment. Mar. Drugs 2010, 8, 705–727. [Google Scholar] [CrossRef]
- Karner, M.B.; DeLong, E.F.; Karl, D.M. Archaeal dominance in the mesopelagic zone of the Pacific Ocean. Nature 2001, 409, 507–510. [Google Scholar]
- Koga, Y.; Morii, H. Biosynthesis of ether-type polar lipids in Archaea and evolutionary considerations. Microbiol. Mol. Biol. Rev. 2007, 71, 97–120. [Google Scholar] [CrossRef]
- Han, X.; Gross, R.W. Global analyses of cellular lipidomes directly from crude extracts of biological samples by ESI mass spectrometry. J. Lipid Res. 2003, 44, 1071–1079. [Google Scholar] [CrossRef]
- Harkewicz, R.; Dennis, E.A. Applications of mass spectrometry to lipids and membranes. Annu. Rev. Biochem. 2011, 80, 301–325. [Google Scholar] [CrossRef]
- Bergé, J.-P.; Barnathan, G. Fatty acids from lipids of marine organisms: Molecular biodiversity, roles as biomarkers, biologically active compounds, and economical aspects. Adv. Biochem. Eng. Biothchnol. 2005, 96, 49–125. [Google Scholar]
- Mozaffarian, D.; Rimm, E.B. Fish intake, contaminants, and human health: Evaluating the risks and the benefits. JAMA 2006, 296, 1885–1899. [Google Scholar] [CrossRef]
- Calon, F. Omega-3 polyunsaturated fatty acids in Alzheimer’s disease: Key questions and partial answers. Curr. Alzheimer Res. 2011, 8, 470–478. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Evolutionary aspects of diet: The omega-6/omega-3 ratio and the brain. Mol. Neurobiol. 2011, 44, 203–215. [Google Scholar] [CrossRef]
- Lu, F.S.; Nielsen, N.S.; Timm-Heinrich, M.; Jacobsen, C. Oxidative stability of marine phospholipids in the liposomal form and their applications. Lipids 2011, 46, 3–23. [Google Scholar]
- Wijendran, V.; Huang, M.-C.; Diau, G.-Y.; Boehm, G.; Nathanielsz, P.W.; Brenna, J.T. Efficacy of dietary arachidonic acid provided as triglyceride or phospholipid as substrates for brain arachidonic acid accretion in Baboon Neonates. Pediatr. Res. 2002, 51, 265–272. [Google Scholar] [CrossRef]
- Peng, J.; Larondelle, Y.; Pham, D.; Ackman, R.G.; Rollin, X. Polyunsaturated fatty acid profiles of whole body phospholipids and triacylglycerols in anadromous and landlocked Atlantic salmon (Salmo salar L.) fry. Comp. Biochem. Physiol. 2003, 134, 335–348. [Google Scholar]
- Okuyama, H.; Orikasa, Y.; Nishida, T.; Watanabe, K.; Morita, N. Bacterial genes responsible for the biosynthesis of Eicosapentaenoic and Docosahexaenoic acids and their heterologous expression. Appl. Environ. Microbiol. 2007, 73, 665–670. [Google Scholar] [CrossRef]
- De Carvalho, C.C.C.R. Adaptation of Rhodococcus erythropolis cells for growth and bioremediation under extreme conditions. Res. Microbiol. 2012, 163, 125–136. [Google Scholar] [CrossRef]
- Russell, N.J.; Nichols, D.S. Polyunsaturated fatty acids in marine bacteria-A dogma rewritten. Microbiology 1999, 145, 767–779. [Google Scholar] [CrossRef]
- Valentine, R.C.; Valentine, D.L. Omega-3 fatty acids in cellular membranes: A unified concept. Prog. Lipid Res. 2004, 43, 383–402. [Google Scholar]
- Okuyama, H.; Orikasa, Y.; Nishida, T. Significance of antioxidative functions of eicosapentaenoic and docosahexaenoic acids in marine microorganisms. Appl. Environ. Microbiol. 2008, 74, 570–574. [Google Scholar]
- Miyashita, K.; Nara, E.; Ota, T. Oxidative stability of polyunsaturated fatty acids in an aqueous solution. Biosci. Biotechnol. Biochem. 1993, 57, 1638–1640. [Google Scholar] [CrossRef]
- Yazu, K.; Yamamoto, Y.; Niki, E.; Ukegawa, K. Mechanism of lower oxidizability of eicosapentaenoate than linoleate in aqueous micelles. Lipids 1998, 33, 597–600. [Google Scholar] [CrossRef]
- Lovern, J.A. Fat metabolism in fishes: The fats of some plankton crustacea. Biochem. J. 1935, 30, 387–390. [Google Scholar]
- Dalsgaard, J.; St John, M.; Kattner, G.; Muller-Navarra, D.; Hagen, W. Fatty acid trophic markers in the pelagic marine environment. Adv. Mar. Biol. 2003, 46, 225–340. [Google Scholar] [CrossRef]
- Sinensky, M. Homeoviscous adaptation-A homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc. Natl. Acad. Sci. USA 1974, 71, 522–525. [Google Scholar] [CrossRef]
- MacElroy, M. Some comments on the evolution of extremophiles. Biosytems 1974, 6, 74–75. [Google Scholar] [CrossRef]
- Bowers, K.; Mesbah, N.; Wiegel, J. Biodiversity of poly-extremophilic bacteria: Does combining the extremes of high salt, alkaline pH and elevated temperature approach a physico-chemical boundary for life? Saline Syst. 2009, 5, 9. [Google Scholar]
- Delong, E.F.; Yayanos, A.A. Properties of the glucose-transport system in some deep-sea bacteria. Appl. Environ. Microbiol. 1987, 53, 527–532. [Google Scholar]
- Kuypers, M.M.M.; Blokker, P.; Erbacher, J.; Kinkel, H.; Pancost, R.D.; Schouten, S.; Sinninghe Damsté, J.S. Massive expansion of marine Archaea during a mid-cretaceous oceanic anoxic event. Science 2001, 293, 92–95. [Google Scholar] [CrossRef]
- Lipp, J.S.; Morono, Y.; Inagaki, F.; Hinrichs, K.-U. Significant contribution of Archaea to extant biomass in marine subsurface sediments. Nature 2008, 454, 991–994. [Google Scholar]
- Canganella, F.; Wiegel, J. Extremophiles: From abyssal to terrestrial ecosystems and possibly beyond. Naturwissenschaften 2011, 98, 253–279. [Google Scholar] [CrossRef]
- Thomas, D.N.; Dieckmann, G.S. Antarctic Sea Ice-A habitat for extremophiles. Science 2002, 295, 641–644. [Google Scholar] [CrossRef] [Green Version]
- Metz, J.G.; Roessler, P.; Facciotti, D.; Levering, C.; Dittrich, F.; Lassner, M.; Valentine, R.; Lardizabal, K.; Domergue, F.; Yamada, A.; Yazawa, K.; Knauf, V.; Browse, J. Production of polyunsaturated fatty acids by polyketide synthases in both prokaryotes and eukaryotes. Science 2001, 293, 290–293. [Google Scholar] [CrossRef]
- Bowman, J.P.; Gosink, J.J.; McCammon, S.A.; Lewis, T.E.; Nichols, D.S.; Nichols, P.D.; Skerratt, J.H.; Staley, J.T.; McMeekin, T.A. Colwellia demingiae sp. nov., Colwellia hornerae sp. nov., Colwellia rossensis sp. nov. and Colwellia psychrotropica sp. nov.: Psychrophilic Antarctic species with the ability to synthesize docosahexaenoic acid (22:6ω3). Int. J. Syst. Bacteriol. 1998, 48, 1171–1180. [Google Scholar]
- Shulse, C.N.; Allen, E.E. Widespread occurrence of secondary lipid biosynthesis potential in microbial lineages. PLoS One 2011, 6, e20146. [Google Scholar] [CrossRef]
- De Rosa, M.; Gambacorta, A.; Huber, R.; Lanzotti, V.; Nicolaus, B.; Stetter, K.O.; Trincone, A. Microbiology of Extreme Environments and Its Potential for Biotechnology; da Costa, M.S., Duarte, J.C., Williams, R.A.D., Eds.; Springer: New York, NY, USA, 1989; pp. 167–173. [Google Scholar]
- Wilson, Z.E.; Brimble, M.A. Molecules derived from the extremes of life. Nat. Prod. Rep. 2009, 26, 44–71. [Google Scholar] [CrossRef]
- Stetter, K.O. Extremophiles and their adaptation to hot environments. FEBS Lett. 1999, 452, 22–25. [Google Scholar] [CrossRef]
- Uda, I.; Sugai, A.; Itoh, Y.H.; Itoh, T. Variation in molecular species of polar lipids from Thermoplasma acidophilum depends on growth temperature. Lipids 2001, 36, 103–105. [Google Scholar] [CrossRef]
- Nicolas, J. A molecular dynamics study of an archaeal tetraether lipid membrane: Comparison with a dipalmitoylphosphatidylcholine lipid bilayer. Lipids 2005, 40, 1023–1030. [Google Scholar] [CrossRef]
- Van de Vossenberg, J.L.C.M.; Driessen, A.J.M.; Grant, D.; Konings, W.N. Lipid membranes from halophilic and alkali-halophilic Archaea have a low H+ and Na+ permeability at high salt concentration. Extremophiles 1999, 3, 253–257. [Google Scholar] [CrossRef]
- Vreeland, R.H.; Anderson, R.; Murray, R.G. Cell wall and phospholipid composition and their contribution to the salt tolerance of Halomonas elongata. J. Bacteriol. 1984, 160, 879–883. [Google Scholar]
- Russell, N.J. Adaptive modifications in membranes of halotolerant and halophilic microorganisms. J. Bioenerg. Biomembr. 1989, 21, 93–113. [Google Scholar] [CrossRef]
- Coker, J.A.; DasSarma, P.; Kumar, J.; Müller, J.; DasSarma, S. Transcriptional profiling of the model Archaeon Halobacterium sp. NRC-1: Responses to changes in salinity and temperature. Saline Sys. 2007, 3, 6. [Google Scholar] [CrossRef]
- McElhaney, R.N. The effects of alterations in the physical state of the membrane lipids on the ability of Acholeoplasma laidlawii B to grow at different temperatures. J. Mol. Biol. 1974, 84, 145–157. [Google Scholar] [CrossRef]
- Fang, J.; Barcelona, M.J.; Nogi, Y.; Kato, C. Biochemical implications and geochemical significance of novel phospholipids of the extremely barophilic bacteria from the Marianas Trench at 11,000 m. Deep-Sea Res. 2000, 47, 1173–1182. [Google Scholar] [CrossRef]
- Son, B.; Kim, J.; Choi, H. A new diacylgalactolipid containing 4Z-16:1 from the marine cyanobacterium Oscillatoria sp. Lipids 2001, 36, 427–429. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Srebnik, M. Natural halogenated fatty acids: Their analogues and derivatives. Prog. Lipid Res. 2002, 41, 315–367. [Google Scholar] [CrossRef]
- Wu, M.; Milligan, K.E.; Gerwick, W.H. Three new malyngamides from the marine cyanobacterium Lyngbya majuscula. Tetrahedron 1997, 53, 15983–15990. [Google Scholar]
- Kaneda, T. Iso- and Anteiso-fatty acids in bacteria: Biosynthesis, function, and taxonomic significance. Microbiol. Rev. 1991, 55, 288–302. [Google Scholar]
- Wollenweber, H.W.; Rietschel, E.T.; Hofstad, T.; Weintraub, A.; Lindbert., A.A. Nature, type of linkage, quantity, and absolute configuration of (3-hydroxy) fatty acids in lipopolysaccharides from Bacteroides fragilis NCTC 9343 and related strains. J. Bacteriol. 1980, 144, 898–903. [Google Scholar]
- Sanchez, L.M.; Wong, W.R.; Riener, R.M.; Schulze, C.J.; Linington, R.G. Examining the fish microbiome: Vertebrate-derived bacteria as an environmental niche for the discovery of unique marine natural products. Plos One 2012, 7, e35398. [Google Scholar]
- Spiteller, G. Furan fatty acids: Occurrence, synthesis, and reactions. Are furan fatty acids responsible for the cardioprotective effects of a fish diet? Lipids 2005, 40, 755–771. [Google Scholar] [CrossRef]
- Shirasaka, N.; Nishi, K.; Shimizu, S. Biosynthesis of furan fatty acids (F-acids) by a marine bacterium, Shewanella putrefaciens. Biochim. Biophys. Acta 1997, 1346, 253–260. [Google Scholar] [CrossRef]
- Poralla, K.; König, W.A. The occurrence of ω-cycloheptane fatty acids in a thermo-acidophilic bacillus. FEMS Microbiol. Lett. 1983, 16, 303–306. [Google Scholar]
- Kates, M.; Moldoveanu, N.; Stewart, L.C. On the revised structure of the major phospholipid of Halobacterium salinarium. Biochim. Biophys. Acta 1993, 1169, 46–53. [Google Scholar]
- Aries, E.; Doumenq, P.; Artaud, J.; Molinet, J.; Bertrand, J.C. Occurrence of fatty acids linked to non-phospholipid compounds in the polar fraction of a marine sedimentary extract from Carteau cove, France. Org. Geochem. 2001, 32, 193–197. [Google Scholar] [CrossRef]
- Shirasaka, N.; Nishi, K.; Shimizu, S. Occurrence of a furan fatty acid in marine bacteria. Biochim. Biophys. Acta 1995, 1258, 225–227. [Google Scholar]
- Alvarez, H.M.; Pucci, O.H.; Steinbüchel, A. Lipid storage compounds in marine bacteria. Appl. Microbiol. Biotechnol. 1997, 47, 132–139. [Google Scholar] [CrossRef]
- Nakano, M.; Iehata, S.; Tanaka, R.; Maeda, H. Extracellular neutral lipids produced by the marine bacteria Marinobacter sp. Biocontrol Sci. 2012, 17, 69–75. [Google Scholar] [CrossRef]
- De Carvalho, C.C.C.R.; ds Fonseca, M.M.R. Degradation of hydrocarbons and alcohols at different temperatures and salinities by Rhodococcus erythropolis DCL14. FEMS Microbiol. Ecol. 2005, 51, 389–399. [Google Scholar] [CrossRef]
- Decho, A.W. Microbial biofilms in intertidal systems: An overview. Cont. Shelf Res. 2000, 20, 1257–1273. [Google Scholar] [CrossRef]
- Otto Ortega-Morales, B.; Jesus Chan-Bacab, M.; del Carmen De la Rosa-Garcia, S.; Carlos Camacho-Chab, J. Valuable processes and products from marine intertidal microbial communities. Curr. Opin. Biotechnol. 2010, 21, 346–352. [Google Scholar] [CrossRef]
- Freese, E.; Rütters, H.; Köster, J.; Rullkötter, J.; Sass, H. Gammaproteobacteria as a possible source of eicosapentaenoic acid in anoxic intertidal sediments. Microb. Ecol. 2009, 57, 444–454. [Google Scholar] [CrossRef]
- Sherr, E.B.; Sherr, B.F.; Albright, L.J. Bacteria: Link or sink? Science 1987, 235, 88. [Google Scholar]
- Phillips, N.W. Role of different microbes and substrates as potential suppliers of specific, essential nutrients to marine detritivores. Bull. Mar. Sci. 1984, 35, 283–298. [Google Scholar]
- Ahlgren, G.; Lundstedt, L.; Brett, M.; Forsberg, C. Lipid composition and food quality of some freshwater phytoplankton for cladoceran zooplankters. J. Plankton Res. 1990, 12, 809–818. [Google Scholar] [CrossRef]
- Volkman, J.V. Sterols in microorganisms. Appl. Microbiol. Biotechnol. 2003, 60, 495–506. [Google Scholar]
- Brett, M.; Müller-Navarra, D. The role of highly unsaturated fatty acids in aquatic foodweb processes. Freshw. Biol. 1997, 38, 483–499. [Google Scholar] [CrossRef]
- Albers, C.S.; Kattner, G.; Hagen, W. The compositions of wax esters, triacylglycerols and phospholipids in Arctic and Antarctic copepods: Evidence of energetic adaptations. Mar. Chem. 1996, 55, 347–358. [Google Scholar] [CrossRef]
- Yazawa, K. Production of eicosapentaenoic acid from marine bacteria. Lipids 1996, 31, S297–S300. [Google Scholar] [CrossRef]
- Nichols, D.; Bowman, J.; Sanderson, K.; Nichols, C.M.; Lewis, T.; McMeekin, T.; Nichols, P.D. Developments with Antarctic microorganisms: Culture collections, bioactivity screening, taxonomy, PUFA production and cold-adapted enzymes. Curr. Opin. Biotechnol. 1999, 10, 240–246. [Google Scholar] [CrossRef]
- Wallis, J.G.; Watts, J.L.; Browse, J. Polyunsaturated fatty acid synthesis: What will they think of next? Trends Biochem. Sci. 2002, 27, 467–473. [Google Scholar] [CrossRef]
- Ustach, J.F. Algae, bacteria and detritus as food for the harpacticoid copepod, Heteropsyllus pseudonunni Coull and Palmer. J. Exp. Mar. Biol. Ecol. 1982, 64, 203–214. [Google Scholar] [CrossRef]
- Sogard, S.M. Utilization of meiofauna as a food source by a grassbed fish, the spotted dragonet Callionymus pauciradiatus. Mar. Ecol. Prog. Ser. 1984, 17, 183–191. [Google Scholar] [CrossRef]
- Decho, A.W. Water-cover influences on diatom ingestion rates by meiobenthic copepods. Mar. Ecol. Prog. Ser. 1986, 33, 139–146. [Google Scholar] [CrossRef]
- Norsker, N.-H.; Støttrup, J.G. The importance of dietary HUFAs for fecundity and HUFA content in the harpacticoid, Tisbe holothuriae Humes. Aquaculture 1994, 125, 155–166. [Google Scholar] [CrossRef]
- De Troch, M.; Mees, J.; Wakwabi, E. Diets of abundant fishes from beach seine catches in seagrass beds of a tropical bay (Gazi Bay, Kenya). Belg. J. Zool. 1998, 128, 135–154. [Google Scholar]
- Buffan-Dubau, E.; Carman, K.R. Diel feeding behavior of meiofauna and their relationships with microalgal resources. Limnol. Oceanogr. 2000, 45, 381–395. [Google Scholar] [CrossRef]
- De Troch, M.; Boeckx, P.; Cnudde, C.; Van Gansbeke, D.; Vanreusel, A.; Vincx, M.; Caramujo, M.J. Bioconversion of fatty acids at the basis of marine food webs: Insights from a compound-specific stable isotope analysis. Mar. Ecol. Prog. Ser. 2012, 465, 53–67. [Google Scholar] [CrossRef]
- Stevens, C.J.; Limén, H.; Pond, D.W.; Gélinas, Y.; Juniper, S.K. Ontogenetic shifts in the trophic ecology of two alvinocaridid shrimp species at hydrothermal vents on the Mariana Arc, western Pacific Ocean. Mar. Ecol. Prog. Ser. 2008, 356, 225–237. [Google Scholar] [CrossRef] [Green Version]
- Šajbidor, J.; Dobroñová, S.; Čertík, M. Arachidonic acid production by Mortierella sp. S-17 influence of C/N ratio. Biotechnol. Lett. 1990, 12, 455–456. [Google Scholar] [CrossRef]
- Nichols, D.S.; Nichols, P.D.; McMeekin, T.A. Polyunsaturated fatty acids in Antarctic bacteria. Antarct. Sci. 1993, 5, 149–160. [Google Scholar]
- Yano, Y.; Nakayama, A.; Yoshida, K. Distribution of polyunsaturated fatty acids in bacteria present in intestines of deep-sea fish and shallow-sea poikilothermic animals. Appl. Environ. Microbiol. 1997, 63, 2572–2577. [Google Scholar]
- Caron, D.A. Symbiosis and Mixotrophy among Pelagic Microorganisms. In Microbial Ecology of the Oceans; Kirchman, D.L., Ed.; Wiley-Liss: New York, NY, USA, 2000; pp. 495–523. [Google Scholar]
- Sherr, E.B.; Sherr, B.F. Heterotrophic dinoflagellates: A significant component of microzooplankton biomass and major grazers of diatoms in the sea. Mar. Ecol. Prog. Ser. 2007, 352, 187–197. [Google Scholar]
- Strom, S.L. Bacterivory: Interactions between Bacteria and Their Grazers. In Microbial Ecology of the Oceans; Kirchman, D.L., Ed.; Wiley-Liss: New York, NY, USA, 2000; pp. 286–351. [Google Scholar]
- Caron, D.A.; Goldman, J.C. Nutrient Regeneration. In Ecology of Marine Protozoa; Capriulo, G.M., Ed.; Oxford University Press: New York, NY, USA, 1990; pp. 283–306. [Google Scholar]
- Stoecker, D.K.; McDowell, C. Predation on protozoa: Its importance to zooplankton. J. Plankton Res. 1990, 12, 891–908. [Google Scholar] [CrossRef]
- Gifford, D.J.; Dagg, M.J. The microzooplankton-mesozooplankton link: Consumption of planktonic protozoa by the calanoid copepods Acartia tonsa Dana and Neocalanus plumchrus Murkukawa. Mar. Microb. Food Webs 1991, 5, 161–177. [Google Scholar]
- Fauré-Fremiet, E. Contribution à la connaissance des infusoires planctoniques. Bull. Biol. Fr. Bel. 1924, 6 (Suppl.), 1–171. [Google Scholar]
- Pomeroy, L.R. The ocean’s food web, a changing paradigm. Bioscience 1974, 24, 499–504. [Google Scholar] [CrossRef]
- Azam, F.; Fenchel, T.; Field, J.G.; Gray, J.S.; Meyer-Reil, L.A.; Thingstad, F. The ecological role of water-column microbes in the sea. Mar. Ecol. Prog. Ser. 1983, 10, 257–263. [Google Scholar] [CrossRef]
- Sherr, E.; Sherr, B.F. Role of microbes in pelagic food webs: A revised concept. Limnol. Oceanogr. 1988, 33, 1225–1227. [Google Scholar] [CrossRef]
- Li, W.K.W.; Rao, D.V.S.; Harrison, W.G.; Smith, J.C.; Cullen, J.J.; Irwin, B.; Platt, T. Autotrophic picoplankton in the tropical ocean. Science 1983, 219, 292–295. [Google Scholar]
- Stockner, J.G.; Antia, N.J. Algal picoplankton from marine and freshwater ecosystems: A multidisciplinary perspective. Can. J. Fish. Aquat. Sci. 1986, 43, 2472–2503. [Google Scholar] [CrossRef]
- Weisse, T. Dynamics of autotrophic picoplankton in marine and freshwater ecosystems. Adv. Microb. Ecol. 1993, 13, 327–369. [Google Scholar] [CrossRef]
- Desvilettes, C.; Bec, A. Formation and Transfer of Fatty Acids in Aquatic Microbial Food Webs: Role of Heterotrophic Protists. In Lipids in Aquatic Ecosystems; Kainz, M., Brett, M.T., Arts, M.T., Eds.; Springer: New York, NY, USA, 2009; pp. 25–42. [Google Scholar]
- Breteler, W.C.M.K.; Schogt, N.; Baas, M.; Schouten, S.; Kraay, G.W. Trophic upgrading of food quality by protozoans enhancing copepod growth: Role of essential lipids. Mar. Biol. 1999, 135, 191–198. [Google Scholar] [CrossRef]
- Bec, A.; Martin-Creuzburg, D.; Elert, E.V. Trophic upgrading of autotrophic picoplankton by the heterotrophic nanoflagellate Paraphysomonas sp. Limnol. Oceanogr. 2006, 51, 1699–1707. [Google Scholar]
- Sanders, R.W.; Wickham, S.A. Planktonic protozoa and metazoa: Predation, food quality and population control. Mar. Microb. Food Webs 1993, 7, 197–223. [Google Scholar]
- Veloza, A.; Chu, F.-L.; Tang, K. Trophic modification of essential fatty acids by heterotrophic protists and its effects on the fatty acid composition of the copepod Acartia tonsa. Mar. Biol. 2006, 148, 779–788. [Google Scholar] [CrossRef]
- Zhukova, N.V.; Kharlamenko, V.I. Sources of essential fatty acids in the marine microbial loop. Aquat. Microb. Ecol. 1999, 17, 153–157. [Google Scholar]
- Vera, A.; Desvilettes, C.; Bec, A.; Bourdier, G. Fatty acid composition of freshwater heterotrophic flagellates: An experimental study. Aquat. Microb. Ecol. 2001, 25, 271–279. [Google Scholar]
- Broglio, E.; Jónasdóttir, S.H.; Calbet, A.; Jakobsen, H.H.; Saiz, E. Effect of heterotrophic versus autotrophic food on feeding and reproduction of the calanoid copepod Acartia tonsa: Relationship with prey fatty acid composition. Aquat. Microb. Ecol. 2003, 31, 267–278. [Google Scholar] [CrossRef]
- Bec, A.; Desvilettes, C.; Vera, A.; Fontvielle, D.; Bourdier, G. Nutritional value of different food sources for the bennthic daphnidae Simocephalus vetulus: Role of fatty acids. Arch. Hydrobiol. 2003, 156, 145–163. [Google Scholar] [CrossRef]
- Ratledge, C. Fatty acid biosynthesis in microorganisms being used for single cell oil production. Biochimie 2004, 86, 807–815. [Google Scholar] [CrossRef]
- Kobayashi, T.; Sakaguchi, K.; Matsuda, T.; Abe, E.; Hama, Y.; Hayashi, M.; Honda, D.; Okita, Y.; Sugimoto, S.; Okino, N.; et al. Increase of eicosapentaenoic acid in Thraustochytrids through thraustochytrid ubiquitin promoter-driven expression of a fatty acid Δ5 desaturase gene. Appl. Environ. Microbiol. 2011, 77, 3870–3876. [Google Scholar]
- Parrish, C.C.; Whiticar, M.; Puvanendran, V. Is w6 docosapentaenoic acid an essential fatty acid during early ontogeny in marine fauna? Limnol. Oceanogr. 2007, 52, 476–479. [Google Scholar]
- Raghukumar, S. Thraustochytrid marine protists: Production of PUFAs and other emerging technologies. Mar. Biotechnol. 2008, 10, 631–640. [Google Scholar] [CrossRef]
- Volkman, J.K. Sterols and other triterpenoids: Source specificity and evolution of biosynthetic pathways. Org. Geochem. 2005, 36, 139–159. [Google Scholar] [CrossRef]
- Martin-Creuzburg, D.; Elert, E.V. Ecological Significance of Sterols in Aquatic Food Webs. In Lipids in Aquatic Ecosystems; Kainz, M., Brett, M.T., Arts, M.T., Eds.; Springer: New York, NY, USA, 2009; pp. 43–64. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
De Carvalho, C.C.C.R.; Caramujo, M.J. Lipids of Prokaryotic Origin at the Base of Marine Food Webs. Mar. Drugs 2012, 10, 2698-2714. https://doi.org/10.3390/md10122698
De Carvalho CCCR, Caramujo MJ. Lipids of Prokaryotic Origin at the Base of Marine Food Webs. Marine Drugs. 2012; 10(12):2698-2714. https://doi.org/10.3390/md10122698
Chicago/Turabian StyleDe Carvalho, Carla C. C. R., and Maria José Caramujo. 2012. "Lipids of Prokaryotic Origin at the Base of Marine Food Webs" Marine Drugs 10, no. 12: 2698-2714. https://doi.org/10.3390/md10122698
APA StyleDe Carvalho, C. C. C. R., & Caramujo, M. J. (2012). Lipids of Prokaryotic Origin at the Base of Marine Food Webs. Marine Drugs, 10(12), 2698-2714. https://doi.org/10.3390/md10122698






