Chemical and Genetic Diversity of Nodularia spumigena from the Baltic Sea
Abstract
:1. Introduction
2. Results and Discussion
2.1. Non-Ribosomal Peptides and Peptide Profiles of Baltic Nodularia spumigena
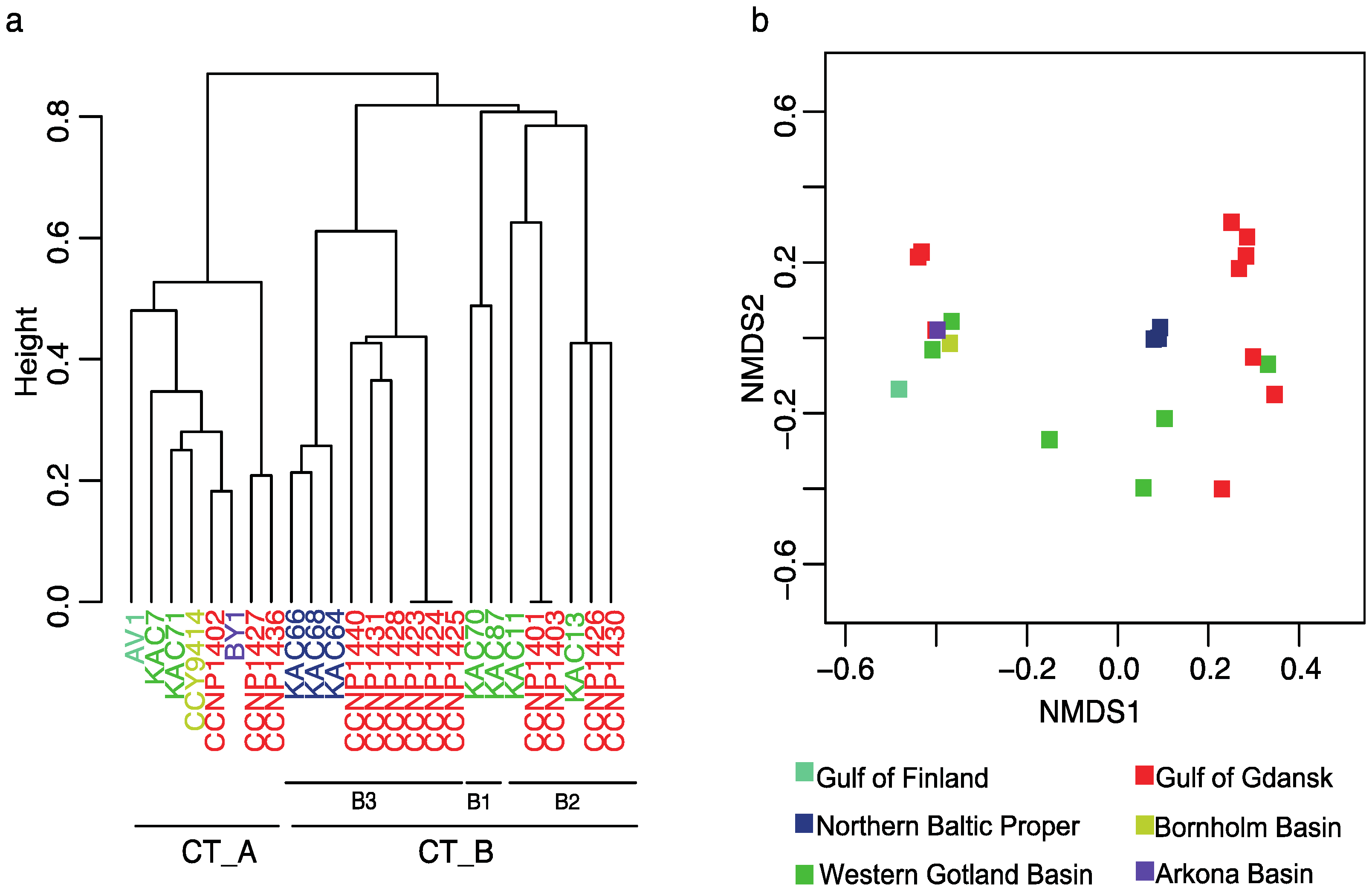
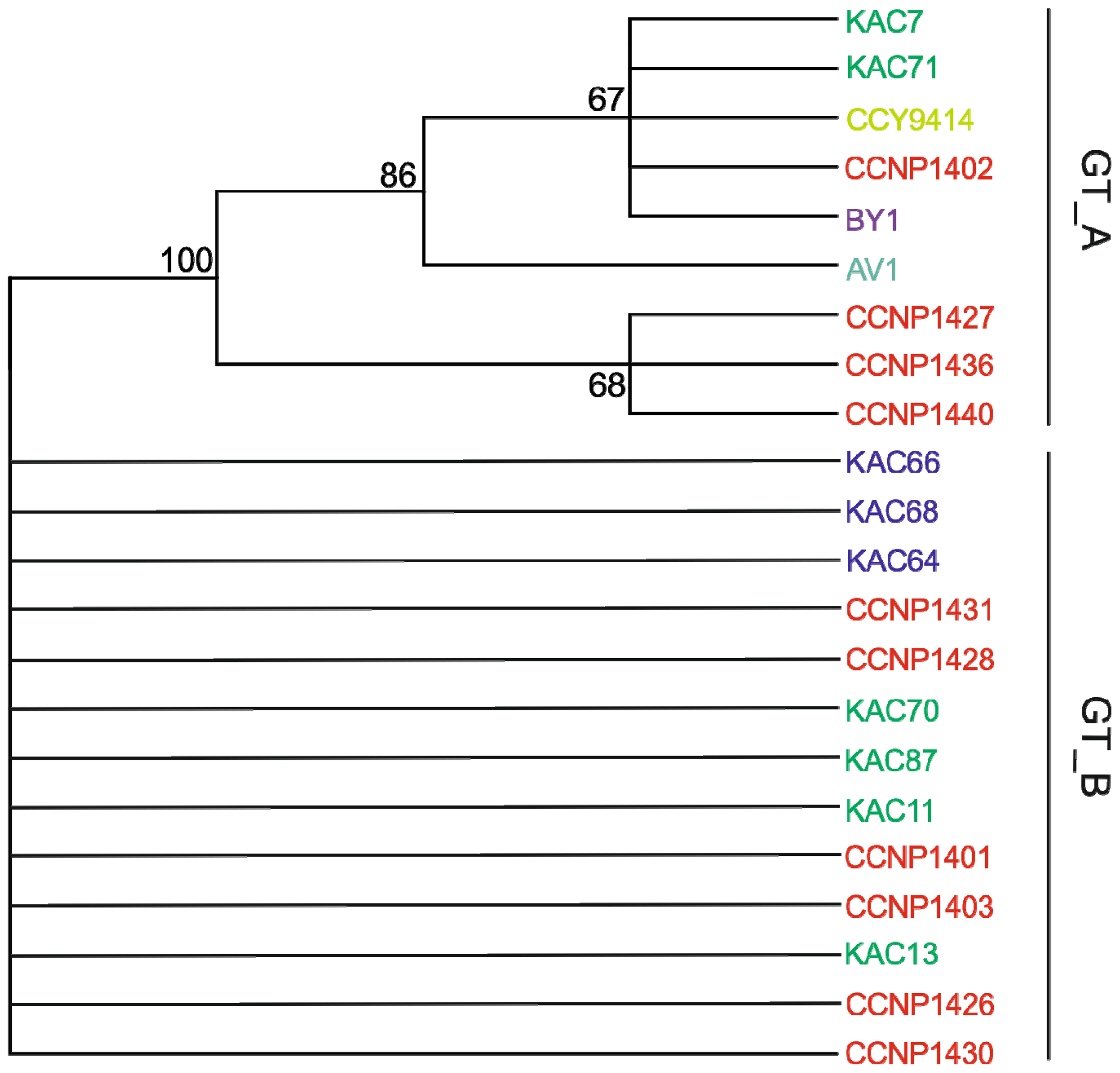
2.2. PC-IGS Sequences and Phylogenetic Analyses of Baltic Nodularia spumigena
2.3. N. spumigena Chemotypes vs. Genotypes
3. Materials and Methods
3.1. Cultivation of Nodularia spumigena Strains
3.2. Extraction and LC-MS/MS Analysis of Peptides
3.3. DNA Extraction and PC-IGS Sequencing
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AcSer | acetylserine |
| Agm | agmatine |
| Bu | butanoic acid |
| Hex | hexanoic acid |
| Hpla | hydroxyphenyl lactic acid |
| Hph | homophenylalanine |
| Hty | homotyrosine |
| MePro | metylproline |
| MetO | methionine sufloxide |
| Met(O2) | methionine sulfone |
| MeHty | methylhomotyrosine |
| Oct | octanoic acid |
References
- Jones, G.J.; Blackburn, S.I.; Parker, N.S. A toxic bloom of Nodularia spumigena Mertens in Orielton Lagoon, Tasmania. Aust. J. Mar. Freshw. Res. 1994, 45, 787–800. [Google Scholar] [CrossRef]
- John, J.; Kemp, A. Cyanobacterial blooms in the wetlands of the Perth region, taxonomy and distribution: An overview. J. R. Soc. West. Aust. 2006, 89, 51–56. [Google Scholar]
- McGregor, G.B.; Stewart, I.; Sendall, B.C.; Sadler, R.; Reardon, K.; Carter, S.; Wruck, D.; Wickramasinghe, W. First report of a toxic Nodularia spumigena (Nostocales/Cyanobacteria) bloom in sub-tropical Australia. Phycological and public health investigations. Int. J. Environ. Res. Public Health 2012, 9, 2396–2411. [Google Scholar] [CrossRef] [PubMed]
- Sivonen, K.; Kononen, K.; Carmichael, W.; Dahlem, A.M.; Rinehart, K.L.; Kiviranta, J.; Niemela, S.I. Occurrence of the hepatotoxic cyanobacterium Nodularia spumigena in the Baltic Sea and structure of the toxin. Appl. Environ. Microbiol. 1989, 55, 1990–1995. [Google Scholar] [PubMed]
- Karjalainen, M.; Engström-Öst, J.; Korpinen, S.; Peltonen, H.; Pääkkönen, J.P.; Rönkkönen, S.; Suikkanen, S.; Viitasalo, M. Ecosystem consequences of cyanobacteria in the northern Baltic Sea. AMBIO 2007, 36, 195–202. [Google Scholar] [CrossRef]
- Sotton, B.; Domaizon, I.; Anneville, O.; Cattanéo, F.; Guillard, J. Nodularin and cylindrospermopsin: A review of their effects on fish. Rev. Fish Biol. Fisher. 2015, 25, 1–19. [Google Scholar] [CrossRef]
- Mazur-Marzec, H.; Kaczkowska, M.J.; Błaszczyk, A.; Akcaalan, R.; Spoof, L.; Meriluoto, J. Diversity of peptides produced by Nodularia spumigena from various geographical regions. Mar. Drugs 2013, 11, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Lehtimäki, J.; Lyra, C.; Suomalainen, S.; Sundman, P.; Rouhiainen, L.; Paulin, L.; Salkinoja-Salonen, M.; Sivonen, K. Characterization of Nodularia strains, cyanobacteria from brackish waters, by genotypic and phenotypic methods. Int. J. Syst. Evol. Microbiol. 2000, 50, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Congestri, R.; Capucci, E.; Albertano, P. Morphometric variability of the genus Nodularia (Cyanobacteria) in Baltic natural communities. Aquat. Microb. Ecol. 2003, 32, 251–259. [Google Scholar] [CrossRef]
- Komárek, J.; Hübel, M.; Hübel, H.; Šmarda, J. The Nodularia studies 2. Taxonomy. Algol. Stud. 1993, 68, 1–25. [Google Scholar]
- Laamanen, M.J.; Gugger, M.F.; Lehtimäki, J.; Haukka, K.; Sivonen, K. Diversity of toxic and non-toxic Nodularia isolates (Cyanobacteria) and filaments from the Baltic Sea. Appl. Environ. Microbiol. 2001, 67, 4638–4647. [Google Scholar] [CrossRef] [PubMed]
- Barker, G.L.; Handley, B.A.; Vacharapiyasophon, P.; Stevens, J.R.; Hayes, P.K. Allele-specific PCR shows that genetic exchange occurs among genetically diverse Nodularia (Cyanobacteria) filaments in the Baltic Sea. Microbiology 2000, 146, 2865–2875. [Google Scholar] [CrossRef] [PubMed]
- Řeháková, K.; Mareš, J.; Lukkešová, A.; Zapomělová, E.; Bernardová, K.; Hrouzek, P. Nodularia (Cyanobacteria, Nostocaceae): A phylogenetically uniform genus with variable phenotypes. Phytotaxa 2014, 172, 235–246. [Google Scholar] [CrossRef]
- Bertos-Fortis, M.; Klotz, F.; Mazur-Marzec, H.; Legrand, C. Phenotypic plasticity in brackish water cyanobacteria: Survival at any cost under salinity changes. Evolution 2016. submitted. [Google Scholar]
- Moffitt, M.C.; Neilan, B.A. Characterization of the nodularin synthetase gene cluster and proposed theory of the evolution of cyanobacterial hepatotoxins. Appl. Environ. Microbiol. 2004, 70, 6353–6362. [Google Scholar] [CrossRef] [PubMed]
- Rouhiainen, L.; Jokela, J.; Fewer, D.P.; Urmann, M.; Sivonen, K. Two alternative starter modules for the non-ribosomal biosynthesis of specific anabaenopeptin variants in Anabaena (Cyanobacteria). Chem. Biol. 2010, 17, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.; Kehr, J.K.; Mainz, A.; Dehm, D.; Petras, D.; Süssmuth, R.D.; Dittmann, E. Biochemical dissection of the natural diversification of microcystin provides lessons for synthetic biology of NRPS. Cell. Chem. Biol. 2016, 23, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Amoutzias, G.D.; Chaliotis, A.; Mossialos, D. Discovery strategies of bioactive compounds synthesized by nonribosomal peptide synthetases and type-I polyketide synthases derived from marine microbiomes. Mar. Drugs 2016, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Briand, E.; Bormans, M.; Gugger, M.; Dorrestein, P.C.; Gerwick, W.H. Changes in secondary metabolic profiles of Microcystis aeruginosa strains in response to intraspecific interactions. Environ. Microbiol. 2016, 18, 384–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujii, K.; Sivonen, K.; Adachi, K.; Noguchi, K.; Shimizu, Y.; Sano, H.; Hirayama, K.; Suzuki, M.; Harada, K.-I. Comparative study of toxic and non-toxic cyanobacterial products: Novel peptides from toxic Nodularia spumigena AV1. Tetrahedron Lett. 1997, 38, 5529–5532. [Google Scholar] [CrossRef]
- Fewer, D.P.; Jokela, J.; Rouhiainen, L.; Wahlsten, M.; Koskenniemi, K.; Stal, L.; Sivonen, K. The non-ribosomal assembly and frequent occurrence of the protease inhibitor spumigin in the bloom-forming cyanobacterium Nodularia spumigena. Mol. Microbiol. 2009, 73, 924–937. [Google Scholar] [CrossRef] [PubMed]
- Fewer, D.P.; Jokela, J.; Paukku, E.; Österholm, J.; Wahlsten, M.; Permi, P.; Aitio, O.; Rouhiainen, L.; Gomeez-Saez, G.V.; Sivonen, K. New structural variants of aeruginosins produced by the toxic bloom forming cyanobacterium Nodularia spumigena. PLoS ONE 2013, 8, e73618. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Budnjo, A.; Jokela, J.; Haug, B.E.; Fewer, D.P.; Wahlsten, M.; Rouhiainen, L.; Permi, P.; Fossen, T.; Sivonen, K. Pseudoaeruginosis, non-ribosomal peptides in Nodularia spumigena. ACS Chem. Biol. 2014, 10, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, K.L.; Harada, K.I.; Namikoshi, M.; Chen, C.; Harvis, C.A.; Munroe, M.H.G.; Blunt, J.W.; Mulligan, P.E.; Beasley, V.R.; Dahlem, A.M.; et al. Nodularin, microcystin and the configuration of Adda. J. Am. Chem. Soc. 1988, 110, 8557–8558. [Google Scholar] [CrossRef]
- Spoof, L.; Błaszczyk, A.; Meriluoto, J.; Cegłowska, M.; Mazur-Marzec, H. Structures and activity of new anabaenopeptins produced by Baltic Sea cyanobacteria. Mar. Drugs 2016, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Welker, M.; Christiansen, G.; van Döhren, H. Diversity of coexisting Planktothrix (Cyanobacteria) chemotypes deduced by mass spectral analysis of microcystins and other oligopeptides. Arch. Microbiol. 2004, 182, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.; Saker, M.L.; Moreira, C.; Welker, M.; Fastner, J.; Vasconcelos, V.M. Peptide diversity in strains of the cyanobacterium Microcystis aeruginosa isolated from Portuguese water supplies. Appl. Microbiol. Biotechnol. 2009, 82, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Rohrlack, T.; Skulberg, R.; Skulberg, O.M. Distribution of oligopeptidechemotypes of the cyanobacterium Planktothrix and their persistence in selected lakes in Fennoscandia. J. Phycol. 2009, 44, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Kurmayer, R.; Blom, J.F.; Deng, L.; Pernthaler, J. Integrating phylogeny, geographic niche partitioning and secondary metabolite synthesis in bloom-forming Planktothrix. ISME J. 2015, 9, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Agha, R.; Quesada, A. Oligopeptides as biomarkers of cyanobacterial subpopulations. Toward an understanding of their biological role. Toxins 2014, 6, 1929–1950. [Google Scholar] [CrossRef] [PubMed]
- Neilan, B.A.; Jacobs, D.; Goodman, A.E. Genetic Diversity and phylogeny of toxic cyanobacteria determined by DNA polymorphisms within the phycocyanin locus. Appl. Environ. Microbiol. 1995, 61, 3875–3883. [Google Scholar] [PubMed]
- Voß, B.; Bolhuis, H.; Fewer, D.P.; Kopf, M.; Möke, F.; Haas, F.; El-Shehawy, R.; Hayes, P.; Bergman, B.; Sivonen, K.; et al. Insights into the physiology and ecology of the brackish-water-adapted cyanobacterium Nodularia spumigena CCY9414 based on a genome-transcriptome analysis. PLoS ONE 2013, 8, e60224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ersmark, K.; Del Valle, J.R.; Hanessian, S. Chemistry and Biology of the aeruginosin family of serine protease inhibitors. Angew. Chem. Int. Ed. 2008, 47, 1202–1223. [Google Scholar] [CrossRef] [PubMed]
- Czarnecki, O.; Henning, M.; Lippert, I.; Welker, M. Identyfication of peptide metabolites of Microcystis (Cyanobacteria) that inhibit trypsin-like activity in planktonic herbivorous Daphnia (Cladocera). Environ. Microbiol. 2006, 8, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Okita, Y.; Matsuda, H.; Murakami, M. Aeruginosins, protease inhibitors from the cyanobacterium Microcystis aeruginosa. Tetrahedron 1999, 55, 10971–10988. [Google Scholar] [CrossRef]
- Mount, D.W. Bioinformatics: Sequence and Genome Analysis, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Habor, NY, USA, 2004. [Google Scholar]
- Bolch, C.J.S.; Orr, P.T.; Jones, G.J.; Blackburn, S.I. Genetic, morphological and toxicological variation among globally distributed strains of Nodularia (Cyanobacteria). J. Phycol. 1999, 35, 339–355. [Google Scholar] [CrossRef]
- Janson, S.; Granéli, E. Phylogenetic analyses of nitrogen-fixing cyanobacteria from Baltic Sea reveal sequence anomalies in the phycocyanin operon. Int. J. Syst. Evol. Microbiol. 2002, 52, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Lyra, C.; Laamanen, M.; Lehtimäki, J.M.; Surakka, A.; Sivonen, K. Benthic cyanobacteria of the genus Nodularia are non-toxic, without vacuoles, able to glide and genetically more diverse than planktonic Nodularia. Int. J. Syst. Evol. Microbiol. 2005, 55, 555–569. [Google Scholar] [CrossRef]
- Rounge, T.B.; Rohrlack, T.; Decenciere, B.; Edvardsen, B.; Kristensen, T.; Jakobsen, K.S. Subpopulation differentiation associated with nonribosomal peptide synthetase gene cluster dynamics in the cyanobacterium Planktothrix spp. J. Phycol. 2010, 46, 645–652. [Google Scholar] [CrossRef]
- Sogge, H.; Rohrlack, T.; Rounge, T.B.; Sønstebe, J.H.; Tooming-Klunderud, A.; Kristensen, T.; Jakobsen, K.S. Gene flow, recombination, and selection in cyanobacteria: Population structure of geographically related Planktothrix freshwater strains. Appl. Environ. Microbiol. 2013, 79, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Tooming-Klunderud, A.; Fewer, D.P.; Rohrlack, T.; Jokela, J.; Rouhiainen, L.; Sivonen, K.; Kristensen, T.; Jakobsen, K.J. Evidence for positive selection acting on microcystin synthetase adenylation domains in three cyanobacterial genera. BMC Evol. Biol. 2008, 8, 256. [Google Scholar] [CrossRef] [PubMed]
- Suikkanen, S.; Fistarol, G.O.; Granéli, E. Allelopathic effects of the Baltic cyanobacteria Nodularia spumigena, Aphanizomenon flos-aquae and Anabaena lemmermannii on algal monocultures. J. Exp. Mar. Biol. Ecol. 2004, 308, 85–101. [Google Scholar] [CrossRef]
- Kotai, J. Introduction for Preparation of Modified Nutrient Solution Z8 for Algae; Norwegian Institute for Water Research: Oslo, Norway, 1972; p. 5. [Google Scholar]
- CRAN—Package Vegan. Available online: http://CRAN.R-project.org/package=vegan (accessed on 5 November 2016).
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
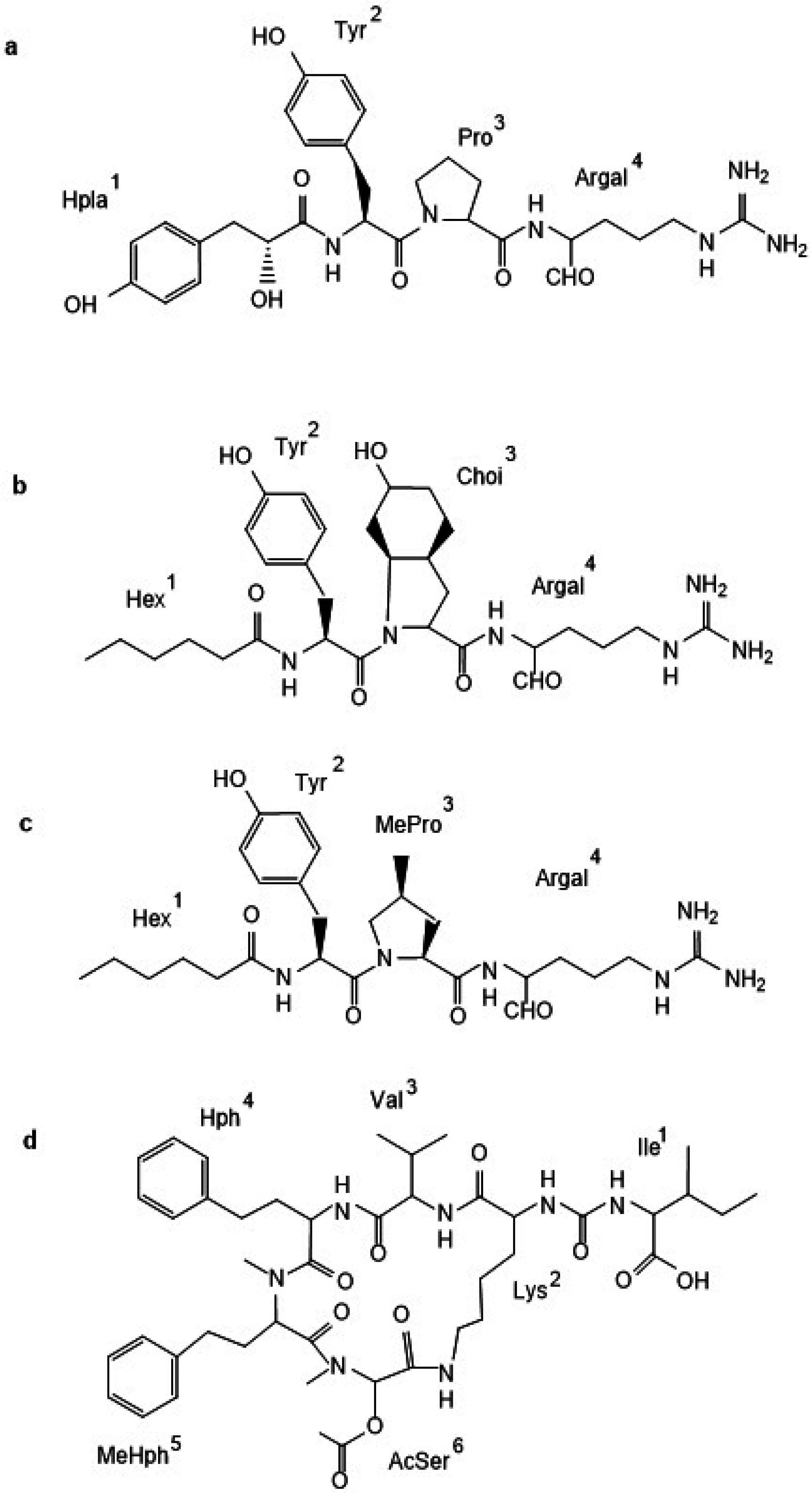
| Chemotype Cluster | N. spumigena Strain | Peptide m/z | Amino Acids Components | |||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||
| CT_A | CCNP1402, BY1, CCY9414, AV1, CCNP1427, CCNP1436, KAC7, KAC71 | 932 | Ile | Lys | MetO | Hph | MeHty | MetO |
| 916a | Ile | Lys | MetO | Hph | MeHty | Met | ||
| 914 | Ile | Lys | MetO | Hph | MeHty | AcSer | ||
| 900b | Ile | Lys | Met | Hph | MeHty | Met | ||
| 898a | Ile | Lys | Met | Hph | MeHty | AcSer | ||
| 898b | Ile | Lys | MetO | Hph | MeHph | AcSer | ||
| 884a | Ile | Lys | Met | Hph | MeHph | Met | ||
| 882a | Ile | Lys | Met | Hph | MeHph | AcSer | ||
| 882b | Ile | Lys | Ile | Hph | MeHty | Met | ||
| 880 | Ile | Lys | Ile | Hph | MeHty | AcSer | ||
| 872 | Ile | Lys | MetO | Hph | MeHty | Ser | ||
| 856a | Ile | Lys | Met | Hph | MeHty | Ser | ||
| *840 | Ile | Lys | Met | Hph | MeHph | Ser | ||
| CCNP1402, CCY9414, AV1, KAC71 | 930 | Ile | Lys | MetO2 | Hph | MeHty | AcSer | |
| CCNP1402, BY1, CCY9414, CCNP1427, CCNP1436, KAC7, KAC71 | 856b | Ile | Lys | MetO | Hph | MeHph | Ser | |
| CCNP1402, BY1, AV1, CCNP1427, CCNP1436, KAC7, KAC71 | *822 | Ile | Lys | Ile | Hph | MeHph | Ser | |
| KAC71 | 824 | Ile | Lys | Val | Hph | MeHty | Ser | |
| AV1 | 868 | Ile | Lys | Val | Hph | MeHty | Met | |
| 866 | Ile | Lys | Val | Hph | MeHty | AcSer | ||
| *864 | Ile | Lys | Ile | Hph | MeHph | AcSer | ||
| *850 | Ile | Lys | Val | Hph | MeHph | AcSer | ||
| CT_B1 | KAC70, KAC87 | 884c | Ile | Lys | Val | Hph | MeHty | MetO |
| 868 | Ile | Lys | Val | Hph | MeHty | Met | ||
| 866 | Ile | Lys | Val | Hph | MeHty | AcSer | ||
| *852 | Ile | Lys | Val | Hph | MeHph | Met | ||
| 824 | Ile | Lys | Val | Hph | MeHty | Ser | ||
| *808a | Ile | Lys | Val | Hph | MeHph | Ser | ||
| KAC87 | *850 | Ile | Lys | Val | Hph | MeHph | AcSer | |
| CT_B2 | KAC 11 | 808b | Ile | Lys | Ile | Hty | MeAla | Phe |
| 794 | Ile | Lys | Val | Hty | MeAla | Phe | ||
| CCNP1401, CCNP1403, CCNP1426, CCNP1430, KAC13 | 842 | Phe | Lys | Ile | Hty | MeAla | Phe | |
| 828 | Phe | Lys | Val | Hty | MeAla | Phe | ||
| CT_B3 | CCNP1440, CCNP1431, CCNP1428, CCNP1423, CCNP1424, CCNP1425, KAC64, KAC66, KAC68 | 934 | Phe | Lys | Val | Hty | MeHty | MetO |
| 918 | Phe | Lys | Val | Hph | MeHty | MetO | ||
| 902 | Phe | Lys | Val | Hph | MeHty | Met | ||
| 900a | Phe | Lys | Val | Hph | MeHty | AcSer | ||
| 884b | Phe | Lys | Val | Hph | MeHph | AcSer | ||
| CCNP1423, CCNP1424, CCNP1425, CCNP1428, CCNP1431, CCNP1440, KAC64, KAC66 | 916b | Phe | Lys | Val | Hty | MeHty | AcSer | |
| CCNP1431, CCNP1440, KAC64, KAC66, KAC68 | *886 | Phe | Lys | Val | Hph | MeHph | Met | |
| KAC66, KAC68 | 858 | Phe | Lys | Val | Hph | MeHTyr | Ser | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazur-Marzec, H.; Bertos-Fortis, M.; Toruńska-Sitarz, A.; Fidor, A.; Legrand, C. Chemical and Genetic Diversity of Nodularia spumigena from the Baltic Sea. Mar. Drugs 2016, 14, 209. https://doi.org/10.3390/md14110209
Mazur-Marzec H, Bertos-Fortis M, Toruńska-Sitarz A, Fidor A, Legrand C. Chemical and Genetic Diversity of Nodularia spumigena from the Baltic Sea. Marine Drugs. 2016; 14(11):209. https://doi.org/10.3390/md14110209
Chicago/Turabian StyleMazur-Marzec, Hanna, Mireia Bertos-Fortis, Anna Toruńska-Sitarz, Anna Fidor, and Catherine Legrand. 2016. "Chemical and Genetic Diversity of Nodularia spumigena from the Baltic Sea" Marine Drugs 14, no. 11: 209. https://doi.org/10.3390/md14110209






