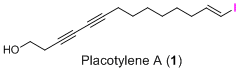
Placotylene A, an Inhibitor of the Receptor Activator of Nuclear Factor-κB Ligand-Induced Osteoclast Differentiation, from a Korean Sponge Placospongia sp.
Abstract
:1. Introduction

2. Results and Discussion
2.1. Structure Elucidation

2.2. Effects of 1 and 2 on Osteoclast Differentiation



3. Experimental Section
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction, Isolation and Characterization of Placotylenes A (1) and B (2)
| No. | δC | δH (J in Hz) b | COSY | HMBC |
|---|---|---|---|---|
| 1-OH | 1.96 brs | |||
| 1 | 60.4, CH2 | 3.75 t (6.0) | 2 | 2, 3 |
| 2 | 23.6, CH2 | 2.53 t (6.0) | 1 | 1, 3, 4, 5 |
| 3 | 73.8, C | |||
| 4 | 67.1, C | |||
| 5 | 65.1, C | |||
| 6 | 78.1, C | |||
| 7 | 19.0, CH2 | 2.25 t (6.0) | 8 | 4, 5, 6, 9 |
| 8 | 28.5, CH2 | 1.51 m | 7, 9 | 6, 7, 9 |
| 9 | 28.1, CH2 | 1.38 m | 8, 10 | 8, 10 |
| 10 | 28.1, CH2 | 1.29 m | 9, 11 | 9, 11 |
| 11 | 28.3, CH2 | 1.39 m | 10, 12 | 12, 13 |
| 12 | 35.9, CH2 | 2.05 m | 11, 13 | 11, 13, 14 |
| 13 | 146.5, CH | 6.50 dt (14.3, 7.2) | 12, 14 | 11, 12, 18 |
| 14 | 74.5, CH | 5.98 d (14.3) | 13 | 12, 13 |
| No. | δC | δH (J in Hz) b | COSY | HMBC |
|---|---|---|---|---|
| 1 | 61.5, CH2 | 3.62 t (6.6) | 2 | 2, 3 |
| 2 | 24.1, CH2 | 2.44 t (6.6) | 1 | 1, 3, 4, 5 |
| 3 | 75.2, C | |||
| 4 | 67.4, C | |||
| 5 | 66.5, C | |||
| 6 | 78.3, C | |||
| 7 | 19.8, CH2 | 2.26 t (6.6) | 8 | 4, 5, 6, 9 |
| 8 | 29.5, CH2 | 1.51 m | 7, 9 | 6, 7, 9 |
| 9 | 29.7, CH2 | 1.43 m | 8, 10 | 8, 10 |
| 10 | 29.7, CH2 | 1.36 m | 9, 11 | 9, 11 |
| 11 | 29.0, CH2 | 1.45 m | 10, 12 | 12, 13 |
| 12 | 35.7, CH2 | 2.15 m | 11, 13 | 11, 13, 14 |
| 13 | 142.5, CH | 6.19 dt (7.2, 7.2) | 12, 14 | 11, 12, 18 |
| 14 | 82.8, CH | 6.27 d (7.2) | 13 | 12, 13 |
3.4. Osteoclast Differentiation
3.5. Cytotoxicity Assay
3.6. Quantitative Real-Time PCR Analysis
3.7. Western Blotting Analysis
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gribble, G.W. Natural organohalogens: A new frontier for medicinal agents? J. Chem. Educ. 2004, 81, 1441–1449. [Google Scholar] [CrossRef]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 1997, 14, 259–302. [Google Scholar]
- Ko, J.; Morinaka, B.I.; Molinski, T.F. Faulknerynes A–C from a Bahamian sponge Diplastrella sp.: Stereoassignment by critical application of two exciton coupled CD methods. J. Org. Chem. 2010, 76, 894–901. [Google Scholar]
- Lerch, M.L.; Harper, M.K.; Faulkner, D.J. Brominated polyacetylenes from the Philippines sponge Diplastrella sp. J. Nat. Prod. 2003, 66, 667–670. [Google Scholar]
- Bourguet-Kondracki, M.L.; Rakotoatisoa, M.T.; Martin, M.T.; Guyot, M. Brominated polyacetylenic acids from the marine sponge Xestospongia muta: Inhibitors of HIV protease. Tetrahedron Lett. 1992, 33, 225–226. [Google Scholar]
- Fusetani, N.; Li, H.-Y.; Tamura, K.; Matsunaga, S. Antifungal brominated Cl8 acetylenic acids from the marine sponge, Petrosia volcano Hoshino. Tertrahedron 1993, 49, 1203–1210. [Google Scholar] [CrossRef]
- Kim, H.; Chin, J.; Choi, H.; Baek, K.; Lee, T.G.; Park, S.E.; Wang, W.; Hahn, D.; Yang, I.; Lee, J.; et al. Phosphoiodyns A and B, unique phosphorus-containing iodinated polyacetylenes from a Korean sponge Placospongia sp. Org. Lett. 2013, 15, 100–103. [Google Scholar] [CrossRef]
- Hwang, B.S.; Lee, K.; Yang, C.; Jeong, E.J.; Rho, J.-R. Characterization and anti-inflammatory effects of iodinated acetylenic acids isolated from the Marine Sponges Suberites mammilaris and Suberites japonicus. J. Nat. Prod. 2013, 76, 2355–2359. [Google Scholar]
- Manolagas, S.C. Birth and death of bone cells: Basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr. Rev. 2000, 21, 115–137. [Google Scholar]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Asagiri, M.; Takayanagi, H. The molecular understanding of osteoclast differentiation. Bone 2007, 40, 251–264. [Google Scholar]
- Ross, F.P.; Teitelbaum, S.L. αvβ3 and macrophage colony-stimulating factor: partners in osteoclast biology. Immunol. Rev. 2005, 208, 88–105. [Google Scholar] [CrossRef]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and activation of the transcription factor NFATc1 (NFATc2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef]
- Crotti, T.N.; Flannery, M.; Walsh, N.C.; Fleming, J.D.; Goldring, S.R.; McHugh, K.P. NFATc1 regulation of the human β3 integrin promoter in osteoclast differentiation. Gene 2006, 372, 92–102. [Google Scholar] [CrossRef]
- Matsumoto, M.; Kogawa, M.; Wada, S.; Takayanagi, H.; Tsujimoto, M.; Katayama, S.; Hisatake, K.; Nogi, Y. Essential role of p38 mitogen-activated protein kinase in cathepsin K gene expression during osteoclastogenesis through association of NFATc1 and PU.1. J. Biol. Chem. 2004, 279, 45969–45979. [Google Scholar] [CrossRef]
- Yagi, M.; Miyamoto, T.; Sawatani, Y.; Iwamoto, K.; Hosogane, N.; Fujita, N.; Morita, K.; Ninomiya, K.; Suzuki, T.; Miyamoto, K.; et al. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J. Exp. Med. 2005, 202, 345–351. [Google Scholar] [CrossRef]
- Ren, H.; Krasovskiy, A.; Knochel, P. Stereoselective Preparation of Functionalized Acyclic Alkenylmagnesium Reagents Using i-PrMgCl LiCl. Org. Lett. 2004, 6, 4215–4217. [Google Scholar]
- Nakashima, T.; Takayanagi, H. New regulation mechanisms of osteoclast differentiation. Ann. N. Y. Acad. Sci. 2012, 1240, E13–E18. [Google Scholar]
- Choi, S.W.; Son, Y.J.; Yun, J.M.; Kim, S.H. Fisetin inhibits osteoclast differentiation via downregulation of p38 and c-Fos-NFATc1 signaling pathways. Evid. Based Complement. Alternat. Med. 2012, 2012, 810563:1–810563:9. [Google Scholar]
- Rozen, S.; Skaletsky, H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 2000, 132, 365–386. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yeon, J.T.; Choi, S.W.; Park, K.I. Glutaredoxin 2 isoform b (Glrx2b) promotes RANKL-induced osteoclastogenesis through activation of the p38-MAPK signaling pathway. Biochem. Mol. Biol. Rep. 2012, 45, 171–176. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kim, H.; Kim, K.-J.; Yeon, J.-T.; Kim, S.H.; Won, D.H.; Choi, H.; Nam, S.-J.; Son, Y.-J.; Kang, H. Placotylene A, an Inhibitor of the Receptor Activator of Nuclear Factor-κB Ligand-Induced Osteoclast Differentiation, from a Korean Sponge Placospongia sp. Mar. Drugs 2014, 12, 2054-2065. https://doi.org/10.3390/md12042054
Kim H, Kim K-J, Yeon J-T, Kim SH, Won DH, Choi H, Nam S-J, Son Y-J, Kang H. Placotylene A, an Inhibitor of the Receptor Activator of Nuclear Factor-κB Ligand-Induced Osteoclast Differentiation, from a Korean Sponge Placospongia sp. Marine Drugs. 2014; 12(4):2054-2065. https://doi.org/10.3390/md12042054
Chicago/Turabian StyleKim, Hiyoung, Kwang-Jin Kim, Jeong-Tae Yeon, Seong Hwan Kim, Dong Hwan Won, Hyukjae Choi, Sang-Jip Nam, Young-Jin Son, and Heonjoong Kang. 2014. "Placotylene A, an Inhibitor of the Receptor Activator of Nuclear Factor-κB Ligand-Induced Osteoclast Differentiation, from a Korean Sponge Placospongia sp." Marine Drugs 12, no. 4: 2054-2065. https://doi.org/10.3390/md12042054
APA StyleKim, H., Kim, K.-J., Yeon, J.-T., Kim, S. H., Won, D. H., Choi, H., Nam, S.-J., Son, Y.-J., & Kang, H. (2014). Placotylene A, an Inhibitor of the Receptor Activator of Nuclear Factor-κB Ligand-Induced Osteoclast Differentiation, from a Korean Sponge Placospongia sp. Marine Drugs, 12(4), 2054-2065. https://doi.org/10.3390/md12042054