Shrimp Lipids: A Source of Cancer Chemopreventive Compounds
Abstract
:1. Introduction
2. Chemoprevention
2.1. Definition of Chemoprevention
2.2. Types of Chemopreventive Activities
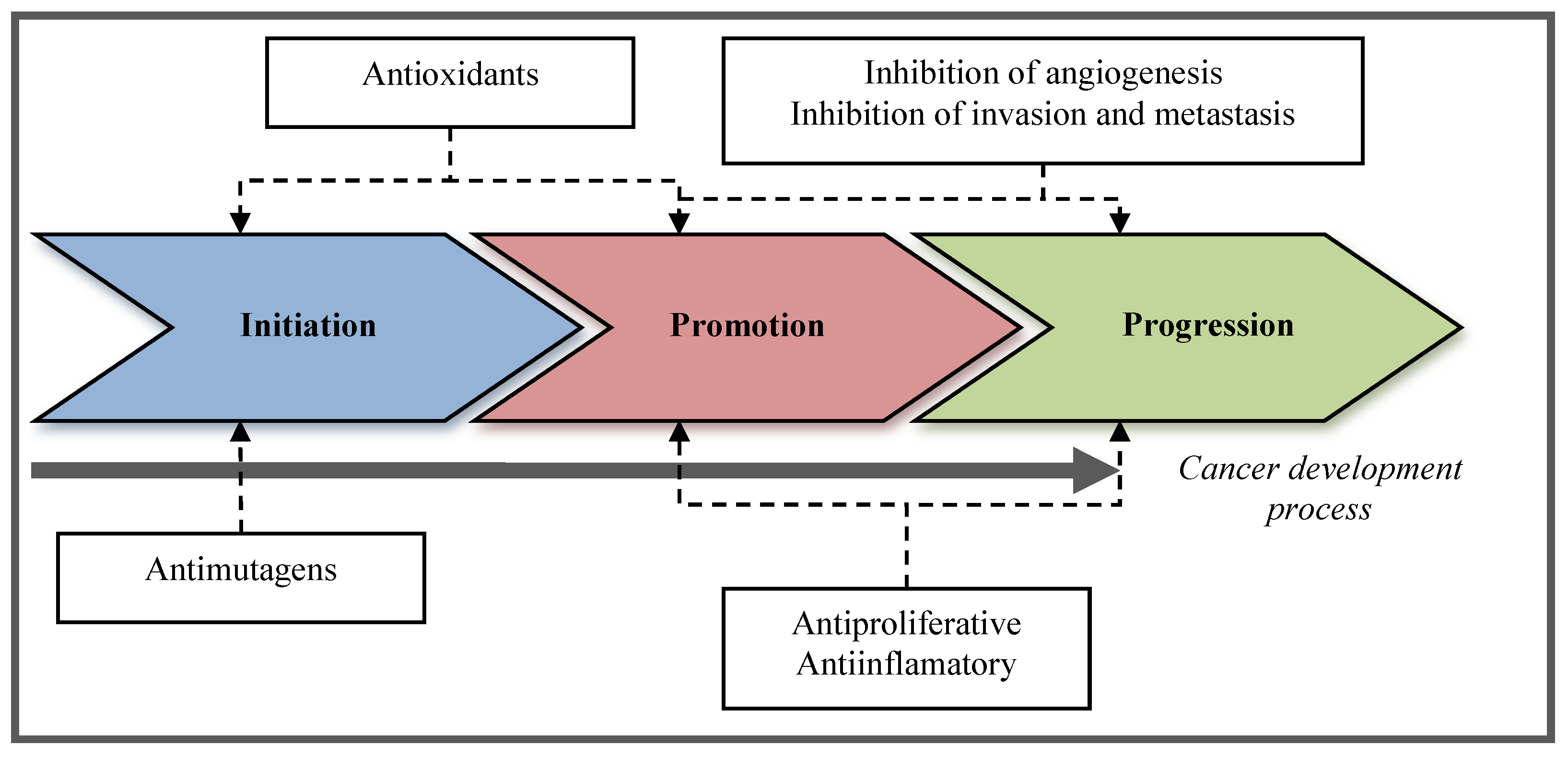
3. Chemopreventive Compounds in the Lipidic Fraction of Shrimp
3.1. Lipidic Content of Shrimp Muscle
3.2. Carotenoids
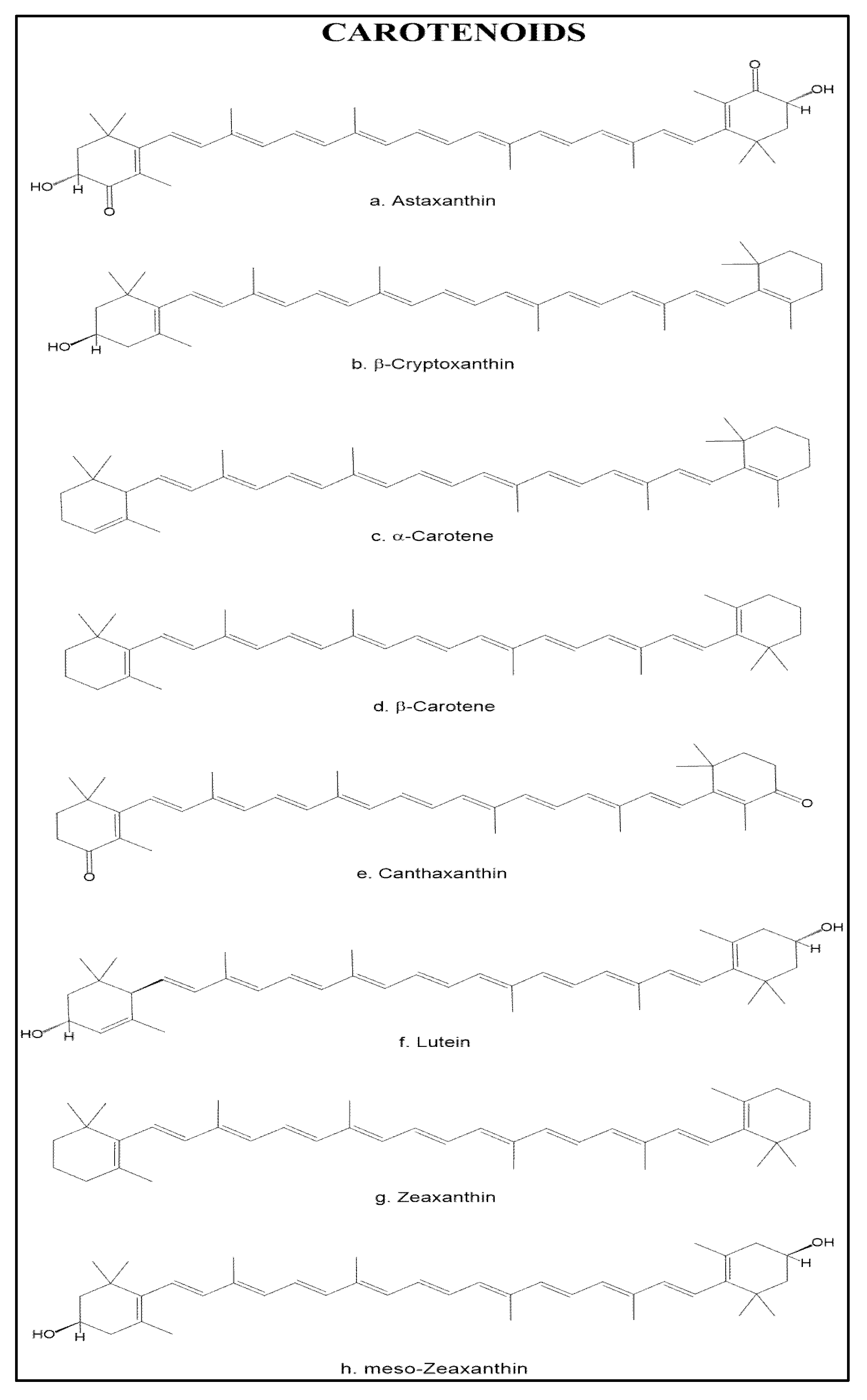

3.2.1. Antioxidant Mechanism
3.2.2. Intervention in Cell Cycle
3.2.3. Antimutagenic Activity
3.2.4. Anti-Inflammatory Mechanism and Tumor Immunity
3.3. Polyunsaturated Fatty Acids
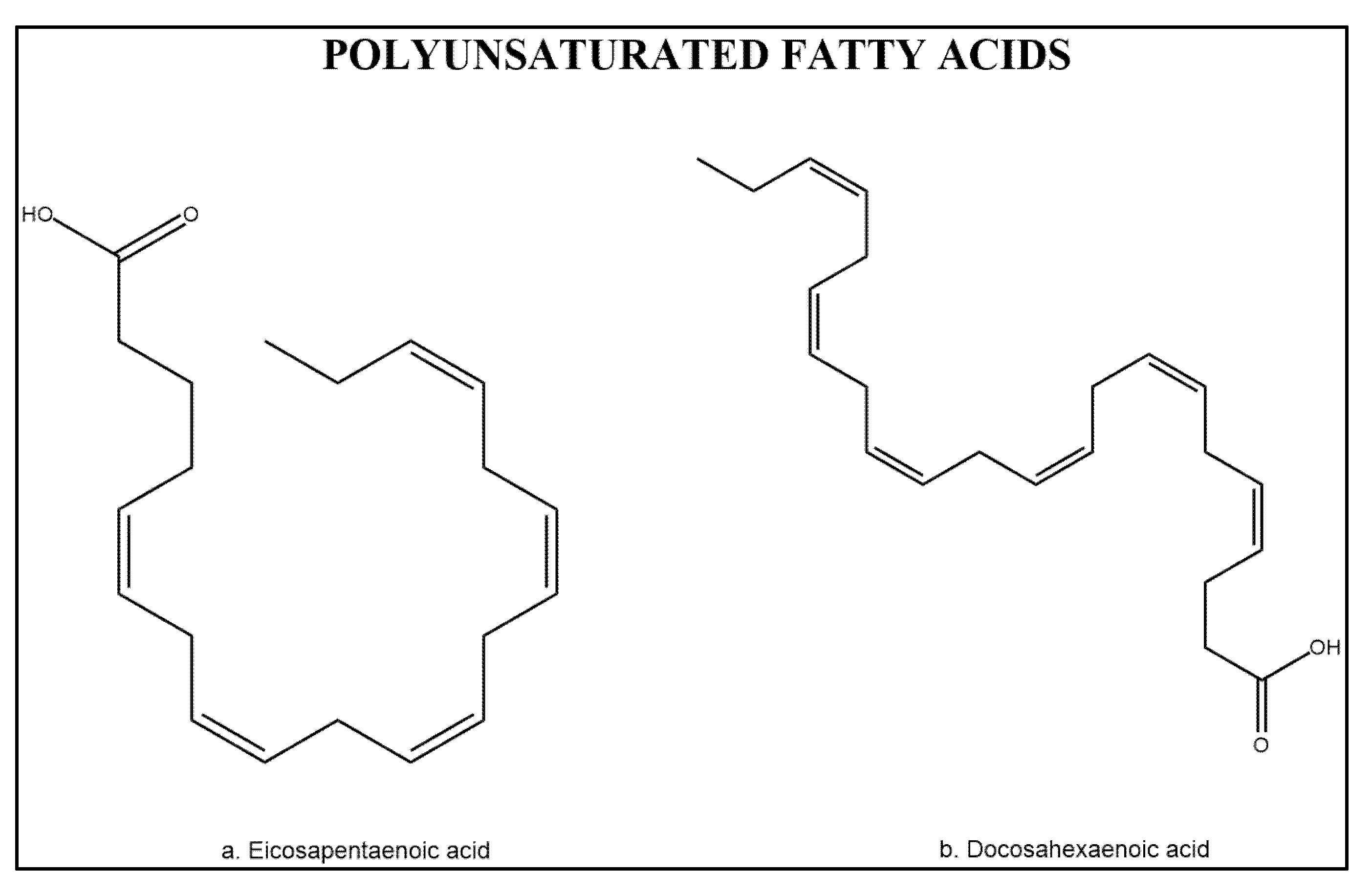
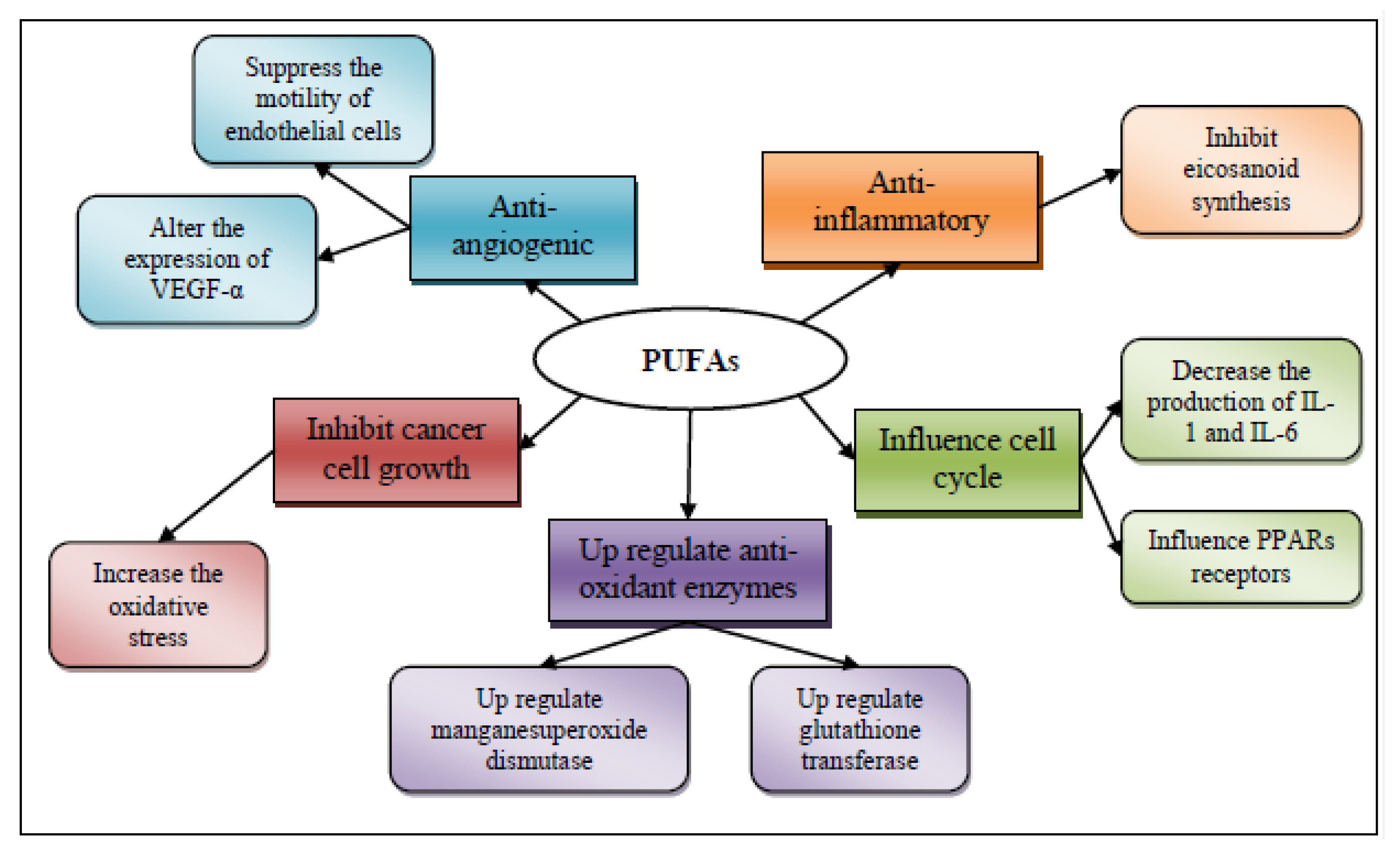
3.3.1. Anti-Inflammatory Effect of Polyunsaturated Fatty Acids
3.3.2. Influence in Transcription Factor Activity
3.3.3. Increased or Decreased Production of Free Radicals and Radical Oxygen Species
3.3.4. Antiangiogenic Potential
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Oksuz, A.; Ozylmaz, A.; Aktas, M.; Gercek, G.; Motte, J. A comparative study on proximate, mineral and fatty acid compositions of deep seawater rose shrimp (Parapenaus longirostris, Lucas 1846) and red shrimp (Plesionika martia, A. Milne-Edwards, 1883). J. Anim. Vet. Adv. 2009, 8, 183–189. [Google Scholar]
- Gillett, R. Global Study of Shrimp Fisheries; FAO: Rome, Italy, 2008; Volume 475. [Google Scholar]
- FAO, Cultured Aquatic Species Information Programme. Penaeus Vannamei. Cultured Aquatic Species Information Programme; FAO Fisheries and Aquaculture Department: Rome, Italy, 2006.
- Silva, E.; Seidman, C.; Tian, J.; Hudgins, L.; Sacks, F.; Breslow, J. Effects of shrimp consumption on plasma lipoproteins. Am. J. Clin. Nutr. 1996, 64, 712–717. [Google Scholar]
- Wilson-Sanchez, G.; Moreno-Félix, C.; Velazquez, C.; Plascencia-Jatomea, M.; Acosta, A.; Machi-Lara, L.; Aldana-Madrid, M.L.; Ezquerra-Brauer, J.M.; Robles-Zepeda, R.; Burgos-Hernandez, A. Antimutagenicity and antiproliferative studies of lipidic extracts from white shrimp (Litopenaeus vannamei). Mar. Drugs 2010, 8, 2795–809. [Google Scholar] [CrossRef]
- De Rosenzweig-Pasquel, L.J.; Babbitt, J.K. Isolation and partial characterization of a natural antioxidant from shrimp (Pandalus jordani). J. Food Sci. 1991, 56, 143–145. [Google Scholar]
- Sowmya, R.; Sachindra, N.M. Evaluation of antioxidant activity of carotenoid extract from shrimp processing byproducts by in vitro assays and in membrane model system. Food Chem. 2012. [Google Scholar] [CrossRef]
- Jackson, C.; Esnouf, M.; Winzor, D.; Duewer, D. Defining and measuring biological activity: Applying the principles of metrology. Accredit. Qual. Assur. 2007, 12, 283–294. [Google Scholar] [CrossRef]
- Tsao, A.S.; Kim, E.S.; Hong, W.K. Chemoprevention of cancer. CA Cancer J. Clin. 2004, 54, 150–180. [Google Scholar] [CrossRef]
- Jemal, A.; Bray, F.; Center, M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef]
- Brenner, C.; Duggan, D. Oncogenomics: Molecular Approaches to Cancer; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Bray, F.; Møller, B. Predicting the future burden of cancer. Nat. Rev. Cancer 2006, 6, 63–74. [Google Scholar] [CrossRef]
- American Cancer Society, Cancer Prevention & Early Detection Facts & Figures; American Cancer Society: Atlanta, GA, USA, 2010.
- Society, A.C. Cancer Facts & Figures; American Cancer Society: Atlanta, GA, USA, 2011. [Google Scholar]
- Robbins, S.L.; Kumar, V.; Cotran, R.S. Patologia Humana; Elsevier: Madrid, Spain, 2004. [Google Scholar]
- Carreca, I.; Balducci, L.; Extermann, M. Cancer in the older person. Cancer Treat. Rev. 2005, 31, 380–402. [Google Scholar] [CrossRef]
- Slattery, M.L.; Edwards, S.; Curtin, K.; Ma, K.; Edwards, R.; Holubkov, R.; Schaffer, D. Physical activity and colorectal cancer. Am. J. Epidemiol. 2003, 158, 214–224. [Google Scholar] [CrossRef]
- Nerurkar, P.; Ray, R.B. Bitter melon: Antagonist to cancer. Pharm. Res. 2010, 27, 1049–1053. [Google Scholar] [CrossRef]
- Wang, Y.K.; He, H.L.; Wang, G.F.; Wu, H.; Zhou, B.C.; Chen, X.L.; Zhang, Y.Z. Oyster (Crassostrea gigas) hydrolysates produced on a plant scale have antitumor activity and immunostimulating effects in BALB/c mice. Mar. Drugs 2010, 8, 255–268. [Google Scholar] [CrossRef]
- Pelayo-Zaldivar, C. Las frutas y hortalizas como alimentos funcionales. Contactos 2003, 47, 12–19. [Google Scholar]
- Ramawat, K.G.; Goyal, S. Natural Products in Cancer Chemoprevention and Chemotherapy. In Herbal Drugs: Ethnomedicine to Modern Medicine; Ramawat, K.G., Ed.; Springer: Berlin, Germany, 2009. [Google Scholar]
- Pezzuto, J.M.; Kosmeder, J.W., II; Park, E.-J.; Lee, S.K.; Cuendet, M.; Gills, J.; Bhat, K.; Grubjesic, S.; Hye-Sung Park; Mata-Greenwood, E.; et al. Characterization of Natural Product Chemopreventive Agents. In Cancer Chemoprevention; Kellof, G.J., Hawk, E.T., Sigman, C.C., Eds.; Humana Press: Totowa, NJ, USA, 2005; Volume 2. [Google Scholar]
- Manoharan, S.; Singh, R.B.; Balakrishnan, S. Chemopreventive mechanisms of natural products in oral, mammary and skin carcinogenesis: An overview. Open Nutraceuticals J. 2009, 2, 52–63. [Google Scholar] [CrossRef]
- Chipault, J.R. Antioxidants for Use in Foods. In Autoxidation and Antioxidants; Lundberg, W.O., Ed.; Wiley: New York, NY, USA, 1962; Volume 2, pp. 477–542. [Google Scholar]
- Brambilla, D.; Mancuso, C.; Scuderi, M.R.; Bosco, P.; Cantarella, G.; Lempereur, L.; di Benedetto, G.; Pezzino, S.; Bernardini, R. The role of antioxidant supplement in immune system, neoplastic, and neurodegenerative disorders: A point of view for an assessment of the risk/benefit profile. Nutr. J. 2008, 7, 29. [Google Scholar] [CrossRef]
- Kim, S.K.; Thomas, N.V.; Li, X. Anticancer compounds from marine macroalgae and their application as medicinal foods. Adv. Food Nutr. Res. 2011, 64, 213–224. [Google Scholar] [CrossRef]
- Shankel, D.M.; Pillai, S.P.; Telikepalli, H.; Menon, S.R.; Pillai, C.A.; Mitscher, L.A. Role of antimutagens/anticarcinogens in cancer prevention. Biofactors 2000, 12, 113–121. [Google Scholar] [CrossRef]
- Coussens, L.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Rose, D.P.; Connolly, J.M. Regulation of tumor angiogenesis by dietary fatty acids and eicosanoids. Nutr. Cancer 2000, 37, 119–127. [Google Scholar] [CrossRef]
- De Kok, T.; van Breda, S.; Manson, M. Mechanisms of combined action of different chemopreventive dietary compounds. Eur. J. Nutr. 2008, 47, 51–59. [Google Scholar] [CrossRef]
- Thomson, C.A.; LeWinn, K.; Newton, T.R.; Alberts, D.S.; Martinez, M.E. Nutrition and diet in the development of gastrointestinal cancer. Curr. Oncol. Rep. 2003, 5, 192–202. [Google Scholar] [CrossRef]
- Stankevicins, L.; Aiub, C.; Maria, L.C.; Lobo-Hajdu, G.; Felzenszwalb, I. Genotoxic and antigenotoxic evaluation of extracts from Arenosclera brasiliensis, a Brazilian marine sponge. Toxicol. In Vitro 2008, 22, 1869–1877. [Google Scholar] [CrossRef]
- De Vries, D.J.; Beart, P.M. Fishing for drugs from the sea: Status and strategies. Trends Pharmacol. Sci. 1995, 16, 275–279. [Google Scholar] [CrossRef]
- Lordan, S.; Ross, R.P.; Stanton, C. Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic diseases. Mar. Drugs 2011, 9, 1056–1100. [Google Scholar] [CrossRef]
- Munro, M.H.G.; Blunt, J.W. MarinLit, a Marine Literature Database, version 13.5; Marine Chemistry Group, University of Canterbury: Christchurch, New Zealand, 2007. [Google Scholar]
- Suarez-Jimenez, G.M.; Burgos-Hernandez, A.; Ezquerra-Brauer, J.M. Bioactive peptides and depsipeptides with anticancer potential: Sources from marine animals. Mar. Drugs 2012, 10, 963–986. [Google Scholar]
- Davis, P.F.; He, Y.; Furneaux, R.H.; Johnston, P.S.; Rüger, B.M.; Slim, G.C. Inhibition of angiogenesis by oral ingestion of powdered shark cartilage in a rat model. Microvasc. Res. 1997, 54, 178–182. [Google Scholar] [CrossRef]
- Moore, K.S.; Wehrli, S.; Roder, H.; Rogers, M.; Forrest, J.N.; McCrimmon, D.; Zasloff, M. Squalamine: An aminosterol antibiotic from the shark. Proc. Natl. Acad. Sci. USA 1993, 90, 1354–1358. [Google Scholar]
- Burgos-Hernandez, A.; Peña-Sarmiento, M.; Moreno-Ochoa, F. Mutagencity and antimutagencity studies of lipidic extracts from yellowtail fish (Seriola lalandi), lisa fish (Mugil cephalus) and cazón fish (Mustelus lunulatus). Food Chem. Toxicol. 2002, 40, 1469–1474. [Google Scholar]
- Moreno-Felix, C.; Wilson-Sanchez, G.; Cruz-Ramirez, S.G.; Velazquez-Contreras, C.; Plascencia-Jatomea, M.; Acosta, A.; Machi-Lara, L.; Aldana-Madrid, M.L.; Ezquerra-Brauer, J.M.; Rocha-Alonzo, F.; et al. Bioactive lipidic extracts from octopus (Paraoctopus limaculatus): Antimutagenicity and antiproliferative studies. Evid. Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Jimeno, J.; Faircloth, G.; Sousa-Faro, J.M.; Scheuer, P.; Rinehart, K. New marine derived anticancer therapeutics—A journey from the sea to clinical trials. Mar. Drugs 2004, 2, 14–29. [Google Scholar] [CrossRef]
- Sindhu, S.; Sherief, P.M. Extraction, characterization, antioxidant and anti-inflammatory properties of carotenoids from the shell waste of arabian red shrimp Aristeus alcocki, ramadan 1938. Open Conf. Proc. J. 2011, 2, 95–103. [Google Scholar]
- Ezquerra-Brauer, J.M.; Brignas-Alvarado, L.; Burgos-Hernández, A.; Rouzaud-Sández, O. Control de la Composición Química y Atributos de Calidad de Camarones Cultivados. In Avances en Nutrición Acuícola VII, Proceedings of the Memorias del VII Simposium Internacional de Nutrición Acuícola, Hermosillo, Sonora, México, 16–19 November 2004; Suárez, L.E., Ricque Marie, D., Nieto López, M.G., Villarreal, D., Scholz, U., González, M., Eds.; Universidad Autónoma de Nuevo León: Monterrey, México, 2004. [Google Scholar]
- Meyers, S.P. Papel del Carotenoide Astaxantina en Nutrición de Especies Acuáticas. In Avances en Nutrición Acuícola IV, Proceedings of the Memorias del IV Simposium Internacional de Nutrición Acuícola, La Paz, Baja California Sur, México, 2000; Civera-Cerecedo, R., Pérez-Estrada, C.J., Ricque-Marie, D., Cruz-Suárez, L.E., Eds.; Universidad Autónoma de Nuevo León: Monterrey, México, 2004; pp. 473–491. [Google Scholar]
- Latscha, T. The Role of Astaxanthin in Shrimp Pigmentation. In Advances in Tropical Aquaculture; Aquacop IFREMER Actes de Collegue: Tahiti, French Polynesia, 1989; Volume 9, pp. 319–325. [Google Scholar]
- Olson, J. Absorption, transport, and metabolism of carotenoids in humans. Pure Appl. Chem. 1994, 66, 1011–1016. [Google Scholar] [CrossRef]
- Latscha, T. The role of astaxanthin in shrimp pigmentation. Adv. Trop. Aquac. 1989, 9, 319–325. [Google Scholar]
- Liang, J.; Tian, Y.-X.; Yang, F.; Zhang, J.-P.; Skibsted, L.H. Antioxidant synergism between carotenoids in membranes. Astaxanthin as a radical transfer bridge. Food Chem. 2009, 115, 1437–1442. [Google Scholar]
- Maoka, T. Carotenoids in marine animals. Mar. Drugs 2011, 9, 278–293. [Google Scholar]
- Nawar, W.W. Lipids. In Food Chemistry, 3rd ed.; Fennema, O.R., Ed.; Marcel Dekker: New York, NY, USA, 1996; pp. 225–320. [Google Scholar]
- Hardman, W.E. (n-3) Fatty acids and cancer therapy. J. Nutr. 2004, 134, 3427S–3430S. [Google Scholar]
- Akoh, C.C.; Min, D.B. Food Lipids: Chemistry, Nutrition, and Biotechnology, 2nd ed.; Marcel Dekker, Inc.: New York, NY, USA, 2002; p. 464. [Google Scholar]
- Cahú, T.B.; Santos, S.D.; Mendes, A.; Córdula, C.R.; Chavante, S.F.; Carvalho, L.B., Jr.; Nader, H.B.; Bezerra, R.S. Recovery of protein, chitin, carotenoids and glycosaminoglycans from Pacific white shrimp (Litopenaeus vannamei) processing waste. Process Biochem. 2012, 47, 570–577. [Google Scholar]
- Nishino, H.; Murakosh, M.; Ii, T.; Takemura, M.; Kuchide, M.; Kanazawa, M.; Mou, X.Y.; Wada, S.; Masuda, M.; Ohsaka, Y.; et al. Carotenoids in cancer chemoprevention. Cancer Metastasis Rev. 2002, 21, 257–264. [Google Scholar]
- Rock, C.L. Carotenoids and Cancer. In Carotenoids; Britton, G., Pfander, H., Liaaen-Jensen, S., Eds.; Birkhäuser Verlag: Berlin, Germany, 2009; Volume 5, pp. 269–286. [Google Scholar]
- Santamaria, L.; Bianchi, A.; Arnaboldi, A.; Andreoni, L. Prevention of the benzo(a)pyrene photocarcinogenic effect by beta-carotene and canthaxanthine. Preliminary study. Boll. Chim. Farm. 1980, 119, 745–748. [Google Scholar]
- Santamaria, L.; Bianchi, A. Cancer chemoprevention by supplemental carotenoids in animals and humans. Prev. Med. 1989, 18, 603–623. [Google Scholar] [CrossRef]
- Chew, B.P.; Mathison, B.D.; Hayek, M.G.; Massimino, S.; Reinhart, G.A.; Park, J.S. Dietary astaxanthin enhances immune response in dogs. Vet. Immunol. Immunopathol. 2011, 140, 199–206. [Google Scholar]
- Arredondo-Figueroa, J.L.; Pedroza-Islas, R.; Ponce-Palafox, J.T.; Vernon-Carter, E.J. Pigmentation of Pacific white shrimp (Litopenaeus vannamei, Boone 1931) with esterified and saponified carotenoids from red chili (Capsicum annuum) in comparison to astaxanthin. Rev. Mex. Ing. Quim. 2003, 2, 101–108. [Google Scholar]
- Sánchez-Camargo, A.P.; Almeida Meireles, M.Â.; Lopes, B.L.F.; Cabral, F.A. Proximate composition and extraction of carotenoids and lipids from Brazilian redspotted shrimp waste (Farfantepenaeus paulensis). J. Food Eng. 2011, 102, 87–93. [Google Scholar] [CrossRef]
- Mezzomo, N.; Maestri, B.; dos Santos, R.L.; Maraschin, M.; Ferreira, S.R.S. Pink shrimp (P. brasiliensis and P. paulensis) residue: Influence of extraction method on carotenoid concentration. Talanta 2011, 85, 1383–1391. [Google Scholar] [CrossRef]
- Firdous, A.; Sindhu, E.; Ramnath, V.; Kuttan, R. Anti-mutagenic and anti-carcinogenic potential of the carotenoid meso-zeaxanthin. Asian Pac. J. Cancer Prev. 2010, 11, 1795–1800. [Google Scholar]
- Babu, C.M.; Chakrabarti, R.; Surya Sambasivarao, K.R. Enzymatic isolation of carotenoid-protein complex from shrimp head waste and its use as a source of carotenoids. LWT Food Sci. Technol. 2008, 41, 227–235. [Google Scholar] [CrossRef]
- Vílchez, C.; Forján, E.; Cuaresma, M.; Bédmar, F.; Garbayo, I.; Vega, J.M. Marine carotenoids: Biological functions and commercial applications. Mar. Drugs 2011, 9, 319–333. [Google Scholar] [CrossRef]
- Linnewiel, K.; Ernst, H.; Caris-Veyrat, C.; Ben-Dor, A.; Kampf, A.; Salman, H.; Danilenko, M.; Levy, J.; Sharoni, Y. Structure activity relationship of carotenoid derivatives in activation of the electrophile/antioxidant response element transcription system. Free Radic. Biol. Med. 2009, 47, 659–667. [Google Scholar] [CrossRef]
- Tsuchiya, M.; Scita, G.; Freisleben, H.-J.; Kagan, V.E.; Packer, L. Antioxidant radical-scavenging activity of carotenoids and retinoids compared to α-tocopherol. Methods Enzymol. 1992, 213, 460–472. [Google Scholar]
- Miki, W. Biological functions and activities of animal carotenoids. Pure Appl. Chem. 1991, 63, 141–146. [Google Scholar] [CrossRef]
- Burton, G.W. Antioxidant action of carotenoids. J. Nutr. 1989, 119, 109–111. [Google Scholar]
- Paiva, S.A.; Russell, R.M. Beta-carotene and other carotenoids as antioxidants. J. Am. Coll. Nutr. 1999, 18, 426–433. [Google Scholar] [CrossRef]
- Bendich, A.; Canfield, L.; Krinsky, N.; Olson, J. Biological functions of dietary carotenoids. Ann. N. Y. Acad. Sci. 1993, 691, 61–67. [Google Scholar]
- Naguib, Y.M.A. Antioxidant activities of astaxanthin and related carotenoids. J. Agric. Food Chem. 2000, 48, 1150–1154. [Google Scholar] [CrossRef]
- Nishida, Y.; Yamashita, E.; Miki, W. Quenching activities of common hydrophilic and lipophilic antioxidants against singlet oxygen using chemiluminescence detection system. Carotenoid Sci. 2007, 11, 16–20. [Google Scholar]
- Martínez, A.; Rodríguez-Gironés, M.A.; Barbosa, A.S.; Costas, M. Donator acceptor map for carotenoids, melatonin and vitamins. J. Phys. Chem. A 2008, 112, 9037–9042. [Google Scholar] [CrossRef]
- Goto, S.; Kogure, K.; Abe, K.; Kimata, Y.; Kitahama, K.; Yamashita, E.; Terada, H. Efficient radical trapping at the surface and inside the phospholipid membrane is responsible for highly potent antiperoxidative activity of the carotenoid astaxanthin. Biochim. Biophys. Acta 2001, 1512, 251–258. [Google Scholar]
- Wolf, A.M.; Asoh, S.; Hiranuma, H.; Ohsawa, I.; Iio, K.; Satou, A.; Ishikura, M.; Ohta, S. Astaxanthin protects mitochondrial redox state and functional integrity against oxidative stress. J. Nutr. Biochem. 2010, 21, 381–389. [Google Scholar] [CrossRef]
- Jaswir, I.; Kobayashi, M.; Koyama, T.; Kotake-Nara, E.; Nagao, A. Antioxidant behaviour of carotenoids highly accumulated in HepG2 cells. Food Chem. 2012, 132, 865–872. [Google Scholar] [CrossRef]
- Palozza, P. Prooxidant actions of carotenoids in biologic systems. Nutr. Rev. 1998, 56, 257–265. [Google Scholar] [CrossRef]
- Young, A.J.; Lowe, G.M. Antioxidant and prooxidant properties of carotenoids. Arch. Biochem. Biophys. 2001, 385, 20–27. [Google Scholar] [CrossRef]
- Zhang, P.; Omaye, S.T. Antioxidant and prooxidant roles for β-carotene, α-tocopherol and ascorbic acid in human lung cells. Toxicol. In Vitro 2001, 15, 13–24. [Google Scholar] [CrossRef]
- Kurihara, H.; Koda, H.; Asami, S.; Kiso, Y.; Tanaka, T. Contribution of the antioxidative property of astaxanthin to its protective effect on the promotion of cancer metastasis in mice treated with restraint stress. Life Sci. 2002, 70, 2509–2520. [Google Scholar] [CrossRef]
- Augusti, P.R.; Conterato, G.M.M.; Somacal, S.; Sobieski, R.; Spohr, P.R.; Torres, J.V.; Charão, M.F.; Moro, A.M.; Rocha, M.P.; Garcia, S.C.; et al. Effect of astaxanthin on kidney function impairment and oxidative stress induced by mercuric chloride in rats. Food Chem. Toxicol. 2008, 46, 212–219. [Google Scholar] [CrossRef]
- Tyson, J.; Novák, B. Cell Cycle Controls. In Computational Cell Biology; Fall, C.P., Marland, E.S., Wagner, J.M., Tyson, J.J., Marsden, J.E., Sirovich, L., Wiggins, S., Eds.; Springer: New York, NY, USA, 2002; Volume 20, pp. 261–284. [Google Scholar]
- Schafer, K.A. The cell cycle: A review. Vet. Pathol. 1998, 35, 461–478. [Google Scholar] [CrossRef]
- Vermeulen, K.; van Bockstaele, D.R.; Berneman, Z.N. The cell cycle: A review of regulation, deregulation and therapeutic targets in cancer. Cell. Prolif. 2003, 36, 131–149. [Google Scholar]
- Clurman, B.E.; Roberts, J.M. Cell cycle and cancer. J. Natl. Cancer Inst. 1995, 87, 1499–1501. [Google Scholar] [CrossRef]
- Cheng, H.-C.; Chien, H.; Liao, C.-H.; Yang, Y.-Y.; Huang, S.-Y. Carotenoids suppress proliferating cell nuclear antigen and cyclin D1 expression in oral carcinogenic models. J. Nutr. Biochem. 2007, 18, 667–675. [Google Scholar] [CrossRef]
- Stivala, L.A.; Savio, M.; Cazzalini, O.; Pizzala, R.; Rehak, L.; Bianchi, L.; Vannini, V.; Prosperi, E. Effect of beta-carotene on cell cycle progression of human fibroblasts. Carcinogenesis 1996, 17, 2395–2401. [Google Scholar] [CrossRef]
- Stivala, L.A.; Savio, M.; Quarta, S.; Scotti, C.; Cazzalini, O.; Rossi, L.; Scovassi, I.A.; Pizzala, R.; Melli, R.; Bianchi, L.; et al. The antiproliferative effect of beta-carotene requires p21waf1/cip1 in normal human fibroblasts. Eur. J. Biochem. 2000, 267, 2290–2296. [Google Scholar] [CrossRef]
- Palozza, P.; Serini, S.; Maggiano, N.; Angelini, M.; Boninsegna, A.; Di Nicuolo, F.; Ranelletti, F.O.; Calviello, G. Induction of cell cycle arrest and apoptosis in human colon adenocarcinoma cell lines by beta-carotene through down-regulation of cyclin A and Bcl-2 family proteins. Carcinogenesis 2002, 23, 11–18. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, W.-E.; Hu, L.; Zhao, L.; Huang, J. Carotenoids inhibit proliferation and regulate expression of peroxisome proliferators-activated receptor gamma (PPARγ) in K562 cancer cells. Arch. Biochem. Biophys. 2011, 512, 96–106. [Google Scholar]
- Sacha, T.; Zawada, M.; Hartwich, J.; Lach, Z.; Polus, A.; Szostek, M.; Zdzitowska, E.; Libura, M.; Bodzioch, M.; Dembińska-Kieć, A.; et al. The effect of β-carotene and its derivatives on cytotoxicity, differentiation, proliferative potential and apoptosis on the three human acute leukemia cell lines: U-937, HL-60 and TF-1. Biochim. Biophys. Acta 2005, 1740, 206–214. [Google Scholar]
- Guruvayoorappan, C.; Kuttan, G. β-Carotene down-regulates inducible nitric oxide synthase gene expression and induces apoptosis by suppressing bcl-2 expression and activating caspase-3 and p53 genes in B16F-10 melanoma cells. Nutr. Res. 2007, 27, 336–342. [Google Scholar] [CrossRef]
- Cui, Y.; Lu, Z.; Bai, L.; Shi, Z.; Zhao, W.-E.; Zhao, B. β-Carotene induces apoptosis and up-regulates peroxisome proliferator-activated receptor γ expression and reactive oxygen species production in MCF-7 cancer cells. Eur. J. Cancer 2007, 43, 2590–2601. [Google Scholar] [CrossRef]
- Nagaraj, S.; Rajaram, M.G.; Arulmurugan, P.; Baskaraboopathy, A.; Karuppasamy, K.; Jayappriyan, K.R.; Sundararaj, R.; Rengasamy, R. Antiproliferative potential of astaxanthin-rich alga Haematococcus pluvialis Flotow on human hepatic cancer (HepG2) cell line. Biomed. Prev. Nutr. 2012. [Google Scholar] [CrossRef]
- Nagendraprabhu, P.; Sudhandiran, G. Astaxanthin inhibits tumor invasion by decreasing extracellular matrix production and induces apoptosis in experimental rat colon carcinogenesis by modulating the expressions of ERK-2, NFkB and COX-2. Investig. New Drugs 2011, 29, 207–224. [Google Scholar] [CrossRef]
- Song, X.D.; Zhang, J.J.; Wang, M.R.; Liu, W.B.; Gu, X.B.; Lv, C.J. Astaxanthin induces mitochondria-mediated apoptosis in rat hepatocellular carcinoma CBRH-7919 cells. Biol. Pharm. Bull. 2011, 34, 839–844. [Google Scholar]
- Karas, M.; Amir, H.; Fishman, D.; Danilenko, M.; Segal, S.; Nahum, A.; Koifmann, A.; Giat, Y.; Levy, J.; Sharoni, Y. Lycopene interferes with cell cycle progression and insulin-like growth factor I signaling in mammary cancer cells. Nutr. Cancer 2000, 36, 101–111. [Google Scholar] [CrossRef]
- Nahum, A.; Hirsch, K.; Danilenko, M.; Watts, C.K.; Prall, O.W.; Levy, J.; Sharoni, Y. Lycopene inhibition of cell cycle progression in breast and endometrial cancer cells is associated with reduction in cyclin D levels and retention of p27(Kip1) in the cyclin E-cdk2 complexes. Oncogene 2001, 20, 3428–3436. [Google Scholar] [CrossRef]
- Bhagavathy, S.; Sumathi, P.; Madhushree, M. Antimutagenic assay of carotenoids from green algae Chlorococcum humicola using Salmonella typhimurium TA98, TA100 and TA102. Asian Pac. J. Trop. Dis. 2011, 1, 308–316. [Google Scholar] [CrossRef]
- González de Mejı́a, E.; Quintanar-Hernández, J.A.; Loarca-Piña, G. Antimutagenic activity of carotenoids in green peppers against some nitroarenes. Mutat. Res. 1998, 416, 11–19. [Google Scholar] [CrossRef]
- Azuine, M.A.; Goswami, U.C.; Kayal, J.J.; Bhide, S.V. Antimutagenic and anticarcinogenic effects of carotenoids and dietary palm oil. Nutr. Cancer 1992, 17, 287–295. [Google Scholar] [CrossRef]
- Merriman, R.L.; Bertram, J.S. Reversible inhibition by retinoids of 3-methylcholanthrene-induced neoplastic transformation in C3H/10T1/2 clone 8 cells. Cancer Res. 1979, 39, 1661–1666. [Google Scholar]
- Aidoo, A.; Lyncook, L.; Lensing, S.; Bishop, M.; Wamer, W. In-vivo antimutagenic activity of beta-carotene in rat spleen lymphocytes. Carcinogenesis 1995, 16, 2237–2241. [Google Scholar] [CrossRef]
- Hussein, G.; Sankawa, U.; Goto, H.; Matsumoto, K.; Watanabe, H. Astaxanthin, a carotenoid with potential in human health and nutrition. J. Nat. Prod. 2006, 69, 443–449. [Google Scholar] [CrossRef]
- Chew, B.P.; Park, J.S. Carotenoid action on the immune response. J. Nutr. 2004, 134, 257S–261S. [Google Scholar]
- Chew, B.P. Role of Carotenoids in the Immune Response. J. Dairy Sci. 1993, 76, 2804–2811. [Google Scholar] [CrossRef]
- Wang, C.J.; Chou, M.Y.; Lin, J.K. Inhibition of growth and development of the transplantable C-6 glioma cells inoculated in rats by retinoids and carotenoids. Cancer Lett. 1989, 48, 135–142. [Google Scholar]
- Kim, J.H.; Na, H.J.; Kim, C.K.; Kim, J.Y.; Ha, K.S.; Lee, H.; Chung, H.T.; Kwon, H.J.; Kwon, Y.G.; Kim, Y.M. The non-provitamin A carotenoid, lutein, inhibits NF-kappaB-dependent gene expression through redox-based regulation of the phosphatidylinositol 3-kinase/PTEN/Akt and NF-kappaB-inducing kinase pathways: Role of H(2)O(2) in NF-kappaB activation. Free Radic. Biol. Med. 2008, 45, 885–896. [Google Scholar] [CrossRef]
- Park, J.S.; Mathison, B.D.; Hayek, M.G.; Massimino, S.; Reinhart, G.A.; Chew, B.P. Astaxanthin stimulates cell-mediated and humoral immune responses in cats. Vet. Immunol. Immunopathol. 2011, 144, 455–461. [Google Scholar]
- De Moura, A.; Torres, R.; Mancini, J.; Tenuta, A. Characterization of the lipid portion of pink shrimp commercial samples. Arch. Latinoam Nutr. 2002, 52, 207–211. [Google Scholar]
- Kher-un-Nisa; Sultana, R. Chariation in the proximate composition of shrimp, Fenneropenaeus penicillatus at different stages of maturity. Pak. J. Biochem. Mol. Biol. 2010, 43, 135–139. [Google Scholar]
- Bougnoux, P.; Hajjaji, N.; Maheo, K.; Couet, C.; Chevalier, S. Fatty acids and breast cancer: Sensitization to treatments and prevention of metastatic re-growth. Prog. Lipid Res. 2010, 49, 76–86. [Google Scholar] [CrossRef]
- Sahena, F.; Zaidul, I.; Jinap, S.; Saari, N.; Jahurul, H.; Abbas, K.; Norulaini, N. PUFAs in fish: Extraction, fractionation, importance in health. Compr. Rev. Food Sci. Food Safety 2009, 8, 59–74. [Google Scholar] [CrossRef]
- Dyerberg, J.; Bang, H.O. Haemostatic function and platelet polyunsaturated fatty acids in Eskimos. Lancet 1979, 2, 433–435. [Google Scholar] [CrossRef]
- Dyerberg, J. Linolenate-derived polyunsaturated fatty acids and prevention of atherosclerosis. Nutr. Rev. 1986, 44, 125–134. [Google Scholar]
- Chlebowski, R.T.; Blackburn, G.L.; Thomson, C.A.; Nixon, D.W.; Shapiro, A.; Hoy, M.K.; Goodman, M.T.; Giuliano, A.E.; Karanja, N.; McAndrew, P.; et al. Dietary fat reduction and breast cancer outcome: Interim efficacy results from the Women’s Intervention Nutrition Study. J. Natl. Cancer Inst. 2006, 98, 1767–1776. [Google Scholar]
- Augustsson, K.; Michaud, D.S.; Rimm, E.B.; Leitzmann, M.F.; Stampfer, M.J.; Willett, W.C.; Giovannucci, E. A prospective study of intake of fish and marine fatty acids and prostate cancer. Cancer Epidemiol. Biomark. Prev. 2003, 12, 64–67. [Google Scholar]
- Shannon, J.; King, I.B.; Moshofsky, R.; Lampe, J.W.; Gao, D.L.; Ray, R.M.; Thomas, D.B. Erythrocyte fatty acids and breast cancer risk: A case-control study in Shanghai, China. Am. J. Clin. Nutr. 2007, 85, 1090–1097. [Google Scholar]
- Caygill, C.P.; Hill, M.J. Fish, n-3 fatty acids and human colorectal and breast cancer mortality. Eur. J. Cancer Prev. 1995, 4, 329–332. [Google Scholar] [CrossRef]
- De Deckere, E.A. Possible beneficial effect of fish and fish n-3 polyunsaturated fatty acids in breast and colorectal cancer. Eur. J. Cancer Prev. 1999, 8, 213–221. [Google Scholar]
- Virtanen, J.K.; Mozaffarian, D.; Chiuve, S.E.; Rimm, E.B. Fish consumption and risk of major chronic disease in men. Am. J. Clin. Nutr. 2008, 88, 1618–1625. [Google Scholar]
- Simon, J.A.; Fong, J.; Bernert, J.T.; Browner, W.S. Serum fatty acids and the risk of fatal cancer. MRFIT research group. Multiple risk factor intervention trial. Am. J. Epidemiol. 1998, 148, 854–858. [Google Scholar]
- Williams, C.D.; Whitley, B.M.; Hoyo, C.; Grant, D.J.; Iraggi, J.D.; Newman, K.A.; Gerber, L.; Taylor, L.A.; McKeever, M.G.; Freedland, S.J. A high ratio of dietary n-6/n-3 polyunsaturated fatty acids is associated with increased risk of prostate cancer. Nutr. Res. 2011, 31, 1–8. [Google Scholar]
- Manson, J.E.; Bassuk, S.S.; Lee, I.M.; Cook, N.R.; Albert, M.A.; Gordon, D.; Zaharris, E.; Macfadyen, J.G.; Danielson, E.; Lin, J.; et al. The VITamin D and OmegA-3 TriaL (VITAL): Rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp. Clin. Trials 2012, 33, 159–171. [Google Scholar]
- Larsson, S.C.; Kumlin, M.; Ingelman-Sundberg, M.; Wolk, A. Dietary long-chain n-3 fatty acids for the prevention of cancer: A review of potential mechanisms. Am. J. Clin. Nutr. 2004, 79, 935–945. [Google Scholar]
- Hull, M.A. Omega-3 polyunsaturated fatty acids. Best Pract.Res.Clin.Gastroenterol. 2011, 25, 547–554. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, C.; Jie, B. Various dietary polyunsaturated fatty acids modulate acrylamide-induced preneoplatic urothelial proliferation and apoptosis in mice. Exp. Toxicol. Pathol. 2010, 62, 9–16. [Google Scholar] [CrossRef]
- Nicosia, S.; Patrono, C. Eicosanoid biosynthesis and action: Novel opportunities for pharmacological intervention. FASEB J. 1989, 3, 1941–1948. [Google Scholar]
- Bordoni, A.; Di Nunzio, M.; Danesi, F.; Biagi, P.L. Polyunsaturated fatty acids: From diet to binding to ppars and other nuclear receptors. Genes Nutr. 2006, 1, 95–106. [Google Scholar]
- Nie, D.; Che, M.; Grignon, D.; Tang, K.; Honn, K.V. Role of Eicosanoids in Prostate Cancer Progression. In Prostate Cancer: New Horizons in Research and Treatment; Cher, M.L., Raz, A., Honn, K.V., Eds.; Kluwer Academic Publishers: New York, NY, USA, 2002. [Google Scholar]
- Marks, F.; Müller-Decker, K.; Fürstenberger, G. A causal relationship between unscheduled eicosanoid signaling and tumor development: Cancer chemoprevention by inhibitors of arachidonic acid metabolism. Toxicology 2000, 153, 11–26. [Google Scholar]
- Bunn, P.A., Jr.; Keith, R.L. The future of cyclooxygenase-2 inhibitors and other inhibitors of the eicosanoid signal pathway in the prevention and therapy of lung cancer. Clin. Lung Cancer 2002, 3, 271–277. [Google Scholar] [CrossRef]
- Dubois, R.N.; Abramson, S.B.; Crofford, L.; Gupta, R.A.; Simon, L.S.; van de Putte, L.B.; Lipsky, P.E. Cyclooxygenase in biology and disease. FASEB J. 1998, 12, 1063–1073. [Google Scholar]
- Kremer, J.M. Clinical studies of omega-3 fatty acid supplementation in patients who have rheumatoid arthritis. Rheum. Dis. Clin. N. Am. 1991, 17, 391–402. [Google Scholar]
- Nielsen, G.L.; Faarvang, K.L.; Thomsen, B.S.; Teglbjaerg, K.L.; Jensen, L.T.; Hansen, T.M.; Lervang, H.H.; Schmidt, E.B.; Dyerberg, J.; Ernst, E. The effects of dietary supplementation with n-3 polyunsaturated fatty acids in patients with rheumatoid arthritis: A randomized, double blind trial. Eur. J. Clin. Investig. 1992, 22, 687–691. [Google Scholar]
- Galarraga, B.; Ho, M.; Youssef, H.M.; Hill, A.; McMahon, H.; Hall, C.; Ogston, S.; Nuki, G.; Belch, J.J. Cod liver oil (n-3 fatty acids) as an non-steroidal anti-inflammatory drug sparing agent in rheumatoid arthritis. Rheumatology 2008, 47, 665–669. [Google Scholar] [CrossRef]
- Pidgeon, G.P.; Lysaght, J.; Krishnamoorthy, S.; Reynolds, J.V.; O’Byrne, K.; Nie, D.; Honn, K.V. Lipoxygenase metabolism: Roles in tumor progression and survival. Cancer Metastasis Rev. 2007, 26, 503–524. [Google Scholar]
- Greene, E.R.; Huang, S.; Serhan, C.N.; Panigrahy, D. Regulation of inflammation in cancer by eicosanoids. Prostaglandins Other Lipid Mediat. 2011, 96, 27–36. [Google Scholar] [CrossRef]
- Gogos, C.A.; Ginopoulos, P.; Salsa, B.; Apostolidou, E.; Zoumbos, N.C.; Kalfarentzos, F. Dietary omega-3 polyunsaturated fatty acids plus vitamin E restore immunodeficiency and prolong survival for severely ill patients with generalized malignancy: A randomized control trial. Cancer 1998, 82, 395–402. [Google Scholar] [CrossRef]
- Kim, W.; Khan, N.A.; McMurray, D.N.; Prior, I.A.; Wang, N.; Chapkin, R.S. Regulatory activity of polyunsaturated fatty acids in T-cell signaling. Progr. Lipid Res. 2010, 49, 250–261. [Google Scholar] [CrossRef]
- Yaqoob, P.; Calder, P. Effects of dietary lipid manipulation upon inflammatory mediator production by murine macrophages. Cell. Immunol. 1995, 163, 120–128. [Google Scholar] [CrossRef]
- Mangelsdorf, D.J.; Thummel, C.; Beato, M.; Herrlich, P.; Schütz, G.; Umesono, K.; Blumberg, B.; Kastner, P.; Mark, M.; Chambon, P.; et al. The nuclear receptor superfamily: The second decade. Cell 1995, 83, 835–839. [Google Scholar]
- Peters, J.M.; Gonzalez, F.J. Regulation of Squamous Cell Carcinoma Carcinogenesis by Peroxisome Proliferator-Activated Receptors. In Signaling Pathways in Squamous Cancer; Glick, A.B., van Maes, C., Eds.; Springer: New York, NY, USA, 2011; pp. 223–240. [Google Scholar]
- Fajas, L.; Debril, M.B.; Auwerx, J. Peroxisome proliferator-activated receptor-gamma: From adipogenesis to carcinogenesis. J. Mol. Endocrinol. 2001, 27, 1–9. [Google Scholar] [CrossRef]
- Peters, J.M.; Gonzalez, F.J. Sorting out the functional role(s) of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) in cell proliferation and cancer. Biochim. Biophys. Acta 2009, 1796, 230–241. [Google Scholar]
- Lee, J.Y.; Hwang, D.H. Docosahexaenoic acid suppresses the activity of peroxisome proliferator-activated receptors in a colon tumor cell line. Biochem. Biophy. Res. Commun. 2002, 298, 667–674. [Google Scholar] [CrossRef]
- Nair, J.; Vaca, C.E.; Velic, I.; Mutanen, M.; Valsta, L.M.; Bartsch, H. High dietary omega-6 polyunsaturated fatty acids drastically increase the formation of etheno-DNA base adducts in white blood cells of female subjects. Cancer Epidemiol. Biomark.Prev. 1997, 6, 597–601. [Google Scholar]
- Takahashi, M.; Tsuboyama-Kasaoka, N.; Nakatani, T.; Ishii, M.; Tsutsumi, S.; Aburatani, H.; Ezaki, O. Fish oil feeding alters liver gene expressions to defend against PPARalpha activation and ROS production. Am. J. Physiol.Gastrointest Liver Physiol. 2002, 282, G338–G348. [Google Scholar]
- Chajès, V.; Sattler, W.; Stranzl, A.; Kostner, G.M. Influence of n-3 fatty acids on the growth of human breast cancer cells in vitro: Relationship to peroxides and vitamin-E. Breast Cancer Res. Treat. 1995, 34, 199–212. [Google Scholar]
- Sato, S.; Sato, S.; Kawamoto, J.; Kurihara, T. Differential roles of internal and terminal double bonds in docosahexaenoic acid: Comparative study of cytotoxicity of polyunsaturated fatty acids to HT-29 human colorectal tumor cell line. Prostaglandins Leukot. Essent. Fat. Acids 2011, 84, 31–37. [Google Scholar] [CrossRef]
- Jenkinson, A.M.; Collins, A.R.; Duthie, S.J.; Wahle, K.W.; Duthie, G.G. The effect of increased intakes of polyunsaturated fatty acids and vitamin E on DNA damage in human lymphocytes. FASEB J. 1999, 13, 2138–2142. [Google Scholar]
- Srivastava, A.; Laidler, P.; Davies, R.P.; Horgan, K.; Hughes, L.E. The prognostic significance of tumor vascularity in intermediate-thickness (0.76–4.0 mm thick) skin melanoma. A quantitative histologic study. Am. J. Pathol. 198, 133, 419–423. [Google Scholar]
- Heimann, R.; Ferguson, D.; Powers, C.; Recant, W.M.; Weichselbaum, R.R.; Hellman, S. Angiogenesis as a predictor of long-term survival for patients with node-negative breast cancer. J. Natl. Cancer Inst. 1996, 88, 1764–1769. [Google Scholar] [CrossRef]
- Kanayasu, T.; Morita, I.; Nakao-Hayashi, J.; Asuwa, N.; Fujisawa, C.; Ishii, T.; Ito, H.; Murota, S. Eicosapentaenoic acid inhibits tube formation of vascular endothelial cells in vitro. Lipids 1991, 26, 271–276. [Google Scholar] [CrossRef]
- Vibet, S.; Mahéo, K.; Goré, J.; Hardy, T.; Bougnoux, P.; Tranquart, F.; Goupille, C. Potentiation of antitumoral and antiangiogenic actions of docetaxel by docosahexaenoic acid (DHA): Impact on micro- and macro-vascularization. EJC Suppl. 2008, 6, 124. [Google Scholar]
- Jiang, W.G.; Bryce, R.P.; Mansel, R.E. Gamma linolenic acid regulates gap junction communication in endothelial cells and their interaction with tumour cells. Prostaglandins Leukot. Essent. Fat. Acids 1997, 56, 307–316. [Google Scholar] [CrossRef]
- Tevar, R.; Jho, D.H.; Babcock, T.; Helton, W.S.; Espat, N.J. Omega-3 fatty acid supplementation reduces tumor growth and vascular endothelial growth factor expression in a model of progressive non-metastasizing malignancy. J. Parenter. Enter. Nutr. 2002, 26, 285–289. [Google Scholar] [CrossRef]
- Biondo, P.D.; Brindley, D.N.; Sawyer, M.B.; Field, C.J. The potential for treatment with dietary long-chain polyunsaturated n-3 fatty acids during chemotherapy. J. Nutr. Biochem. 2008, 19, 787–796. [Google Scholar] [CrossRef]
- Pardini, R.S. Nutritional intervention with omega-3 fatty acids enhances tumor response to anti-neoplastic agents. Chem. Biol. Interact. 2006, 162, 89–105. [Google Scholar] [CrossRef]
- Burns, C.P.; Spector, A.A. Membrane fatty acid modification in tumor cells: A potential therapeutic adjunct. Lipids 1987, 22, 178–184. [Google Scholar] [CrossRef]
- Burns, C.P.; Halabi, S.; Clamon, G.H.; Hars, V.; Wagner, B.A.; Hohl, R.J.; Lester, E.; Kirshner, J.J.; Vinciguerra, V.; Paskett, E. Phase I clinical study of fish oil fatty acid capsules for patients with cancer cachexia: Cancer and leukemia group B study 9473. Clin. Cancer Res. 1999, 5, 3942–3947. [Google Scholar]
- Atkinson, T.G.; Murray, L.; Berry, D.M.; Ruthig, D.J.; Meckling-Gill, K.A. DHA feeding provides host protection and prevents fibrosarcoma-induced hyperlipidemia while maintaining the tumor response to araC in Fischer 344 rats. Nutr. Cancer 1997, 28, 225–235. [Google Scholar] [CrossRef]
- Cha, M.C.; Meckling, K.A.; Stewart, C. Dietary docosahexaenoic acid levels influence the outcome of arabinosylcytosine chemotherapy in L1210 leukemic mice. Nutr. Cancer 2002, 44, 176–181. [Google Scholar] [CrossRef]
- Horie, T.; Nakamaru, M.; Masubuchi, Y. Docosahexaenoic acid exhibits a potent protection of small intestine from methotrexate-induced damage in mice. Life Sci. 1998, 62, 1333–1338. [Google Scholar]
- Gómez de Segura, I.A.; Valderrábano, S.; Vázquez, I.; Vallejo-Cremades, M.T.; Gómez-García, L.; Sánchez, M.; de Miguel, E. Protective effects of dietary enrichment with docosahexaenoic acid plus protein in 5-fluorouracil-induced intestinal injury in the rat. Eur. J. Gastroenterol. Hepatol. 2004, 16, 479–485. [Google Scholar] [CrossRef]
- Ogilvie, G.K.; Fettman, M.J.; Mallinckrodt, C.H.; Walton, J.A.; Hansen, R.A.; Davenport, D.J.; Gross, K.L.; Richardson, K.L.; Rogers, Q.; Hand, M.S. Effect of fish oil, arginine, and doxorubicin chemotherapy on remission and survival time for dogs with lymphoma: A double-blind, randomized placebo-controlled study. Cancer 2000, 88, 1916–1928. [Google Scholar] [CrossRef]
- Pardini, R.S.; Wilson, D.; Schiff, S.; Bajo, S.A.; Pierce, R. Nutritional intervention with omega-3 fatty acids in a case of malignant fibrous histiocytoma of the lungs. Nutr. Cancer 2005, 52, 121–129. [Google Scholar] [CrossRef]
- Xin-Xin, L.; Jian-Chun, Y.; Wei-Ming, K.; Quan, W.; Zhi-Qiang, M.; Hai-Liang, F.; Bei, G.; Yu-Qin, L. ω-3 Polyunsaturated fatty acid enhance chemotherapy sensitivity by inhibiting NF-κB pathway. ESPEN J. 2011, 6, e36–e40. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
López-Saiz, C.-M.; Suárez-Jiménez, G.-M.; Plascencia-Jatomea, M.; Burgos-Hernández, A. Shrimp Lipids: A Source of Cancer Chemopreventive Compounds. Mar. Drugs 2013, 11, 3926-3950. https://doi.org/10.3390/md11103926
López-Saiz C-M, Suárez-Jiménez G-M, Plascencia-Jatomea M, Burgos-Hernández A. Shrimp Lipids: A Source of Cancer Chemopreventive Compounds. Marine Drugs. 2013; 11(10):3926-3950. https://doi.org/10.3390/md11103926
Chicago/Turabian StyleLópez-Saiz, Carmen-María, Guadalupe-Miroslava Suárez-Jiménez, Maribel Plascencia-Jatomea, and Armando Burgos-Hernández. 2013. "Shrimp Lipids: A Source of Cancer Chemopreventive Compounds" Marine Drugs 11, no. 10: 3926-3950. https://doi.org/10.3390/md11103926



