Multiple Beneficial Lipids Including Lecithin Detected in the Edible Invasive Mollusk Crepidula fornicata from the French Northeastern Atlantic Coast
Abstract
:1. Introduction
2. Results and Discussion
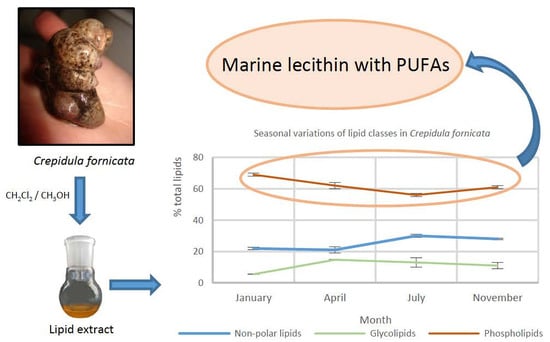
2.1. Lipid Content and Lipid Class Composition at the Four Seasons
| Collection Season | Total Lipids | Non-Polar Lipids | Glycolipids | Phospholipids |
|---|---|---|---|---|
| Winter (January) | 5.3 ± 0.3 | 21.9 ± 0.8 | 5.5 ± 0.1 | 69 ± 1 |
| Spring (April) | 2.7 ± 0.2 | 21 ± 2 | 14.7 ± 0.2 | 62 ± 2 |
| Summer (July) | 3.3 ± 0.3 | 30 ± 1 | 13 ± 3 | 56 ± 1 |
| Autumn (November) | 3.1 ± 0.1 | 28.0 ± 0.4 | 11 ± 2 | 61 ± 1 |
2.2. Phospholipid Class Composition at the Four Seasons
| Phospholipid Class | Winter | Spring | Summer | Autumn |
|---|---|---|---|---|
| Cardiolipin | 6.0 ± 0.1 | 8 ± 1 | 10.1 ± 0.3 | 6.0 ± 0.6 |
| Phosphatidylethanolamine | 11.7 ± 0.3 | 2.1 ± 0.1 | 2.9 ± 0.1 | nd |
| Ceramide aminoethylphosphonate | 15.8 ± 0.2 | 11 ± 2 | 9.6 ± 0.3 | 3.7 ± 0.4 |
| Phosphatidylserine | 1.0 ± 0.1 | 1.0 ± 0.1 | nd | nd |
| Lysophosphatidylethanolamine | nd | nd | nd | 1.5 ± 0.2 |
| Phosphatidylcholine | 63.9 ± 0.3 | 76 ± 1 | 70.0 ± 0.8 | 88.9 ± 0.1 |
| Undetermined | 1.6 ± 0.1 | 2.1 ± 0.7 | 7.3 ± 0.1 | -- |
2.3. Phospholipid Fatty Acid Composition in Winter
| Fatty Acids (Symbol) | ECL a | Abundance (wt %) |
|---|---|---|
| Saturated Fatty Acids (SFAs) | ||
| 14:0 | 14.00 | 1.13 ± 0.02 |
| 4,8,12-Me3-13:0 | 14.49 | 1.18 ± 0.03 |
| 15:0 | 15.00 | 0.62 ± 0.04 |
| i-16:0 | 15.60 | 1.04 ± 0.03 |
| 16:0 | 16.00 | 11.7 ± 0.6 |
| i-17:0 | 16.64 | 2.20 ± 0.06 |
| ai-17:0 | 16.73 | 1.36 ± 0.02 |
| 17:0 | 17.00 | 1.49 ± 0.08 |
| i-18:0 | 17.64 | 0.34 ± 0.01 |
| 18:0 | 18.00 | 5.49 ± 0.09 |
| br-20:0 | 18.38 | 0.24 ± 0.01 |
| Total SFAs | -- | 27 ± 1 |
| Monounsaturated fatty acids (MUFAs) | ||
| 9-16:1 | 15.74 | 1.90 ± 0.02 |
| 7-Me-8-16:1 | 16.12 | 0.21 ± 0.01 |
| 7-Me-6(Z)-16:1 | 16.20 | 0.22 ± 0.01 |
| 7-Me-6(E)-16:1 | 16.53 | 0.95 ± 0.05 |
| 9-18:1 | 17.72 | 4.2 ± 0.1 |
| 11-18:1 | 17.80 | 2.71 ± 0.09 |
| 11-20:1 | 19.68 | 4.85 ± 0.02 |
| 13-20:1 | 19.73 | 2.64 ± 0.05 |
| br-21:1 | 20.32 | 0.64 ± 0.01 |
| Total MUFAs | 18.3 ± 0.3 | |
| Polyunsaturated fatty acids (PUFAs) | ||
| 18:4n-3 | 17.54 | 1.11 ± 0.04 |
| 18:2n-6 | 17.66 | 2.11 ± 0.01 |
| 20:4n-6 | 19.24 | 7.64 ± 0.03 |
| 20:5n-3 | 19.34 | 9.4 ± 0.1 |
| 20:2n-9 | 19.52 | 1.13 ± 0.04 |
| 20:2n-7 | 19.63 | 1.52 ± 0.02 |
| 22:6n-3 | 21.12 | 7.3 ± 0.3 |
| 22:4n-6 | 21.19 | 1.34 ± 0.03 |
| 22:5n-3 | 21.28 | 3.8 ± 0.1 |
| 22:3n-6 | 21.34 | 0.36 ± 0.02 |
| 22:2n-9,15 | 21.40 | 1.86 ± 0.01 |
| 22:2n-7,15 | 21.46 | 6.19 ± 0.08 |
| Total PUFAs | 43.8 ± 0.8 | |
| Fatty aldehyde dimethylacetals (DMAs) | ||
| 16:0 | 16.48 | 0.63 ± 0.02 |
| br1-17:0 | 17.12 | 1.14 ± 0.02 |
| br2-17:0 | 17.22 | 1.00 ± 0.04 |
| 17:0 | 17.48 | 0.47 ± 0.06 |
| br1-18:0 | 18.10 | 0.95 ± 0.09 |
| br2-18:0 | 18.22 | 0.24 ± 0.04 |
| 18:0 | 18.48 | 3.51 ± 0.05 |
| br-19:0 | 19.22 | 0.24 ± 0.01 |
| 20:1 | 20.17 | 2.48 ± 0.03 |
| Total DMAs | 10.7 ± 0.4 | |
2.4. Free Sterol Composition in Winter
| Systematic Names | Trivial Names | % Sterol Fraction |
|---|---|---|
| 24-nor-Cholesta-5,22E-dien-3β-ol | 24-nor-Dehydrocholesterol | 1.24 ± 0.07 |
| 24-nor-5α-Cholest-22E-en-3β-ol | 24-nor-Dehydrocholestanol | 0.69 ± 0.02 |
| Cholesta-5,22Z-dien-3β-ol | 22Z-Dehydrocholesterol | 2.85 ± 0.04 |
| Cholesta-5,22E-dien-3β-ol | 22E-Dehydrocholesterol | 8.33 ± 0.08 |
| 5α-Cholest-22E-en-3β-ol | 22-Dehydrocholestanol | 3.13 ± 0.03 |
| 5α-Cholest-5-en-3β-ol | Cholesterol | 31.29 ± 0.04 |
| 5α-Cholestan-3β-ol | Cholestanol | 7.21 ± 0.06 |
| 24-Methylcholesta-5,22E-dien-3β-ol | Brassicasterol/Crinosterol | 18.6 ± 0.1 |
| X1 (Δ° C28:0) | -- | 0.32 ± 0.01 |
| 24-Methylcholesta-5,24(28)-dien-3β-ol | 24-Methylenecholesterol | 7.32 ± .04 |
| 24-Methylcholest-5-en-3β-ol | Campesterol 22,23-Dihydrobrassicasterol | 6.72 ± 0.08 |
| X2 (Δ° C29:2) | -- | 0.54 ± 0.02 |
| 24-Ethylcholest-5,22E-dien-3β-ol | Poriferasterol/Stigmasterol | 0.45 ± 0.02 |
| 24-Ethyl-5α-cholest-22E-en-3β-ol | Poriferastanol/Stigmastanol | 0.43 ± 0.01 |
| 24-Ethylcholest-5-en-3β-ol | β-Sitosterol/Clionasterol | 4.62 ± 0.07 |
| 24-Ethylcholesta-5,24(28)-dien-3β-ol | Fucosterol | 5.13 ± 0.06 |
| X3 (Δ° C30:0) | -- | 0.32 ± 0.03 |
| X4 (Δ° C30:0) | -- | 0.37 ± 0.03 |
| 22,23-Methylene-23,24-dimethylcholest-5-en-3β-ol | Gorgosterol | 0.38 ± 0.01 |
3. Experimental Section
3.1. Specimen Collection
3.2. Chemicals
3.3. Lipid Extraction and Separation of Lipid Classes
3.4. Phospholipid Determination by HPLC-ELSD
3.5. Preparation of Fatty Acid Methyl Esters, N-Acyl Pyrrolidides and Sterol Acetates
3.6. Gas Chromatography-Mass Spectrometry Analyses
3.7. Statistical Expression of Data
4. Conclusions
| Lipids | Biological Effects | References * |
|---|---|---|
| Lecithin (polyunsaturated FAs) | Protective factor against colon cancer | [50,51,52] |
| Treatment of psoriasis | [53] | |
| CAEP | Implications in some hemocyte functions | [31] |
| Cardiolipin | Optimization of mitochondrial respiratory performance | [34] |
| n-3 Polyunsaturated FAs (EPA, DHA) | Human health benefits | [9,10,11,12,13,14,15] |
| n-3 Docosapentaenoic acid (DPA) | Inhibition of platelet aggregation | [35] |
| Influence to endothelial cell migration ability | [35] | |
| Diunsaturated NMI FAs | Resistance to oxydative stress and microbial lipases | [36,37] |
| Plasmalogens | Anti oxydative stress | [37,38,39,40] |
| Phytosterols | Lowering cholesterol effects | [44,45,46,47] |
| Action on cardiovascular disease | [47] | |
| Anti-inflammatory properties | [47] |
Acknowledgments
Author Contributions
Abbreviations
| CAEP | ceramide aminoethylphosphonate |
| DHA | n-3 docosahexaenoic acid |
| DMA(s) | dimethylacetal(s) |
| DPA | n-3 docosapentaenoic acid |
| ELSD | evaporative light scattering detector |
| EPA | n-3 eicosapentaenoic acid |
| FA(s) | fatty acid(s) |
| FAME(s) | fatty acid methyl ester(s) |
| GC-MS | gas chromatography-mass spectrometry |
| NAP | N-acyl pyrrolidide(s) |
| NMI | non-methylene-interrupted |
| PC | phosphatidylcholine |
| PUFA(s) | polyunsaturated fatty acid(s) |
| PL(s) | phospholipid(s) |
| SA(s) | sterol acetate(s) |
Conflicts of Interest
References
- Minchin, D.; McGrath, D.; Duggan, C.B. The slipper limpet, Crepidula fornicata (L.), in Irish waters, with a review of its occurrence in the Northeastern Atlantic. J. Conchol. 1995, 35, 249–256. [Google Scholar]
- Blanchard, M. Spread of the slipper limpet Crepidula fornicata (L. 1758) in Europe. Current state and consequences. Sci. Mar. 1997, 61, 109–118. [Google Scholar]
- Thieltges, D.; Strasser, M.; Reise, K. How bad are invaders in coastal waters? The case of the American slipper limpet Crepidula fornicata in Western Europe. Biol. Invasions 2006, 8, 1673–1680. [Google Scholar]
- Viard, F.; Ellien, C.; Dupont, L. Dispersal ability and invasion success of Crepidula fornicata in a single gulf: Insights from genetic markers and larval-dispersal model. Helgol. Mar. Res. 2006, 60, 144–152. [Google Scholar] [CrossRef]
- Thieltges, D.W.; Strasser, M.; van Beusekom, J.E.E.; Reise, K. Too cold to prosper—Winter mortality prevents population increase of the introduced American slipper limpet Crepidula fornicata in northern Europe. J. Exp. Mar. Biol. Ecol. 2004, 311, 375–391. [Google Scholar] [CrossRef]
- De Montaudouin, X.; Audemard, C.; Labourg, P.-J. Does the slipper limpet (Crepidula fornicata, L.) impair oyster growth and zoobenthos biodiversity? A revisited hypothesis. J. Exp. Mar. Biol. Ecol. 1999, 235, 105–124. [Google Scholar]
- Decottignies, P.; Beninger, P.G.; Rincé, Y.; Robins, R.J.; Riera, P. Exploitation of natural food sources by two sympatric, invasive suspension-feeders, Crassostrea gigas and Crepidula fornicata. Mar. Ecol. Prog. Ser. 2007, 334, 179–192. [Google Scholar] [CrossRef]
- Frésard, M.; Boncoeur, J. Controlling the biological invasion of a commercial fishery by a space competitor: A bioeconomic model with reference to the Bay of St-Brieuc scallop fishery. Agric. Resour. Econ. Rev. 2006, 35, 78–97. [Google Scholar]
- Bergé, J.P.; Barnathan, G. Recent advances in fatty acids from lipids of marine organisms: Molecular biodiversity, roles as biomarkers, biologically-active compounds and economical aspects. Adv. Biochem. Eng. Biotechnol 2005, 96, 49–125. [Google Scholar] [PubMed]
- Russell, F.D.; Bürgin-Maunder, C.S. Distinguishing health benefits of eicosapentaenoic and docosahexaenoic acids. Mar. Drugs 2012, 10, 2535–2559. [Google Scholar] [CrossRef] [PubMed]
- Pereira, H.; Barreira, L.; Figueiredo, F.; Custodio, L.; Vizetto-Duarte, C.; Polo, C.; Resek, E.; Engelen, A.; Varela, J. Polyunsaturated fatty acids of marine macroalgae: Potential for nutritional and pharmaceutical applications. Mar. Drugs 2012, 10, 1920–1935. [Google Scholar]
- Baum, S.J.; Kris-Etherton, P.M.; Willett, W.C.; Lichtenstein, A.H.; Rudel, L.L.; Maki, K.C.; Whelan, J.; Ramsden, C.E.; Block, R.C. Fatty acids in cardiovascular health and disease: A comprehensive update. J. Clin. Lipidol. 2012, 6, 216–234. [Google Scholar] [CrossRef] [PubMed]
- Lorente-Cebrian, S.; Costa, A.G.; Navas-Carretero, S.; Zabala, M.; Martinez, J.A.; Moreno-Aliaga, M.J. Role of omega-3 fatty acids in obesity, metabolic syndrome, and cardiovascular diseases: A review of the evidence. J. Physiol. Biochem. 2013, 69, 633–651. [Google Scholar] [CrossRef] [PubMed]
- Jing, K.; Wu, T.; Lim, K. Omega-3 polyunsaturated fatty acids and cancer. Anticancer Agents Med. Chem. 2013, 13, 1162–1177. [Google Scholar] [CrossRef] [PubMed]
- Eckert, G.P.; Lipka, U.; Muller, W.E. Omega-3 fatty acids in neurodegenerative diseases: Focus on mitochondria. Prostaglandins Leukot. Essent. Fatty Acids 2013, 88, 105–114. [Google Scholar] [CrossRef]
- Kattner, G.; Hagen, W.; Grave, M.; Albers, C. Exceptional lipids and fatty acids in the pteropod Clione limacina (Gastropoda) from both polar oceans. Mar. Chem. 1998, 61, 219–228. [Google Scholar] [CrossRef]
- Falk-Petersen, S.; Sargent, J.R.; Kwasniewski, S.; Gulliksen, B.; Millar, R.-M. Lipids and fatty acids in Clione limacina and Limacina helicina in Svalbard waters and the Arctic Ocean: Trophic implications. Polar Biol. 2001, 24, 163–170. [Google Scholar] [CrossRef]
- Saito, H. Lipid and FA composition of the pearl oyster Pinctada fucata martensii: Influence of season and maturation. Lipids 2004, 39, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Dunstan, G.A.; Volkman, J.K.; Barrett, S.M. The effect of lyophilization on the solvent extraction of lipid classes, fatty acids, and sterols from the oyster Crassostrea gigas. Lipids 1993, 28, 937–944. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Y.; Sinclair, A.J. Seasonal variations of lipid content and composition in Perna viridis. Lipids 2007, 42, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Jiang, J.; Xue, C.H.; Zhang, B.; Xu, J.C. Seasonal changes in phospholipids of mussel (Mytilus edulis Linné). J. Sci. Food Agric. 2003, 83, 133–135. [Google Scholar] [CrossRef]
- Murphy, K.J.; Mooney, B.D.; Manna, N.J.; Nichols, P.D.; Sinclair, A.J. Lipid, FA, and sterol composition of New Zealand green lipped mussel (Perna canaliculus) and Tasmanian blue mussel (Mytilus edulis). Lipids 2002, 37, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Aono, H. Characteristics of lipid and fatty acid of marine gastropod Turbo cornutus: High levels of arachidonic and n-3 docosapentaenoic acid. Food Chem. 2014, 145, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Beninger, P.G. Seasonal variations of the major lipid classes in relation to the reproductive activity of two species of clams raised in a common habitat: Tapes decussatus L. (Jereys 1863) and T. philippinarum (Adams & Reeve 1850). J. Exp. Mar. Biol. Ecol. 1984, 79, 79–90. [Google Scholar] [CrossRef]
- Fernandez-Reiriz, M.J.; Labarta, U.; Babarro, J.M.F. Comparative allometries in growth and chemical composition of mussel (Mytilus galloprovincialis Lmk) cultured in two zones in the Ria sada (Galicia, NW Spain). J. Shell. Res. 1996, 15, 349–353. [Google Scholar]
- Matsubara, T. The structure and distribution of ceramide aminoethylphosphonates in the oyster (Ostrea gigas). Biochim. Biophys. Acta 1975, 388, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Hanuš, L.O.; Levitsky, D.O.; Shkrob, I.; Dembitsky, V.M. Plasmalogens, fatty acids and alkyl glyceryl ethers of marine and freshwater clams and mussels. Food Chem. 2009, 116, 491–498. [Google Scholar] [CrossRef]
- Zhukova, N.V. Lipids and fatty acids of nudibranch mollusks: Potential sources of bioactive compounds. Mar. Drugs 2014, 12, 4578–4592. [Google Scholar] [CrossRef] [PubMed]
- Le Grand, F.; Kraffe, E.; Marty, Y.; Donaghy, L.; Soudant, P. Membrane phospholipid composition of hemocytes in the Pacific oyster Crassostrea gigas and the Manila clam Ruditapes philippinarum. Comp. Biochem. Physiol. Part A 2011, 159, 383–391. [Google Scholar] [CrossRef]
- Kostetsky, E.Y.; Velansky, P.V. Phospholipids of sea worms, mollusks, and arthropods. Russ. J. Mar. Biol. 2009, 35, 187–199. [Google Scholar] [CrossRef]
- Kraffe, E.; Soudant, P.; Marty, Y.; Kervarec, N. Docosahexaenoic acid- and eicosapentaenoic acid-enriched cardiolipin in the Manila clam Ruditapes philippinarum. Lipids 2005, 40, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Schlame, M.; Rua, D.; Greenberg, M.L. The biosynthesis and functional role of cardiolipin. Prog. Lipid Res. 2000, 39, 257–288. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Cameron-Smith, D.; Garg, M.; Sinclair, A.J. Docosapentaenoic acid (22:5n-3): A review of its biological effects. Prog. Lipid Res. 2011, 50, 28–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnathan, G. Non-methylene-interrupted fatty acids from marine invertebrates: Occurrence, characterization and biological properties. Biochimie 2009, 91, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Kraffe, E.; Soudant, P.; Marty, Y. Fatty acids of serine, ethanolamine, and choline plasmalogens in some marine bivalves. Lipids 2004, 39, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Nagan, N.; Zoeller, R.A. Plasmalogens: Biosynthesis and functions. Prog. Lipid Res. 2001, 40, 199–229. [Google Scholar] [CrossRef] [PubMed]
- Brites, P.; Waterham, H.R.; Wanders, R.J.A. Functions and biosynthesis of plasmalogens in health and disease. Biochim. Biophys. Acta 2004, 1636, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, T. The role of plasmalogen in the oxidative stability of neutral lipids and phospholipids. J. Agric. Food Chem. 2010, 58, 2554–2561. [Google Scholar] [CrossRef] [PubMed]
- Voogt, P.A. Investigations of the capacity of synthesizing 3β-sterols in mollusca. V. The biosynthesis and composition of 3 β-sterols in the mesogastropods Crepidula fornicata and Natica cataena. Comp. Biochem. Physiol. Part B 1971, 39, 139–149. [Google Scholar]
- Withers, N.; Kokke, W.C.M.C.; Fenical, W.; Djerassi, C. Sterol patterns of cultured zooxanthellae isolated from marine invertebrates: Synthesis of gorgosterol and 23-desmethylgorgosterol by aposymbiotic algae. Proc. Natl. Acad. Sci. USA 1982, 79, 3764–3768. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, H.; Ohnishi, M.; Ogawa, S. Differences in sterol composition between male and female gonads of dominant limpet species. Lipids 2009, 44, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Brufau, G.; Canela, M.A.; Rafecas, M. Phytosterols: Physiologic and metabolic aspects related to cholesterol-lowering properties. Nutr. Res. 2008, 28, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Saeidnia, S.; Manayi, A.; Gohari, A.R.; Abdollahi, M. The story of beta-sitosterol—A review. Eur. J. Med. Plants 2014, 4, 590–609. [Google Scholar] [CrossRef]
- Do, H.Q.; van Landeghem, L.; Wielgosz-Collin, G.; Takoudju, M.; Huvelin, J.M.; Kornprobst, J.M.; Bard, J.M.; Barnathan, G.; Nazih, H. Unusual sterolic mixture, and 24-isopropylcholesterol, from the sponge Ciocalypta sp. reduce cholesterol uptake and basolateral secretion in Caco-2 cells. J. Cell. Biochem. 2009, 106, 659–665. [Google Scholar]
- Patch, C.S.; Tapsell, L.C.; Williams, P.G.; Gordon, M. Plant sterols as dietary adjuvants in the reduction of cardiovascular risk: Theory and evidence. Vasc. Health Risk Manag. 2006, 2, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Dagorn, F.; Dumay, J.; Wielgosz-Collin, G.; Rabesaotra, V.; Viau, M.; Monniot, C.; Biard, J.F.; Barnathan, G. Phospholipid distribution and phospholipid fatty acids of the tropical tunicates Eudistoma sp. and Leptoclinides uniorbis. Lipids 2010, 45, 253–261. [Google Scholar] [CrossRef]
- Barnathan, G.; Genin, E.; Nongonierma, R.; Al-Lihaibi, S.; Velosaotsy, N.E.; Kornprobst, J.M. Phospholipid fatty acids and sterols of two Cinachyrella from Saudi Arabia Red Sea. Comparative study with Cinachyrella sponges species from other origins. Comp. Biochem. Physiol. B 2003, 135, 297–308. [Google Scholar]
- Fernandes, G.B.; Alberici, R.M.; Pereira, G.G.; Cabral, E.C.; Eberlin, M.N.; Barrera-Arellano, D. Direct characterization of commercial lecithins by easy ambient sonic-spray ionization mass spectrometry. Food Chem. 2012, 135, 1855–1860. [Google Scholar] [CrossRef] [PubMed]
- Helmerich, G.; Koehler, J. Comparison of methods for the quantitative determination of phospholipids in lecithins and flour improvers. J. Agric. Food Chem. 2003, 51, 6645–6651. [Google Scholar] [CrossRef] [PubMed]
- Canty, D.J.; Zeisel, S.H. Lecithin and choline in human health and disease. Nutr. Rev. 1994, 52, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Küllenberg, D.; Taylor, L.A.; Schneider, M.; Massing, U. Health effects of dietary phospholipids. Lipids Health Dis. 2012, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, K.; Hossain, Z.; Takahashi, K. Marine phosphatidylcholine suppresses 1,2-dimethylhydrazine-induced colon carcinogenesis in rats by inducing apoptosis. Nutr. Res. 2008, 28, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Dupont, P. Traitement du psoriasis par la lécithine marine. Phytothérapie 2006, 4, 15–22. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dagorn, F.; Buzin, F.; Couzinet-Mossion, A.; Decottignies, P.; Viau, M.; Rabesaotra, V.; Barnathan, G.; Wielgosz-Collin, G. Multiple Beneficial Lipids Including Lecithin Detected in the Edible Invasive Mollusk Crepidula fornicata from the French Northeastern Atlantic Coast. Mar. Drugs 2014, 12, 6254-6268. https://doi.org/10.3390/md12126254
Dagorn F, Buzin F, Couzinet-Mossion A, Decottignies P, Viau M, Rabesaotra V, Barnathan G, Wielgosz-Collin G. Multiple Beneficial Lipids Including Lecithin Detected in the Edible Invasive Mollusk Crepidula fornicata from the French Northeastern Atlantic Coast. Marine Drugs. 2014; 12(12):6254-6268. https://doi.org/10.3390/md12126254
Chicago/Turabian StyleDagorn, Flore, Florence Buzin, Aurélie Couzinet-Mossion, Priscilla Decottignies, Michèle Viau, Vony Rabesaotra, Gilles Barnathan, and Gaëtane Wielgosz-Collin. 2014. "Multiple Beneficial Lipids Including Lecithin Detected in the Edible Invasive Mollusk Crepidula fornicata from the French Northeastern Atlantic Coast" Marine Drugs 12, no. 12: 6254-6268. https://doi.org/10.3390/md12126254