Diversity of Peptides Produced by Nodularia spumigena from Various Geographical Regions
Abstract
:1. Introduction
| m/z | Oligopeptide structure | Peptide no. | Peptide name | Baltic Sea | Iznik Lake | Australian waters | |||||||||||||||||||||
| CCNP 1401 | CCNP1403 | B15a | CCNP1402 | KAC66 | BY1 | CCNP 1423 | CCNP 1424 | CCNP 1425 | Node 2 | Nodg 3 | Nodh 2 | NSBL-05 | NSBL-06 | NSLA-01 | NSOR-02 | NSGL-01 | NSKR-07 | NSBR-01 | NSPH-02 | ||||||||
| Spumigins | |||||||||||||||||||||||||||
| 655 | (Hpla + 42)-Hty-Pro-Arg | 1 | ◦ | ◦ | ◦ | ||||||||||||||||||||||
| 653 | (Hpla + 42)-Hty-MePro-Argal | 2 | ◦ | ◦ | ◦ | ||||||||||||||||||||||
| 641 | (Hpla + 42)-Hty-Pro-Argol | 3 | ● | ● | ○ | ||||||||||||||||||||||
| 639 | (Hpla + 42)-Hty-Pro-Argal | 4 | ● | ● | ● | ||||||||||||||||||||||
| 627 | Hpla-Hty-MePro-Arg | 5 | B | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ||||||||||||||||
| 613 | Hpla-Hty-MePro-Argol | 6 | A | ○ | ◦ | ◦ | ◦ | ○ | ◦ | ◦ | ◦ | ◦ | |||||||||||||||
| 613 | Hpla-Hty-Pro-Arg | 7 | C | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ||||||||||||||||||
| 611 | Hpla-Hty-MePro-Argal | 8 | E | ◦ | ◦ | ◦ | ● | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ||||||||||||
| 599 | Hpla-Hty-Pro-Argol | 9 | D | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ○ | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ||||
| 597 | Hpla-Hty-Pro-Argal | 10 | F | ○ | ○ | ◦ | ◦ | ○ | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ○ | ◦ | ◦ | ◦ | ○ | ◦ | ◦ | ◦ | ||||
| 595 | Hpla-Hph-MePro-Argal | 11 | G | ◦ | ○ | ◦ | ◦ | ◦ | |||||||||||||||||||
| 583 | Hpla-Tyr-Pro-Argal | 12 | ◦ | ◦ | ◦ | ||||||||||||||||||||||
| 583 | Hpla-Hty-MePro-Agm | 13 | ◦ | ◦ | ◦ | ||||||||||||||||||||||
| 581 | Hpla-Hph-Pro-Argal | 14* | H | ||||||||||||||||||||||||
| 575 | (Hpla + 42)-Leu-Pro-Argal | 15 | ◦ | ◦ | ◦ | ||||||||||||||||||||||
| 535 | Hpla-Leu-Pro-Argol | 16* | I | ||||||||||||||||||||||||
| 470 | Hpla-Hty-MePro-NH2 | 17 | ◦ | ◦ | ◦ | ||||||||||||||||||||||
| 457 | Hpla-Hty-Pro-OH | 18 | ◦ | ◦ | ◦ | ◦ | ◦ | ||||||||||||||||||||
| Aeruginosins | |||||||||||||||||||||||||||
| 603 | -Choi-Arg | 19 | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | |||||||||
| 589 | -Choi-Argol | 20 | ◦ | ◦ | ◦ | ○ | ● | ◦ | ○ | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ○ | ○ | ◦ | ||||||||
| 587 | -Choi-Argal | 21 | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ● | ● | ● | ● | ● | ● | ● | ◦ | |||||
| 559 | -Choi-Agm | 22 | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ||||||||||||||
| Nodularins | |||||||||||||||||||||||||||
| 825 | Cyclo[MeAsp-Arg-Adda-Glu-Mdhb] | 23 | NOD | ● | ● | ● | ● | ● | ● | ○ | ○ | ○ | ● | ● | ● | ● | ● | ● | ● | ● | ● | ||||||
| 811 | Cyclo[Asp-Arg-Adda-Glu-Mdhb] | 24 | [dMeAsp3] | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | ○ | ○ | ○ | ◦ | ◦ | ◦ | ◦ | ○ | ◦ | ◦ | ◦ | ● | ||||||
| NOD | |||||||||||||||||||||||||||
| Anabaenopeptins (Nodulapeptins) | |||||||||||||||||||||||||||
| 934 | Phe-CO-[Lys-Val-Hty-MeHty-MetO] | 25 | ◦ | ○ | ○ | ○ | |||||||||||||||||||||
| 932 | Ile-CO-[Lys-MetO-Hph-MeHty-MetO] | 26 | ◦ | ◦ | |||||||||||||||||||||||
| 930 | Ile-CO-[Lys-MetO2-Hph-MeHty-AcSer] | 27 | NP A | ||||||||||||||||||||||||
| 918 | Phe-CO-[Lys-Val-Hph-MeHty-MetO] | 28 | NP 917 | ◦ | ○ | ○ | ○ | ||||||||||||||||||||
| 916 | Ile-CO-[Lys-MetO-Hph-MeHty-Met] | 29 | ○ | ◦ | ◦ | ◦ | ◦ | ||||||||||||||||||||
| 916 | Phe-CO-[Lys-Val-Hty-MeHty-AcSer] | 30 | ◦ | ○ | ○ | ○ | |||||||||||||||||||||
| 914 | Ile-CO-[Lys-MetO-Hph-MeHty-AcSer] | 31 | NP B | ● | ● | ◦ | ◦ | ◦ | |||||||||||||||||||
| 902 | Phe-CO-[Lys-Val-Hph-MeHty-Met] | 32 | NP 901 | ◦ | ○ | ○ | ○ | ||||||||||||||||||||
| 900 | Phe-CO-[Lys-Val-Hph-MeHty-AcSer] | 33 | NP 899 | ◦ | ○ | ○ | ○ | ||||||||||||||||||||
| 900 | Ile-CO-[Lys-Met-Hph-MeHty-Met] | 34 | [Met6] | ◦ | ◦ | ||||||||||||||||||||||
| NP C | |||||||||||||||||||||||||||
| 898 | Ile-CO-[Lys-Met-Hph-MeHty-AcSer] | 35 | NP C | ◦ | ○ | ○ | ○ | ◦ | |||||||||||||||||||
| 898 | Ile-CO-[Lys-MetO-Hph-MeHph-AcSer] | 36 | [MeHph5] | ● | ○ | ○ | ○ | ○ | |||||||||||||||||||
| NP B | |||||||||||||||||||||||||||
| 884 | Ile-CO-[Lys-Met-Hph-MeHph-Met] | 37 | ◦ | ||||||||||||||||||||||||
| 884 | Phe-CO-[Lys-Val-Hph-MeHph-AcSer] | 38 | ◦ | ○ | ○ | ○ | |||||||||||||||||||||
| 882 | Ile-CO-[Lys-Met-Hph-MeHph-AcSer] | 39 | ● | ◦ | |||||||||||||||||||||||
| 882 | Ile-CO-[Lys-Ile-Hph-MeHty-Met] | 40 | ● | ||||||||||||||||||||||||
| 880 | Ile-CO-[Lys-Ile-Hph-MeHty-AcSer] | 41 | ◦ | ◦ | ◦ | ◦ | ◦ | ||||||||||||||||||||
| 872 | Ile-CO-[Lys-MetO-Hph-MeHty-Ser] | 42 | [Ser6] | ◦ | ◦ | ◦ | ◦ | ◦ | |||||||||||||||||||
| NP B | |||||||||||||||||||||||||||
| 856 | Ile-CO-[Lys-Met-Hph-MeHty-Ser] | 43 | ◦ | ||||||||||||||||||||||||
| 856 | Ile-CO-[Lys-MetO-Hph-MeHph-Ser] | 44 | ◦ | ◦ | ◦ | ◦ | ◦ | ||||||||||||||||||||
| 842 | Phe-CO-[Lys-Ile-Hty-MeAla-Phe] | 45 | ● | ● | ● | ||||||||||||||||||||||
| 828 | Phe-CO-[Lys-Val-Hty-MeAla-Phe] | 46 | AP D | ◦ | ◦ | ◦ | |||||||||||||||||||||
| 808 | Ile-CO-[Lys-Ile-Hty-MeAla-Phe] | 47 | ○ | ○ | ◦ | ◦ | ○ | ◦ | ◦ | ◦ | |||||||||||||||||
| Unknown peptides | |||||||||||||||||||||||||||
| 829 | Unknown | 48 | ○ | ○ | ◦ | ◦ | ◦ | ◦ | ◦ | ◦ | |||||||||||||||||
| 576 | Unknown | 49 | ● | ● | ● | ● | ● | ● | ● | ● | |||||||||||||||||
| 562 | Unknown | 50 | ◦ | ◦ | ○ | ◦ | ◦ | ◦ | ○ | ||||||||||||||||||
2. Results and Discussion
2.1.Linear Peptides: Spumigins and Aeruginosins
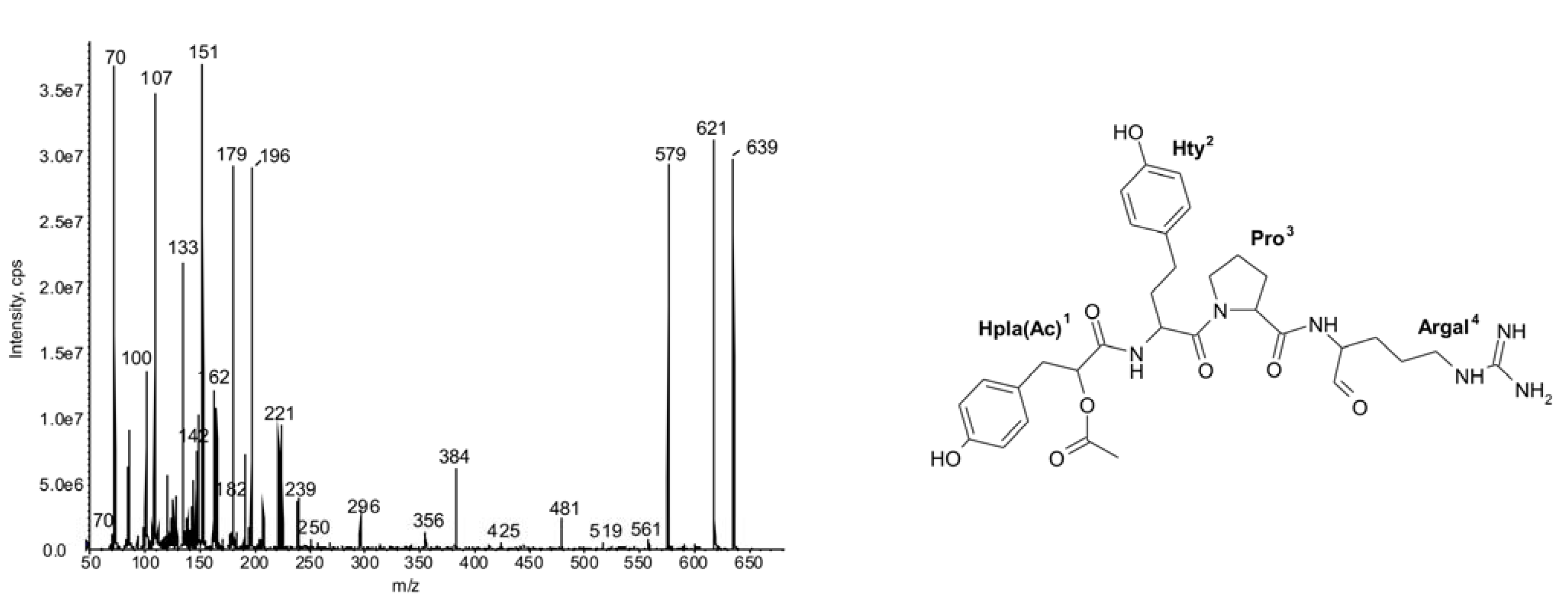
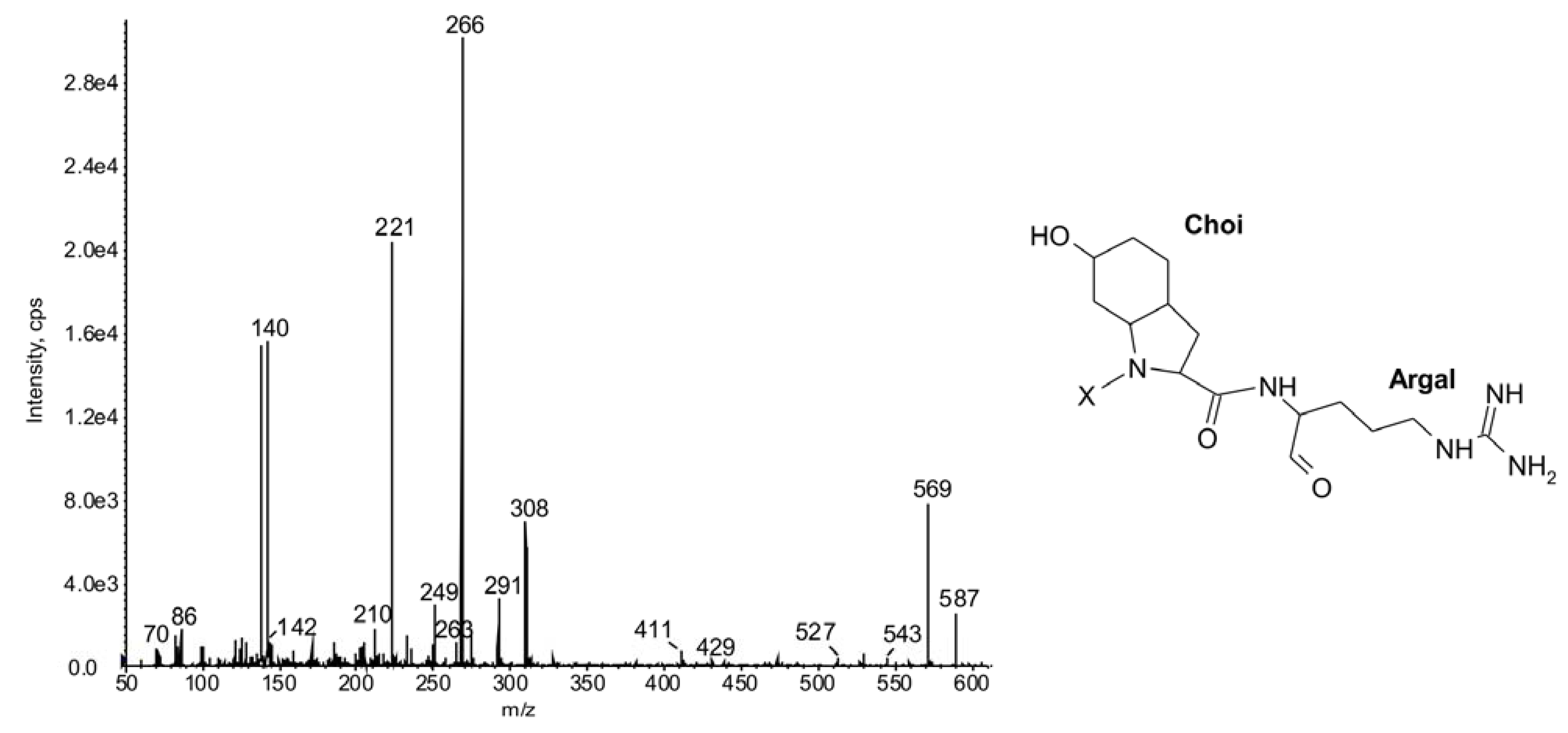
2.2. Cyclic Peptides: Nodularins and Anabaenopeptins
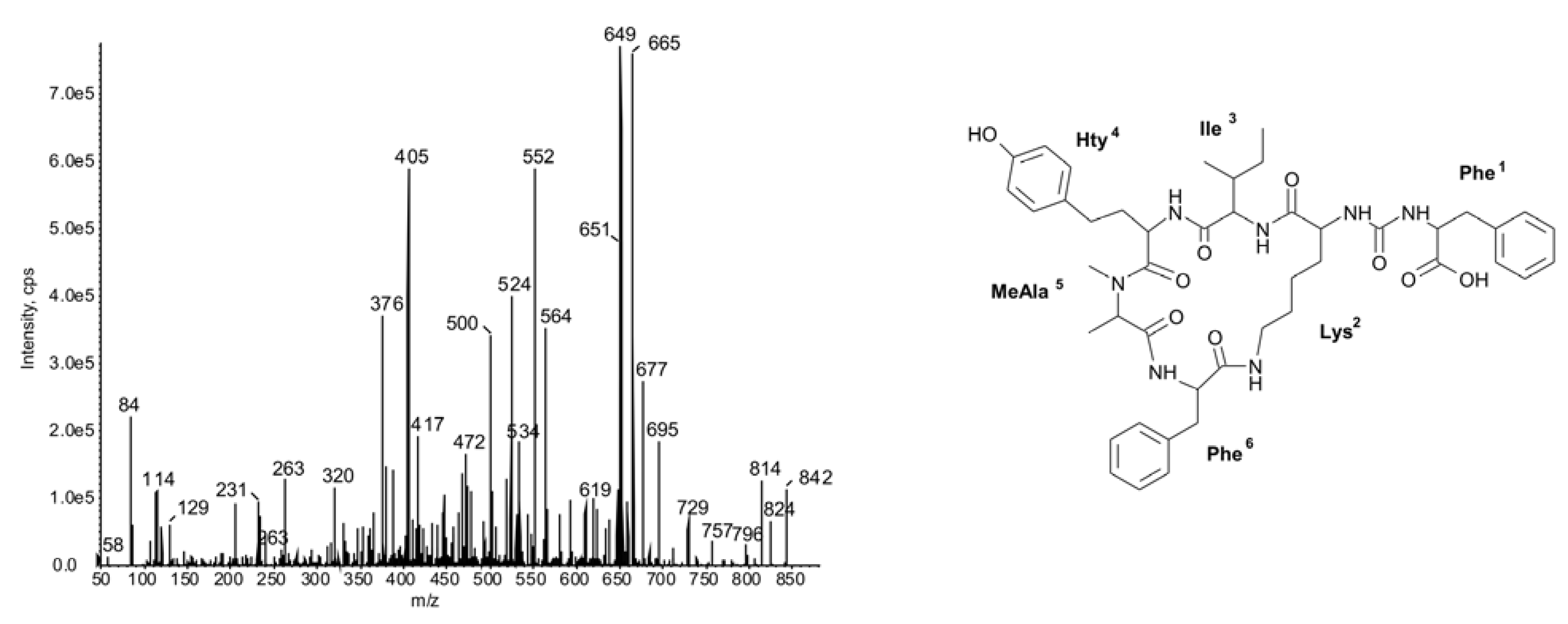
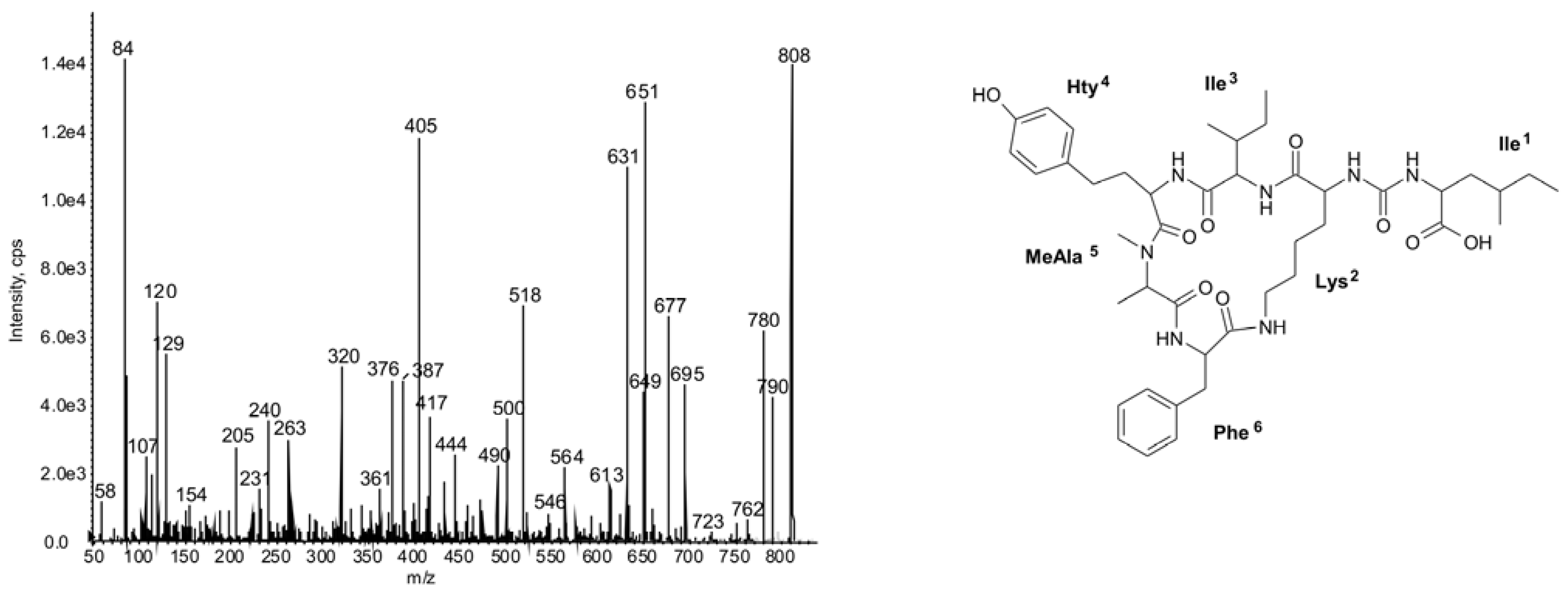
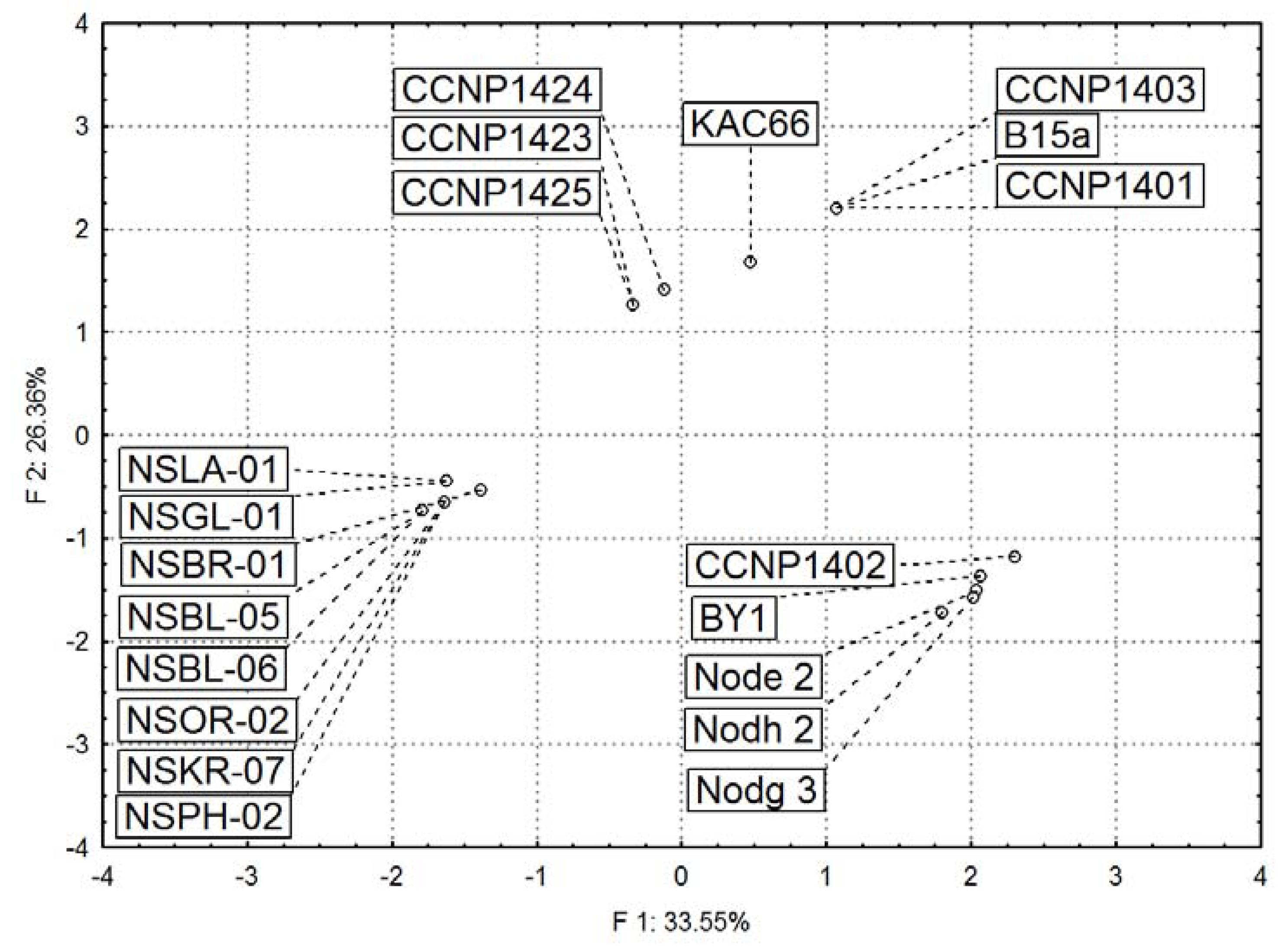
2.3. Diversity in Peptidomic Profiles of N. spumigena Strains
3. Experimental Section
3.1. Source of Cyanobacteria and Culture Conditions
3.2. Extraction and LC-MS/MS Analyses
3.3. Statistical Analyses
4. Conclusions
Acknowledgments
Supplementary Files
References
- Welker, M.; von Döhren, H. Cyanobacterial peptides—Nature’s own combinatorial biosynthesis. FEMS Microbiol. Rev. 2006, 30, 530–563. [Google Scholar] [CrossRef]
- Ishida, K.; Christiansen, G.; Yoshida, W.Y.; Kurmayer, R.; Welker, M.; Valls, N.; Bonjoch, J.; Hertweck, C.; Börner, T.; Hemscheidt, T.; et al. Biosynthesis and structure of aeruginoside 126A and 126B, cyanobacterial peptide glycosides bearing a 2-carboxy-6-hydroxyoctahydroindole moiety. Chem. Biol. 2007, 14, 565–576. [Google Scholar] [CrossRef]
- Fewer, D.P.; Jokela, J.; Rouhiainen, L.; Wahlsten, M.; Koskenniemi, K.; Stal, L.J.; Sivonen, K. The non-ribosomal assembly and frequent occurrence of the protease inhibitors spumigins in the bloom-forming cyanobacterium Nodularia spumigena. Mol. Microbiol. 2009, 73, 924–937. [Google Scholar] [CrossRef]
- Rouhiainen, L.; Jokela, J.; Fewer, D.P.; Urmann, M.; Sivonen, K. Two alternative starter modules for the non-ribosomal biosynthesis of specific anabaenopeptin variants in Anabaena (Cyanobacteria). Chem. Biol. 2010, 17, 265–273. [Google Scholar] [CrossRef]
- Schmidt, E.W.; Donia, M.S. Cyanobactin ribosomally synthesized peptides—A case of deep metagenome mining. Methods Enzymol. 2009, 458, 575–596. [Google Scholar]
- Sivonen, K.; Leikoski, N.; Fewer, D.P.; Jokela, J. Cyanobactin-ribosomal cyclic peptides produced by cyanobacteria. Appl. Microbiol. Biotechnol. 2010, 86, 1213–1225. [Google Scholar] [CrossRef]
- Ziemert, N.; Ishida, K.; Liaimer, A.; Hertweck, C.; Dittmann, E. Ribosomal synthesis of tricyclic depsipeptides in bloom-forming cyanobacteria. Angew. Chem. Int. Ed. 2008, 47, 7756–7759. [Google Scholar]
- Rounge, T.B.; Rohrlack, T.; Nederbragt, A.J.; Kristensen, T.; Jakobsen, K.S. A genome-wide analysis of nonribosomal peptide synthetase gene clusters and their peptides in a Planktothrix rubescens strain. BMC Genomics 2009, 10, 1–11. [Google Scholar]
- Dittmann, E.; Neilan, B.A.; Börner, T. Molecular biology of peptide and polyketide biosynthesis in cyanobacteria. Appl. Microbiol. Biotechnol. 2001, 57, 467–473. [Google Scholar]
- Moffitt, M.C.; Neilan, B.A. The expansion of mechanistic and organismic diversity associated with non-ribosomal peptides. FEMS Microbiol. Lett. 2000, 191, 159–167. [Google Scholar] [CrossRef]
- Harada, K.-I.; Fujii, K.; Shimada, T.; Suzuki, M.; Sano, H.; Adach, K. Two cyclic peptides, anabaenopeptins, a third group of bioactive compounds from the cyanobacterium Anabaena flos-aquae NRC 525-17. Tetrahedron Lett. 1995, 36, 1511–1514. [Google Scholar] [CrossRef]
- Fujii, K.; Mayumi, T.; Noguchi, K.; Kashiwagi, T.; Akashi, S.; Sivonen, K.; Hirayama, K.; Harada, K.-I. Mass spectrometry studies of peptides from cyanobacteria under FAB MS/MS conditions. J. Mass. Spectrom. Soc. Jpn. 2000, 48, 56–64. [Google Scholar] [CrossRef]
- Welker, M.; Christiansen, G.; van Döhren, H. Diversity of coexisting Planktothrix (Cyanobacteria) chemotypes deduced by mass spectral analysis of microcystins and other oligopeptides. Arch. Microbiol. 2004, 182, 288–298. [Google Scholar] [CrossRef]
- Welker, M.; Brunke, M.; Preussel, K.; Lippert, I.; van Döhren, H. Diversity and distribution of Microcystis (Cyanobacteria) oligopeptide chemotypes from natural communities studied by single-colony mass spectrometry. Microbiology 2004, 150, 1785–1796. [Google Scholar] [CrossRef]
- Ohta, T.; Sueoka, E.; Iida, N.; Komori, A.; Suganuma, M.; Nishiwaki, R.; Tatematsu, M.; Kim, S.J.; Carmichael, W.W.; Fujiki, H. Nodularin, a potent inhibitor of protein phosphatases 1 and 2A, a new environmental carcinogen in male F344 rat liver. Cancer Res. 1994, 54, 6402–6406. [Google Scholar]
- Ishida, K.; Okita, Y.; Matsuda, H.; Okino, T.; Murakami, M. Aeruginosins, protease inhibitors from the cyanobacterium Microcystis aeruginosa. Tetrahedron 1999, 55, 10971–10988. [Google Scholar] [CrossRef]
- Reshef, V.; Carmeli, S. Protease inhibitors from a water bloom of the cyanobacterium Microcystis aeruginosa. Tetrahedron 2001, 57, 2885–2894. [Google Scholar] [CrossRef]
- Rohrlack, T.; Christoffersen, K.; Kaebernick, M.; Neilan, B.A. Cyanobacterial protease inhibitor microviridin J causes a lethal molting disruption in Daphnia pulicaria. Appl. Environ. Microbiol. 2004, 70, 5047–5050. [Google Scholar] [CrossRef]
- Czarnecki, O.; Henning, M.; Lippert, I.; Welker, M. Identification of peptide metabolites of Microcystis (Cyanobacteria) that inhibit trypsin-like activity in planktonic herbivorous Daphnia (Cladocera). Environ. Microbiol. 2006, 8, 77–87. [Google Scholar] [CrossRef]
- Gesner-Apter, S.; Carmeli, S. Protease inhibitors from a water bloom of the cyanobacterium Microcystis aeruginosa. J. Nat. Prod. 2009, 72, 1429–1436. [Google Scholar] [CrossRef]
- Radau, G.; Gebel, J.; Rauh, D. New cyanopeptide-derived low molecular weight thrombin inhibitors. Arch. Pharm. 2003, 336, 372–380. [Google Scholar]
- Ersmark, K.; Del Valle, J.R.; Hanessian, S. Chemistry and biology of the aeruginosin family of serine protease inhibitors. Angew. Chem. Int. Ed. 2008, 47, 1202–1223. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Sinha, S.P. Biotechnology and industrial significance of cyanobacterial secondary metabolites. Biotechnol. Adv. 2009, 27, 521–539. [Google Scholar] [CrossRef]
- Silva-Stenico, M.E.; Silva, C.S.; Lorenzi, A.S.; Shishido, T.K.; Etchegaray, A.; Lira, S.P.; Moraes, L.A.; Fiore, M.F. Non-ribosomal peptides produced by Brazilian cyanobacterial isolates with antimicrobial activity. Microbiol. Res. 2010, 166, 161–175. [Google Scholar]
- Rinehart, K.L.; Harada, K.; Namikoshi, M.; Chen, C.; Harvis, C.A.; Munroe, M.H.G.; Blunt, J.W.; Mulligan, P.E.; Beasley, V.R.; Dahlem, A.M.; et al. Nodularin, microcystin and the configuration of Adda. J. Am. Chem. Soc. 1988, 110, 8557–8558. [Google Scholar]
- Namikoshi, M.; Choi, B.W.; Sakai, R.; Sun, F.; Rinehart, K.L.; Carmichael, W.W.; Evans, W.R.; Cruz, P.; Munro, M.H.G.; Blunt, J.W. New nodularin: A general method for structure assignment. J. Org. Chem. 1994, 59, 2349–2357. [Google Scholar]
- Fujii, K.; Sivonen, K.; Adachi, K.; Noguchi, K.; Sano, H.; Hirayama, K.; Suzuki, M.; Harada, K.-I. Comparative study of toxic and non-toxic cyanobacteria products: Novel peptides from toxic Nodularia spumigena AV1. Tetrahedron Lett. 1997, 38, 5525–5528. [Google Scholar]
- Beattie, K.A.; Kaya, K.; Codd, G.A. The cyanobacterium Nodularia PCC 7804, of freshwater origin, produces [L-Har2] nodularin. Phytochemistry 2000, 54, 57–61. [Google Scholar]
- Gehringer, M.M.; Adler, L.; Roberts, A.A.; Moffitt, M.C.; Mihali, T.K.; Mills, T.J.; Fieker, C.; Neilan, B.A. Nodularin, a cyanobacterial toxin, is synthesized in planta by symbiotic Nostoc sp. ISME J. 2012, 6, 1834–1847. [Google Scholar] [CrossRef]
- Kaasalainen, U.; Fewer, D.P.; Jokele, J.; Wahlsten, M.; Sivonen, K.; Rikkinen, J. Cyanobacteria produce a high variety of hepatotoxic peptides in lichen symbiosis. Proc. Natl. Acad. Sci. USA 2012, 109, 5886–5891. [Google Scholar]
- Ploutno, A.; Shoshan, M.; Carmeli, S. Three novel protease inhibitors from a natural bloom of the cyanobacterium Microcystis aeruginosa. J. Nat. Prod. 2002, 65, 973–978. [Google Scholar] [CrossRef]
- Ishida, K.; Welker, M.; Christiansen, G.; Cadel-Six, S.; Bouchier, C.; Dittmann, E. Plasticity and evolution of aeruginosin biosynthesis in cyanobacteria. Appl. Environ. Microbiol. 2009, 75, 2017–2026. [Google Scholar]
- Murakami, M.; Ishida, K.; Okino, T.; Okira, Y.; Matsuda, H.; Yamaguchi, K. Aeruginosins 98-A and B, trypsyn inhibitors from the blue-green alga Microcystis aeruginosa (NIES-98). Tetrahedron Lett. 1995, 36, 2785–2788. [Google Scholar]
- Hanessian, S.; Tremblay, M.; Petersen, J.F.W. The N-acyloxyiminium ion aza-prins route to octahydroindoles: Total synthesis and structural confirmation of the antithrombotic marine natural product oscillarin. J. Am. Chem. Soc. 2004, 126, 6064–6071. [Google Scholar] [CrossRef]
- Cadel-Six, S.; Dauga, C.; Castets, A.M.; Rippka, R.; Bouchier, C.; de Marsac, N.T.; Welker, M. Halogenase genes in nonribosomal peptide synthetase gene clusters of Microcystis (Cyanobacteria): Sporadic distribution and evolution. Mol. Biol. Evol. 2008, 25, 2031–2041. [Google Scholar] [CrossRef]
- Welker, M.; Maršálek, B.; Šejnohová, L.; von Döhren, H. Detection and identification of oligopeptides in Microcystis (Cyanobacteria) colonies: Toward an understanding of metabolic diversity. Peptides 2006, 27, 2090–2103. [Google Scholar] [CrossRef]
- Pearson, L.; Mihali, T.; Moffitt, M.; Kellmann, R.; Neilan, B. On the chemistry, toxicology and genetics of the cyanobacterial toxins, microcystin, nodularin, saxitoxin and cylindrospermopin. Mar. Drugs 2010, 8, 1650–1680. [Google Scholar] [CrossRef]
- Bolch, C.J.S.; Orr, P.T.; Jones, G.J.; Blackburn, S.I. Genetic, morphological, and toxicological variation among globally distributed strains of Nodularia (Cyanobacteria). J. Phycol. 1999, 35, 339–355. [Google Scholar] [CrossRef]
- Moffitt, M.C.; Blackburn, S.I.; Neilan, B.A. rRNA sequences reflect the ecophysiology and define the toxic cyanobacteria of the genus Nodularia. Int. J. Syst. Evol. Microbiol. 2001, 51, 505–512. [Google Scholar]
- Erhard, M.; von Döhren, H.; Jungblut, P.R. Rapid identification of the new anabaenopeptin G from Planktothrix agardhii HUB 011 using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 1999, 13, 337–342. [Google Scholar]
- Mayumi, T.; Kato, H.; Kawasaki, Y.; Harada, K.-I. Formation of diagnostic product ions from cyanobacterial cyclic peptides by the two-bond fission mechanism using ion trap liquid chromatography/multi-stage mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 1025–1033. [Google Scholar]
- Puddick, J.; Prinsep, M.R. MALDI-TOF mass spectrometry of cyanobacteria: A global approach to the discovery of novel secondary metabolites. Chem. N. Z. 2008, 72, 68–71. [Google Scholar]
- Schumacher, M.; Wilson, N.; Tabudravu, J.N.; Edwards, C.; Lawton, L.A.; Motti, C.; Wright, A.D.; Diederich, M.; Jaspars, M. New nodulapeptins from Nodularia spumigena KAC 66. Tetrahedron 2011, 68, 1622–1628. [Google Scholar]
- Christiansen, G.; Philmus, B.; Hemscheidt, T.; Kurmayer, R. Genetic variation of adenylation domains of the anabaenopeptin synthesis operon and evolution of the substrate promiscuity. J. Bacteriol. 2011, 193, 3822–3831. [Google Scholar] [CrossRef]
- Lehtimäki, J.; Lyra, C.; Suomalainen, S.; Sundman, P.; Rouhiainen, L.; Paulin, L.; Salkinoja-Salonen, M.; Sivonen, K. Characterization of Nodularia strains, cyanobacteria from brackish waters, by genotypic and phenotypic methods. Int. J. Syst. Evol. Microbiol. 2000, 50, 1043–1053. [Google Scholar] [CrossRef]
- Hayes, P.K.; Barker, G.L.A. Genetic diversity within Baltic Sea populations of Nodularia (Cyanobacteria). J. Phycol. 1997, 33, 919–923. [Google Scholar]
- Laamanen, M.J.; Gugger, M.F.; Lehtimäki, J.; Haukka, K.; Sivonen, K. Diversity of toxic and non-toxic Nodularia isolates (Cyanobacteria) and filaments from the Baltic Sea. Appl. Environ. Microbiol. 2001, 67, 4638–4647. [Google Scholar] [CrossRef]
- Fastner, J.; Erhard, M.; von Döhren, H. Determination of oligopeptide diversity within a natural population of Microcystis spp. (Cyanobacteria) by typing single colonies by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Appl. Environ. Microbiol. 2001, 7, 5069–5076. [Google Scholar]
- Yépremian, C.; Gugger, M.F.; Briand, E.; Catherine, A.; Berger, C.; Quiblier, C.; Bernard, C. Microcystin ecotypes in a perennial Planktothrix agardhii bloom. Water Res. 2007, 41, 4446–4456. [Google Scholar] [CrossRef]
- Rohrlack, T.; Edvardsen, B.; Skulberg, R.; Halstvedt, B.; Utkilen, H.C.; Ptacnik, R.; Skulberg, O.M. Oligopeptide chemotypes of the toxic freshwater cyanobacterium Planktothrix can form subpopulations with dissimilar ecological traits. Limnol. Oceanogr. 2008, 53, 1279–1293. [Google Scholar] [CrossRef]
- Martins, J.; Saker, M.L.; Moreira, C.; Welker, M.; Fastner, J.; Vasconcelos, V.M. Peptide diversity in strains of the cyanobacterium Microcystis aeruginosa isolated from Portuguese water supplies. Appl. Microbiol. Biotechnol. 2009, 82, 951–961. [Google Scholar] [CrossRef]
- Haande, S.; Ballot, A.; Rohrlack, T.; Fastner, J.; Wiedner, C.; Edvardsen, B. Diversity of Microcystis aeruginosa isolates (Chroococcales, Cyanobacteria) from East-African water bodies. Arch. Microbiol. 2007, 188, 15–25. [Google Scholar] [CrossRef]
- Rantala, A.; Fewer, D.P.; Hisbergues, M.; Rouhiainen, L.; Vaitomaa, J.; Börner, T.; Sivonen, K. Phylogenetic evidence for the early evolution of microcystin synthesis. Proc. Natl. Acad. Sci. USA 2004, 101, 568–573. [Google Scholar]
- Moffitt, M.C.; Neilan, B.A. Characterization of the nodularin synthetase gene cluster and proposed theory of the evolution of cyanobacterial hepatotoxins. Appl. Environ. Microbiol. 2004, 70, 6353–6362. [Google Scholar] [CrossRef]
- Rounge, T.B.; Rohrlack, T.; Decenciere, B.; Edvardsen, B.; Kristensen, T.; Jakobsen, K.S. Subpopulation differentiation associated with nonribosomal peptide synthetase gene cluster dynamics in the cyanobacterium Planktothrix spp. J. Phycol. 2010, 46, 645–652. [Google Scholar] [CrossRef]
- Suikkanen, S.; Fistarol, G.O.; Granéli, E. Allelopathic effects of the Baltic cyanobacteria Nodularia spumigena, Aphanizomenon flos-aquae and Anabaena lemmermannii on algal monocultures. J. Exp. Mar. Biol. Ecol. 2004, 308, 85–101. [Google Scholar] [CrossRef]
- Schatz, D.; Keren, Y.; Vardi, A.; Sukenik, A.; Carmeli, S.; Börner, T.; Dittmann, E.; Kaplan, A. Towards clarification of the biological role of microcystins, a family of cyanobacterial toxins. Environ. Microbiol. 2007, 9, 965–970. [Google Scholar] [CrossRef]
- Sedmak, B.; Carmeli, S.; Eleršek, T. Non-toxic” cyclic peptides induce lysis of cyanobacteria—An effective cell population density control mechanism in cyanobacterial blooms. Microbiol. Ecol. 2008, 56, 201–209. [Google Scholar] [CrossRef]
- Sønstebø, J.H.; Rohrlack, T. Possible implication of chytrid parasitism for population subdivision in freshwater cyanobacteria of the genus Planktothrix. Appl. Environ. Microbiol. 2011, 77, 1344–1351. [Google Scholar] [CrossRef]
- Sano, T.; Usui, T.; Ueda, K.; Osada, H.; Kaya, K. Isolation of new protein phosphatase inhibitors from two cyanobacteria species, Planktothrix sp. J. Nat. Prod. 2001, 64, 1052–1055. [Google Scholar] [CrossRef]
- Sano, T.; Kaya, K. Oscillamide Y, a chymotrypsin inhibitor from toxic Oscillatoria agardhii. Tetrahedron Lett. 1995, 36, 5933–5936. [Google Scholar]
- Murakami, M.; Suzuki, S.; Itou, Y.; Kodani, S.; Ishida, K. New anabaenopeptins, potent carboxypeptidase—A inhibitors from the cyanobacterium Aphanizomenon flos-aquae. J. Nat. Prod. 2000, 83, 1280–1282. [Google Scholar]
- Bubik, A.; Sedmak, B.; Novinec, M.; Lenarčič, B.; Lah, T.T. Cytotoxic and peptidase inhibitory activities of selected non-hepatotoxic cyclic peptides from cyanobacteria. Biol. Chem. 2008, 389, 1339–1346. [Google Scholar]
- Samples Availability: Available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mazur-Marzec, H.; Kaczkowska, M.J.; Blaszczyk, A.; Akcaalan, R.; Spoof, L.; Meriluoto, J. Diversity of Peptides Produced by Nodularia spumigena from Various Geographical Regions. Mar. Drugs 2013, 11, 1-19. https://doi.org/10.3390/md11010001
Mazur-Marzec H, Kaczkowska MJ, Blaszczyk A, Akcaalan R, Spoof L, Meriluoto J. Diversity of Peptides Produced by Nodularia spumigena from Various Geographical Regions. Marine Drugs. 2013; 11(1):1-19. https://doi.org/10.3390/md11010001
Chicago/Turabian StyleMazur-Marzec, Hanna, Monika J. Kaczkowska, Agata Blaszczyk, Reyhan Akcaalan, Lisa Spoof, and Jussi Meriluoto. 2013. "Diversity of Peptides Produced by Nodularia spumigena from Various Geographical Regions" Marine Drugs 11, no. 1: 1-19. https://doi.org/10.3390/md11010001
APA StyleMazur-Marzec, H., Kaczkowska, M. J., Blaszczyk, A., Akcaalan, R., Spoof, L., & Meriluoto, J. (2013). Diversity of Peptides Produced by Nodularia spumigena from Various Geographical Regions. Marine Drugs, 11(1), 1-19. https://doi.org/10.3390/md11010001







