Combined Effects of Nitrogen Concentration and Seasonal Changes on the Production of Lipids in Nannochloropsis oculata
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optical Density (OD) and Dry Weight (DW)
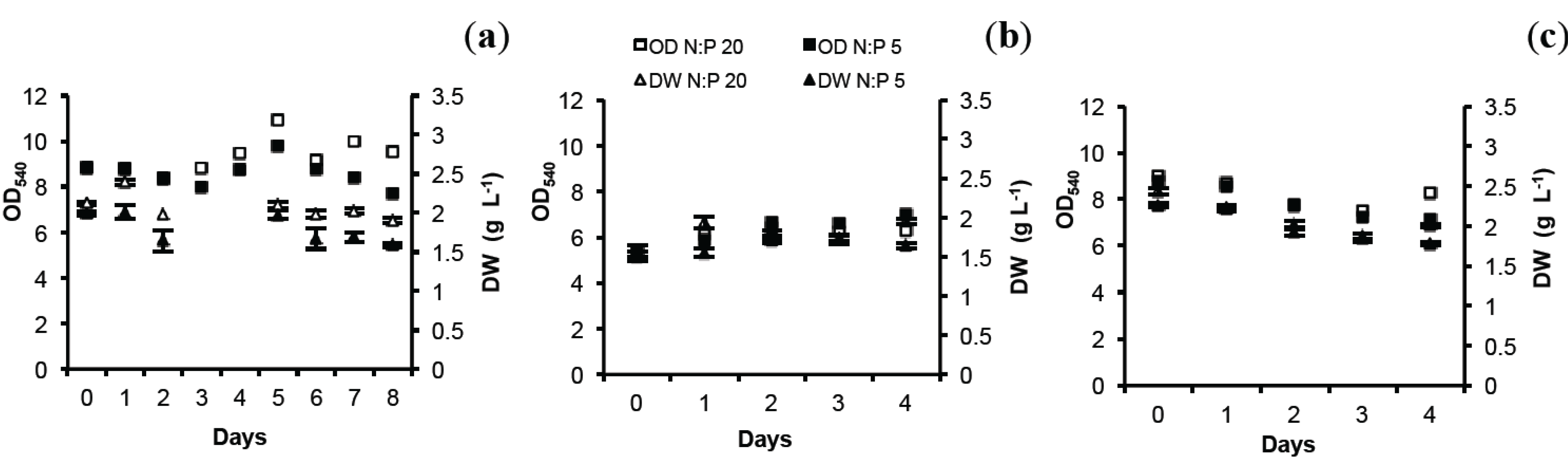
2.2. Total Lipids (TL) and Total Protein (TP)

2.3. Fatty Acid (FA) Profiles
| Autumn 2008 | |||||||||||||||
| Days | 0 | 2 | 5 | 7 | 8 | ||||||||||
| FA | N:P 20 | N:P 5 | N:P 20 | N:P 5 | N:P 20 | N:P 5 | N:P 20 | N:P 5 | N:P 20 | N:P 5 | |||||
| C14:0 | 7.90 | 7.75 | 7.4 | 7.25 | 7.00 | 6.80 | 7.40 | 5.85 | 7.45 | 5.90 | |||||
| C16:0 | 39.80 | 39.40 | 36.75 | 43.30 | 31.00 | 39.75 | 40.50 | 43.30 | 39.80 | 43.95 | |||||
| C16:1 | 29.10 | 27.75 | 31.35 | 27.65 | 32.65 | 29.50 | 28.5 | 28.65 | 28.35 | 28.40 | |||||
| C18:1 | 5.35 | 7.20 | 5.20 | 7.15 | 4.50 | 8.30 | 5.20 | 8.45 | 3.80 | 8.80 | |||||
| C18:2 | 1.95 | 1.75 | 1.90 | 1.60 | 2.60 | 1.65 | 1.70 | 1.40 | 1.90 | 1.35 | |||||
| C20:4 | 4.30 | 3.60 | 4.75 | 2.95 | 6.60 | 3.65 | 4.35 | 3.10 | 5.00 | 2.90 | |||||
| C20:5 | 9.15 | 8.95 | 10.55 | 7.65 | 13.40 | 7.80 | 9.85 | 6.90 | 11.15 | 6.30 | |||||
| Spring 2009 | Spring 2009 | ||||||||||||||
| Days | 2 | 4 | 4 | ||||||||||||
| FA | N:P 20 | N:P 5 | N:P 20 | N:P 5 | N:P 20 | N:P 2.5 | |||||||||
| C14:0 | 7.10 | 5.90 | 7.0 | 5.40 | 6.70 | 5.30 | |||||||||
| C16:0 | 31.20 | 37.80 | 35.80 | 45.10 | 29.80 | 45.40 | |||||||||
| C16:1 | 26.50 | 27.40 | 27.20 | 27.90 | 30.40 | 27.00 | |||||||||
| C18:1 | 4.90 | 6.30 | 4.90 | 8.60 | 4.60 | 9.10 | |||||||||
| C18:2 | 3.50 | 2.40 | 2.90 | 1.30 | 3.50 | 1.40 | |||||||||
| C20:4 | 5.20 | 4.10 | 4.30 | 2.60 | 4.70 | 2.50 | |||||||||
| C20:5 | 18.70 | 14.00 | 15.60 | 7.00 | 17.50 | 7.40 | |||||||||

2.4. Biomass Productivity (BP) and Lipid Productivity (LP)
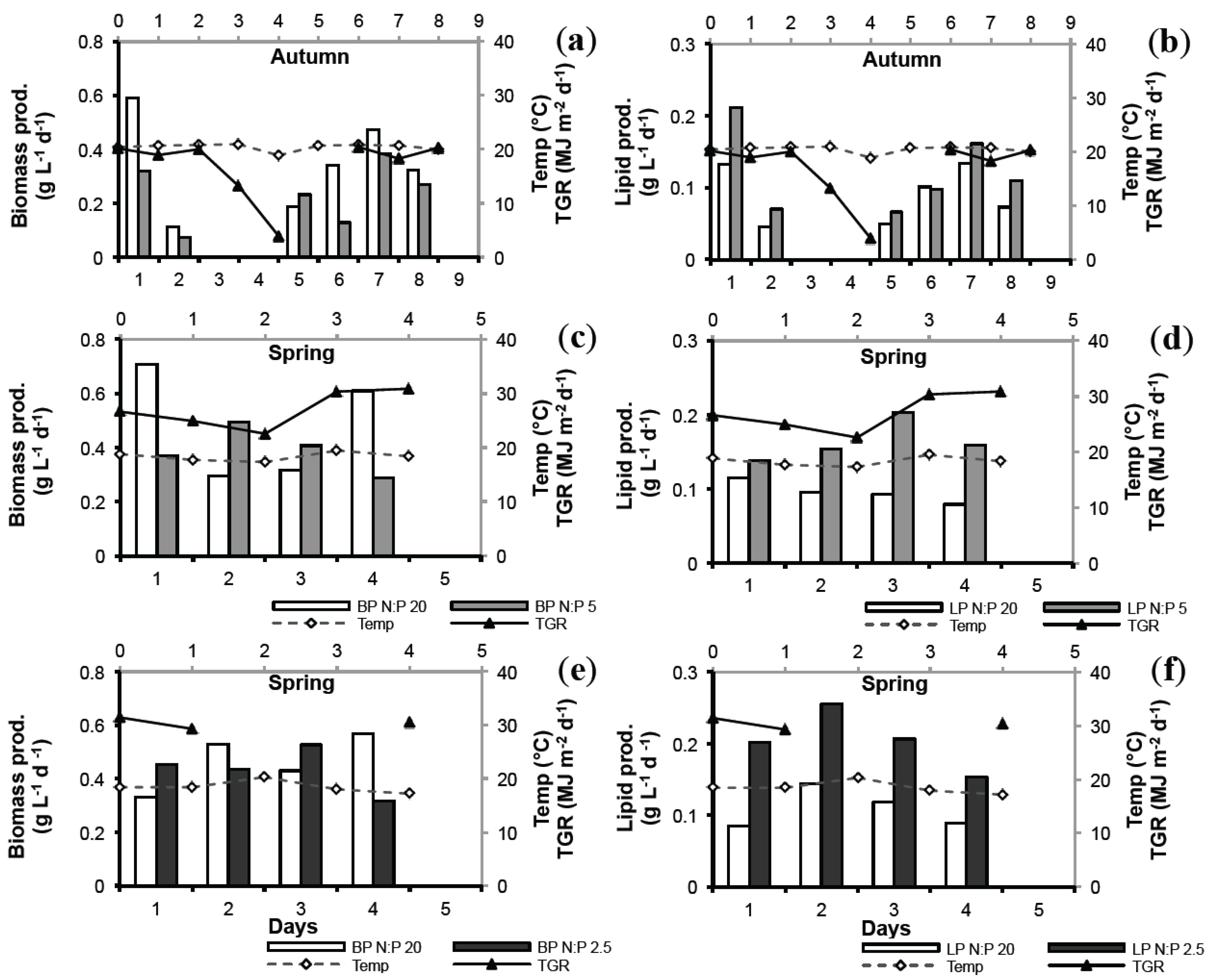
| Nutrient Stress | Average BP (g L−1 day−1) | Average LP (g L−1 day−1) | ||
|---|---|---|---|---|
| Control | Treatment | Control | Treatment | |
| N:P 5 autumn | 0.34 | 0.24 | 0.09 | 0.12 |
| N:P 5 spring | 0.48 | 0.39 | 0.10 | 0.16 |
| N:P 2.5 spring | 0.47 | 0.43 | 0.11 | 0.21 |
| Parameter | N-sufficiency (control) | N-limitation | |
|---|---|---|---|
| N:P | 20 | 5 | 2.5 |
| Season | Autumn, Spring | Autumn, Spring | Spring |
| N | 2000 µM | 500 µM | 250 µM |
| P | 100 µM | 100 µM | 100 µM |
| Harvesting rate | 15%–22%, 22%, 22%–33% | 15%–22%, 22% | 22%–33% |
| Study | Dilution Rate | PBR Volume (L) | Variable | N-sufficiency (g L−1 day−1) | N-limitation (g L−1 day−1) | N-starvation (g L−1 day−1) |
|---|---|---|---|---|---|---|
| Present study | 30%–33% | 1374 | BP | 0.48 | 0.43 | n.a. |
| LP | 0.11 | 0.21 | n.a. | |||
| Rodolfi et al. [21] | 40% | 110 | BP | 0.36 | 0.22 | 0.30 |
| LP | 0.12 | 0.11 | 0.20 | |||
| Bondioli et al. [33] | 44% | 590 | BP | n.a. | n.a. | 0.33 * |
| LP | n.a. | n.a. | 0.22 * |
| Treatment | Cautious Scenario | Best-Case Scenario | ||
|---|---|---|---|---|
| Volumetric (g L−1 day−1) | Annual Yield (t ha−1 year−1) | Volumetric (g L−1 day−1) | Annual Yield (t ha−1 year−1) | |
| BP | ||||
| N-sufficiency | 0.30–0.40 | 19–26 | 0.40–0.50 | 26–32 |
| N-limitation | 0.25–0.35 | 16–22 | 0.35–0.45 | 22–29 |
| LP | ||||
| N-sufficiency | 0.05–0.10 | 3.0–6.0 | 0.10–0.12 | 6.0–8.0 |
| N-limitation | 0.12–0.15 | 8.0–10 | 0.15–0.20 | 10–13 |
2.5. Lab vs. Large Scale
2.6. Seasonal Variation

3. Experimental Section
3.1. Photobioreactors (PBRs) and Experimental Set Up
3.2. Analyses Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Schenk, P.M.; Thomas-Hall, S.R.; Stephens, E.; Marx, U.C.; Mussgnug, J.H.; Posten, C.; Kruse, O.; Hankamer, B. Second generation biofuels: High-efficiency microalgae for biodiesel production. BioEnergy Res. 2008, 1, 20–43. [Google Scholar]
- Meng, X.; Yang, J.; Xu, X.; Zhang, L.; Nie, Q.; Xian, M. Biodiesel production from oleaginous microorganisms. Renewable Energy 2009, 34, 1–5. [Google Scholar] [CrossRef]
- Dismukes, G.C.; Carrieri, D.; Bennette, N.; Ananyev, G.M.; Posewitz, M.C. Aquatic phototrophs: efficient alternatives to land-based crops for biofuels. Curr. Opin. Biotechnol. 2008, 19, 235–240. [Google Scholar]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sust. Energ. Rev. 2010, 14, 217–232. [Google Scholar]
- Tredici, M. Photobiology of microalgae mass cultures: Understanding the tools for the next green revolution. Biofuels 2010, 1, 143–162. [Google Scholar] [CrossRef]
- Becker, W. 18 Microalgae in Human and Animal Nutrition. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Richmond, A., Ed.; Blackwell Science Ltd.: Oxford, UK, 2004; pp. 312–352. [Google Scholar]
- Renaud, S.M.; Parry, D.L.; Thinh, L.V.; Kuo, C.; Padovan, A.; Sammy, N. Effect of light-intensity on the proximate biochemical and fatty-acid composition of Isochrysis sp. and Nannochloropsis oculata for use in tropical aquaculture. J. Appl. Phycol. 1991, 3, 43–53. [Google Scholar] [CrossRef]
- Dhert, P.; Rombaut, G.; Suantika, G.; Sorgeloos, P. Advancement of rotifer culture and manipulation techniques in Europe. Aquaculture 2001, 200, 129–146. [Google Scholar] [CrossRef]
- Ferreira, M.; Coutinho, P.; Seixas, P.; Fabregas, J.; Otero, A. Enriching Rotifers with “Premium” Microalgae. Nannochloropsis gaditana. Mar. Biotechnol. 2009, 11, 585–595. [Google Scholar] [CrossRef]
- Sukenik, A.; Carmeli, Y.; Berner, T. Regulation of fatty-acid composition by irradiance level in the eustigmatophyte Nannochloropsis sp. J. Phycol. 1989, 25, 686–692. [Google Scholar]
- Zittelli, G.C.; Lavista, F.; Bastianini, A.; Rodolfi, L.; Vincenzini, M.; Tredici, M.R. Production of eicosapentaenoic acid by Nannochloropsis sp. cultures in outdoor tubular photobioreactors. J. Biotechnol. 1999, 70, 299–312. [Google Scholar] [CrossRef]
- Zou, N.; Richmond, A. Effect of light-path length in outdoor flat plate reactors on output rate of cell mass and of EPA in Nannochloropsis sp. J. Biotechnol. 1999, 70, 351–356. [Google Scholar] [CrossRef]
- Pulz, O.; Gross, W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004, 65, 635–648. [Google Scholar] [CrossRef]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. Cell Mol. Biol. 2008, 54, 621–639. [Google Scholar] [CrossRef]
- Yu, E.T.; Zendejas, F.J.; Lane, P.D.; Gaucher, S.; Simmons, B.A.; Lane, T.W. Triacylglycerol accumulation and profiling in the model diatoms Thalassiosira pseudonana and Phaeodactylum tricornutum (Bacilariophyceae) during starvation. J. App. Phycol. 2009, 21, 669–681. [Google Scholar] [CrossRef]
- Aatola, H.; Larmi, M.; Sarjovaara, T. Hydrotreated vegetable oil (HVO) as a renewable diesel fuel: trade-off between NOx, particulate emission, and fuel consumption of a heavy duty engine. SAE Int. J. Engines 2009, 1, 1251–1262. [Google Scholar]
- Shifrin, N.S.; Chisholm, S.W. Phytoplankton lipids—Interspecific differences and effects of nitrate, silicate and light-dark cycles. J. Phycol. 1981, 17, 374–384. [Google Scholar] [CrossRef]
- Suen, Y.; Hubbard, J.S.; Holzer, G.; Tornabene, T.G. Total lipid production of the green-alga Nannochloropsis sp. QII under different nitrogen regimes. J. Phycol. 1987, 23, 289–296. [Google Scholar] [CrossRef]
- Takagi, M.; Watanabe, K.; Yamaberi, K.; Yoshida, T. Limited feeding of potassium nitrate for intracellular lipid and triglyceride accumulation of Nannochloris sp. UTEX LB1999. Appl. Microbiol. Biotechnol. 2000, 54, 112–117. [Google Scholar] [CrossRef]
- Rodolfi, L.; Chini Zittelli, G.; Bassi, N.; Padovani, G.; Biondi, N.; Bonini, G.; Tredici, M.R. Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 2009, 102, 100–112. [Google Scholar] [CrossRef]
- Gouveia, L.; Oliveira, A.C. Microalgae as a raw material for biofuels production. J. Ind. Microbiol. Biotechnol. 2009, 36, 269–274. [Google Scholar] [CrossRef]
- Roncarati, A.; Meluzzi, A.; Acciarri, S.; Tallarico, N.; Melotti, P. Fatty acid composition of different microalgae strains (Nannochloropsis sp., Nannochloropsis oculata (Droop) Hibberd, Nannochloris atomus Butcher and Isochrysis sp.) according to the culture phase and the carbon dioxide concentration. J. World Aquacult. Soc. 2004, 3, 401–411. [Google Scholar]
- Chiu, S.Y.; Kao, C.Y.; Tsai, M.T.; Ong, S.C.; Chen, C.H.; Lin, C.S. Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration. Bioresour. Technol. 2009, 100, 833–838. [Google Scholar] [CrossRef]
- Hu, C.; Li, M.; Li, J.; Zhu, Q.; Liu, Z. Variation of lipid and fatty acid compositions of the marine microalga Pavlova viridis (Prymnesiophyceae) under laboratory and outdoor culture conditions. World J. Microbiol. Biotechnol. 2007, 24, 1209–1214. [Google Scholar]
- Olofsson, M.; Lamela, T.; Nilsson, E.; Berge, J.P.; del Pino, V.; Uronen, P.; Legrand, C. Seasonal variation of lipids and fatty acids of the microalgae Nannochloropsis oculata grown in outdoor large-scale photobioreactors. Energies 2012, 5, 1577–1592. [Google Scholar] [CrossRef]
- Lynch, D.V.; Thompson, G.A. Low Temperature-induced alterations in the chloroplast and microsomal-membranes of Dunaliella salina. Plant Physiol. 1982, 69, 1369–1375. [Google Scholar] [CrossRef]
- Thompson, P.A.; Guo, M.X.; Harrison, P.J.; Whyte, J.N.C. Effects of variation in temperature. II. on the fatty-acid composition of 8 species of marine-phytoplankton. J. Phycol. 1992, 28, 488–497. [Google Scholar]
- Sukenik, A.; Yamaguchi, Y.; Livne, A. Alterations in lipid molecular-species of the marine eustigmatophyte Nannochloropsis sp. J. Phycol. 1993, 29, 620–626. [Google Scholar] [CrossRef]
- Renaud, S.M.; Thinh, L.V.; Lambrinidis, G.; Parry, D.L. Effect of temperature on growth, chemical composition and fatty acid composition of tropical Australian microalgae grown in batch cultures. Aquaculture 2002, 211, 195–214. [Google Scholar] [CrossRef]
- Takagi, M.; Karseno; Yoshida, T. Effect of salt concentration on intracellular accumulation of lipids and triacylglyceride in marine microalgae Dunaliella cells. J. Biosci. Bioeng. 2006, 101, 223–226. [Google Scholar] [CrossRef]
- Bartley, M.L.; Boeing, W.J.; Corcoran, A.A.; Holguin, F.O.; Schaub, T. Effects of salinity on growth and lipid accumulation of biofuel microalga Nannochloropsis salina and invading organisms. Biomass Bioenerg. 2013, 54, 83–88. [Google Scholar] [CrossRef]
- Xu, Z.B.; Yan, X.J.; Pei, L.Q.; Luo, Q.J.; Xu, J.L. Changes in fatty acids and sterols during batch growth of Pavlova viridis in photobioreactor. J. Appl. Phycol. 2008, 20, 237–243. [Google Scholar] [CrossRef]
- Huerlimann, R.; de Nys, R.; Heimann, K. Growth, lipid content, productivity, and fatty acid composition of tropical microalgae for scale-up production. Biotechnol. Bioeng. 2010, 107, 245–257. [Google Scholar] [CrossRef]
- Bondioli, P.; Della Bella, L.; Rivolta, G.; Chini Zittelli, G.; Bassi, N.; Rodolfi, L.; Casini, D.; Prussi, M.; Chiaramonti, D.; Tredici, M.R. Oil production by the marine microalgae Nannochloropsis sp. F&M-M24 and Tetraselmis suecica F&M-M33. Bioresour. Technol. 2012, 114, 567–572. [Google Scholar] [CrossRef]
- Li, M.; Gong, R.M.; Rao, X.J.; Liu, Z.L.; Wang, X.M. Effects of nitrate concentration on growth and fatty acid composition of the marine microalga Pavlova viridis (Prymnesiophyceae). Ann. Microbiol. 2005, 55, 51–55. [Google Scholar]
- Weldy, C.S.; Huesemann, M. Lipid production by Dunaliella salina in batch culture: Effects of nitrogen limitation and light intensity. US Dep. Energy J. Undergrad. Res. 2007, 7, 115–122. [Google Scholar]
- Gordillo, F.J.L.; Goutx, M.; Figueroa, F.L.; Niell, F.X. Effects of light intensity, CO2 and nitrogen supply on lipid class composition of Dunaliella viridis. J. Appl. Phycol. 1998, 10, 135–144. [Google Scholar] [CrossRef]
- Simionato, D.; Block, M.A.; La Rocca, N.; Jouhet, J.; Marechal, E.; Finazzi, G.; Morosinotto, T. The response of Nannochloropsis gaditana to nitrogen starvation includes de novo biosynthesis of triacylglycerols, a decrease of chloroplast galactolipids, and reorganization of the photosynthetic apparatus. Eukaryot. Cell 2013, 12, 665–676. [Google Scholar] [CrossRef]
- El-Baky, H.H.A.; El-Baz, F.K.; El-Baroty, G.S. Production of lipids rich in omega 3 fatty acids from the halotolerant alga Dunaliella salina. Biotechnology 2004, 3, 102–108. [Google Scholar]
- Su, C.-H.; Chien, L.-J.; Gomes, J.; Lin, Y.-S.; Yu, Y.-K.; Liou, J.-S.; Syu, R.-J. Factors affecting lipid accumulation by Nannochloropsis oculata in a two-stage cultivation process. J. Appl. Phycol. 2010, 23, 903–908. [Google Scholar]
- Pal, D.; Khozin-Goldberg, I.; Cohen, Z.; Boussiba, S. The effect of light, salinity, and nitrogen availability on lipid production by Nannochloropsis sp. Appl. Microbiol. Biotechnol. 2011, 90, 1429–1441. [Google Scholar] [CrossRef]
- Fabregas, J.; Abalde, J.; Cabezas, B.; Herrero, C. Changes in protein, carbohydrates and gross energy in the marine microalga Dunaliella tertiolecta (Butcher) by nitrogen concentrations as nitrate, nitrite and urea. Aquacult. Eng. 1989, 8, 223–239. [Google Scholar] [CrossRef]
- Harrison, P.; Thompson, P.; Calderwood, G. Effects of nutrient and light limitation on the biochemical composition of phytoplankton. J. Appl. Phycol. 1990, 2, 45–56. [Google Scholar] [CrossRef]
- Kilham, S.S.; Kreeger, D.A.; Goulden, C.E.; Lynn, S.G. Effects of nutrient limitation on biochemical constituents of Ankistrodesmus falcatus. Freshwater Biol. 1997, 38, 591–596. [Google Scholar] [CrossRef]
- Dunstan, G.A.; Volkman, J.K.; Barrett, S.M.; Garland, C.D. Changes in the lipid-composition and maximization of the polyunsaturated fatty-acid content of 3 microalgae grown in mass-culture. J. Appl. Phycol. 1993, 5, 71–83. [Google Scholar] [CrossRef]
- Hu, H.H.; Gao, K.S. Optimization of growth and fatty acid composition of a unicellular marine picoplankton, Nannochloropsis sp., with enriched carbon sources. Biotechnol. Lett. 2003, 25, 421–425. [Google Scholar] [CrossRef]
- Fabregas, J.; Maseda, A.; Dominguez, A.; Otero, A. The cell composition of Nannochloropsis sp. changes under different irradiances in semicontinuous culture. World J. Microbiol. Biotechnol. 2004, 20, 31–35. [Google Scholar] [CrossRef]
- European Standard. In Automotive Fuels—Fatty Acid Methyl Esters (FAME) for Diesel Engines—Requirements and Test Methods; EN 14214; Standardiserings-Kommissionen I Sverige Publications: Paramus, NJ, USA, 2008.
- Richmond, A. Biological Principles of Mass Cultivation. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Blackwell Publishing Ltd.: Oxford, UK, 2004; pp. 125–177. [Google Scholar]
- Fisher, T.; Minnaard, J.; Dubinsky, Z. Photoacclimation in the marine alga Nannochloropsis sp. (Eustigmatophyte): A kinetic study. J. Plankton Res. 1996, 18, 1797–1818. [Google Scholar] [CrossRef]
- Zou, N.; Zhang, C.W.; Cohen, Z.; Richmond, A. Production of cell mass and eicosapentaenoic acid (EPA) in ultrahigh cell density cultures of Nannochloropsis sp. (Eustigmatophyceae). Eur. J. Phycol. 2000, 35, 127–133. [Google Scholar] [CrossRef]
- Griffiths, M.J.; Hille, R.P.; Harrison, S.T.L. Lipid productivity, settling potential and fatty acid profile of 11 microalgal species grown under nitrogen replete and limited conditions. J. Appl. Phycol. 2011, 24, 989–1001. [Google Scholar] [CrossRef]
- Sheehan, J.; Dunahay, T.; Benemann, J.; Roessler, P. A Look Back at the US Department of Energy’s Aquatic Species Program: Biodiesel from Algae; Close-Out Report NREL/TP-580-24190; National Renewable Energy Laboratory: Golden, CO, USA, 1998. [Google Scholar]
- Li, Y.; Horsman, M.; Wang, B.; Wu, N.; Lan, C.Q. Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl. Microbiol. Biotechnol. 2008, 81, 629–636. [Google Scholar] [CrossRef]
- Yamaberi, K.; Takagi, M.; Yoshida, T. Nitrogen depletion for intracellular triglyceride accumulation to enhance liquefaction yield of marine microalgal cells into a fuel oil. J. Mar. Biotechnol. 1998, 6, 44–48. [Google Scholar]
- Illman, A.M.; Scragg, A.H.; Shales, S.W. Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzyme Microb. Technol. 2000, 27, 631–635. [Google Scholar] [CrossRef]
- Matsukawa, R.; Hotta, M.; Masuda, Y.; Chihara, M.; Karube, I. Antioxidants from carbon dioxide fixing Chlorella sorokiniana. J. Appl. Phycol. 2000, 12, 263–267. [Google Scholar] [CrossRef]
- Feng, Y.J.; Li, C.; Zhang, D.W. Lipid production of Chlorella vulgaris cultured in artificial wastewater medium. Bioresource Technol. 2011, 102, 101–105. [Google Scholar] [CrossRef]
- Moheimani, N.R.; Borowitzka, M.A. The long-term culture of the coccolithophore Pleurochrysis carterae (Haptophyta) in outdoor raceway ponds. J. Appl. Phycol. 2006, 18, 703–712. [Google Scholar] [CrossRef]
- Quinn, J.C.; Yates, T.; Douglas, N.; Weyer, K.; Butler, J.; Bradley, T.H.; Lammers, P.J. Nannochloropsis production metrics in a scalable outdoor photobioreactor for commercial applications. Bioresource Technol. 2012, 117, 164–171. [Google Scholar]
- Moazami, N.; Ashori, A.; Ranjbar, R.; Tangestani, M.; Eghtesadi, R.; Nejad, A.S. Large-scale biodiesel production using microalgae biomass of Nannochloropsis. Biomass Bioenerg. 2012, 39, 449–453. [Google Scholar] [CrossRef]
- Weyer, K.M.; Bush, D.R.; Darzins, A.; Willson, B.D. Theoretical maximum algal oil production. BioEnergy Res. 2010, 3, 204–213. [Google Scholar] [CrossRef]
- James, G.O.; Hocart, C.H.; Hillier, W.; Chen, H.C.; Kordbacheh, F.; Price, G.D.; Djordjevic, M.A. Fatty acid profiling of Chlamydomonas reinhardtii under nitrogen deprivation. Bioresource Technol. 2011, 102, 3343–3351. [Google Scholar] [CrossRef]
- Ortiz-Marquez, J.C.F.; Do Nascimento, M.; Zehr, J.P.; Curatti, L. Genetic engineering of multispecies microbial cell factories as an alternative for bioenergy production. Trends Biotechnol. 2013, 31, 521–529. [Google Scholar] [CrossRef]
- Eaton, A.; Clesceri, L.; Rice, R.; Greenberg, A.; Franson, M. Standard Methods for the Examination of Water and Wastewate; American Public Health Association (APHA), American Water Works Association (AWWA) and Water Environment Federation (WEF): Washington, DC, USA, 2005. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Phys. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Herbert, B.N.; Gould, H.J.; Chain, E.B. Crystal protein of Bacillus thuringiensis var. tolworthi—subunit sttructure and toxicity to Pieris brassicae. Eur. J. Biochem. 1971, 24, 366–375. [Google Scholar] [CrossRef]
- Dumay, J.; Barthomeuf, C.; Bergé, J. How enzymes may be helpful for upgrading fish by-products: Enhancement of fat extraction. J. Aquat. Food Prod. Technol. 2004, 13, 69–84. [Google Scholar]
- Clima Tiempo Meteored. Meteorology portal by Alpred SL. Available online: http://clima.meteored.com/clima-en-faro+aeropuerto-085540.html (accessed on 22 February 2011).
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Olofsson, M.; Lamela, T.; Nilsson, E.; Bergé, J.-P.; Del Pino, V.; Uronen, P.; Legrand, C. Combined Effects of Nitrogen Concentration and Seasonal Changes on the Production of Lipids in Nannochloropsis oculata. Mar. Drugs 2014, 12, 1891-1910. https://doi.org/10.3390/md12041891
Olofsson M, Lamela T, Nilsson E, Bergé J-P, Del Pino V, Uronen P, Legrand C. Combined Effects of Nitrogen Concentration and Seasonal Changes on the Production of Lipids in Nannochloropsis oculata. Marine Drugs. 2014; 12(4):1891-1910. https://doi.org/10.3390/md12041891
Chicago/Turabian StyleOlofsson, Martin, Teresa Lamela, Emmelie Nilsson, Jean-Pascal Bergé, Victória Del Pino, Pauliina Uronen, and Catherine Legrand. 2014. "Combined Effects of Nitrogen Concentration and Seasonal Changes on the Production of Lipids in Nannochloropsis oculata" Marine Drugs 12, no. 4: 1891-1910. https://doi.org/10.3390/md12041891
APA StyleOlofsson, M., Lamela, T., Nilsson, E., Bergé, J.-P., Del Pino, V., Uronen, P., & Legrand, C. (2014). Combined Effects of Nitrogen Concentration and Seasonal Changes on the Production of Lipids in Nannochloropsis oculata. Marine Drugs, 12(4), 1891-1910. https://doi.org/10.3390/md12041891




