Influence of Environmental Factors on the Paralytic Shellfish Toxin Content and Profile of Alexandrium catenella (Dinophyceae) Isolated from the Mediterranean Sea
Abstract
:1. Introduction
2. Results and Discussion
2.1. Toxin Content and Profile of ACT03 Strain

| Temperature (°C) | Salinity (psu) | GTX3 | GTX4 | GTX5 | C1 | C2 | C3 | C4 |
|---|---|---|---|---|---|---|---|---|
| 12 | 10 | - | - | - | - | 100 | - | - |
| 12 | 15 | - | - | - | - | 100 | - | - |
| 12 | 20 | - | - | - | - | 100 | - | - |
| 12 | 25 | - | - | - | - | 100 | - | - |
| 12 | 30 | - | - | - | - | 100 | - | - |
| 12 | 35 | - | - | 31 | - | 69 | - | - |
| 12 | 40 | - | - | - | - | 57.3 | - | 42.7 |
| 18 | 10 | - | - | - | - | 100 | - | - |
| 18 | 15 | - | - | - | - | 80.8 | - | 19.2 |
| 18 | 20 | - | 18.5 | 24.1 | - | 47.8 | - | 9.6 |
| 18 | 25 | - | - | - | - | 100 | - | - |
| 18 | 30 | 0.1 | 13.4 | 55.8 | 0.3 | 24 | 1.1 | 5.2 |
| 18 | 35 | - | 12.8 | 57.8 | 0.3 | 22.9 | 1.2 | 4.9 |
| 18 | 40 | - | 10.2 | 66.7 | - | 17.6 | - | 5.5 |
| 21 | 10 | - | 9.4 | 51.6 | - | 33.3 | - | 5.7 |
| 21 | 15 | - | 10.5 | 59.5 | - | 27.6 | - | 2.4 |
| 21 | 20 | - | 11 | 58.7 | - | 28.1 | - | 2.3 |
| 21 | 25 | - | 11.5 | 61.3 | - | 26.1 | - | 1.1 |
| 21 | 30 | 0.4 | 14.6 | 56.1 | 0.3 | 24.5 | 1.4 | 2.8 |
| 21 | 35 | - | 12.1 | 62.3 | - | 21.7 | - | 3.9 |
| 21 | 40 | - | 11.3 | 66.8 | - | 16.3 | - | 5.6 |
| 27 | 15 | - | - | - | - | 100 | - | - |
| 27 | 20 | - | - | - | - | 100 | - | - |
| 27 | 25 | - | 18.9 | 59 | - | 18.8 | - | 3.3 |
| 27 | 30 | 0.6 | 23.7 | 54.6 | 0.5 | 15.6 | 2.7 | 2.2 |
| 27 | 35 | 0.5 | 25.2 | 52.4 | 0.6 | 15.3 | 2.8 | 3.3 |
| 27 | 40 | - | 23.2 | 60 | 0.2 | 11.4 | 1 | 4.2 |
| 30 | 30 | - | 19.7 | 66.8 | - | 6.7 | 5.6 | 1.1 |
| 30 | 35 | - | 35 | 71.8 | - | 7.3 | - | 1.1 |
| 30 | 40 | - | 40 | 60.6 | - | 10.4 | - | 1.9 |
2.2. Influence of Salinity
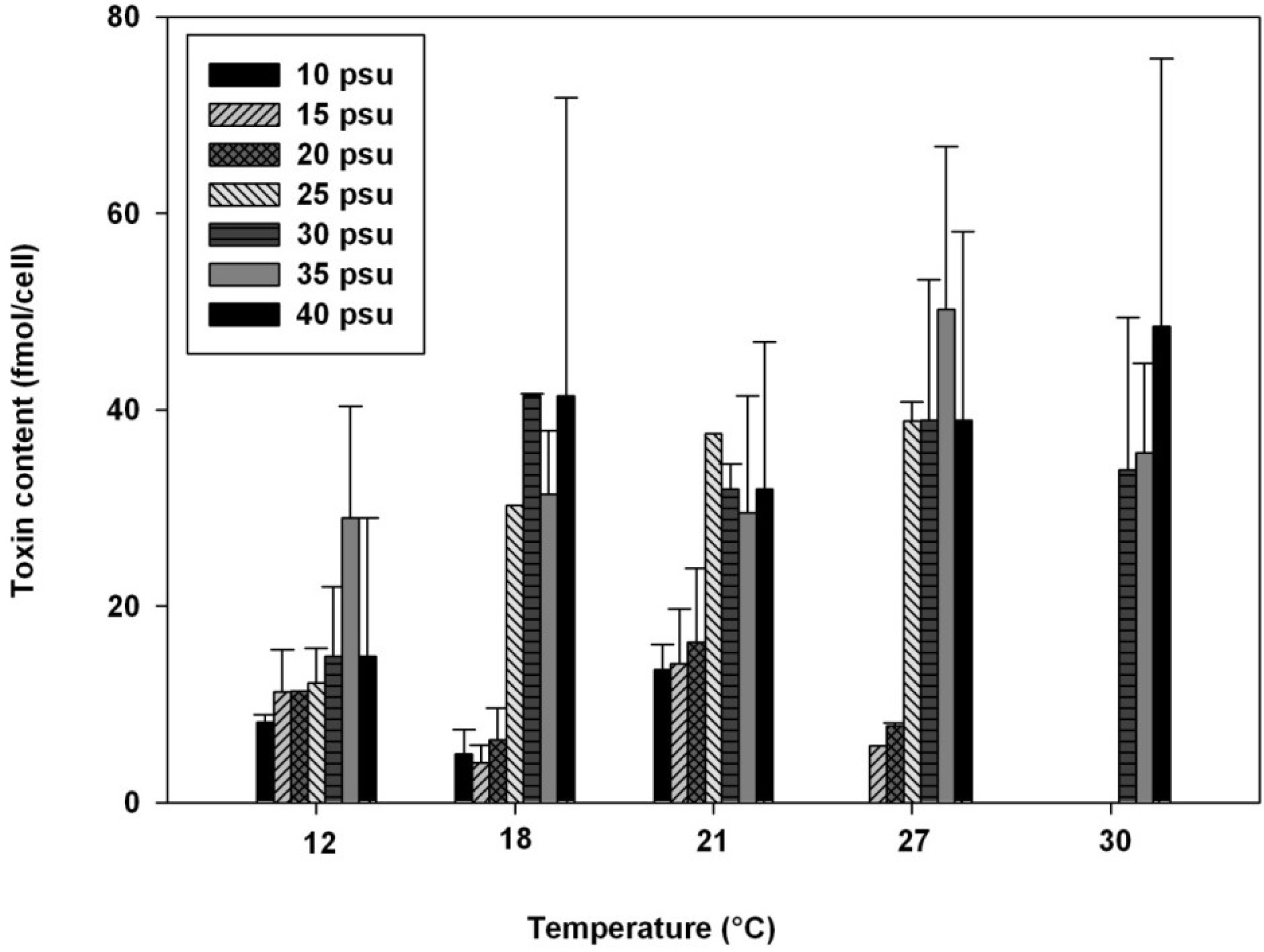
2.3. Influence of Temperature
2.4. Influence of Irradiance on Toxin Content
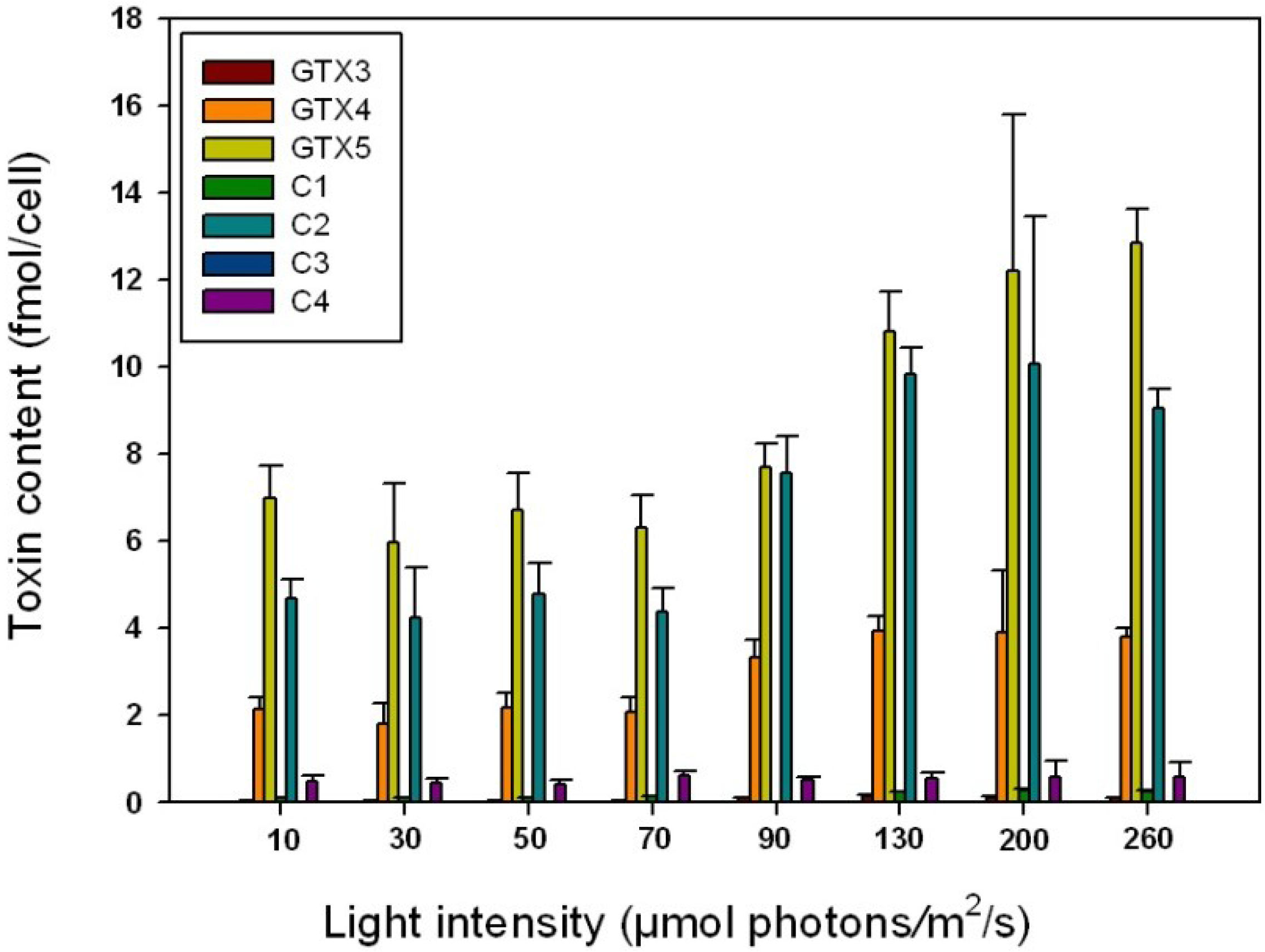
| Irradiance (μmol photons/m2/s) | GTX3 | GTX4 | GTX5 | C1 | C2 | C4 |
|---|---|---|---|---|---|---|
| 10 | 1.3 | 65.2 | 12.7 | - | 19.9 | 0.9 |
| 30 | 2.1 | 62.1 | 14.8 | - | 20.2 | 0.7 |
| 50 | 1.4 | 61 | 15.8 | - | 20.6 | 0.9 |
| 70 | 1.6 | 61.1 | 17 | - | 19.5 | 0.8 |
| 90 | 0.6 | 62.9 | 16.9 | - | 18.4 | 1.2 |
| 130 | 1 | 61.2 | 16.9 | - | 19.5 | 1.4 |
| 200 | 1.1 | 63.4 | 16 | - | 18.5 | 1 |
| 260 | 0.7 | 63.4 | 16.4 | - | 17.9 | 1.6 |
2.5. Relationship between Growth and Alexandrium catenella Toxicity
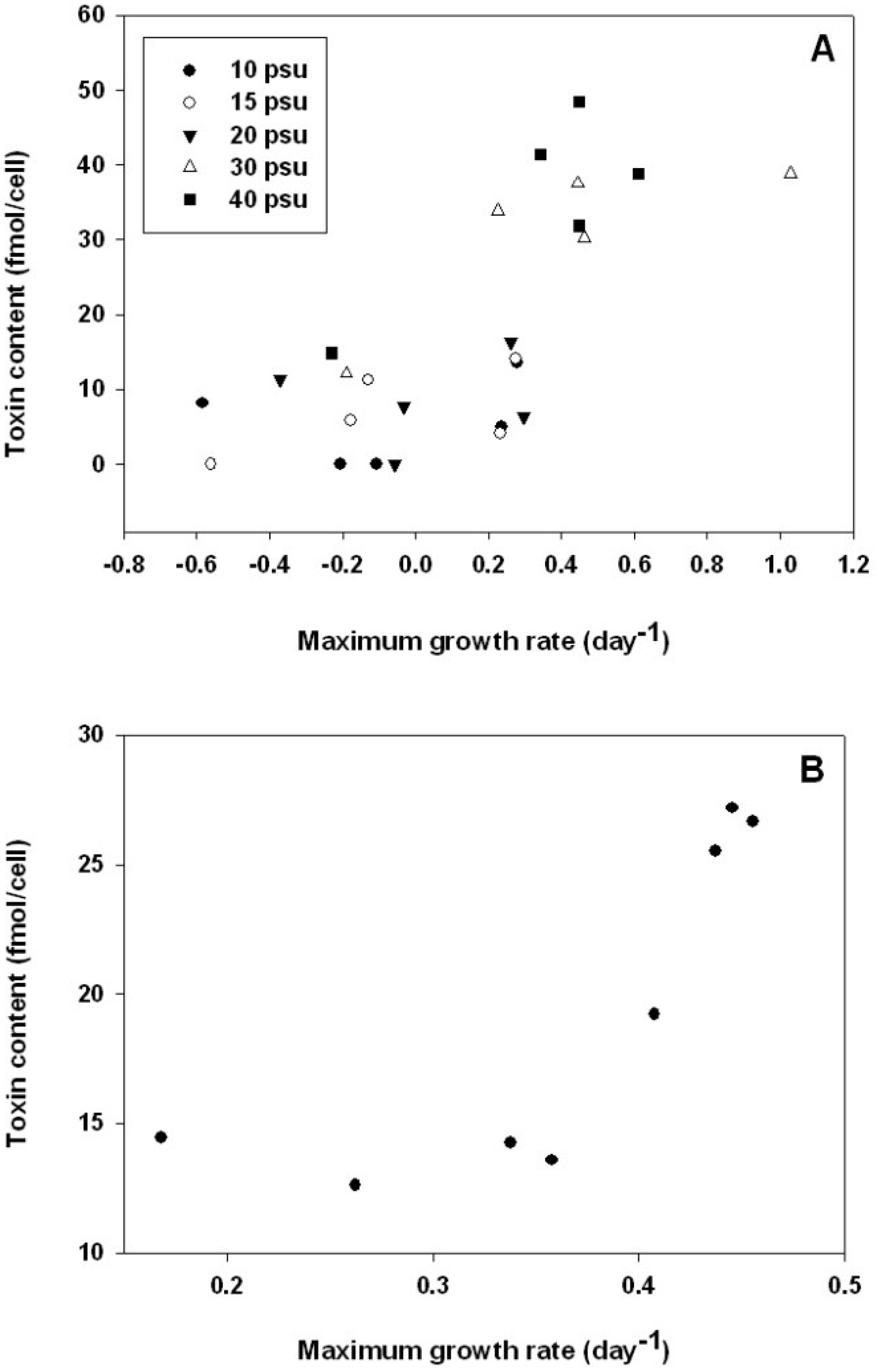
3. Experimental Section
3.1. Culture of A. catenella
3.2. Toxin Quantification by Liquid Chromatography/Fluorescence Detection
3.3. Statistics
4. Conclusion
| Species and studied area | Temp | Sal | Irradiance | Toxins | Toxin content | Reference |
|---|---|---|---|---|---|---|
| (°C) | (psu) | (μmol/m2/s) | (molar basis in %) | (fmol/cell) | ||
| Mediterranean waters | ||||||
| Alexandrium catenella ATTL01,02 (Thau, France) | 15 | 31 | 200 | PT: C1,2 > GTX1,4 > GTX2,3 | 5.3–44.3 fgEqSTX/cell | Lilly et al. (2002) [21] |
| Alexandrium catenella ACT03 (Thau, France) | 21–27 | 35–40 | 90–130 | PT: GX5 > C2 > C4 > GTX4 > GTX3 ≈ C1 ≈ C3 | 2.9–50.3 | This study |
| Alexandrium minutum (Greek coastal waters) | 20 | 37.5 | 60 | PT: GTX1; GTX4 | - | Ignatiades et al. (2007) [29] |
| Alexandrium minutum (Spain) | 17.5 | 35 | - | PT: GTX4 > GTX1 > GTX3; GTX2 | 0.1–0.4 | Dacosta et al. (2008) [31] |
| Asian waters | ||||||
| Alexandrium catenella (Japan) | 15 | 50 | - | PT: C2 (70.3%); NeoSTX(19.9%); C1 ≈ GTX1 ≈ STX | 34.5 ± 23 | Sekiguchi et al. (2001) [48] |
| Alexandrium catenella (Hong Kong) | 20–23 | 33 | - | PT: GTX4 > GTX3 > GTX1 > GTX6 > Neo STX; Cx | - | Siu et al. (1997) [36] |
| Alexandrium catenella (China) | 23.5 | - | 120 | PT: C1,2 (80%–90%) and GTX1,4 (5%–15%) | - | Xu et al. (2012) [35] |
| Alexandrium catenella (East China Sea) | 20 | - | 80 | PT: C1,2 (predominant)/trace amounts of GTX1,3,4,5,6 | 4–14 | Li et al. (2011) [34] |
| Alexandrium tamarense (Japan) | 15 | - | - | PT: C1,2 > GTX1,4 > GTX2,3/traces of NeoSTX and STX | - | Ichimi et al. (2002) [49] |
| Alexandrium tamarense (ATHK01, Hong Kong) | 22 | - | 220 | PT: GTX1,4; GTX2,3; STX | 1.64 | He et al. (2010) [50] |
| Alexandrium tamarense HK9301 (Hong Kong) | 23+ | 25–30 | 80–220 | PT: C1,2 > GTX4 > GTX5 > GTX1 > C3,4 | 15–85 | Wang and Hsieh (2005) [14] |
| Alexandrium tamarense (Southeast China sea) | 12–24 | 15–30 | 60 | PT: C1,2 (60%–80%); GTX5 (15%–30%); traces of GTX1,3,4 | 8–55 | Wang et al. (2006) [51] |
| Alexandrium tamarense AtPA01 (Malaysia) | 25+ | 10–30 | 140+ | PT: GTX1,4 (25%–40%); NeoSTX (30%–40%) | 0.1–0.8 | Lim and Ogata (2005) [7] |
| Alexandrium peruvianum ApKS01 (Malaysia) | 25+ | 10–30 | 140+ | PT: GTX1,4,5,6 | 0.25–0.75 | Lim and Ogata (2005) [7] |
| Alexandrium minutum (Malaysia) | 25+ | 10–30 | 140+ | PT: GTX1,4 (95%); MT: GTX2,3; traces of STX; NeoSTX | 0.27–2.08 | Lim and Ogata (2005) [7] |
| Alexandrium minutum T1 (Taiwan) | 25 | 15 | 120 | PT: GTX1,4 > GT2,3 | - | Hwang and Lu (2001) [11] |
| Alexandrium minutum (Taiwan) | 20–22 | - | 60 | PT: GTX1,4 or GTX2,3 depending on the strains | - | Chou et al. (2004) [52] |
| Alexandrium affine (Vietnam) | 24 | 30 | 25 | PT: GTX1,4; NeoSTX; STX | 1–2.28 | Nguyen-Ngoc (2004) [53] |
| Alexandrium tamiyavanichi (Japan) | 25 | 30 | 100 | PT: C2; GTX4/MT: C3,4; GTX2,3,5; STX; NeoSTX | - | Oh et al. (2009) [54] |
| Gymnodinium catenatum (Australia) | - | - | - | PT: C1,2; C3,4; B1,2; NeoSTX | - | Hallegraeff et al. (2012) [55] |
| Pyrodinium bahamense (Philippines) | - | - | - | PT: STX (85%–98%)/MT: dcSTX; B1 | 54–298 | Gedaria et al. (2007) [47] |
| North American Atlantic waters | ||||||
| Alexandrium fundyense (Casco Bay, Maine) | 15 | - | 200 | PT: GTX3,4; NeoSTX; STX ; C2 MT: GTX1,2,5; dcGTX3; C1 | ≈141 | Poulton et al. (2005) [15] |
| Alexandrium fundyense MI (Gulf of Maine, USA) | 20 | 30 | 100 | PT: GTX2,3; GTX1,4 > STX > NeoSTX | 244 | Etheridge and Roesler (2005) [8] |
| Alexandrium tamarense Pr18b (Canada) | 10–30 | 25 | 40–470 | PT: C1,2 (64.0%), GTX1,2,3,4 (1.7%); STX-NeoSTX (16.2%–17.8%) | 140–450 | Parkhill and Cembella (1999) [20] |
| South American waters | ||||||
| Alexandrium catenella (South of Chile) | 12–14 | 30 | 50 | PT: C1,2; GTX1,4/scarcity of absence of STX | 41.4–295.5 | Varela et al. (2012) [37] |
| Alexandrium catenella (South of Chile) | 15 | - | 50 | PT: GTX1,4 > GTX2,3, (they represent more than 98%) | - | Aguilera-Belmonte et al. (2011) [56] |
| Alexandrium tamarense (Argentina, Brazil, Chile) | - | - | - | PT: C1,2 (predominant); GTX1,4/MT: STX | 17–261 | Montoya et al. (2010) [57] |
| Alexandrium tamarense (Southern Brazil) | 20 | - | 250 | PT: C1,2 (30%–84%); GTX1,4 (6.6%–55%); GTX2,3 (<29%); NeoSTX (<24%) | - | Persich et al. (2006) [58] |
| European Atlantic waters | ||||||
| Alexandrium tamarense (Coast of Greenland) | 10 | - | 30 | PT: GTX1,2,3,4 | - | Baggesen et al. (2012) [59] |
| Alexandrium minutum (Irish coastal waters) | 15 | - | - | PT: GTX3 (>80%) and GTX2 | 14 pg/cell | Touzet et al. (2007) [60] |
| Alexandrium minutum AM89BM (France) | 18 | 25 | 100 | PT: GTX2,3 (>97%) | ≈30.4 | Grzebyk et al. (2003) [6] |
| Alexandrium minutum (Fleet Lagoon, UK) | 17 | - | 120 | PT: GTX3 (54%); GTX2 (10%); STX (36%) | 5.6–16.8 | Nascimento et al. (2005) [61] |
Acknowledgments
References
- Hallegraeff, G.M. A review of harmful algal blooms and their apparent global increase. Phycologia 1993, 32, 79–99. [Google Scholar] [CrossRef]
- Anderson, D.M.; Alpermann, T.J.; Cembella, A.D.; Collos, Y.; Masseret, E.; Montresor, M. The globally distributed genus Alexandrium: Multifaceted roles in marine ecosystems and impacts on human health. Harmful Algae 2012, 14, 10–35. [Google Scholar] [CrossRef]
- Cembella, A.D. Ecophysiology and Metabolism of Paralytic Shellfish Toxins in Marine Microalgae. In Physiological Ecology of Harmful Algal Blooms; Anderson, D.M., Cembella, A.D., Hallegraeff, G.M., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 1998; pp. 381–403. [Google Scholar]
- Oshima, Y. Chemical and Enzymatic Transformation of Paralytic Shellfish Toxins in Marine Organisms. In Harmful Marine Algal Blooms; Lassus, P., Arzul, G., Erard, E., Gentien, P., Marcaillou, C., Eds.; Lavoisier/Intercept: Paris, France, 1995; pp. 475–480. [Google Scholar]
- Hamasaki, K.; Horie, M.; Tokimitsu, A.; Toda, A.; Taguchi, S. Variability in toxicity of the dinoflagellate Alexandrium tamarense isolated from Hiroshima Bay, Western Japan, as a reflection of changing environmental conditions. J. Plankton Res. 2001, 23, 271–278. [Google Scholar] [CrossRef]
- Grzebyk, D.; Béchemin, C.; Ward, C.; Vérité, C.; Codd, G.; Maestrini, S.Y. Effects of salinity and two coastal waters on the growth and toxin content of the dinoflagellate Alexandrium minutum. J. Plankton Res. 2003, 25, 1185–1199. [Google Scholar] [CrossRef]
- Lim, P.T.; Ogata, T. Salinity effect on growth and toxin production of four tropical Alexandrium species (Dinophyceae). Toxicon 2005, 45, 699–710. [Google Scholar] [CrossRef]
- Etheridge, S.M.; Roesler, C. Effects of temperature, irradiance, and salinity on photosynthesis, growth rates, total toxicity, and toxin composition for Alexandrium fundyense isolates from the Gulf of Maine and Bay of Fundy. Deep-Sea Res. II 2005, 52, 2491–2500. [Google Scholar] [CrossRef]
- Ogata, T.; Ishimaru, T.; Kodama, M. Effect of water temperature and light intensity on growth rate and toxicity change in Protogonyaulax tamarensis. Mar. Biol. 1987, 95, 217–220. [Google Scholar] [CrossRef]
- Anderson, D.M.; Kulis, D.M.; Sullivan, J.J.; Hall, S.; Lee, C. Dynamics and physiology of saxitoxin production by the dinoflagellate Alexandrium spp. Mar. Biol. 1990, 104, 511–524. [Google Scholar] [CrossRef]
- Hwang, D.F.; Lu, Y.H. Influence of environmental and nutritional factor on growth, toxicity, and toxin profile of dinoflagellate Alexandrium minutum. Toxicon 2000, 38, 1491–1503. [Google Scholar] [CrossRef]
- Béchemin, C.; Grzebyk, D.; Hachame, F.; Hummert, C.; Maestrini, S.Y. Effect of different nitrogen/phosphorus nutrient ratios on the toxin content in Alexandrium minutum. Aquat. Microb. Ecol. 1999, 20, 157–165. [Google Scholar] [CrossRef]
- John, E.H.; Flynn, K.J. Growth dynamic and toxicity of Alexandrium fundyense (Dinophyceae): The effect of changing N:P supply ratios on internal toxin and nutrient levels. Eur. J. Phycol. 2000, 35, 11–23. [Google Scholar]
- Wang, D.Z.; Hsieh, D.P.H. Dynamics of C2 toxin and chlorophyll a formation in the dinoflagellate Alexandrium tamarense during large scale cultivation. Toxicon 2005, 39, 1533–1536. [Google Scholar] [CrossRef]
- Poulton, N.J.; Keafer, B.A.; Anderson, D.M. Toxin variability in natural populations of Alexandrium fundyense in Casco Bay, Maine evidence of nitrogen limitation. Deep-Sea Res. II 2005, 52, 2501–2521. [Google Scholar] [CrossRef]
- Boyer, G.L.; Sullivan, J.J.; Andersen, R.J.; Harrison, P.J.; Taylor, F.J.R. Effects of nutrient limitation on toxin production and composition in the marine dinoflagellate Protogonyaulax tamarensis. Mar. Biol. 1987, 96, 123–128. [Google Scholar] [CrossRef]
- Flynn, K.; Franco, J.M.; Fernandez, P.; Reguera, B.; Zapata, M.; Wood, G.; Flynn, K.J. Changes in toxin content, biomass and pigments of the dinoflagellate Alexandrium minutum during nitrogen refeeding and growth into nitrogen or phosphorus stress. Mar. Ecol. Prog. Ser. 1994, 111, 99–109. [Google Scholar] [CrossRef]
- Hall, S. Toxins and toxicity of Protogonyaulax from the northeast Pacific. Ph.D. Thesis, University of Alaska, Fairbanks, AK, USA, 1982. [Google Scholar]
- Franco, J.M.; Fernandez, P.; Reguera, B. Toxin profiles of natural populations and cultures of Alexandrium minutum Halim from Galician (Spain) coastal waters. J. Appl. Phycol. 1994, 6, 275–279. [Google Scholar] [CrossRef]
- Vila, M.; Garces, E.; Maso, M.; Camp, J. Is the distribution of the toxic dinoflagellate Alexandrium catenella expanding along the NW Mediterranean coast? Mar. Ecol. Prog. Ser. 2001, 222, 73–83. [Google Scholar] [CrossRef]
- Lilly, E.L.; Kulis, D.M.; Gentien, P.; Anderson, D.M. Paralytic shellfish poisoning toxins in France linked to a human-introduced strain of Alexandrium catenella from the western Pacific: Evidence from DNA and toxin analysis. J. Plankton Res. 2002, 24, 443–452. [Google Scholar] [CrossRef]
- Lugliè, A.; Giacobbe, M.G.; Sannio, A.; Fiocca, F.; Sechi, N. First record of the dinoflagellate Alexandrium catenella (Whedon & Kofoid) Balech (Dinophyta), a potential producer of paralytic shellfish poisoning, in Italian waters (Sardinia, Tyrrhenian Sea). Bocconea 2003, 16, 1045–1052. [Google Scholar]
- Turki, S.; Balti, N.; Ben Janet, H. First bloom of dinoflagellate Alexandrium catenella in Bizerte lagoon (northern Tunisia). Harmful Algae News 2007, 35, 7–9. [Google Scholar]
- Genovesi, B.; Laabir, M.; Masseret, E.; Collos, Y.; Vaquer, A.; Grzebyk, D. Dormancy and germination patterns in resting cysts of Alexandrium tamarense species complex (Dinophyceae) can facilitate bloom formation in a shallow lagoon (Thau, southern France). J. Plankton Res. 2009, 31, 1209–1224. [Google Scholar] [CrossRef]
- Masselin, P.; Amzil, Z.; Abadie, E.; Nézan, E.; Le Bec, C.; Chiantella, C.; Truquet, P. Paralytic shellfish poisoning on the French Mediterranean coast in the autumn 1998: Alexandrium tamarense complex (Dinophyceae) as causative agent. In Harmful Algae Blooms; Hallegraeff, G.M., Blackburn, S.I., Bolch, C.J., Lewis, R.J., Eds.; JOC-UNESCO: Paris, France, 2000; pp. 407–410. [Google Scholar]
- Chambouvet, A.; Laabir, M.; Sengco, M.; Vaquer, A.; Guillou, L. Genetic diversity of Amoebophryidae (Syndiniales) during Alexandrium catenella/tamarense (Dinophyceae) blooms in Thau lagoon (Mediterranean Sea, France). Res. Microbiol. 2011, 162, 959–968. [Google Scholar]
- Plus, M.; Chapelle, A.; Ménesguen, A.; Deslous-Paoli, J.M.; Auby, I. Modelling seasonal dynamics of biomasses and nitrogen contents in a seagrass meadow (Zostera noltii Hornem.): Application to the Thau lagoon (French Mediterranean coast). Ecol. Modell. 2003, 16, 213–238. [Google Scholar]
- Cembella, A.D.; Sullivan, J.J.; Boyer, G.L.; Taylor, F.J.R.; Andersen, R.J. Variation in paralytic shellfish toxin composition within the Protogonyaulax tamarensis/catenella species complex; red tide dinoflagellates. Biochem. Ecol. Syst. 1987, 15, 171–186. [Google Scholar]
- Ignatiades, L.; Gotsis-Skretas, O.; Metaxatos, A. Field and culture studies on the ecophysiology of the toxic dinoflagellate Alexandrium minutum (Halim) present in Greek coastal waters. Harmful Algae 2007, 6, 153–165. [Google Scholar] [CrossRef]
- Laabir, M.; Amzil, Z.; Lassus, P.; Masseret, E.; Tapilatu, Y.; de Vargas, R.; Grzebyk, D. Viability, growth and toxicity of Alexandrium catenella and Alexandrium minutum (Dinophyceae) following ingestion and gut passage in the oyster Crassostrea gigas. Aquat. Living Resour. 2007, 20, 51–57. [Google Scholar] [CrossRef]
- Da Costa, R.M.; Pereira, L.C.; Fernandez, F. Effects of toxic Alexandrium minutum strains on the feeding and survival rates of pelagic marine copepods Acartia grani and Euterpina acutifrons. Hydrobiologia 2008, 614, 55–63. [Google Scholar] [CrossRef]
- Pistocchi, R.; Guerrini, F.; Pezzolesi, L.; Riccardi, M.; Vanucci, S.; Ciminiello, P.; Dell’Aversano, C.; Forino, M.; Fattorusso, E.; Tartaglione, L.; et al. Toxin levels and profiles in microalgae from the north-western adriatic sea-15 years of studies on cultured species. Mar. Drugs 2012, 10, 140–162. [Google Scholar] [CrossRef]
- Ciminiello, P.; Fattorusso, E.; Forino, M.; Montresor, M. Saxitoxin and neosaxitoxin as toxic principles of Alexandrium andersoni (Dinophyceae) from the Gulf of Naples, Italy. Toxicon 2000, 38, 1871–1877. [Google Scholar] [CrossRef]
- Li, T.S.; Yu, R.C.; Zhou, M.J. Short-term effects of different nitrogen substrates on growth and toxin production of dinoflagellate Alexandrium catenella Balech (strain ACDH). Harmful Algae 2011, 12, 46–54. [Google Scholar] [CrossRef]
- Xu, J.; Ho, A.Y.T.; He, L.; Yin, K.; Hung, C.; Choi, N.; Lam, P.K.S.; Wud, R.; Anderson, D.M.; Harrison, P. Effects of inorganic and organic nitrogen and phosphorus on the growth and toxicity of two Alexandrium species from Hong Kong. Harmful Algae 2012, 16, 89–97. [Google Scholar] [CrossRef]
- Siu, G.; Young, M.; Chan, D.K.O. Environmental and nutritional factors which regulate population dynamics and toxin production in the dinoflagellate Alexandrium catenella. Hydrobiologia 1997, 352, 117–140. [Google Scholar] [CrossRef]
- Varela, D.; Paredes, J.; Alves-de-Souza, C.; Seguel, M.; Sfeir, A.; Frangopulos, M. Intraregional variation among Alexandrium catenella (Dinophyceae) strainsfrom southern Chile: Morphological, toxicological and genetic diversity. Harmful Algae 2012, 15, 8–18. [Google Scholar] [CrossRef]
- Laabir, M.; Jauzein, C.; Genovesi, B.; Masseret, E.; Grzebyk, D.; Cecchi, P.; Vaquer, A.; Perrin, Y.; Colos, Y. Influence of temperature, salinity and irradiance on the growth and cell yield of the harmful red tide dinoflagellate Alexandrium catenella colonizing Mediterranean waters. J. Plankton Res. 2011, 33, 1550–1563. [Google Scholar] [CrossRef]
- Kodama, M. Possible Links between Bacteria and Toxin Production in Algal Blooms. In Toxic Marine Phytoplankton; Graneli, E.P., Sundstrom, B., Edler, L., Anderson, D.M., Eds.; Elsevier: New York, NY, USA, 1990; pp. 52–61. [Google Scholar]
- Parkhill, J.P.; Cembella, A.D. Effects of salinity, light and inorganic nitrogen on growth and toxigenicity of the marine dinoflagellate Alexandrium tamarense from north eastern Canada. J. Plankton Res. 1999, 21, 939–955. [Google Scholar] [CrossRef]
- Toulza, E.; Shin, M.-S.; Blanc, G.; Audic, S.; Laabir, M.; Collos, Y.; Claverie, J.-M.; Grzebyk, D. Gene expression in proliferating cells of the dinoflagellate Alexandrium catenella (Dinophyceae). Appl. Environ. Microbiol. 2010, 76, 4521–4529. [Google Scholar]
- Harrison, P.J.; Waters, R.E.; Taylor, F.J.R. A broad-spectrum artificial seawater medium for coastal and open ocean phytoplankton. J. Phycol. 1980, 16, 28–35. [Google Scholar]
- Yamaguchi, M.; Honjo, T. Effect of temperature, salinity and irradiance on the growth of the noxious red tide flagellate Gymnodinium nagasakiense (Dinophyceae). Nippon Suisan Gakkaishi 1989, 55, 2029–2036. [Google Scholar] [CrossRef]
- Kim, D.I.; Matsuyama, Y.; Nagasoe, S.; Yamaguch, M.; Yoon, Y.H.; Oshima, Y.; Imada, N.; Honjo, T. Effects of temperature, salinity and irradiance on the growth of the harmful red tide dinoflagellate Cochlodinium polykrikoides Margalef (Dinophyceae). J. Plankton Res. 2004, 26, 61–66. [Google Scholar] [CrossRef]
- Guillard, R.L.R. Division Rates. In Handbook of Phycological Methods. Cultures Methods and Growth Measurements; Stein, J.R., Ed.; Cambridge University Press: Cambridge, UK, 1973; pp. 290–311. [Google Scholar]
- Oshima, Y. Post-column Derivatization HPLC Methods for Paralytic Shellfish Poisons. In Manual on Harmful Marine Microalgae; Hallegraeff, G.M., Anderson, D.M., Cembella, A.D., Eds.; UNESCO: Paris, France, 1995; pp. 81–94. [Google Scholar]
- Gedaria, A.I.; Luckas, B.; Reinhardt, K.; Azanza, R.V. Growth response and toxin concentration of cultured Pyrodinium bahamense var. compressum to varying salinity and temperature conditions. Toxicon 2007, 50, 518–529. [Google Scholar] [CrossRef]
- Sekiguchi, K.; Ogata, T.; Kaga, Y.; Yoshida, M.; Fukuyo, Y.; Kodama, M. Accumulation of paralytic shellfish toxins in the scallop Patinopecten yessoensis caused by the dinoflagellate Alexandrium catenella in Otsuchi Bay, Iwate Prefecture, northern Pacific coast of Japan. Fish. Sci. 2001, 67, 1157–1162. [Google Scholar] [CrossRef]
- Ichimi, K.; Suzuki, T.; Ito, A. Variety of PSP toxin profiles in various culture strains of Alexandrium tamarense and change of toxin profile in natural A. tamarense population. J. Exp. Mar. Biol. Ecol. 2002, 273, 51–60. [Google Scholar] [CrossRef]
- He, H.; Chen, F.; Li, H.; Xiang, W.; Li, Y.; Jiang, Y. Effect of iron on growth, biochemical composition and paralytic shellfish poisoning toxins production of Alexandrium tamarense. Harmful Algae 2010, 9, 98–104. [Google Scholar] [CrossRef]
- Wang, D.Z.; Zhang, S.G.; Gu, H.F.; Chan, L.L.; Hong, H.S. Paralytic shellfish toxin profiles and toxin variability of the genus Alexandrium (Dinophyceae) isolated from the Southeast China Sea. Toxicon 2006, 48, 138–151. [Google Scholar] [CrossRef]
- Chou, H.G.; Chen, Y.M.; Chen, C.Y. Variety of PSP toxins in four culture strains of Alexandrium minutum collected from southern Taiwan. Toxicon 2004, 43, 337–340. [Google Scholar] [CrossRef]
- Nguyen-Ngoc, L. An autoecological study of the potentially toxic dinoflagellate Alexandrium affine isolated from Vietnamese waters. Harmful Algae 2004, 3, 117–129. [Google Scholar] [CrossRef]
- Oh, S.J.; Matsuyama, Y.; Nagai, S.; Itakura, S.; Yoon, Y.H.; Yang, H.S. Comparative study on the PSP component and toxicity produced by Alexandrium tamiyavanichii (Dinophyceae) strains occurring in Japanese coastal water. Harmful Algae 2009, 8, 362–368. [Google Scholar] [CrossRef]
- Hallegraeff, G.M.; Blackburn, S.I.; Doblin, M.A.; Bolch, C.J.S. Global toxicology, ecophysiology and population relationships of the chain forming PST dinoflagellate Gymnodinium catenatum. Harmful Algae 2012, 14, 130–143. [Google Scholar] [CrossRef]
- Aguilera-Belmonte, A.; Inostroza, I.; Franco, J.; Riobo, P.; Gomez, P. The growth, toxicity and genetic characterization of seven strains of Alexandrium catenella (Whedon and Kofoid) Balech 1985 (Dinophyceae) isolated during the 2009 summer outbreak in southern Chile. Harmful Algae 2011, 12, 105–112. [Google Scholar] [CrossRef]
- Montoya, N.G.; Fulco, V.K.; Carignan, M.O.; Carreto, J.I. Toxin variability in cultured and natural populations of Alexandrium tamarense from southern South America—Evidences of diversity and environmental regulation. Toxicon 2010, 56, 1408–1418. [Google Scholar] [CrossRef]
- Persich, G.; Kulis, D.; Lilly, E.; Anderson, D.; Garcia, V.M.T. Probable origin and toxin profile of Alexandrium tamarense (Lebour) Balech from southern Brazil. Harmful Algae 2006, 5, 36–44. [Google Scholar] [CrossRef]
- Baggesen, C.; Moestrup, Ø.; Daugbjerg, N.; Krock, B.; Cembella, A.D.; Madsen, S. Molecular phylogeny and toxin profiles of Alexandrium tamarense (Lebour) Balech (Dinophyceae) from the west coast of Greenland. Harmful Algae 2012, 19, 108–116. [Google Scholar] [CrossRef] [Green Version]
- Touzet, N.; Franco, J.M.; Raine, R. Characterization of nontoxic and toxin producing strains of Alexandrium minutum (Dinophyceae) in Irish Coastal waters. Appl. Environ. Microbiol. 2007, 73, 3333–3342. [Google Scholar] [CrossRef]
- Nascimento, S.M.; Duncan, A.; Purdie, D.A.; Lilly, E.L.; Larsen, J.; Morris, S. Toxin profile, pigment composition and large subunit rDNAphylogentic analysis of an Alexandrium minutum (Dinophycea) strain isolated from the Fleet lagoon, United Kingdom. J. Phycol. 2005, 41, 343–353. [Google Scholar] [CrossRef]
- Boczar, B.A.; Beitler, M.K.; Liston, J.; Sullivan, J.J.; Cattolico, R.A. Paralytic shellfish toxins in Protogonyaulax tamarensis and Protogonyaulax catenella in axenic culture. Plant Physiol. 1988, 88, 1285–1290. [Google Scholar] [CrossRef]
- Alpermann, T.J.; Tillmann, U.; Beszteri, B.; Cembella, A.D.; John, U. Phenotypic variation and genotypic diversity in a planktonic population of the toxigenic marine dinoflagellate Alexandrium tamarense (Dinophycea). J. Phycol. 2010, 46, 18–32. [Google Scholar] [CrossRef] [Green Version]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Laabir, M.; Collos, Y.; Masseret, E.; Grzebyk, D.; Abadie, E.; Savar, V.; Sibat, M.; Amzil, Z. Influence of Environmental Factors on the Paralytic Shellfish Toxin Content and Profile of Alexandrium catenella (Dinophyceae) Isolated from the Mediterranean Sea. Mar. Drugs 2013, 11, 1583-1601. https://doi.org/10.3390/md11051583
Laabir M, Collos Y, Masseret E, Grzebyk D, Abadie E, Savar V, Sibat M, Amzil Z. Influence of Environmental Factors on the Paralytic Shellfish Toxin Content and Profile of Alexandrium catenella (Dinophyceae) Isolated from the Mediterranean Sea. Marine Drugs. 2013; 11(5):1583-1601. https://doi.org/10.3390/md11051583
Chicago/Turabian StyleLaabir, Mohamed, Yves Collos, Estelle Masseret, Daniel Grzebyk, Eric Abadie, Véronique Savar, Manoella Sibat, and Zouher Amzil. 2013. "Influence of Environmental Factors on the Paralytic Shellfish Toxin Content and Profile of Alexandrium catenella (Dinophyceae) Isolated from the Mediterranean Sea" Marine Drugs 11, no. 5: 1583-1601. https://doi.org/10.3390/md11051583






