Nutritional and Chemical Composition and Antiviral Activity of Cultivated Seaweed Sargassum naozhouense Tseng et Lu
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition of Cultivated S. naozhouense
| Components | Values |
|---|---|
| Ash | 35.18 |
| Protein | 11.20 |
| Lipid | 1.06 |
| Total carbohydrate | 47.73 |
| Total water-soluble carbohydrate | 29.74 |
| Water-soluble polysaccharide | 21.01 |
| Total dietary fiber | 4.83 |
2.2. Amino Acid Composition
| Amino acids | Contents | Amino acids | Contents |
|---|---|---|---|
| Aspartic acid | 8.39 | Tyrosine | 2.95 |
| Threonine | 3.93 | Phenylalanine | 4.38 |
| Serine | 3.21 | Histidine | 1.07 |
| Glutamic acid | 13.21 | Lysine | 3.66 |
| Proline | 3.30 | Arginine | 4.20 |
| Glycine | 4.38 | Tryptophan | 0.89 |
| Alanine | 5.27 | Total | 76.97 |
| Valine | 4.64 | EAA | 36.35 |
| Methionine | 2.41 | NEAA | 40.62 |
| Cysteine | 0.54 | EAA/NEAA | 0.89 |
| Isoleucine | 4.02 | EAAI | 66.24 |
| Leucine | 6.52 |
2.3. Fatty Acid Composition
| Fatty acids | Methyl esters (%) | Fatty acids | Methyl esters (%) |
|---|---|---|---|
| C6:0 | 0.44 | C18:3ω3 | 0.25 |
| C8:0 | 0.49 | C20:1 | 0.53 |
| C12:0 | 0.34 | C20:3ω6 | 2.95 |
| C14:0 | 6.7 | C20:4ω6 | 9.61 |
| C15:0 | 0.3 | C20:5ω3 | 1.38 |
| C16:0 | 24.61 | C22:0 | 0.21 |
| C16:1 | 3.55 | SAFA | 33.63 |
| C16:2ω6 | 0.22 | MUFA | 10.42 |
| C18:0 | 0.54 | PUFA | 18.84 |
| C18:1 | 6.34 | PUFAω6 | 13.24 |
| C18:2trans | 3.97 | PUFAω3 | 1.63 |
| C18:2ω6cis | 0.46 | Ratioω6/ω3 | 8.12 |
2.4. Mineral Contents
| Minerals | Contents |
|---|---|
| K | 4170 |
| Na | 3250 |
| P | 120 |
| Ca | 66.98 |
| Fe | 147 |
| Zn | 9.08 |
| Mn | 5.84 |
| Cu | 0.36 |
| Cd | 0.17 |
2.5. Properties of Polysaccharide
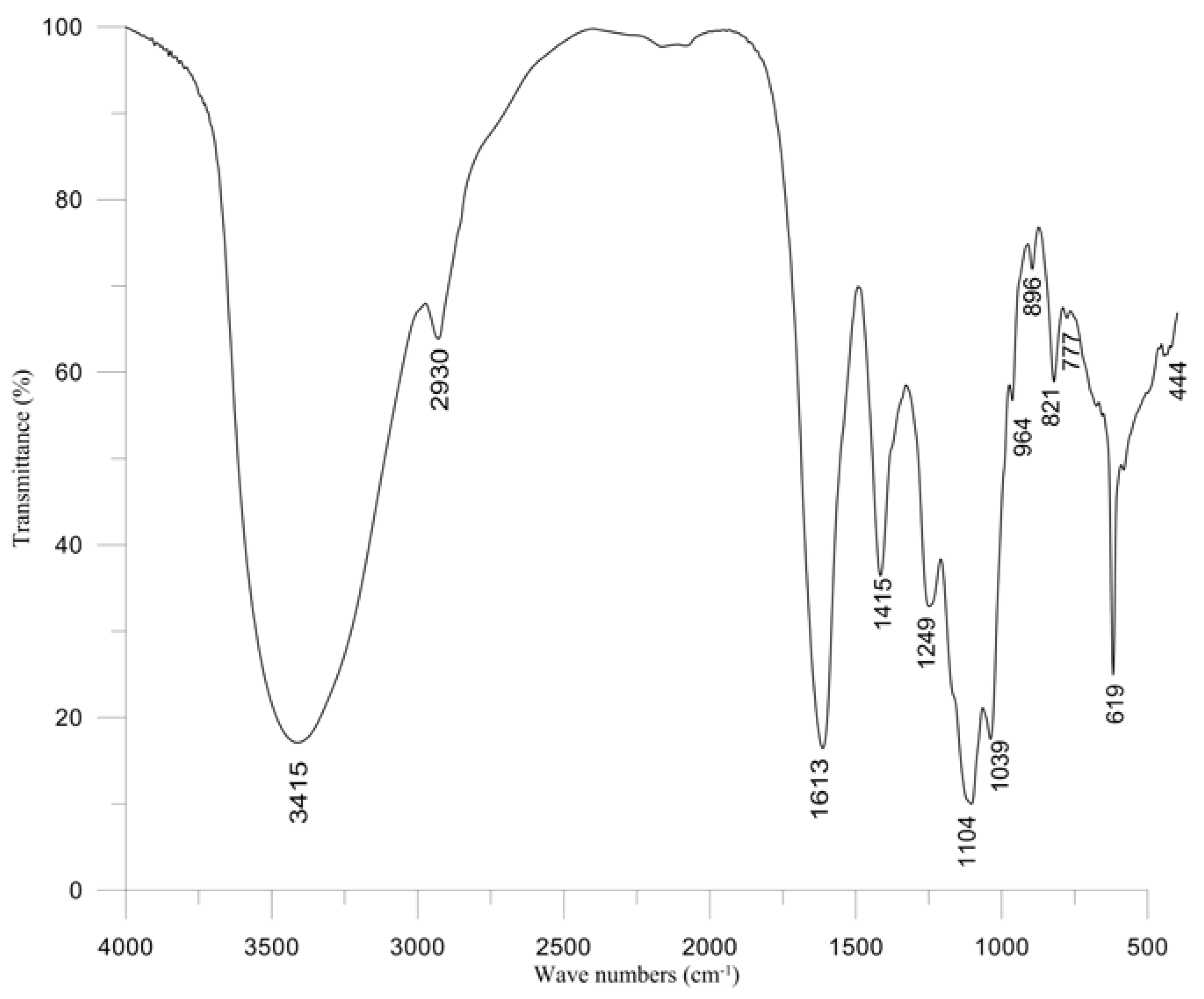
3. Experimental Section
3.1. Algal Material
3.2. Chemical Composition
3.3. Preparation of Polysaccharides
3.4. FT-IR Spectroscopy
4. Conclusions
Acknowledgments
References
- Gupta, S.; Abu-Ghannam, N. Bioactive potential and possible health effects of edible brown seaweeds. Trends Food Sci. Technol. 2011, 22, 315–326. [Google Scholar] [CrossRef]
- Pereira, L. A Review of the Nutrient Composition of Selected Edible Seaweeds Ecology. In Seaweed: Nutrient Composition and Medicinal Uses; Pomin, V.H., Ed.; Nova Science Publishers Inc.: Now York, NY, USA, 2011; pp. 15–47. [Google Scholar]
- Ortiz, J.; Romero, N.; Robert, P.; Araya, J.; Lopez-Hernández, J.; Bozzo, C.; Navarrete, E.; Osori, A.; Rios, A. Dietary fiber, amino acid, fatty acid and tocopherol contents of the edible seaweeds Ulva lactuca and Durvillaea antarctica. Food Chem. 2006, 99, 98–104. [Google Scholar] [CrossRef]
- Besada, V.; Andrade, J.M.; Schultze, F.; González, J.J. Heavy metals in edible seaweeds commercialized for human consumption. J. Mar. Syst. 2009, 75, 303–315. [Google Scholar]
- Cruz-Suárez, L.E.; León, A.; Peña-Rodríguez, A.; Rodríguez-Peña, G.; Moll, B.; Ricque-Marie, D. Shrimp/Ulva co-culture: A sustainable alternative to diminish the need for artificial feed and improve shrimp quality. Aquaculture 2010, 301, 64–68. [Google Scholar] [CrossRef]
- Fleurence, J. Seaweed proteins: Biochemical, nutritional aspects and potential uses. Trends Food Sci. Technol. 1999, 10, 25–28. [Google Scholar] [CrossRef]
- Lee, J.B.; Takeshita, A.; Hayashi, K.; Hayashi, T. Structures and antiviral activities of polysaccharides from Sargassum trichophyllum. Carbohydr. Polym. 2011, 86, 995–999. [Google Scholar] [CrossRef]
- Denis, C.; Morancais, M.; Li, M.; Deniaud, E.; Gaudin, P.; Wielgosz-Collin, G.; Barnathan, G.; Jaouen, P.; Fleurence, J. Study of the chemical composition of edible red macroalgae Grateloupia turuturu from Brittany (France). Food Chem. 2010, 119, 913–917. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Cornish, M.L.; Garbary, D.J. Antioxidants from macroalgae: Potential applications in human health and nutrition. Algae 2010, 25, 155–171. [Google Scholar] [CrossRef]
- Rupérez, P.; Saura-Calixto, F. Dietary fiber and physicochemical properties of edible Spanish seaweeds. Eur. Food Res. Technol. 2001, 212, 349–354. [Google Scholar] [CrossRef]
- Lahaye, M.; Kaeffer, B. Seaweed dietary fibres: Structure, physic-chemical and biological properties relevant to intestinal physiology. Sci. Aliment. 1997, 17, 619–639. [Google Scholar]
- Yan, X.; Nagata, T.; Fan, X. Antioxidative activities in some common seaweeds. Plant Foods Hum. Nutr. 1998, 52, 253–262. [Google Scholar] [CrossRef]
- Bhaskar, N.; Miyashita, K. Lipid composition of Padina tetratomatica (Dictyotales, Pheophyta), a brown seaweed of the west coast of India. Indian J. Fish. 2005, 52, 263–268. [Google Scholar]
- Mabeau, S.; Fleurence, J. Seaweed in food products: Biochemical and nutritional aspects. Trends Food Sci. Technol. 1993, 4, 103–107. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Wu, W.H.; Wang, J.; Lan, M.B. Antioxidant properties of polysaccharide from the brown seaweed Sargassum graminifolium (Turn.), and its effects on calcium oxalate crystallization. Mar. Drugs 2012, 10, 119–130. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Zhang, Z.; Zhang, H.; Niu, X. Structural studies on a novel fucogalactan sulfated from the brown seaweed Laminaria japonica. Int. J. Biol. Macromol. 2010, 47, 126–131. [Google Scholar] [CrossRef]
- Editorial Committee of Chinese Materia MedicaState Administration of Traditional Chinese MedicineChinese Materia Medica; Shanghai Science and Technology Press: Shanghai, China, 1999; pp. 435–483.
- Wang, B.; Huang, H.; Xiong, H.P.; Xie, E.Y.; Li, Z.M. Analysis on nutrition constituents of Sargassum naozhouense sp. nov. Food Res. Dev. 2010, 31, 195–197. [Google Scholar]
- Zhu, W.; Ooi, V.E.C.; Ang, P.O.J.; Chan, P.K.S. Isolation and characterization of a sulfated polysaccharide from the brown alga Sargassum patens and determination of its anti-herpes activity. Biochem. Cell Biol. 2003, 81, 25–33. [Google Scholar] [CrossRef]
- Witvrouw, M.; de Clercq, E. Sulfated polysaccharides extracted from sea algae as potential antiviral drugs. Gen. Pharmacol. 1997, 29, 497–511. [Google Scholar] [CrossRef]
- Zhao, M.J. Nutrition evaluation of the edible seaweed. Fish. Sci. 1990, 28–31. [Google Scholar]
- Chem, S.; Wang, W.; Liu, H.; Li, C. Chemical constituents in Sargassum henslowianum and its nutrition evaluation. Food Res. Dev. 2010, 31, 154–156. [Google Scholar]
- Dai, Z.; Hong, Y.; Zhang, Y.; Zhang, H. Evaluation on nutritional components of Sargassum fusiforme. J. Fish. China 2002, 26, 382–384. [Google Scholar]
- Li, B.F. Marine Food Nutrition and Health Characteristics. In Marine Health Food; Chemical Industry Press: Beijing, China, 2009; pp. 51–72. [Google Scholar]
- Sánchez-Machado, D.I.; López-Cervantes, J.; López-Hernández, J.; Paseiro-Losada, P. Fatty acids, total lipid, protein and ash contents of processed edible seaweeds. Food Chem. 2004, 85, 439–444. [Google Scholar] [CrossRef]
- Li, D. Non-Energy Nutrients: Mineral. In Food Nutrition; Chemical Industry Press: Beijing, China, 2011; pp. 140–146. [Google Scholar]
- Ortega-Calvo, J.J.; Mazuelos, C.; Hermosín, B.; Sáiz-Jiménez, C. Chemical composition of Spirulina and eukaryotica algae food products marked in Spain. J. Appl. Phycol. 1993, 5, 425–435. [Google Scholar] [CrossRef]
- Indegaard, M.; Minsaas, J. Seaweed Resources in EUROPE: Uses and Potential. In Animal and Human Nutrition; Guiry, M.D., Blunden, G., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 1991; pp. 21–64. [Google Scholar]
- Chandía, N.P.; Matsuhiro, B.; Vásquez, A.E. Alginic acids in Lessonia trabeculata: Characterization by formic acid hydrolysis and FT-IR spectroscopy. Carbohydr. Polym. 2001, 46, 81–87. [Google Scholar] [CrossRef]
- Asare, S.O. Seasonal changes in sulphate and 3,6-anhydrogalactose content of phycocolloids from two red algae. Bot. Mar. 1980, 23, 595–598. [Google Scholar]
- Stancioff, D.J.; Stanley, N.F. Infrared and chemical studies on algal polysaccharides. Proc. Int. Seaweed Symp. 1969, 6, 595–609. [Google Scholar]
- Chandía, N.P.; Matsuhiro, B. Characterization of a fucoidan from Lessonia vadosa (Phaeophyta) and its anticoagulant and elicitor properties. Int. J. Biol. Macromol. 2008, 42, 235–240. [Google Scholar] [CrossRef]
- Coen, D.M. Antiviral drug resistance in herpes simplex virus. Adv. Exp. Med. Biol. 1996, 394, 49–57. [Google Scholar]
- Ernst, M.E.; Franey, R.J. Acyclovir- and ganciclovir-induced neutrotoxicity. Ann. Pharmacother. 1998, 32, 111–113. [Google Scholar] [CrossRef]
- Wijesekara, I.; Pangestuti, R.; Kim, S.K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011, 84, 14–21. [Google Scholar] [CrossRef]
- Chen, J.H.; Lim, J.D.; Sohn, E.H.; Choi, Y.S.; Han, E.T. Growth-inhibitory effect of a fucoidan from brown seaweed Undaria pinnatifida on plasmodium parasites. Parasitol. Res. 2009, 104, 245–250. [Google Scholar] [CrossRef]
- Hemmingson, J.A.; Falshaw, R.; Furneaux, R.H.; Thompson, K. Structure and antiviral activity of the galactofucan sulfates extracted from Undaria pinnatifida (Phaeophyta). J. Appl. Phycol. 2006, 18, 185–193. [Google Scholar] [CrossRef]
- Queiroz, K.C.S.; Medeiros, V.P.; Queiroz, L.S.; Abreu, L.R.D.; Rocha, H.A.O.; Ferreira, C.V.; Jucá, M.B.; Aoyama, H.; Leite, E.L. Inhibition of reverse transcriptase activity of HIV by polysaccharides of brown algae. Biomed. Pharmacother. 2008, 62, 303–307. [Google Scholar] [CrossRef]
- Dalian Polytechnic UniversitySouth China University of TechnologyNorthwest University of Light IndustryZhengzhou University of Light IndustryHubei University of TechnologyQiqihar University of Light IndustrySouth China Agricultural UniversityShengyang Agricultural UniversityFood Analysis; China Light Industry Press: Beijing, China, 2006; pp. 99–206.
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugar and related substance. Anal. Chem. 1956, 28, 350–356. [Google Scholar]
- Chinese Center for Disease Control and Prevention, Determination of Amino Acids in Foods; Ministry of Health of the People’s Republic of China: Beijing, China, 2003; pp. 117–119.
- Association of Official Analytical Chemists, Official Methods of Analysis, 18th ed; Association of Official Analytical Chemists: Washington, DC, USA, 2006; pp. 2–247.
- Ye, H.; Wang, K.; Zhou, C.; Liu, J.; Zeng, X. Purification, antitumor and antioxidant activities in vitro of polysaccharides from the brown seaweed Sargassum pallidum. Food Chem. 2008, 111, 428–432. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, M.; Zhang, H.; Wang, Y.; Nie, S.; Li, C. Quantification of total polysaccharides and triterpenoids in Ganoderma lucidum and Ganoderma atrum by near infrared spectroscopy and chemometrics. Food Chem. 2012, 135, 268–275. [Google Scholar] [CrossRef]
- Haslin, C.; Lahaye, M.; Pellegrini, M. Chemical composition and structure of sulphated water-soluble cell-wall polysaccharides from the gametic, carposporic and tetrasporic stages of Asparagopsis armata Harvey (Rhodophyta, Bonnemaisoniaceae). Bot. Mar. 2000, 43, 475–482. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Peng, Y.; Zheng, J.; Huang, R.; Wang, Y.; Xu, T.; Zhou, X.; Liu, Q.; Zeng, F.; Ju, H.; Yang, X.; et al. Polyhydroxy steroids and saponins from China Sea starfish Asterina pectinifera and their biological activities. Chem. Pharm. Bull. 2010, 58, 856–858. [Google Scholar] [CrossRef]
- Che, C.T. Marine products as a source of antiviral drugs leads. Drug Dev. Res. 1991, 23, 201–218. [Google Scholar] [CrossRef]
- Samples Availability: Available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Peng, Y.; Xie, E.; Zheng, K.; Fredimoses, M.; Yang, X.; Zhou, X.; Wang, Y.; Yang, B.; Lin, X.; Liu, J.; et al. Nutritional and Chemical Composition and Antiviral Activity of Cultivated Seaweed Sargassum naozhouense Tseng et Lu. Mar. Drugs 2013, 11, 20-32. https://doi.org/10.3390/md11010020
Peng Y, Xie E, Zheng K, Fredimoses M, Yang X, Zhou X, Wang Y, Yang B, Lin X, Liu J, et al. Nutritional and Chemical Composition and Antiviral Activity of Cultivated Seaweed Sargassum naozhouense Tseng et Lu. Marine Drugs. 2013; 11(1):20-32. https://doi.org/10.3390/md11010020
Chicago/Turabian StylePeng, Yan, Enyi Xie, Kai Zheng, Mangaladoss Fredimoses, Xianwen Yang, Xuefeng Zhou, Yifei Wang, Bin Yang, Xiuping Lin, Juan Liu, and et al. 2013. "Nutritional and Chemical Composition and Antiviral Activity of Cultivated Seaweed Sargassum naozhouense Tseng et Lu" Marine Drugs 11, no. 1: 20-32. https://doi.org/10.3390/md11010020





