Diatom Milking: A Review and New Approaches
Abstract
:1. Introduction
- manufacture of fertilizers [28],
| Phylum | Class | Taxonomy | Oil Content (% d.w.) | High Value Molecules | Reference |
|---|---|---|---|---|---|
| Chlorophyta | Chlorodendrophyceae | Tetraselmis suecica | 15–32 | Carotenoids, chlorophyll, tocopherol, lipids | [40] |
| Chlorophyta | Chlorophyceae | Ankistrodesmus sp. | 28–40 | Mycosporine-like amino acids, polysaccharides | [17] |
| Chlorophyta | Chlorophyceae | Dunaliella salina | 10 | Carotenoid, β carotene, mycosporine-like amino acids, sporopollenin | [41] |
| Chlorophyta | Chlorophyceae | Dunaliella tertiolecta | 36–42 | Carotenoid, β carotene, mycosporine-like amino acids | [42] |
| Chlorophyta | Chlorophyceae | Neochloris oleoabundans | 35–65 | Fatty acids, starch | [43] |
| Chlorophyta | Trebouxiophyceae | Botryococcus braunii | 29–75 | Isobotryococcene, botryococcene, triterpenes | [44] |
| Chlorophyta | Trebouxiophyceae | Chlorella vulgaris | 58 | Neutral lipids | [45] |
| Chlorophyta | Trebouxiophyceae | Chlorella emersonii | 34 | Neutral lipids | [46] |
| Chlorophyta | Trebouxiophyceae | Chlorella protothecoides | 15–55 | Eicosapentaenoic acid (EPA), ascorbic acid | [47] |
| Chlorophyta | Trebouxiophyceae | Chlorella minutissima | 57 | C16- and C18-lipids | [48] |
| Heterokontophyta | Bacillariophyceae | Nitzschia laevi | 28–69 | EPA | [49] |
| Heterokontophyta | Coscinodiscophyceae | Thalassiosira pseudonana | 21–31 | Glycosylglycerides, neutral lipids, TAG | [50] |
| Heterokontophyta | Labrynthulomycetes | Schizochytrium limacinum | 50–77 | Docosahexaenoic acid (DHA) | [51] |
| Myzozoa | Peridinea | Crypthecodinium cohnii | 20 | DHA, Starch | [52] |
| Ochrophyta | Coscinodiscophyceae | Cyclotella sp. | 42 | Neutral lipids | [53] |
| Ochrophyta | Eustigmatophyceae | Nannochloropsis sp. | 46–68 | EPA, TAG, ω-3 LC-PUFA | [54] |
“No harvesting method has yet been identified as being efficient, reliable and at reasonable cost”.[71]

“Plenty of organisms use those same inputs—All photosynthetic microalgae, for example. But you can’t milk them like a cow. You have to crush them”.[79]
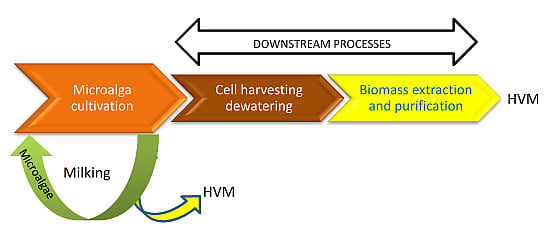
- Milking as the removal of a specific set of products without killing the organism, in such a way that it can be milked again at a later time.
- Extraction as the removal of a specific set of products without concern about the survival of the organism, generally leaving an organic residue.
- Secretion or spontaneous oozing as the active dumping of a specific set of products by an organism into its surrounding environment.
2. Development of Alternative Processes to Extract and Harvest High Value Molecules (HVM)
| Milking Process | Microorganism | Advantages | Disadvantages | Ability to Keep Cells Alive |
|---|---|---|---|---|
| Biocompatible organic solvents | Microalgae [67,100] | Improvement of lipid production Positive effect on growth | Not environmentally friendly Possible toxic mechanism | Yes, when using hydrophobic solvents |
| Pulsed electric field (PEF) | Yeast [103] Microalgae [104,105,106,107] Cyanobacteria [108] | High extraction yield Adjustable PEF parameters Not an energy-intensive process Large-scale process demonstrated Continuous process | Effect of electric pulsation is size dependent | Yes, but depends on the PEF parameters |
| Spontaneous oozing | Microalgae [109] Bacteria [110,111,112] Cyanobacteria [113,114,115] | Not an energy-intensive process Possibility of scaling up Application in solar panels | Slow oozing of HVM | Yes, it is a natural mechanism |
| Mechanical methods | ||||
| -sonication | Microalgae [116,117] Cyanobacteria [118,119,120] | Improvement of lipid recovery | Cellular damage apoptosis Thickness of the cell wall | No |
| -pressure | Microalgae [this work] | Not an energy-intensive process Weak pressure to be used (below 750 µN) | Large-scale process not demonstrated Process needs to be improved | Yeswhen using low pressure (< 750 µN) |
| -centrifugation | Diatoms [work in progress] | Continuous process Application in solar panels | Requires energy | Not yet tested |
| Membrane-bound protein pumps | Bacteria [121,122] | Oozing of HVM Lower toxicity of overexpressed HVM High rate growth Possibility of scaling up | Metabolism engineering Organic phase needed for solubilization of water insoluble HVM | Yes |
2.1. Pulsed Electric Field
2.2. Spontaneous Oozing

2.3. Mechanical Pressure

2.4. Centrifugation
3. The Design and Functioning of Photobioreactors
4. Diatom Chemobiodiversity
4.1. Strain Selection
4.2. Exploring Biodiversity
| Location | No. | T (°C) | pH | DO % | Conductivity | Latitude | Longitude |
|---|---|---|---|---|---|---|---|
| Lake Sinclair Power Plant | 1 | 23.5 ± 4.2 | 7.0 ± 0.2 | 9 ± 2.52 | 32 ± 3.6 | 33.20 | −83.30 |
| Lake Sinclair, Goat Island | 2 | 21.8 ± 1.3 | 8.5 ± 0.1 | 110 ± 14.1 | 67 ± 6.2 | 33.16 | −83.23 |
| Lake Sinclair at Dam | 3 | 19.9 ± 6.1 | 7.8 ± 0.8 | 69 ± 8.2 | 46 ± 7 | 33.14 | −83.20 |
| Oconee River at Dam | 4 | 23.8±5.1 | 7.1± 2.1 | 61 ± 11.1 | 82.6 ± 1.4 | 33.14 | −83.20 |
| Oconee River Greenway | 5 | 20.2 ± 9 | 7.2 ± 1.6 | 75 ± 2.6 | 78.3 ± 6.9 | 33.08 | −83.21 |
| Fishing Creek | 6 | 22.4 ± 3.2 | 6.3 ± 0.4 | 89 ± 8.2 | 29.2 ± 11.4 | 33.08 | −83.22 |
| Tobler Creek | 7 | 22.1 ± 2.1 | 7.2 ± 0.2 | 45 ± 10.6 | 85 ± 5.3 | 33.12 | −83.27 |
| Andalusia pond | 8 | 23.5 ± 1.8 | 6.8 ± 0.5 | 76 ± 9.6 | 24.3 ± 1.8 | 33.13 | −83.27 |
| Bartram forest pond | 9 | 19.5 ± 3.2 | 7.4 ± 1.3 | 58 ± 12.6 | 78.5 ± 1.6 | 33.02 | −83.21 |
| Savannah River at Port Wentworth, GA | 10 | 25.5 ± 4.4 | 7.6 ± 0.4 | 86 ± 9.7 | 8204 ± 125.4 | 32.17 | −81.16 |

4.3. Toward Dedicated Producers Using Synthetic Biology
| Type of Transporter | Origin | Host Cells | Molecules Transported | Pro/Cons | References |
|---|---|---|---|---|---|
| Resistance-nodulation-cell division (RND) family | Gram-negative bacteria, similarities with cyanobacteria | Escherichia coli | limonene | Pro: increase the excretion of limonene Con: Large tripartite protein complex [239] | [122] |
| ATP-binding cassette (ABC) | Bacteria | Escherichia coli | carotenoids, squalene, botrycoccene | Pro: present in all 5 kingdoms; import or export molecules and ions across cell membranes | [121,240,241] |
| Formate transporter (focA) | Escherichia coli | Escherichia coli | formate | [242] |
5. Understanding of Diatom (Stress) Biology
6. Conclusions and Perspectives
Acknowledgments
Author Contributions
Abbreviations
| DW | dry weight |
| EPA | Eicosapentaenoic acid |
| EROI | energy return on investment |
| FAE | Fatty Acid Ester-type |
| FT-IR | Fast-Fourier InfraRed spectroscopy |
| HVM | high value molecules |
| LED | light emitting diode |
| PBR | photobioreactor |
| PDMS | polydimethylsiloxane |
| PEF | pulsed electric field |
| PUFA | PolyUnsaturated Fatty Acids |
| SE | standard error |
| UVC | ultraviolet C band |
| TAG | TriAcylGlycerol |
Supplementary Information
Conflicts of Interest
References
- Alexandratos, N.; Bruinsma, J. World Agriculture towards 2030/2050: The 2012 Revision; ESA Working paper Rome; FAO: Rome, Italy, 2012. [Google Scholar]
- Mimouni, V.; Ulmann, L.; Pasquet, V.; Mathieu, M.; Picot, L.; Bougaran, G.; Cadoret, J.-P.; Morant-Manceau, A.; Schoefs, B. The potential of microalgae for the production of bioactive molecules of pharmaceutical interest. Curr. Pharm. Biotechnol. 2012, 13, 2733–2750. [Google Scholar] [CrossRef] [PubMed]
- Schoefs, B. Chlorophyll and carotenoid analysis in food products. A practical case-by-case view. TrAC Trends Anal. Chem. 2003, 22, 335–339. [Google Scholar] [CrossRef]
- Plaza, M.; Herrero, M.; Cifuentes, A.; Ibanez, E. Innovative natural functional ingredients from microalgae. J. Agric. Food Chem. 2009, 57, 7159–7170. [Google Scholar] [CrossRef] [PubMed]
- Chisti, Y. Biodiesel from microalgae beats bioethanol. Trends Biotechnol. 2008, 26, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Drum, R.W.; Gordon, R. Star Trek replicators and diatom nanotechnology. Trends Biotechnol. 2003, 21, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Gordon, R.; Sterrenburg, F.A.S.; Sandhage, K. A Special Issue on Diatom Nanotechnology. J. Nanosci. Nanotechnol. 2005, 5, 1–4. [Google Scholar] [CrossRef]
- Gordon, R.; Losic, D.; Tiffany, M.A.; Nagy, S.S.; Sterrenburg, F.A.S. The Glass Menagerie: Diatoms for novel applications in nanotechnology. Trends Biotechnol. 2009, 27, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Gordon, R. Diatoms and nanotechnology: Early history and imagined future as seen through patents. In The Diatoms: Applications for the Environmental and Earth Sciences; Smol, J.P., Stoermer, E.F., Eds.; Cambridge University Press: Cambridge, UK, 2010; Volume 2, pp. 585–602. [Google Scholar]
- Gordon, R.; Seckbach, J. The Science of Algal Fuels: Phycology, Geology, Biophotonics, Genomics and Nanotechnology; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary production of the biosphere: Integrating terrestrial and oceanic components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.J.le B.; Laurens, L.M.L. Microalgae as biodiesel & biomass feedstocks: Review & analysis of the biochemistry, energetics & economics. Energy Environ. Sci. 2010, 3, 554–590. [Google Scholar] [CrossRef]
- Lemoine, Y.; Schoefs, B. Secondary ketocarotenoid astaxanthin biosynthesis in algae: A multifunctional response to stress. Photosynth. Res. 2010, 106, 155–177. [Google Scholar] [CrossRef] [PubMed]
- Heydarizadeh, P.; Poirier, I.; Loizeau, D.; Ulmann, L.; Mimouni, V.; Schoefs, B.; Bertrand, M. Plastids of marine phytoplankton produce bioactive pigments and lipids. Mar. Drugs 2013, 11, 3425–3471. [Google Scholar] [CrossRef] [PubMed]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Leu, S.; Boussiba, S. Advances in the production of high-value products by microalgae. Ind. Biotechnol. 2014, 10, 169–183. [Google Scholar] [CrossRef]
- Priyadarshani, I.; Rath, B. Commercial and industrial applications of micro algae—A review. J. Algal Biomass Util. 2012, 3, 89–100. [Google Scholar]
- Henrikson, R.; Edwards, M. Imagine Our Algae Future: Visionary Algae Architecture and Landscape Designs: International Algae Competition; CreateSpace Independent Publishing Platform: Seattle, WA, USA, 2012. [Google Scholar]
- Henrikson, R.; Edwards, M. Algae Microfarms: For Home, School, Community and Urban Gardens, Rooftop, Mobile and Vertical Farms and Living Buildings; CreateSpace Independent Publishing Platform: Seattle, WA, USA, 2013. [Google Scholar]
- Oswald, W.J. Micro-algae and waste-water treatment. In Micro-Algal Biotechnology; Borowitzka, M.A., Borowitzka, L.J., Eds.; Cambridge University Press: New York, NY, USA, 1988; pp. 305–328. [Google Scholar]
- Oswald, W.J. The role of microalgae in liquid waste treatment and reclamation. In Algae and Human Affairs; Lembi, C.A., Waaland, J.R., Eds.; Cambridge University Press: Cambridge, UK, 1988; pp. 255–281. [Google Scholar]
- De la Noüe, J.; Laliberté, G.; Proulx, D. Algae and wastewater. J. Appl. Phycol. 1992, 4, 247–254. [Google Scholar] [CrossRef]
- Shilton, A.N.; Powell, N.; Guieysse, B. Plant based phosphorus recovery from wastewater via algae and macrophytes. Curr. Opin. Biotechnol. 2012, 23, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra, T.V.; Madhab, M.D.; Shilpi, S.; Joshi, N.V. Algal biofuel from urban wastewater in India: Scope and challenges. Renew. Sust. Energ. Rev. 2013, 21, 767–777. [Google Scholar] [CrossRef]
- Koussa, J.; Chaiboonchoe, A.; Salehi-Ashtiani, K. Computational approaches for microalgal biofuel optimization: A review. BioMed Res. Int. 2014, 2014, 649453. [Google Scholar] [CrossRef] [PubMed]
- Levitan, O.; Dinamarca, J.; Hochman, G.; Falkowski, P.G. Diatoms: A fossil fuel of the future. Trends Biotechnol. 2014, 32, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra, T.V.; Mahapatra, D.M.; Karthick, B.; Gordon, R. Milking diatoms for sustainable energy: Biochemical engineering versus gasoline-secreting diatom solar panels. Ind. Eng. Chem. Res. 2009, 48, 8769–8788. [Google Scholar] [CrossRef]
- Metting, F.B., Jr.; Rayburn, W.R.; Reynaud, P.A. Algae and agriculture. In Algae and Human Affairs; Lembi, C.A., Waaland, J.R., Eds.; Cambridge University Press: Cambridge, UK, 1988; pp. 335–370. [Google Scholar]
- Li, S.; Glombitza, K.W.; Koch, M. Phlorotannins from the brown alga Carpophyllum maschalocarpum. Planta Med. 1989, 55, 610–611. [Google Scholar] [CrossRef]
- Rengasamy, K.R.R.; Kulkarni, M.G.; Stirk, W.A.; van Staden, J. Advances in algal drug research with emphasis on enzyme inhibitors. Biotechnol. Adv. 2014, 32, 1364–1381. [Google Scholar] [CrossRef] [PubMed]
- Becker, E.W. Nutritional properties of microalgae: Potentials and constraints. In CRC Handbook of Microalgal Mass Culture; Richmond, A., Ed.; CRC Press: Boca Raton, FL, USA, 1986; pp. 339–419. [Google Scholar]
- Klok, A.J.; Lamers, P.P.; Martens, D.E.; Draaisma, R.B.; Wijffels, R.H. Edible oils from microalgae: Insights in TAG accumulation. Trends Biotechnol. 2014, 32, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Lane, K.; Derbyshire, E.; Li, W.; Brennan, C. Bioavailability and potential uses of vegetarian sources of omega-3 fatty acids: A review of the literature. Crit. Rev. Food Sci. Nutr. 2014, 54, 572–579. [Google Scholar] [CrossRef] [PubMed]
- De Pauw, N.; Persoone, G. Micro-algae for aquaculture. In Micro-Algal Biotechnology; Borowitzka, M.A., Borowitzka, L.J., Eds.; Cambridge University Press: New York, NY, USA, 1988; pp. 197–221. [Google Scholar]
- Benemann, J.R. Microalgae aquaculture feeds. J. Appl. Phycol. 1992, 4, 233–245. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.L.; Nakamura, M. Expression of human growth hormone by the eukaryotic alga, Chlorella. Curr. Microbiol. 1999, 38, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.A.; Mayfield, S.P. Developing inexpensive malaria vaccines from plants and algae. Appl. Microbiol. Biotechnol. 2014, 98, 1983–1990. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Mendoza, S. Future directions for the development of Chlamydomonas-based vaccines. Expert Rev. Vaccines 2013, 12, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Pérez-López, P.; González-García, S.; Ulloa, R.G.; Sineiro, J.; Feijoo, G.; Teresa Moreira, M. Life cycle assessment of the production of bioactive compounds from Tetraselmis suecica at pilot scale. J. Cleaner Prod. 2014, 64, 323–331. [Google Scholar] [CrossRef]
- Moulin, P.; Lemoine, Y.; Schoefs, B. Modifications of the carotenoid metabolism in plastids: A response to stress conditions. In Handbook of Plant and Crop Stress, 3rd ed.; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 2010; pp. 407–433. [Google Scholar]
- Li, Y.; Horsman, M.; Wu, N.; Lan, C.Q.; Dubois-Calero, N. Biofuels from microalgae. Biotechnol. Prog. 2008, 24, 815–820. [Google Scholar] [PubMed]
- Garibay-Hernández, A.; Vazquez-Duhalt, R.; Serrano-Carreón, L.; Martinez, A. Nitrogen limitation in Neochloris oleoabundans: A reassessment of its effect on cell growth and biochemical composition. Appl. Biochem. Biotechnol. 2013, 171, 1775–1791. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Liu, J.; Tian, G. Growth characteristics of Botryococcus braunii 765 under high CO2 concentration in photobioreactor. Bioresour. Technol. 2011, 102, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Illman, A.M.; Scragg, A.H.; Shales, S.W. Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzyme Microb. Technol. 2000, 27, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Scragg, A.H.; Illman, A.M.; Carden, A.; Shales, S.W. Growth of microalgae with increased calorific values in a tubular bioreactor. Biomass Bioenergy 2002, 23, 67–73. [Google Scholar] [CrossRef]
- Liang, Y.; Sarkany, N.; Cui, Y. Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol. Lett. 2009, 31, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yuan, H.; Yang, J.; Li, B. Optimization of the biomass production of oil algae Chlorella minutissima UTEX2341. Bioresour. Technol. 2011, 102, 9128–9134. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.Y.; Chen, F. Heterotrophic production of eicosapentaenoic acid by microalgae. Biotechnol. Adv. 2003, 21, 273–294. [Google Scholar] [CrossRef] [PubMed]
- Bromke, M.A.; Giavalisco, P.; Willmitzer, L.; Hesse, H. Metabolic analysis of adaptation to short-term changes in culture conditions of the marine diatom Thalassiosira pseudonana. PLoS ONE 2013, 8, e67340. [Google Scholar] [CrossRef] [PubMed]
- Ethier, S.; Woisard, K.; Vaughan, D.; Wen, Z. Continuous culture of the microalgae Schizochytrium limacinum on biodiesel-derived crude glycerol for producing docosahexaenoic acid. Bioresour. Technol. 2011, 102, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Deschamps, P.; Guillebeault, D.; Devassine, J.; Dauvillée, D.; Haebel, S.; Steup, M.; Buléon, A.; Putaux, J.-L.; Slomianny, M.-C.; Colleoni, C.; et al. The heterotrophic dinoflagellate Crypthecodinium cohnii defines a model genetic system to investigate cytoplasmic starch synthesis. Eukaryot. Cell 2008, 7, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.K.; Schuhmann, H.; Schenk, P.M. High lipid induction in microalgae for biodiesel production. Energies 2012, 5, 1532–1553. [Google Scholar] [CrossRef]
- Pal, D.; Khozin-Goldberg, I.; Cohen, Z.; Boussiba, S. The effect of light, salinity, and nitrogen availability on lipid production by Nannochloropsis sp. Appl. Microbiol. Biotechnol. 2011, 90, 1429–1441. [Google Scholar] [CrossRef] [PubMed]
- Cadoret, J.-P.; Bernard, O. La production de biocarburant lipidique avec des microalgues: Promesses et defis/Lipid biofuel production with microalgae: Potential and challenges. J. Soc. Biol. 2008, 202, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A. Progress and recent trends in biodiesel fuels. Energy Conv. Manag. 2009, 50, 14–34. [Google Scholar] [CrossRef]
- Shukla, S.K.; Mohan, R. The contribution of diatoms to worldwide crude oil deposits. In The Science of Algal Fuels: Phycology, Geology, Biophotonics, Genomics and Nanotechnology; Gordon, R., Seckbach, J., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 355–382. [Google Scholar]
- Smetacek, V. Diatoms and the ocean carbon cycle. Protist 1999, 150, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Campbell, D.A.; Irwin, A.J.; Suggett, D.J.; Finkel, Z.V. Ocean acidification enhances the growth rate of larger diatoms. Limnol. Oceanogr. 2014, 59, 1027–1034. [Google Scholar] [CrossRef]
- Hejazi, M.A.; Wijffels, R.H. Milking of microalgae. Trends Biotechnol. 2004, 22, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Orosa, M.; Franqueira, D.; Cid, A.; Abalde, J. Analysis and enhancement of astaxanthin accumulation in Haematococcus pluvialis. Bioresour. Technol. 2005, 96, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Kamath, B.S.; Vidhyavathi, R.; Sarada, R.; Ravishankar, G.A. Enhancement of carotenoids by mutation and stress induced carotenogenic genes in Haematococcus pluvialis mutants. Bioresour. Technol. 2008, 99, 8667–8673. [Google Scholar] [CrossRef] [PubMed]
- Bahadar, A.; Khan, M.B. Progress in energy from microalgae: A review. Renew. Sust. Energy Rev. 2013, 27, 128–148. [Google Scholar] [CrossRef]
- Greenwell, H.C.; Laurens, L.M.L.; Shields, R.J.; Lovitt, R.W.; Flynn, K.J. Placing microalgae on the biofuels priority list: A review of the technological challenges. J. R. Soc. Interface 2010, 7, 703–726. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cheng, L.H.; Xu, X.H.; Zhang, L.; Chen, H.L. Screening of biocompatible organic solvents for enhancement of lipid milking from Nannochloropsis sp. Process Biochem. 2011, 46, 1934–1941. [Google Scholar] [CrossRef]
- Mercer, P.; Armenta, R.E. Developments in oil extraction from microalgae. Eur. J. Lipid Sci. Technol. 2011, 113, 539–547. [Google Scholar] [CrossRef]
- Benemann, J.R.; Weissman, J.C.; Koopman, B.L.; Oswald, W.J. Energy production by microbial photosynthesis. Nature 1977, 268, 19–23. [Google Scholar] [CrossRef]
- De Boer, K.; Moheimani, N.R.; Borowitzka, M.A.; Bahri, P.A. Extraction and conversion pathways for microalgae to biodiesel: A review focused on energy consumption. J. Appl. Phycol. 2012, 24, 1681–1698. [Google Scholar] [CrossRef]
- Montagne, X.; Porot, P.; Aymard, C.; Querleu, C.; Bouter, A.; Lorne, D.; Cadoret, J.-P.; Lombaert-Valot, I.; Petillon, O. Algogroup: Towards a shared vision of the possible deployment of algae to biofuels. Oil Gas Sci. Technol. 2013, 68, 875–898. [Google Scholar] [CrossRef]
- Ahmed, F.; Li, Y.; Schenk, P.M. Algal biorefinery: Sustainable production of biofuels and aquaculture feed? In The Science of Algal Fuels: Phycology, Geology, Biophotonics, Genomics and Nanotechnology; Gordon, R., Seckbach, J., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 21–42. [Google Scholar]
- Bajhaiya, A.K.; Suseela, M.R.; Ramteka, P.W. Approaches and prospectives for algal fuel. In The Science of Algal Fuels: Phycology, Geology, Biophotonics, Genomics and Nanotechnology; Gordon, R., Seckbach, J., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 269–282. [Google Scholar]
- Pattarkine, M.; Pattarkine, V.M. Nanotechnology for algal biofuels. In The Science of Algal Fuels: Phycology, Geology, Biophotonics, Genomics and Nanotechnology; Gordon, R., Seckbach, J., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 147–164. [Google Scholar]
- Riosmena-Rodríguez, R.; Arredondo-Vega, B.O.; Reynoso-Granados, T.; Cordoba, M.; López-Vivas, J.M.; López-Calderon, J.M. Approaches to and perspectives on biodiesel and oil production using algae in Mexico. In The Science of Algal Fuels: Phycology, Geology, Biophotonics, Genomics and Nanotechnology; Gordon, R., Seckbach, J., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 269–282. [Google Scholar]
- Craggs, R.J.; Lundquist, T.; Benemann, J. Wastewater treatment pond algal production for biofuel. In The Science of Algal Fuels: Phycology, Geology, Biophotonics, Genomics and Nanotechnology; Gordon, R., Seckbach, J., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 425–446. [Google Scholar]
- Kumar, S. Sub-and supercritical water based processes for microalgae to biofuels. In The Science of Algal Fuels: Phycology, Geology, Biophotonics, Genomics and Nanotechnology; Gordon, R., Seckbach, J., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 467–494. [Google Scholar]
- Richardson, J.W.; Johnson, M.D.; Outlaw, J.L. Economic comparison of open pond raceways to photo bio-reactors for profitable production of algae for transportation fuels in the Southwest. Algal Res. Biomass Biofuels Bioprod. 2012, 1, 93–100. [Google Scholar]
- Lane, J. Joule’s quest for fuels from CO2, sunlight and water. Available online: http://www.biofuelsdigest.com/bdigest/2014/07/03/joules-quest-for-fuels-from-co2-sunlight-and-water/ (accessed on 21 April 2015).
- Wilkinson, S. Curb global warming: Make waste CO2 into fuel. Chem. Eng. News 1997, 75, 6. [Google Scholar] [CrossRef]
- Jiang, Z.; Xiao, T.; Kuznetsov, V.L.; Edwards, P.P. Turning carbon dioxide into fuel. Phil. Trans. R. Soc. Lond. A: Math. Phys. Eng. Sci. 2010, 368, 3343–3364. [Google Scholar] [CrossRef]
- Ladd, C.; Venter, J.C. 10 Big Questions for Maverick Geneticist J. Craig Venter on America’s Energy Future. Available online: http://www.popularmechanics.com/blogs/science_news/4275738.html (accessed on 8 August 2010).
- Wang, X.W.; Liang, J.R.; Luo, C.S.; Chen, C.P.; Gao, Y.H. Biomass, total lipid production, and fatty acid composition of the marine diatom Chaetoceros muelleri in response to different CO2 levels. Bioresour. Technol. 2014, 161, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Van der Hoeven, M. CO2 Emissions from Fuel Combustion: Highlights, 2013 ed.; International Energy Agency: Paris, France, 2013. [Google Scholar]
- Moheimani, N. Microalgae Culture (3); Algae R&D Center, Murdoch University: Perth, Australia, 2014. [Google Scholar]
- Frenz, J.; Largeau, C.; Casadevall, E. Hydrocarbon recovery by extraction with a biocompatible solvent from free and immobilized cultures of Botryococcus braunii. Enzyme Microb. Technol. 1989, 11, 717–724. [Google Scholar] [CrossRef]
- Zhang, F.; Cheng, L.-H.; Xu, X.-H.; Zhang, L.; Chen, H.-L. Application of membrane dispersion for enhanced lipid milking from Botryococcus braunii FACHB 357. J. Biotechnol. 2013, 165, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Rickman, M.; Davis, R.H.; Pellegrino, J. Fractionation of organic fuel precursors from electrolytes with membranes. Ind. Eng. Chem. Res. 2013, 52, 10530–10539. [Google Scholar] [CrossRef]
- Yadugiri, V.T. Milking diatoms—A new route to sustainable energy. Curr. Sci. 2009, 97, 748–750. [Google Scholar]
- Hildebrand, M.; Davis, A.K.; Smith, S.R.; Traller, J.C.; Abbriano, R. The place of diatoms in the biofuels industry. Biofuels 2012, 3, 221–240. [Google Scholar] [CrossRef]
- Ho, S.-H.; Ye, X.; Hasunuma, T.; Chang, J.-S.; Kondo, A. Perspectives on engineering strategies for improving biofuel production from microalgae—A critical review. Biotechnol. Adv. 2014, 32, 1448–1459. [Google Scholar] [CrossRef] [PubMed]
- Ciesielski, A.; Limited, R.T. An Introduction to Rubber Technology; Rapra Technology Limited: Shrewsbury, UK, 1999. [Google Scholar]
- Svanberg, I.; Sõukand, R.; Łuczaj, Ł.; Kalle, R.; Zyryanova, O.; Dénes, A.; Papp, N.; Nedelcheva, A.; Šeškauskaite, D.; Kołodziejska-Degórska, I.; et al. Uses of tree saps in northern and eastern parts of Europe. Acta Soc. Bot. Pol. 2012, 81, 343–357. [Google Scholar] [CrossRef]
- Nearing, H.; Nearing, S. The Maple Sugar Book: Together with Remarks on Pioneering as a Way of Living in the Twentieth Century; Chelsea Green Publishing: White River Junction, VT, USA, 2000. [Google Scholar]
- Munson, P.J. Still more on the antiquity of maple sugar and syrup in aboriginal eastern North America. J. Ethnobiol. 1989, 9, 159–170. [Google Scholar]
- Haller, J.S., Jr. Sampson of the terebinthinates: Medical history of turpentine. South. Med. J. 1984, 77, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Sauer, T.; Galinski, E.A. Bacterial milking: A novel bioprocess for production of compatible solutes. Biotechnol. Bioeng. 1998, 57, 306–313. [Google Scholar] [PubMed]
- Van-Thuoc, D.; Guzmán, H.; Quillaguamán, J.; Hatti-Kaul, R. High productivity of ectoines by Halomonas boliviensis using a combined two-step fed-batch culture and milking process. J. Biotechnol. 2010, 147, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Gillet, J.-N. Ultrafast molecular dynamics of biofuel extraction for microalgae and bacteria milking: Blocking membrane folding pathways to damaged lipid-bilayer conformations with nanomicelles. J. Biomol. Struct. Dyn. 2015, 33, 690–705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cheng, L.H.; Xu, X.H.; Zhang, L.; Chen, H.L. Technologies of microalgal harvesting and lipid extraction. Prog. Chem. 2012, 24, 2062–2072. [Google Scholar]
- Wijffels, R.H.; Barbosa, M.J. An outlook on microalgal biofuels. Science 2010, 329, 796–799. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi Naghdi, F.; Thomas-Hall, S.R.; Durairatnam, R.; Pratt, S.; Schenk, P.M. Comparative effects of biomass pre-treatments for direct and indirect transesterification to enhance microalgal lipid recovery. Front. Energy Res. 2014, 2, 57. [Google Scholar] [CrossRef]
- Ganeva, V.; Galutzov, B.; Teissie, J. High yield electroextraction of proteins from yeast by a flow process. Anal. Biochem. 2003, 315, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Coustets, M.; Al-Karablieh, N.; Thomsen, C.; Teissie, J. Flow process for electroextraction of total proteins from microalgae. J. Membr. Biol. 2013, 246, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Coustets, M.; Joubert-Durigneux, V.; Hérault, J.; Schoefs, B.; Blanckaert, V.; Garnier, J.-P.; Teissié, J. Optimization of protein electroextraction from microalgae by a flow process. Bioelectrochemistry 2015, 103, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Reep, P.; Green, M.P. Procedure for extracting of lipids from algae without cell sacrifice. US Patent 20120040428 A1, 16 February 2012. [Google Scholar]
- Gateau, H.; Marchand, J.; Schoefs, B. Pulse electric fields allow the biocompatible extraction of molecules from the microalgae Haematococcus pluvialis. In Abstracts, Functional Studies on Model Organisms (EFOR), 6th Annual Meeting, Paris, France; EFOR: Paris, France, 2015. [Google Scholar]
- Sheng, J.; Vannela, R.; Rittmann, B.E. Evaluation of cell-disruption effects of pulsed-electric-field treatment of Synechocystis PCC 6803. Environ. Sci. Technol. 2011, 45, 3795–3802. [Google Scholar] [CrossRef] [PubMed]
- Vinayak, V.; Gordon, R.; Gautam, S.; Rai, A. Discovery of a diatom that oozes oil. Adv. Sci. Lett. 2014, 20, 1256–1267. [Google Scholar] [CrossRef]
- Tsukagoshi, N.; Yoshida, H.; Katsurayama, M.; Udaka, S. Uncoupled release of protein and lipid in a protein-secreting bacterium, Bacillus brevis-47. Biochim. Biophys. Acta 1983, 759, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Raetz, C.R.H. Biosynthesis, secretion and function of lipid A in Gram-negative bacteria. Glycobiology 2001, 11, 872–872. [Google Scholar]
- Wald, M.L. Biotech Company to Patent Fuel-Secreting Bacterium. Available online: http://www.nytimes.com/2010/09/14/science/earth/14fuel.html (accessed on 21 April 2015).
- Liu, X.; Sheng, J.; Curtiss, R., III. Fatty acid production in genetically modified cyanobacteria. Proc. Natl. Acad. Sci. USA 2011, 108, 6899–6904. [Google Scholar] [CrossRef] [PubMed]
- MB-BigB Joule Unlimited’s bacteria secretes diesel fuel. Available online: http://www.alt-energy.info/biofuel/joule-unlimiteds-bacteria-secretes-diesel-fuel/ (accessed on 29 August 2012).
- Robertson, D.E.; Jacobson, S.A.; Morgan, F.; Berry, D.; Church, G.M.; Afeyan, N.B. A new dawn for industrial photosynthesis. Photosynth. Res. 2011, 107, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Araujo, G.S.; Matos, L.J.; Fernandes, J.O.; Cartaxo, S.J.; Goncalves, L.R.; Fernandes, F.A.; Farias, W.R. Extraction of lipids from microalgae by ultrasound application: Prospection of the optimal extraction method. Ultrason. Sonochem. 2013, 20, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Patil, C.; Moholkar, V.S. Mechanistic assessment of microalgal lipid extraction. Ind. Eng. Chem. Res. 2010, 49, 2979–2985. [Google Scholar] [CrossRef]
- Rajasekhar, P.; Fan, L.; Thang, N.; Roddick, F.A. Impact of sonication at 20 kHz on Microcystis aeruginosa, Anabaena circinalis and Chlorella sp. Water Res. 2012, 46, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Joyce, E.M.; King, P.M.; Mason, T.J. The effect of ultrasound on the growth and viability of microalgae cells. J. Appl. Phycol. 2014, 26, 1741–1748. [Google Scholar] [CrossRef]
- Broekman, S.; Pohlmann, O.; Beardwood, E.S.; de Meulenaer, E.C. Ultrasonic treatment for microbiological control of water systems. Ultrason. Sonochem. 2010, 17, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Doshi, R.; Tuan, N.; Chang, G. Transporter-mediated biofuel secretion. Proc. Natl. Acad. Sci. USA 2013, 110, 7642–7647. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, M.J.; Dossani, Z.Y.; Szmidt, H.L.; Chu, H.C.; Lee, T.S.; Keasling, J.D.; Hadi, M.Z.; Mukhopadhyay, A. Engineering microbial biofuel tolerance and export using efflux pumps. Mol. Syst. Biol. 2011, 7, 487. [Google Scholar] [CrossRef] [PubMed]
- Dunahay, T.G.; Jarvis, E.E.; Zeiler, K.G.; Roessler, P.G.; Brown, L.M. Genetic engineering of microalgae for fuel production: Scientific note. Appl. Biochem. Biotechnol. 1992, 34–5, 331–339. [Google Scholar] [CrossRef]
- Coll, J.M. Methodologies for transferring DNA into eukaryotic microalgae. Span. J. Agric. Res. 2006, 4, 316–330. [Google Scholar] [CrossRef]
- León, R.; Fernández, E. Nuclear transformation of eukaryotic microalgae: Historical overview, achievements and problems. Adv. Exp. Med. Biol. 2007, 616, 1–11. [Google Scholar] [PubMed]
- Miyahara, M.; Aoi, M.; Inoue-Kashino, N.; Kashino, Y.; Ifuku, K. Highly efficient transformation of the diatom Phaeodactylum tricornutum by multi-pulse electroporation. Biosci. Biotechnol. Biochem. 2013, 77, 874–876. [Google Scholar] [CrossRef] [PubMed]
- Li, F.J.; Gao, D.W.; Hu, H.H. High-Efficiency nuclear transformation of the oleaginous marine Nannochloropsis species using PCR product. Biosci. Biotechnol. Biochem. 2014, 78, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Hu, H.H. High-efficiency nuclear transformation of the diatom Phaeodactylum tricornutum by electroporation. Mar. Genom. 2014, 16, 63–66. [Google Scholar] [CrossRef]
- Stirke, A.; Zimkus, A.; Balevicius, S.; Stankevic, V.; Ramanaviciene, A.; Ramanavicius, A.; Zurauskiene, N. Permeabilization of yeast Saccharomyces cerevisiae cell walls using nanosecond high power electrical pulses. Appl. Phys. Lett. 2014, 105, 253701. [Google Scholar] [CrossRef]
- Joubert, V.; Cheype, C.; Bonnet, J.; Packan, D.; Garnier, J.-P.; Teissie, J.; Blanckaert, V. Inactivation of Bacillus subtilis var. niger of both spore and vegetative forms by means of corona discharges applied in water. Water Res. 2013, 47, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Antezana Zbinden, M.D.; Sturm, B.S.M.; Nord, R.D.; Carey, W.J.; Moore, D.; Shinogle, H.; Stagg-Williams, S.M. Pulsed electric field (PEF) as an intensification pretreatment for greener solvent lipid extraction from microalgae. Biotechnol. Bioeng. 2013, 110, 1605–1615. [Google Scholar] [CrossRef] [PubMed]
- Deipolyi, A.R.; Golberg, A.; Yarmush, M.L.; Arellano, R.S.; Oklu, R. Irreversible electroporation: Evolution of a laboratory technique in interventional oncology. Diagn. Interv. Radiol. 2014, 20, 147–154. [Google Scholar] [PubMed]
- Sixou, S.; Teissié, J. Specific electropermeabilization of leukocytes in a blood sample and application to large volumes of cells. Biochim. Biophys. Acta 1990, 1028, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Bellard, E.; Teissié, J. Double-pulse approach of electrogenotherapy: An analysis at the single cell level. IEEE Trans. Plasma Sci. 2009, 37, 538–544. [Google Scholar] [CrossRef]
- Rols, M.P.; Teissie, J. Ionic-strength modulation of electrically induced permeabilization and associated fusion of mammalian cells. Eur. J. Biochem. 1989, 179, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fallon, S.; Sheng, J.; Curtiss, R., III. CO2-limitation-inducible Green Recovery of fatty acids from cyanobacterial biomass. Proc. Natl. Acad. Sci. USA 2011, 108, 6905–6908. [Google Scholar] [CrossRef] [PubMed]
- Reppas, N.B.; Ridley, C.P. Methods and Compositions for the Recombinant Biosynthesis of N-Alkanes. United States Patent 7,794,969, 14 September 2010. [Google Scholar]
- Ozkan, A.; Berberoglu, H. Physico-chemical surface properties of microalgae. Colloids Surf. B Biointerfaces 2013, 112, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Ruen-ngam, D.; Shotipruk, A.; Pavasant, P. Comparison of extraction methods for recovery of astaxanthin from Haematococcus pluvialis. Sep. Sci. Technol. 2010, 46, 64–70. [Google Scholar] [CrossRef]
- Zou, T.-B.; Jia, Q.; Li, H.-W.; Wang, C.-X.; Wu, H.-F. Response surface methodology for ultrasound-assisted extraction of astaxanthin from Haematococcus pluvialis. Mar. Drugs 2013, 11, 1644–1655. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Liu, N.; Zhang, J.; Yang, Q.; Hua, S.; Song, H.; Xia, C. Optimization of ultrasound-assisted extraction parameters of chlorophyll from Chlorella vulgaris residue after lipid separation using response surface methodology. J. Food Sci. Technol.-Mysore 2014, 51, 2006–2013. [Google Scholar] [CrossRef]
- Macias-Sánchez, M.D.; Mantell, C.; Rodríguez, M.; Martinez de la Ossa, E.; Lubián, L.M.; Montero, O. Comparison of supercritical fluid and ultrasound-assisted extraction of carotenoids and chlorophyll a from Dunaliella salina. Talanta 2009, 77, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Pasquet, V.; Chérouvrier, J.-R.; Farhat, F.; Thiéry, V.; Piot, J.-M.; Bérard, J.-B.; Kaas, R.; Serive, B.; Patrice, T.; Cadoret, J.-P.; et al. Study on the microalgal pigments extraction process: Performance of microwave assisted extraction. Process Biochem. 2011, 46, 59–67. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Galanakis, C.M.; Brnčić, M.; Orlien, V.; Trujillo, F.J.; Mawson, R.; Knoerzer, K.; Tiwari, B.K.; Barba, F.J. Clean recovery of antioxidant compounds from plant foods, by-products and algae assisted by ultrasounds processing. Modeling approaches to optimize processing conditions. Trends Food Sci. Technol. 2015, 42, 134–149. [Google Scholar] [CrossRef]
- Sterrenburg, F.A.S.; Gordon, R.; Tiffany, M.A.; Nagy, S.S. Diatoms: Living in a constructal environment. In Algae and Cyanobacteria in Extreme Environments. Series: Cellular Origin, Life in Extreme Habitats and Astrobiology, Volume 11; Seckbach, J., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 141–172. [Google Scholar]
- Round, F.E.; Crawford, R.M.; Mann, D.G. The Diatoms, Biology & Morphology of the Genera; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Stoermer, E.F.; Julius, M.L. Centric diatoms. In Freshwater Algae of North America. Ecology and Classification; Wehr, J.D., Sheath, R.G., Eds.; Academic Press: New York, NY, USA, 2003; pp. 559–594. [Google Scholar]
- Bahulikar, R.A.; Kroth, P.G. Localization of EPS components secreted by freshwater diatoms using differential staining with fluorophore-conjugated lectins and other fluorochromes. Eur. J. Phycol. 2007, 42, 199–208. [Google Scholar] [CrossRef]
- Kociolek, J.P.; Stoermer, E.F. A preliminary investigation of the phylogenetic relationships among the freshwater, apical pore field-bearing cymbelloid and gomphonemoid diatoms (Bacillariophyceae). J. Phycol. 1988, 24, 377–385. [Google Scholar] [CrossRef]
- Hamm, C.E.; Merkel, R.; Springer, O.; Jurkojc, P.; Maier, C.; Prechtel, K.; Smetacek, V. Architecture and material properties of diatom shells provide effective mechanical protection. Nature 2003, 421, 841–843. [Google Scholar] [CrossRef] [PubMed]
- Abodeely, J.; Stevens, D.; Ray, A.; Schaller, K.; Newby, D. Algal Supply System Design—Harmonized Version [Report INL/EXT-13–28890]; Idaho National Laboratory (INL): Idaho Falls, ID, USA, 2013. [Google Scholar]
- Anderson, E. A cytological study of the centrifuged whole, half, and quarter eggs of the sea urchin Arbacia punctulata. J. Cell Biol. 1970, 47, 711–733. [Google Scholar] [CrossRef] [PubMed]
- Furnas, M.J. Net in situ growth rates of phytoplankton in an oligotrophic, tropical shelf ecosystem. Limnol. Oceanogr. 1991, 36, 13–29. [Google Scholar] [CrossRef]
- Ketheesan, B.; Nirmalakhandan, N. Development of a new airlift-driven raceway reactor for algal cultivation. Appl. Energy 2011, 88, 3370–3376. [Google Scholar] [CrossRef]
- Matsumoto, H.; Shioji, N.; Hamasaki, A.; Ikuta, Y. Basic study on optimization of raceway-type algal cultivator. J. Chem. Eng. Jpn. 1996, 29, 541–543. [Google Scholar] [CrossRef]
- Cohen, B. Changes Going on in the Algae Production Industry. Available online: http://www.nationalalgaeassociation.com/Changes_going_on_in_the_algae_production_industry.docx (accessed on 23 April 2015).
- Day, J.G.; Stanley, M.S. Biological constraints on the production of microalgal-based biofuels. In The Science of Algal Fuels: Phycology, Geology, Biophotonics, Genomics and Nanotechnology; Gordon, R., Seckbach, J., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 101–130. [Google Scholar]
- McNichol, J.; McGinn, P. Adapting mass algaculture for a northern climate. In The Science of Algal Fuels: Phycology, Geology, Biophotonics, Genomics and Nanotechnology; Gordon, R., Seckbach, J., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 131–146. [Google Scholar]
- Rhodes, C.J. Making fuel from algae: Identifying fact amid fiction. In The Science of Algal Fuels: Phycology, Geology, Biophotonics, Genomics and Nanotechnology; Gordon, R., Seckbach, J., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 177–192. [Google Scholar]
- Bhargava, P.; Mohan, M.K. From isolation of potential microalgal strains to strain engineering for biofuel. In The Science of Algal Fuels: Phycology, Geology, Biophotonics, Genomics and Nanotechnology; Gordon, R., Seckbach, J., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 63–82. [Google Scholar]
- Zittelli, G.C.; Rodolfi, L.; Bassi, N.; Biondi, N.; Tredici, M.R. Photobioreactors for microalgal biofuel production. In Algae for Biofuels and Energy; Springer: Dordrecht, The Netherlands, 2013; pp. 115–131. [Google Scholar]
- Schulze, P.S.C.; Barreira, L.A.; Pereira, H.G.C.; Perales, J.A.; Varela, J.C.S. Light emitting diodes (LEDs) applied to microalgal production. Trends Biotechnol. 2014, 32, 423–431. [Google Scholar] [CrossRef]
- Darko, E.; Heydarizadeh, P.; Schoefs, B.; Sabzalian, M.R. Photosynthesis under artificial light: The shift in primary and secondary metabolism. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130243. [Google Scholar] [CrossRef]
- Reijnders, L. Foreword: The production of algal biofuels. In The Science of Algal Fuels: Phycology, Geology, Biophotonics, Genomics and Nanotechnology; Gordon, R., Seckbach, J., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. xiii–xix. [Google Scholar]
- Dahiya, A.; Todd, J.; McInnis, A. Wastewater treatment integrated with algae production for biofuel. In The Science of Algal Fuels: Phycology, Geology, Biophotonics, Genomics and Nanotechnology; Gordon, R., Seckbach, J., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 447–466. [Google Scholar]
- Gordon, R.; Poulin, B.J. Quitting cold turkey: Rapid oil independence for the USA. In The Science of Algal Fuels: Phycology, Geology, Biophotonics, Genomics and Nanotechnology; Gordon, R., Seckbach, J., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 3–20. [Google Scholar]
- Wang, X.J.; Wang, X.B.; Gascoyne, P.R.C. General expressions for dielectrophoretic force and electrorotational torque derived using the Maxwell stress tensor method. J. Electrost. 1997, 39, 277–295. [Google Scholar] [CrossRef]
- Beskok, Ali.; Department of Mechanical Engineering, Southern Methodist University, Dallas, TX, USA. Personal communication, 2014.
- Zhou, J.W.; Ellis, A.V.; Voelcker, N.H. Recent developments in PDMS surface modification for microfluidic devices. Electrophoresis 2010, 31, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Forry, S.P.; Locascio, L.E. On-chip CO2 control for microfluidic cell culture. Lab Chip 2011, 11, 4041–4046. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, T.; Maerkl, S.J.; Quake, S.R. Microfluidic large-scale integration. Science 2002, 298, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Cui, T.H. Tunable wetting properties of patterned silicon microchannels with varied surface free energy based on layer-by-layer nano self-assembly. J. Micromech. Microeng. 2011, 21, 045015. [Google Scholar] [CrossRef]
- Lim, H.S.; Kim, J.Y.; Kwak, H.S.; Sim, S.J. Integrated microfluidic platform for multiple processes from microalgal culture to lipid extraction. Anal. Chem. 2014, 86, 8585–8592. [Google Scholar] [CrossRef] [PubMed]
- Jeffryes, C.; Campbell, J.; Li, H.Y.; Jiao, J.; Rorrer, G. The potential of diatom nanobiotechnology for applications in solar cells, batteries, and electroluminescent devices. Energy Environ. Sci. 2011, 4, 3930–3941. [Google Scholar] [CrossRef]
- Gordon, R.; Witkowski, A.; Gebeshuber, I.C.; Allen, C.S. The diatoms of Antarctica and their potential roles in nanotechnology. In Antarctica: Time of Change; Masó, M., Ed.; Editions ACTAR: Barcelona, Spain, 2010; pp. 84–95. [Google Scholar]
- Chepurnov, V.A.; Chaerle, P.; Mann, D.G. How to breed diatoms: Examination of two species with contrasting reproductive biology. In The Science of Algal Fuels: Phycology, Geology, Biophotonics, Genomics and Nanotechnology; Gordon, R., Seckbach, J., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 323–340. [Google Scholar]
- Dodson, V.J.; Leblond, J.D. Now you see it, now you don’t: Differences in hydrocarbon production in the diatom Phaeodactylum tricornutum due to growth temperature. J. Appl. Phycol. 2014. [Google Scholar] [CrossRef]
- Poulin, Bryan; Faculty of Business Administration, Lakehead University, Thunder Bay, Canada. Personal communication, 2014.
- Mutanda, T.; Ramesh, D.; Karthikeyan, S.; Kumari, S.; Anandraj, A.; Bux, F. Bioprospecting for hyper-lipid producing microalgal strains for sustainable biofuel production. Bioresour. Technol. 2011, 102, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.E. Artifcial Leaf for Biofuel Production and Harvesting: Transport Phenomena and Energy Conversion. Ph.D. Thesis, University of Texas, Austin, TX, USA, 2013. [Google Scholar]
- Onay, M.; Sonmez, C.; Oktem, H.A.; Yucel, A.M. Thermo-resistant green microalgae for effective biodiesel production: Isolation and characterization of unialgal species from geothermal flora of Central Anatolia. Bioresour. Technol. 2014, 169, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Nikulina, T.V.; Kociolek, J.P. Diatoms from hot springs from Kuril and Sakhalin Islands (Far East, Russia). In The Diatom World; Seckbach, J., Kociolek, J.P., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 333–363. [Google Scholar]
- Quintela, A.; Almeida, S.; Terroso, D.; Ferreira da Silva, E.; Forjaz, V.; Rocha, F. Diatom assemblages of thermal and mineral waters from volcanic environments in São Miguel Island, Azores. Diatom Res. 2013, 28, 407–417. [Google Scholar] [CrossRef]
- Owen, R.B.; Renaut, R.W.; Jones, B. Geothermal diatoms: A comparative study of floras in hot spring systems of Iceland, New Zealand, and Kenya. Hydrobiologia 2008, 610, 175–192. [Google Scholar] [CrossRef]
- Lindemann, S.R.; Moran, J.J.; Stegen, J.C.; Renslow, R.S.; Hutchison, J.R.; Cole, J.K.; Dohnalkova, A.C.; Tremblay, J.; Singh, K.; Malfatti, S.A.; et al. The epsomitic phototrophic microbial mat of Hot Lake, Washington: Community structural responses to seasonal cycling. Front. Microbiol. 2013, 4, article 323. [Google Scholar] [CrossRef]
- Andersen, R.A. Diversity of eukaryotic algae. Biodivers. Conserv. 1992, 1, 267–292. [Google Scholar] [CrossRef]
- Guiry, M.D. How many species of algae are there? J. Phycol. 2012, 48, 1057–1063. [Google Scholar] [CrossRef]
- Grobbelaar, J.U. Physiological and technological considerations for optimising mass algal cultures. J. Appl. Phycol. 2000, 12, 201–206. [Google Scholar] [CrossRef]
- Pienkos, P.T.; Darzins, A. The promise and challenges of microalgal-derived biofuels. Biofuels Bioprod. Biorefin. 2009, 3, 431–440. [Google Scholar] [CrossRef]
- Chagoya, J.C.; Brown, J.; Gomez, M.S.; Zhang, J.; Jiang, Y.; Laverty, K.; Brown, L.; Quigg, A.; Burow, M.D. Media optimization and lipid formation of two native diatoms for cultivation in the Southwest Texas desert. J. Appl. Phycol. 2014, 26, 2075–2085. [Google Scholar] [CrossRef]
- Kim, J.K.; Kottuparambil, S.; Moh, S.H.; Lee, T.K.; Kim, Y.-J.; Rhee, J.-S.; Choi, E.-M.; Kim, B.H.; Yu, Y.J.; Yarish, C. Potential applications of nuisance microalgae blooms. J. Appl. Phycol. 2014. [Google Scholar] [CrossRef]
- Jellyman, P.G.; Clearwater, S.J.; Clayton, J.S.; Kilroy, C.; Blair, N.; Hickey, C.W.; Biggs, B.J.F. Controlling the invasive diatom Didymosphenia geminata: An ecotoxicity assessment of four potential biocides. Arch. Environ. Contam. Toxicol. 2011, 61, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Valdez-Ojeda, R.; González-Muñoz, M.; Us-Vázquez, R.; Narváez-Zapata, J.; Chavarria-Hernandez, J.C.; López-Adrián, S.; Barahona-Pérez, F.; Toledano-Thompson, T.; Garduño-Solórzano, G.; Escobedo-Gracia Medrano, R.M. Characterization of five fresh water microalgae with potential for biodiesel production. Algal Res. 2015, 7, 33–44. [Google Scholar] [CrossRef]
- Kopecky, J.; Schoefs, B.; Loest, K.; Stys, D.; Pulz, O. Microalgae as a source for secondary carotenoid production: A screening study. Arch. Hydrobiol. Suppl. 2000, 133, 153–168. [Google Scholar]
- Meng, Y.; Yao, C.; Xue, S.; Yang, H. Application of Fourier transform infrared (FT-IR) spectroscopy in determination of microalgal compositions. Bioresour. Technol. 2014, 151, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Jungandreas, A.; Fanesi, A.; Wilhelm, C. Surveillance of C-allocation in microalgal cells. Metabolites 2014, 4, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, D.M.; Ramachandra, T.V. Algal biofuel: Bountiful lipid from Chlorococcum sp. proliferating in municipal wastewater. Curr. Sci. 2013, 105, 47–55. [Google Scholar]
- Bougaran, G.; Rouxel, C.; Dubois, N.; Kaas, R.; Grouas, S.; Lukomska, E.; Le Coz, J.-R.; Cadoret, J.-P. Enhancement of neutral lipid productivity in the microalga Isochrysis affinis Galbana (T-Iso) by a mutation-selection procedure. Biotechnol. Bioeng. 2012, 109, 2737–2745. [Google Scholar] [CrossRef] [PubMed]
- Robert, S.; Mansour, M.P.; Blackburn, S.I. Metolachlor-mediated selection of a microalgal strain producing novel polyunsaturated fatty acids. Mar. Biotechnol. 2007, 9, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Kasbi-Chadli, F.; Boquien, C.-Y.; Simard, G.; Ulmann, L.; Mimouni, V.; Leray, V.; Meynier, A.; Ferchaud-Roucher, V.; Champ, M.; Nguyen, P.; et al. Maternal supplementation with n-3 long chain polyunsaturated fatty acids during perinatal period alleviates the metabolic syndrome disturbances in adult hamster pups fed a high-fat diet after weaning. J. Nutr. Biochem. 2014, 25, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Juneau, P.; Dewez, D.; Matsui, S.; Kim, S.G.; Popovic, R. Evaluation of different algal species sensitivity to mercury and metolachlor by PAM-fluorometry. Chemosphere 2001, 45, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Metting, F.B., Jr. Biodiversity and application of microalgae. J. Ind. Microbiol. 1996, 17, 477–489. [Google Scholar] [CrossRef]
- Ramesha, B.T.; Gertsch, J.; Ravikanth, G.; Priti, V.; Ganeshaiah, K.N.; Shaanker, R.U. Biodiversity and chemodiversity: Future perspectives in bioprospecting. Curr. Drug Targets 2011, 12, 1515–1530. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, J.; Dunahay, T.; Benemann, J.; Roessler, P. A Look Back at the U.S. Department of Energy’s Aquatic Species Program: Biodiesel from Algae; Close-Out Report NREL/TP-580-24190; National Renewable Energy Laboratory: Golden, CO, USA, 1998. [Google Scholar]
- Tara Expeditions The Oceanomics Project. Available online: http://oceans.taraexpeditions.org/en/m/science/news/the-oceanomics-project/ (accessed on 21 April 2015).
- Fujii, K.; Matsunobu, S.; Takahashi, Y. Characterization of the new microalgal strains, Oogamochlamys spp., and their potential for biofuel production. Algal Res. 2014, 5, 164–170. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Z.; Yu, C.; Yin, Y.; Zhou, G. Evaluation of the potential of 9 Nannochloropsis strains for biodiesel production. Bioresour. Technol. 2014, 167, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Tale, M.; Ghosh, S.; Kapadnis, B.; Kale, S. Isolation and characterization of microalgae for biodiesel production from Nisargruna biogas plant effluent. Bioresour. Technol. 2014, 169, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Becerra, P.I.; Bustamante, R.O. The effect of herbivory on seedling survival of the invasive exotic species Pinus radiata and Eucalyptus globulus in a Mediterranean ecosystem of Central Chile. For. Ecol. Manag. 2008, 256, 1573–1578. [Google Scholar] [CrossRef]
- Boeuf, G.; Kornprobst, J.-M. Biodiversité et chimiodiversité marines/Biodiversity and marine chemodiversity. Biofutur 2009, 301, 28–32. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Jiraskova, D.; Poulickova, A.; Novak, O.; Sedlakova, K.; Hradecka, V.; Strnad, M. High-throughput screening technology for monitoring phytohormone production in microalgae. J. Phycol. 2009, 45, 108–118. [Google Scholar] [CrossRef]
- Griffiths, M.J.; Harrison, S.T.L. Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J. Appl. Phycol. 2009, 21, 493–507. [Google Scholar] [CrossRef]
- Barbour, M.T.; Gerritsen, J.; Snyder, B.D.; Stribling, J.B. Rapid Bioassessment Protocols for Use in Streams and Wadeable Rivers: Periphyton, Benthic Macroinvertebrates and Fish [EPA 841-B-99-002], 2nd ed.; Office of Water, U.S. Environmental Protection Agency: Washington, DC, USA, 1999. [Google Scholar]
- Zhang, W.; Nielsen, D.R. Synthetic biology applications in industrial microbiology. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa-Yamaguchi, A.; Okami, T.; Kira, N.; Yamaguchi, H.; Ohnishi, K.; Adachi, M. Stable nuclear transformation of the diatom Chaetoceros sp. Phycol. Res. 2011, 59, 113–119. [Google Scholar] [CrossRef]
- Dunahay, T.G.; Jarvis, E.E.; Roessler, P.G. Genetic transformation of the diatoms Cyclotella cryptica and Navicula saprophila. J. Phycol. 1995, 31, 1004–1012. [Google Scholar] [CrossRef]
- Fischer, H.; Robl, I.; Sumper, M.; Kroger, N. Targeting and covalent modification of cell wall and membrane proteins heterologously expressed in the diatom Cylindrotheca fusiformis (Bacillariophyceae). J. Phycol. 1999, 35, 113–120. [Google Scholar] [CrossRef]
- Poulsen, N.; Kröger, N. A new molecular tool for transgenic diatoms. Control of mRNA and protein biosynthesis by an inducible promoter-terminator cassette. FEBS J. 2005, 272, 3413–3423. [Google Scholar] [CrossRef]
- Muto, M.; Fukuda, Y.; Nemoto, M.; Yoshino, T.; Matsunaga, T.; Tanaka, T. Establishment of a genetic transformation system for the marine pennate diatom Fistulifera sp. strain JPCC DA0580-a high triglyceride producer. Mar. Biotechnol. 2013, 15, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Apt, K.E.; Kroth-Pancic, P.G.; Grossman, A.R. Stable nuclear transformation of the diatom Phaeodactylum tricornutum. Mol. General Genet. 1996, 252, 572–579. [Google Scholar]
- Zaslavskaia, L.A.; Lippmeier, J.C.; Kroth, P.G.; Grossman, A.R.; Apt, K.E. Transformation of the diatom Phaeodactylum tricornutum (Bacillariophyceae) with a variety of selectable marker and reporter genes. J. Phycol. 2000, 36, 379–386. [Google Scholar] [CrossRef]
- Niu, Y.-F.; Yang, Z.-K.; Zhang, M.-H.; Zhu, C.-C.; Yang, W.-D.; Liu, J.-S.; Li, H.-Y. Transformation of diatom Phaeodactylum tricornutum by electroporation and establishment of inducible selection marker. BioTechn. Rapid Dispatches 2012. [Google Scholar] [CrossRef]
- Ifuku, K.; Yan, D.; Miyahara, M.; Inoue-Kashino, N.; Yamamoto, Y.Y.; Kashino, Y. A stable and efficient nuclear transformation system for the diatom Chaetoceros gracilis. Photosynth. Res. 2015, 123, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Shimogawara, K.; Fujiwara, S.; Grossman, A.; Usuda, H. High-efficiency transformation of Chlamydomonas reinhardtii by electroporation. Genetics 1998, 148, 1821–1828. [Google Scholar] [PubMed]
- Kilian, O.; Benemann, C.S.E.; Niyogi, K.K.; Vick, B. High-efficiency homologous recombination in the oil-producing alga Nannochloropsis sp. Proc. Natl. Acad. Sci. USA 2011, 108, 21265–21269. [Google Scholar] [CrossRef] [PubMed]
- Radakovits, R.; Jinkerson, R.E.; Fuerstenberg, S.I.; Tae, H.; Settlage, R.E.; Boore, J.L.; Posewitz, M.C. Draft genome sequence and genetic transformation of the oleaginous alga Nannochloropsis gaditana. Nat. Commun. 2013, 4, 2356. [Google Scholar] [CrossRef]
- Radakovits, R.; Jinkerson, R.E.; Darzins, A.; Posewitz, M.C. Genetic engineering of algae for enhanced biofuel production. Eukaryot. Cell 2010, 9, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Guo, X.; Wan, X.; Liang, Z.; Jiang, M. Characterization of a novel thioesterase (PtTE) from Phaeodactylum tricornutum. J. Basic Microbiol. 2011, 51, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Trentacoste, E.M.; Shrestha, R.P.; Smith, S.R.; Gle, C.; Hartmann, A.C.; Hildebrand, M.; Gerwick, W.H. Metabolic engineering of lipid catabolism increases microalgal lipid accumulation without compromising growth. Proc. Natl. Acad. Sci. USA 2013, 110, 19748–19753. [Google Scholar] [CrossRef]
- Parker, K. Metabolic Network Construction Based on the Genome of the Marine Diatom Thalassiosira pseudonana and the Analysis of Genome-wide Transcriptome Data to Investigate Triacylglyceride Accumulation. M.Sc. Thesis, Moss Landing Marine Labs, San Jose State University, San Jose, CA, USA, 2013. [Google Scholar]
- Daboussi, F.; Leduc, S.; Maréchal, A.; Dubois, G.; Guyot, V.; Perez-Michaut, C.; Amato, A.; Falciatore, A.; Juillerat, A.; Beurdeley, M.; et al. Genome engineering empowers the diatom Phaeodactylum tricornutum for biotechnology. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.E.; Dunlop, M.J. Synthetic feedback loop model for increasing microbial biofuel production using a biosensor. Front. Microbiol. 2012, 3, 360. [Google Scholar] [CrossRef] [PubMed]
- Foo, J.L.; Jensen, H.M.; Dahl, R.H.; George, K.; Keasling, J.D.; Lee, T.S.; Leong, S.; Mukhopadhyay, A. Improving microbial biogasoline production in Escherichia coli using tolerance engineering. mBio 2014, 5, e0193214. [Google Scholar] [CrossRef]
- Jin, H.; Chen, L.; Wang, J.; Zhang, W. Engineering biofuel tolerance in non-native producing microorganisms. Biotechnol. Adv. 2014, 32, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Heydarizadeh, P.; Marchand, J.; Chenais, B.; Sabzalian, M.R.; Zahedi, M.; Moreau, B.; Schoefs, B. Functional investigations in diatoms need more than a transcriptomic approach. Diatom Res. 2014, 29, 75–89. [Google Scholar] [CrossRef]
- Kutchan, T.M. Predictive metabolic engineering in plants: Still full of surprises. Trends Biotechnol. 2005, 23, 381–383. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, Y.Q.; Wu, N.; Lan, C.Q. CO2 bio-mitigation using microalgae. Appl. Microbiol. Biotechnol. 2008, 79, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H.; Takatsuka, Y. Mechanisms of RND multidrug efflux pumps. BBA-Proteins Proteomics 2009, 1794, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Higgins, C.F. ABC transporters: From microorganisms to man. Annu. Rev. Cell Biol. 1992, 8, 67–113. [Google Scholar] [CrossRef] [PubMed]
- Rees, D.C.; Johnson, E.; Lewinson, O. ABC transporters: The power to change. Nat. Rev. Mol. Cell Biol. 2009, 10, 218–227. [Google Scholar] [CrossRef]
- Reyes, L.H.; Almario, M.P.; Kao, K.C. Genomic library screens for genes involved in n-butanol tolerance in Escherichia coli. PLoS ONE 2011, 6, e17678. [Google Scholar] [CrossRef] [PubMed]
- Tréguer, P.; Nelson, D.M.; van Bennekom, A.J.; DeMaster, D.J.; Leynaert, A.; Quéguiner, B. The silica balance in the world ocean: A reestimate. Science 1995, 268, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Falkowski, P.G.; Katz, M.E.; Knoll, A.H.; Quigg, A.; Raven, J.A.; Schofield, O.; Taylor, F.J. The evolution of modern eukaryotic phytoplankton. Science 2004, 305, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Sims, P.A.; Mann, D.G.; Medlin, L.K. Evolution of the diatoms: Insights from fossil, biological and molecular data. Phycologia 2006, 45, 361–402. [Google Scholar] [CrossRef] [Green Version]
- Moustafa, A.; Beszteri, B.; Maier, U.G.; Bowler, C.; Valentin, K.; Bhattacharya, D. Genomic footprints of a cryptic plastid endosymbiosis in diatoms. Science 2009, 324, 1724–1726. [Google Scholar] [CrossRef] [PubMed]
- Becker, B.; Hoef-Emden, K.; Melkonian, M. Chlamydial genes shed light on the evolution of photoautotrophic eukaryotes. BMC Evol. Biol. 2008, 8. Article Number 203. [Google Scholar] [CrossRef]
- Armbrust, E.V.; Berges, J.A.; Bowler, C.; Green, B.R.; Martinez, D.; Putnam, N.H.; Zhou, S.; Allen, A.E.; Apt, K.E.; Bechner, M.; et al. The genome of the diatom Thalassiosira pseudonana: Ecology, evolution, and metabolism. Science 2004, 306, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Kociolek, J.P. Diatoms: Unique eukaryotic extremophiles providing insights into planetary change. Proc. Soc. Photo-Opt. Instrum. Eng. (SPIE) 2007, 6694, 66940S. [Google Scholar]
- Bertrand, M.; Schoefs, B.; Siffel, P.; Rohacek, K.; Molnar, I. Cadmium inhibits epoxidation of diatoxanthin to diadinoxanthin in the xanthophyll cycle of the marine diatom Phaeodactylum tricornutum. FEBS Lett. 2001, 508, 153–156. [Google Scholar] [CrossRef]
- Nguyen-Deroche, T.L.N.; Caruso, A.; Le, T.T.; Bui, T.V.; Schoefs, B.; Tremblin, G.; Morant-Manceau, A. Zinc affects differently growth, photosynthesis, antioxidant enzyme activities and phytochelatin synthase expression of four marine diatoms. Sci. World J. 2012, 2012, 982957. [Google Scholar] [CrossRef]
- Masmoudi, S.; Nguyen-Deroche, N.; Caruso, A.; Ayadi, H.; Morant-Manceau, A.; Tremblin, G.; Bertrand, M.; Schoefs, B. Cadmium, copper, sodium and zinc effects on diatoms: From heaven to hell—A review. Cryptogam. Algol. 2013, 34, 185–225. [Google Scholar] [CrossRef]
- Rohacek, K.; Bertrand, M.; Moreau, B.; Jacquette, B.; Caplat, C.; Morant-Manceau, A.; Schoefs, B. Relaxation of the non-photochemical chlorophyll fluorescence quenching in diatoms: Kinetics, components and mechanisms. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369S1, 20130241. [Google Scholar] [CrossRef]
- Cheng, R.-l.; Feng, J.; Zhang, B.-X.; Huang, Y.; Cheng, J.; Zhang, C.-X. Transcriptome and gene expression analysis of an oleaginous diatom under different salinity conditions. Bioenergy Res. 2014, 7, 192–205. [Google Scholar] [CrossRef]
- Gacheva, G.V.; Gigova, L.G. Biological activity of microalgae can be enhanced by manipulating the cultivation temperature and irradiance. Cent. Eur. J. Biol. 2014, 9, 1168–1181. [Google Scholar] [CrossRef]
- Maeda, Y.; Sunaga, Y.; Yoshino, T.; Tanaka, T. Oleosome-associated protein of the oleaginous diatom Fistulifera solaris contains an endoplasmic reticulum-targeting signal sequence. Mar. Drugs 2014, 12, 3892–3903. [Google Scholar] [CrossRef] [PubMed]
- Burrows, E.; Bennette, N.; Carrieri, D.; Dixon, J.; Brinker, A.; Frada, M.; Baldassano, S.; Falkowski, P.; Dismukes, G. Dynamics of lipid biosynthesis and redistribution in the marine diatom Phaeodactylum tricornutum under nitrate deprivation. BioEnergy Res. 2012, 5, 876–885. [Google Scholar] [CrossRef]
- Taguchi, S.; Hirata, J.A.; Laws, E.A. Silicate deficiency and lipid synthesis of marine diatoms. J. Phycol. 1987, 23, 260–267. [Google Scholar] [CrossRef]
- Zhang, L.; Han, J.C.; Yang, G.P.; Zhu, B.H.; Pan, K.H. Association of triacylglyceride content and transcript abundance of genes involving in lipid synthesis of nitrogen deficient Phaeodactylum tricornutum. Chin. J. Oceanol. Limnol. 2014, 32, 397–402. [Google Scholar] [CrossRef]
- Badour, S.S.; Gergis, M.S. Cell division and fat accumulation in Nitzschia sp. grown in continuously illuminated mass cultures. Arch. Mikrobiol. 1965, 51, 94–102. [Google Scholar] [CrossRef]
- Beattie, A.; Percival, E.; Hirst, E.L. Studies on the metabolism of the Chrysophyceae. Comparative structural investigations on leucosin (chrysolaminarin) separated from diatoms and laminarin from the brown algae. Biochem. J. 1961, 79, 531–537. [Google Scholar] [PubMed]
- Huang, B.; Marchand, J.; Moreau, B.; Lukomwska, E.; Bougaran, G.; Cadoret, J.-P.; Schoefs, B. Impact de l’approvisionnement en CO2 sur l’utilisation du carbone chez la diatomée Phaeodactylum tricornutum. In Book of Abstracts of the 33ème Colloque de l’Association des Diatomistes de Langue Française; l’Association des Diatomistes de Langue française: Thonon-les-Bains, France, 2014. [Google Scholar]
- Ho, S.-H.; Huang, S.-W.; Chen, C.-Y.; Hasunuma, T.; Kondo, A.; Chang, J.-S. Characterization and optimization of carbohydrate production from an indigenous microalga Chlorella vulgaris FSP-E. Bioresour. Technol. 2013, 135, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Thomas, W.H.; Dodson, A.N.; Reid, F.M.H. Diatom productivity compared to other algae in natural marine phytoplankton assemblages. J. Phycol. 1978, 14, 250–253. [Google Scholar] [CrossRef]
- Jungandreas, A.; Costa, B.S.; Jakob, T.; von Bergen, M.; Baumann, S.; Wilhelm, C. The acclimation of Phaeodactylum tricornutum to blue and red light does not influence the photosynthetic light reaction but strongly disturbs the carbon allocation pattern. PLoS ONE 2014, 9, e99727. [Google Scholar] [CrossRef] [PubMed]
- Schoefs, B.; Rmiki, N.-E.; Rachadi, J.; Lemoine, Y. Astaxanthin accumulation in Haematococcus requires a cytochrome P450 hydroxylase and an active synthesis of fatty acids. FEBS Lett. 2001, 500, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Klok, A.J.; Martens, D.E.; Wijffels, R.H.; Lamers, P.P. Simultaneous growth and neutral lipid accumulation in microalgae. Bioresour. Technol. 2013, 134, 233–243. [Google Scholar] [CrossRef]
- Yatsu, L.Y.; Jacks, T.J.; Hensarling, T.P. Hensarli.Tp Isolation of spherosomes (oleosomes) from onion, cabbage, and cottonseed tissues. Plant Physiol. 1971, 48, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Davidi, L.; Katz, A.; Pick, U. Characterization of major lipid droplet proteins from Dunaliella. Planta 2012, 236, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Nojima, D.; Yoshino, T.; Maeda, Y.; Tanaka, M.; Nemoto, M.; Tanaka, T. Proteomics analysis of oil body-associated proteins in the oleaginous diatom. J. Proteome Res. 2013, 12, 5293–5301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Q.; Dubacq, J.P.; Alfsen, A. Biochemical and cytological evidence for the stimulation of clathrin-coated (vesicle) formation by exogenous folic acid in Dunaliella salina (Chlorophyta). J. Phycol. 1993, 29, 203–209. [Google Scholar] [CrossRef]
- Hamano, S. On the oil of the living egg of salmon, Oncorhynchus keta. Bull. Jpn. Soc. Sci. Fish. 1951, 17, 47–52. [Google Scholar] [CrossRef]
- Targett-Adams, P.; Chambers, D.; Gledhill, S.; Hope, R.G.; Coy, J.F.; Girod, A.; McLauchlan, J. Live cell analysis and targeting of the lipid droplet-binding adipocyte differentiation-related protein. J. Biol. Chem. 2003, 278, 15998–16007. [Google Scholar] [CrossRef] [PubMed]
- Bostrom, P.; Rutberg, M.; Ericsson, J.; Holmdahl, P.; Andersson, L.; Frohman, M.A.; Boren, J.; Olofsson, S.O. Cytosolic lipid droplets increase in size by microtubule-dependent complex formation. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1945–1951. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, M.; Uchida, T.; Gohara, K. Temporal and spatial variations of lipid droplets during adipocyte division and differentiation. J. Lipid Res. 2007, 48, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Thayil, A.; Jesacher, A.; Grieve, K.; Debarre, D.; Wilson, T.; Booth, M.; Srinivas, S. Characterisation of the dynamic behaviour of lipid droplets in the early mouse embryo using adaptive harmonic generation microscopy. BMC Cell Biol. 2010, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Dou, W.; Zhang, D.; Jung, Y.; Cheng, J.-X.; Umulis, D.M. Label-free imaging of lipid-droplet intracellular motion in early Drosophila embryos using femtosecond-stimulated Raman loss microscopy. Biophys. J. 2012, 102, 1666–1675. [Google Scholar] [CrossRef] [PubMed]
- Long, A.P.; Manneschmidt, A.K.; VerBrugge, B.; Dortch, M.R.; Minkin, S.C.; Prater, K.E.; Biggerstaff, J.P.; Dunlap, J.R.; Dalhaimer, P. Lipid droplet de novo formation and fission are linked to the cell cycle in fission yeast. Traffic 2012, 13, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Dalhaimer, P. Lipid droplet organelle distribution in populations of dividing cells studied by simulation. Phys. Biol. 2013, 10, 036007. [Google Scholar] [CrossRef] [PubMed]
- Baffes, J.; Kose, A.; Ohnsorge, F.; Stocker, M.; Chen, D.; Cosic, D.; Gong, X.; Huidrom, R.; Vashakmadze, E.; Zhang, J.; et al. Understanding the plunge in oil prices: Sources and implications. In Global Economic Prospects: Having Fiscal Space and Using It; World Bank Publications: Washington, DC, USA, 2015; pp. 155–168. [Google Scholar]
- Baumeister, C.; Kilian, L. Understanding the decline in the price of oil since June 2014. Available online: http://papers.ssrn.com/sol3/papers.cfm?abstract_id=2557316 (accessed on 21 April 2015).
- Rahemtulla, K. World Manipulated Into Buying Saudi Oil. Available online: http://www.wallstreetdaily.com/2014/11/07/u-s-saudi-arabia-oil/ (accessed on 21 April 2015).
- Sabino, A.M. New York fracking outlook still gloomy. Nat. Gas Electr. 2015, 31, 20–25. [Google Scholar] [CrossRef]
- Golden, J.M.; Wiseman, H.J. The fracking revolution: Shale gas as a case study in innovation policy. Emory Law J. 2015, 64, 955–1040. [Google Scholar]
- Dale, B.E.; Anderson, J.E.; Brown, R.C.; Csonka, S.; Dale, V.H.; Herwick, G.; Jackson, R.D.; Jordan, N.; Kaffka, S.; Kline, K.L. Take a closer look: Biofuels can support environmental, economic and social goals. Environ. Sci. Technol. 2014, 48, 7200–7203. [Google Scholar] [CrossRef] [PubMed]
- Gendy, T.S.; El-Temtamy, S.A. Commercialization potential aspects of microalgae for biofuel production: An overview. Egypt. J. Pet. 2013, 22, 43–51. [Google Scholar] [CrossRef]
- Aluya, J. Biofuels has become disruptive technology to the energy market. Biofuels 2015, 5, 457–467. [Google Scholar] [CrossRef]
- Kirkby, J.; Carslaw, K.S. Variations of galactic cosmic rays and the Earth’s climate. In Solar Journey: The Significance of Our Galactic Environment for the Heliosphere and Earth: The Significance of Our Galactic Environment for the Heliosphere and Earth; Frisch, P.C., Ed.; Springer: Dordrecht, The Netherlands, 2006; pp. 349–397. [Google Scholar]
- Haigh, J.D. Solar variability and climate. In Space Weather; Lilensten, J., Ed.; Springer: The Netherlands, 2007; Volume 344, pp. 65–81. [Google Scholar]
- Hanslmeier, A. Space weather and climate. In The Sun and Space Weather; Springer: The Netherlands, 2007; Volume 347, pp. 143–173. [Google Scholar]
- Previdi, M.; Polvani, L.M. Climate system response to stratospheric ozone depletion and recovery. Q. J. R. Meteorol. Soc. 2014, 140, 2401–2419. [Google Scholar] [CrossRef]
- O’Riordan, T. Fracking, sustainability, and democracy. Environ. Sci. Policy Sustain. Dev. 2015, 57, 2–3. [Google Scholar]
- Ipsen, D. The lifecycle of oil: Problems and conflicts. In Competition and Conflicts on Resource Use; Hartard, S., Liebert, W., Eds.; Springer: Switzerland, 2015; pp. 61–74. [Google Scholar]
- Upham, P.; Dendler, L. Scientists as policy actors: A study of the language of biofuel research. Environ. Sci. Policy 2015, 47, 137–147. [Google Scholar] [CrossRef]
- Fresewinkel, M.; Rosello, R.; Wilhelm, C.; Kruse, O.; Hankamer, B.; Posten, C. Integration in microalgal bioprocess development: Design of efficient, sustainable, and economic processes. Eng. Life Sci. 2014, 14, 560–573. [Google Scholar] [CrossRef]
- Murphy, D.J. The implications of the declining energy return on investment of oil production. Philos. Trans. R. Soc. Lond. A Math. Phys. Eng. Sci. 2014, 372, 20130126. [Google Scholar] [CrossRef]
- Shen, Y. Carbon dioxide bio-fixation and wastewater treatment via algae photochemical synthesis for biofuels production. RSC Adv. 2014, 4, 49672–49722. [Google Scholar] [CrossRef]
- Wikipedia Artificial photosynthesis. Available online: http://en.wikipedia.org/wiki/Artificial_photosynthesis (accessed on 21 April 2015).
- Mandal, S.; Mallick, N. Microalga Scenedesmus obliquus as a potential source for biodiesel production. Appl. Microbiol. Biotechnol. 2009, 84, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, A. Integrated approach to algae production for biofuel utilizing robust algal species. In The Science of Algal Fuels: Phycology, Geology, Biophotonics, Genomics and Nanotechnology; Gordon, R., Seckbach, J., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 83–100. [Google Scholar]
- Topf, M.; Tavasi, M.; Kinel-Tahan, Y.; Iluz, D.; Dubinsky, Z.; Yehoshua, Y. Algal oils: Biosynthesis and uses. In The Science of Algal Fuels: Phycology, Geology, Biophotonics, Genomics and Nanotechnology; Gordon, R., Seckbach, J., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 193–214. [Google Scholar]
- Shifrin, N.S.; Chisholm, S.W. Phytoplankton lipids: Interspecific differences and effects of nitrate, silicate and light-dark cycles. J. Phycol. 1981, 17, 374–384. [Google Scholar] [CrossRef]
- Chen, Y.-C. The biomass and total lipid content and composition of twelve species of marine diatoms cultured under various environments. Food Chem. 2012, 131, 211–219. [Google Scholar] [CrossRef]
- Pittman, J.K.; Dean, A.P.; Osundeko, O. The potential of sustainable algal biofuel production using wastewater resources. Bioresour. Technol. 2011, 102, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, D.M.; Chanakya, H.; Ramachandra, T. Euglena sp. as a suitable source of lipids for potential use as biofuel and sustainable wastewater treatment. J. Appl. Phycol. 2013, 25, 855–865. [Google Scholar] [CrossRef]
- Mahapatra, D.M.; Chanakya, H.N.; Ramachandra, T.V. Bioremediation and lipid synthesis through mixotrophic algal consortia in municipal wastewater. Bioresour. Technol. 2014, 168, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Fathi, A.A.; Azooz, M.M.; Al-Fredan, M.A. Phycoremediation and the potential of sustainable algal biofuel production using wastewater. Am. J. Appl. Sci. 2013, 10, 189–194. [Google Scholar] [CrossRef]
- Chanakya, H.N.; Mahapatra, D.M.; Sarada, R.; Abitha, R. Algal biofuel production and mitigation potential in India. Mitig. Adapt. Strateg. Glob. Chang. 2013, 18, 113–136. [Google Scholar] [CrossRef]
- Brownbridge, G.; Azadi, P.; Smallbone, A.; Bhave, A.; Taylor, B.; Kraft, M. The future viability of algae-derived biodiesel under economic and technical uncertainties. Bioresour. Technol. 2014, 151, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Mobile Algae Refinery: VALORIE, the Versatile ALgae On-site Raw Ingredient Extractor. Available online: https://www.youtube.com/watch?v=gkoxA74q25c (accessed on 21 April 2015).
- Wikipedia Multi-objective optimization. Available online: https://en.wikipedia.org/wiki/Multi-objective_optimization (accessed on 21 April 2015).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinayak, V.; Manoylov, K.M.; Gateau, H.; Blanckaert, V.; Hérault, J.; Pencréac'h, G.; Marchand, J.; Gordon, R.; Schoefs, B. Diatom Milking: A Review and New Approaches. Mar. Drugs 2015, 13, 2629-2665. https://doi.org/10.3390/md13052629
Vinayak V, Manoylov KM, Gateau H, Blanckaert V, Hérault J, Pencréac'h G, Marchand J, Gordon R, Schoefs B. Diatom Milking: A Review and New Approaches. Marine Drugs. 2015; 13(5):2629-2665. https://doi.org/10.3390/md13052629
Chicago/Turabian StyleVinayak, Vandana, Kalina M. Manoylov, Hélène Gateau, Vincent Blanckaert, Josiane Hérault, Gaëlle Pencréac'h, Justine Marchand, Richard Gordon, and Benoît Schoefs. 2015. "Diatom Milking: A Review and New Approaches" Marine Drugs 13, no. 5: 2629-2665. https://doi.org/10.3390/md13052629