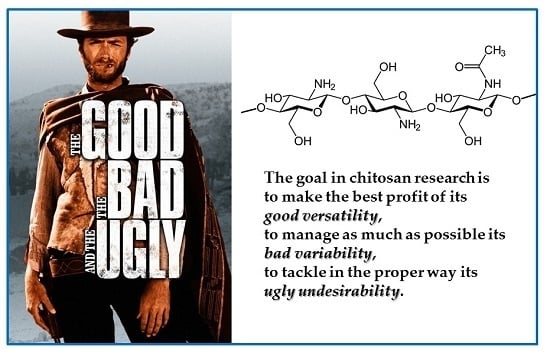
“The Good, the Bad and the Ugly” of Chitosans
Abstract
:1. Introduction
- Chitosan is a linear natural polymer of glucosamine/acetylglucosamine that behaves as a polyelectrolyte with positive charge density at low pH.
- Among the industrial polysaccharides, chitosan is an exception, being the only high molecular weight cationic polyelectrolyte, while polysaccharides are generally either neutral or anionic.
- Chitosan is often claimed to be GRAS (Generally Recognized As Safe) and bioabsorbable.
2. The Beginning
3. A critical Examination of the Physico-Chemical Properties of Chitosan Polymers
3.1. Chitosan as a Copolymer: Acetylation and Substitution
3.2. Chitosan Copolymers: Molecular Weight Determination and Conformation
3.3. Chitosan Solubility
4. Biocompatibility, Antimicrobial Activity and Toxicity (Chemistry vs. Material)
5. Applications of Bacteriostatic Activity of Chitosan and Derivatives
6. Application of Chitosan as Delivery Systems
6.1. Chitosan Microparticles
6.2. Chitosan Nanoparticles
7. Conclusive Remarks
Acknowledgments
Conflicts of Interest
References
- Clark, G.L.; Smith, A.F. X-ray diffraction studies. J. Phys. Chem. 1996, 40, 863–879. [Google Scholar] [CrossRef]
- Ogawa, K. Effect of Heating an Aqueous Suspension of Chitosan on the Crystallinity and Polymorphs. Agric. Biol. Chem. 1991, 55, 2375–2379. [Google Scholar]
- Ogawa, K.; Yui, T.; Okuyama, K. Three D structures of chitosan. Int. J. Biol. Macromol. 2004, 34, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, K.; Noguchi, K.; Miyazawa, T.; Yui, T.; Ogawa, K. Molecular and Crystal Structure of Hydrated hitosan. Macromolecules 1997, 30, 5849–5855. [Google Scholar] [CrossRef]
- Sugano, M.; Fujikawa, T.; Hiratsuji, Y.; Nakashima, K.; Fukuda, N.; Hasegawa, Y. A novel use of chitosan as a hypocholesterolemic agent in rats. Am. J. Clin. Nutr. 1980, 33, 787–793. [Google Scholar] [PubMed]
- Allan, C.R.; Hadwiger, L.A. The fungicidal effect of chitosan on fungi of varying cell wall composition. Exp. Mycol. 1979, 3, 285–287. [Google Scholar] [CrossRef]
- Proceedings of the First International Conference on Chitin/Chitosan; Muzzarelli, R.A.A.; Pariser, E.R. (Eds.) Massachusets Institute of Technology: Boston, MA, USA, 1978.
- Special Issue: Polysaccharide Solution and Gels. Carbohydr. Polym. 1982, 2, 227–324. Available online: http://www.sciencedirect.com/science/journal/01448617/2 (accessed on 13 May 2016).
- New Developments in Industrial Polysaccharides; Crescenzi, V.; Dea, I.C.M.; Stivala, S.S. (Eds.) Gordon and Breach Science Publishers: New York, NY, USA, 1985.
- Domard, A.; Rinaudo, M. Preparation and characterization of fully deacetylated chitosan. Int. J. Biol. Macromol. 1983, 5, 49–52. [Google Scholar] [CrossRef]
- Chandy, T.; Sharma, C.P. Chitosan—As a biomaterial. Biomater. Artif. cells Artif. organs 1990, 18, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.; Tarsi, R.; Filippini, O.; Giovanetti, E.; Biagini, G.; Varaldo, P.E. Antimicrobial properties of N-carboxybutyl chitosan. Antimicrob. Agents Chemother. 1990, 34, 2019–2023. [Google Scholar] [CrossRef] [PubMed]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Chitin & Chitosan: A Global Strategic Business Report. Global Industry Analysts Inc. Available online: http://www.strategyr.com/Chitin_and_Chitosan_Market_Report.asp (accessed on 13 May 2016).
- Stoye, E. Magnificent molecules. Mole 2014, 4. Available online: http://www.rsc.org/eic/sites/default/files/The-Mole-March-2014-corrected.pdf (accessed on 13 May 2016). [Google Scholar]
- Stahel, W.R. The circular economy. Nature 2016, 531, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Chen, X. Sustainability: Don’t waste seafood waste. Nature 2015, 524, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Kerton, F.M.; Liu, Y.; Omari, K.W.; Hawboldt, K. Green chemistry and the ocean-based biorefinery. Green Chem. 2013, 15, 860. [Google Scholar] [CrossRef]
- Chen, X.; Chew, S.L.; Kerton, F.M.; Yan, N. Direct conversion of chitin into a N-containing furan derivative. Green Chem. 2014, 16, 2204. [Google Scholar] [CrossRef]
- Pierson, Y.; Chen, X.; Bobbink, F.D.; Zhang, J.; Yan, N. Acid-Catalyzed Chitin Liquefaction in Ethylene Glycol. ACS Sustain. Chem. Eng. 2014, 2, 2081–2089. [Google Scholar] [CrossRef]
- Bobbink, F.D.; Zhang, J.; Pierson, Y.; Chen, X.; Yan, N. Conversion of chitin derived N-acetyl-d -glucosamine (NAG) into polyols over transition metal catalysts and hydrogen in water. Green Chem. 2015, 17, 1024–1031. [Google Scholar] [CrossRef]
- Cesàro, A.; Bellich, B.; Borgogna, M. Nanoparticles for pharmaceutical and biomedical applications. In Crystallography for Health and Biosciences; Guagliardi, A., Masciocchi, N., Eds.; Insubria University Press: Como, Italy, 2012; pp. 143–161. [Google Scholar]
- Smidsrod, O.; Haug, A.; Whittington, S.G. The molecular basis for some physical properties of polyuronides. Acta Chem. Scand. 1972, 26, 2563–2566. [Google Scholar] [CrossRef]
- Grasdalen, H.; Larsen, B.; Smisrod, O. 13C-N.M.R. studies of monomeric composition and sequence in alginate. Carbohydr. Res. 1981, 89, 179–191. [Google Scholar] [CrossRef]
- Terayama, H. Methods of colloids titration (a new titration between polymer ions). J. Polym. Sci. A 1952, 8, 243–253. [Google Scholar] [CrossRef]
- Balàzs, N.; Sipos, P. Limitations of pH-potentiometric titration for the determination of the degree of deacetylation of chitosan. Carbohydr. Res. 2007, 342, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Domszy, J.G.; Roberts, G.A.F. Evaluation of infrared spectroscopic techniques for analyzing chitosan. Macromol. Chem. Phys. 1985, 186, 1671–1677. [Google Scholar] [CrossRef]
- Miya, M.; Iwamoto, R.; Yoshikawa, S.; Mima, S. IR spectroscopic determination of CONH content in highly deacetylated chitosan. Int. J. Biol. Macromol. 1980, 2, 323–324. [Google Scholar] [CrossRef]
- Moore, G.K.; Roberts, G.A.F. Determination of the degree of N-acetylation of chitosan. Int. J. Biol. Macromol. 1980, 2, 115–116. [Google Scholar] [CrossRef]
- Sabnis, S.; Block, L.H. Improved infrared spectroscopic method for the analysis of degree of N-deacetylation of chitosan. Polym. Bull. 1997, 39, 67–71. [Google Scholar] [CrossRef]
- Sannan, T.; Kurita, K.; Ogura, K.; Iwakura, Y. Studies on chitin: 7. IR spectroscopic determination of degree of deacetylation. Polymer (Guildf). 1978, 19, 458–459. [Google Scholar] [CrossRef]
- Muraki, E.; Yaku, F.; Iyoda, J.; Kojima, H. Measurement of Degree of Deacetylation in d-Glucosamine Oligosaccharides by UV Absorption. Biosci. Biotechnol. Biochem. 1993, 57, 1929–1930. [Google Scholar] [CrossRef]
- Tan, S.C.; Khor, E.; Tan, T.K.; Wong, S.M. The degree of deacetylation of chitosan: Advocating the first derivative UV-spectrophotometry method of determination. Talanta 1998, 45, 713–719. [Google Scholar] [CrossRef]
- Domard, A. Determination of N-acetyl content in chitosan samples by c.d. measurements. Int. J. Biol. Macromol. 1987, 9, 333–336. [Google Scholar] [CrossRef]
- Hirai, A.; Odani, H.; Nakajima, A. Determination of degree of deacetylation of chitosan by H NMR spectroscopy. Polym. Bull. 1991, 26, 87–94. [Google Scholar] [CrossRef]
- Duarte, M.L.; Ferreira, M.C.; Marvao, M.R.; Rocha, J. Determination of the degree of acetylation of chitin materials by 13C CP/MAS NMR spectroscopy. Int. J. Biol. Macromol. 2001, 28, 359–363. [Google Scholar] [CrossRef]
- Raymond, L.; Morin, F.G.; Marcessaurt, R.H. Degree of deacetylation of chitosan using conductometric titration and solid-state NMR. Carbohydr. Res. 1993, 246, 331–336. [Google Scholar] [CrossRef]
- Vårum, K.M.; Anthonsen, M.W.; Grasdalen, H.; Smidsrod, O. Determination of the degree of N-acetylation and the distribution of N-acetyl groups in partially N-deacetylated chitins (chitosans) by high-field NMR spectroscopy. Carbohydr. Res. 1991, 211, 17–23. [Google Scholar] [CrossRef]
- Niola, F.; Basora, N.; Chornet, E.; Vidal, P.F. A rapid method for the determination of the degree of N-acetylation of chitin-chitosan samples by acid hydrolysis and HPLC. Carbohydr. Res. 1993, 238, 1–9. [Google Scholar] [CrossRef]
- Kasaai, M. A review of several reported procedures to determine the degree of N-acetylation for chitin and chitosan using infrared spectroscopy. Carbohydr. Polym. 2008, 71, 497–508. [Google Scholar] [CrossRef]
- Guinesi, L.S.; Cavalheiro, É.T.G. The use of DSC curves to determine the acetylation degree of chitin/chitosan samples. Thermochim. Acta 2006, 444, 128–133. [Google Scholar] [CrossRef]
- Rampino, A. Polysaccharide-based nanoparticles for drug delivery. Ph.D. Thesis, University of Trieste, Trieste, Italy, 2011. [Google Scholar]
- Bovey, F.A.; Mirau, P.A. NMR of Polymers; Academic Press: San Diego, CA, USA, 1996. [Google Scholar]
- Kumirska, J.; Weinhold, M.X.; Sauvageau, J.C.M.; Thöming, J.; Kaczyński, Z.; Stepnowski, P. Determination of the pattern of acetylation of low-molecular-weight chitosan used in biomedical applications. J. Pharm. Biomed. Anal. 2009, 50, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Aiba, S. Studies on chitosan: 3. Evidence for the presence of random and block copolymer structures in partially N-acetylated chitosans. Int. J. Biol. Macromol. 1991, 13, 40–44. [Google Scholar] [CrossRef]
- Cowman, M.K.; Matsuoka, S. Experimental approaches to hyaluronan structure. Carbohydr. Res. 2005, 340, 791–809. [Google Scholar] [CrossRef] [PubMed]
- Furlan, S.; La Penna, G.; Perico, A.; Cesàro, A. Hyaluronan chain conformation and dynamics. Carbohydr. Res. 2005, 340, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.A.; Castile, J.; Smith, A.; Adams, G.G.; Harding, S.E. Macromolecular conformation of chitosan in dilute solution: A new global hydrodynamic approach. Carbohydr. Polym. 2009, 76, 616–621. [Google Scholar] [CrossRef]
- Weinhold, M. Characterization of chitosan using triple detection size-exclusion chromatography and 13 C-NMR spectroscopy. Ph.D. Thesis, Universität Bremen, Bremen, Germany, 2010. [Google Scholar]
- Weinhold, M.X.; Sauvageau, J.C.M.; Keddig, N.; Matzke, M.; Tartsch, B.; Grunwald, I.; Kübel, C.; Jastorff, B.; Thöming, J. Strategy to improve the characterization of chitosan for sustainable biomedical applications: SAR guided multi-dimensional analysis. Green Chem. 2009, 11, 498. [Google Scholar] [CrossRef]
- Kumirska, J.; Weinhold, M.X.; Thöming, J.; Stepnowski, P. Biomedical Activity of Chitin/Chitosan Based Materials—Influence of Physicochemical Properties Apart from Molecular Weight and Degree of N-Acetylation. Polymers (Basel). 2011, 3, 1875–1901. [Google Scholar] [CrossRef]
- Brant, D.A. Novel approaches to the analysis of polysaccharide structures. Curr. Opin. Struct. Biol. 1999, 9, 556–562. [Google Scholar] [CrossRef]
- Cesàro, A.; Bellich, B.; Borgogna, M. Biophysical functionality in polysaccharides: From Lego-blocks to nano-particles. Eur. Biophys. J. 2012, 41, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Nilsen-Nygaard, J.; Strand, S.; Vårum, K.; Draget, K.; Nordgård, C. Chitosan: Gels and Interfacial Properties. Polymers (Basel). 2015, 7, 552–579. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Watts, P.; Smith, A.; Hinchcliffe, M. ChiSys® as a chitosan-based delivery platform for nasal vaccination. In Mucosal Delivery of Biopharmaceuticals: Biology, Challenges and Strategies; das Neves, J., Sarmento, B., Eds.; Springer Science & Business Media: New York, NY, USA, 2014; pp. 499–516. [Google Scholar]
- Ottøy, M.H.; Vårum, K.M.; Smidsrød, O. Compositional heterogeneity of heterogeneously deacetylated chitosans. Carbohydr. Polym. 1996, 29, 17–24. [Google Scholar] [CrossRef]
- Vårum, K.M.; Ottøy, M.H.; Smidsrød, O. Water-solubility of partially N-acetylated chitosans as a function of pH: Effect of chemical composition and depolymerisation. Carbohydr. Polym. 1994, 25, 65–70. [Google Scholar] [CrossRef]
- Korchagina, E.V.; Philippova, O.E. Multichain aggregates in dilute solutions of associating polyelectrolyte keeping a constant size at the increase in the chain length of individual macromolecules. Biomacromolecules 2010, 11, 3457–3466. [Google Scholar] [CrossRef] [PubMed]
- Boucard, N.; David, L.; Rochas, C.; Montembault, A.; Viton, C.; Domard, A. Polyelectrolyte microstructure in chitosan aqueous and alcohol solutions. Biomacromolecules 2007, 8, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Kjøniksen, A.-L.; Iversen, C.; Nyström, B.; Nakken, T.; Palmgren, O. Light Scattering Study of Semidilute Aqueous Systems of Chitosan and Hydrophobically Modified Chitosans. Macromolecules 1998, 31, 8142–8148. [Google Scholar] [CrossRef]
- Ottøy, M.H.; Vårum, K.M.; Christensen, B.E.; Anthonsen, M.W.; Smidsrød, O. Preparative and analytical size-exclusion chromatography of chitosans. Carbohydr. Polym. 1996, 31, 253–261. [Google Scholar] [CrossRef]
- Berth, G.; Dautzenberg, H. The degree of acetylation of chitosans and its effect on the chain conformation in aqueous solution. Carbohydr. Polym. 2002, 47, 39–51. [Google Scholar] [CrossRef]
- Franzén, H.; Draget, K.; Langebäck, J.; Nilsen-Nygaard, J. Characterization and Properties of Hydrogels Made from Neutral Soluble Chitosans. Polymers (Basel). 2015, 7, 373–389. [Google Scholar] [CrossRef]
- Cho, Y.-W.; Jang, J.; Park, C.R.; Ko, S.-W. Preparation and Solubility in Acid and Water of Partially Deacetylated Chitins. Biomacromolecules 2000, 1, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Berth, G.; Dautzenberg, H.; Peter, M.G. Physico-chemical characterization of chitosans varying in degree of acetylation. Carbohydr. Polym. 1998, 36, 205–216. [Google Scholar] [CrossRef]
- Cölfen, H.; Berth, G.; Dautzenberg, H. Hydrodynamic studies on chitosans in aqueous solution. Carbohydr. Polym. 2001, 45, 373–383. [Google Scholar] [CrossRef]
- Anthonsen, M.W.; Vårum, K.M.; Hermansson, A.M.; Smidsrød, O.; Brant, D.A. Aggregates in acidic solutions of chitosans detected by static laser light scattering. Carbohydr. Polym. 1994, 25, 13–23. [Google Scholar] [CrossRef]
- Buhler, E.; Rinaudo, M. Structural and Dynamical Properties of Semirigid Polyelectrolyte Solutions: A Light-Scattering Study. Macromolecules 2000, 33, 2098–2106. [Google Scholar] [CrossRef]
- Philippova, O.E.; Volkov, E.V.; Sitnikova, N.L.; Khokhlov, A.R.; Desbrieres, J.; Rinaudo, M. Two Types of Hydrophobic Aggregates in Aqueous Solutions of Chitosan and Its Hydrophobic Derivative. Biomacromolecules 2001, 2, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Lamarque, G.; Lucas, J.-M.; Viton, C.; Domard, A. Physicochemical behavior of homogeneous series of acetylated chitosans in aqueous solution: Role of various structural parameters. Biomacromolecules 2005, 6, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Schatz, C.; Viton, C.; Delair, T.; Pichot, C.; Domard, A. Typical physicochemical behaviors of chitosan in aqueous solution. Biomacromolecules 2003, 4, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Novoa-Carballal, R.; Riguera, R.; Fernandez-Megia, E. Chitosan hydrophobic domains are favoured at low degree of acetylation and molecular weight. Polymer (Guildf). 2013, 54, 2081–2087. [Google Scholar] [CrossRef]
- Yalpani, M. Selective chemical modification of polysaccharides. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 1980. [Google Scholar]
- Yalpani, M. A survey of recent advances in selective chemical and enzymic polysaccharide modifications. Tetrahedron 1985, 41, 2957–3020. [Google Scholar] [CrossRef]
- Yalpani, M.; Hall, L.D. Some chemical and analytical aspects of polysaccharide modifications. III. Formation of branched-chain, soluble chitosan derivatives. Macromolecules 1984, 17, 272–281. [Google Scholar] [CrossRef]
- Hall, L.D.; Yalpani, M. Formation of branched-chain, soluble polysaccharides from chitosan. J. Chem. Soc. Chem. Commun. 1980, 1153–1154. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R.; Muzzarelli, R.A.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Li, H.; Xiao, Q.; Liu, Y.; Zhu, J.; Du, Y. Water-solubility of chitosan and its antimicrobial activity. Carbohydr. Polym. 2006, 63, 367–374. [Google Scholar] [CrossRef]
- Chen, C.-S.; Liau, W.-Y.; Tsai, G.-J. Antibacterial Effects of N-Sulfonated and N-Sulfobenzoyl Chitosan and Application to Oyster Preservation. J. Food Prot. 1998, 61, 1124–1128. [Google Scholar]
- Kaya, M.; Asan-Ozusaglam, M.; Erdogan, S. Comparison of antimicrobial activities of newly obtained low molecular weight scorpion chitosan and medium molecular weight commercial chitosan. J. Biosci. Bioeng. 2016, 121, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Ravi Kumar, M.N. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Choi, B.-K.; Kim, K.-Y.; Yoo, Y.-J.; Oh, S.-J.; Choi, J.-H.; Kim, C.-Y. In vitro antimicrobial activity of a chitooligosaccharide mixture against Actinobacillus actinomycetemcomitans and Streptococcus mutans. Int. J. Antimicrob. Agents 2001, 18, 553–557. [Google Scholar] [CrossRef]
- İkinci, G.; Şenel, S.; Akıncıbay, H.; Kaş, S.; Erciş, S.; Wilson, C.; Hıncal, A. Effect of chitosan on a periodontal pathogen Porphyromonas gingivalis. Int. J. Pharm. 2002, 235, 121–127. [Google Scholar] [CrossRef]
- Tsai, G.J.; Su, W.H.; Chen, H.C.; Pan, C.L. Antimicrobial activity of shrimp chitin and chitosan from different treatments and applications of fish preservation. Fish. Sci. 2002, 68, 170–177. [Google Scholar] [CrossRef]
- Savard, T.; Beaulieu, C.; Boucher, I.; Champagne, C.P. Antimicrobial Action of Hydrolyzed Chitosan against Spoilage Yeasts and Lactic Acid Bacteria of Fermented Vegetables. J. Food Prot. 2002, 65, 828–833. [Google Scholar]
- Cuero, R.G. Antimicrobial action of exogenous chitosan. EXS 1999, 87, 315–333. [Google Scholar] [PubMed]
- Pelletier, E.; Bonnet, C.; Lemarchand, K. Biofouling growth in cold estuarine waters and evaluation of some chitosan and copper anti-fouling paints. Int. J. Mol. Sci. 2009, 10, 3209–3223. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Camacho, A.P.; Cortez-Rocha, M.O.; Ezquerra-Brauer, J.M.; Graciano-Verdugo, A.Z.; Rodriguez-Félix, F.; Castillo-Ortega, M.M.; Yépiz-Gómez, M.S.; Plascencia-Jatomea, M. Chitosan composite films: Thermal, structural, mechanical and antifungal properties. Carbohydr. Polym. 2010, 82, 305–315. [Google Scholar] [CrossRef]
- Goy, R.C.; de Britto, D.; Assis, O.B.G. A review of the antimicrobial activity of chitosan. 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Raafat, D.; Sahl, H.-G. Chitosan and its antimicrobial potential—A critical literature survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Raafat, D. Chitosan as an Antimicrobial Agent: Modes of Action and Resistance Mechanisms. Ph.D. Thesis, University of Bonn, Bonn, Germany, 2008. [Google Scholar]
- Eaton, P.; Fernandes, J.C.; Pereira, E.; Pintado, M.E.; Xavier Malcata, F. Atomic force microscopy study of the antibacterial effects of chitosans on Escherichia coli and Staphylococcus aureus. Ultramicroscopy 2008, 108, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.; Tsuchida, H.; Nagao, N. N-acetylation in chitosan and the rate of its enzymic hydrolysis. Biomaterials 1989, 10, 574–576. [Google Scholar] [CrossRef]
- Takahashi, T.; Imai, M.; Suzuki, I.; Sawai, J. Growth inhibitory effect on bacteria of chitosan membranes regulated with deacetylation degree. Biochem. Eng. J. 2008, 40, 485–491. [Google Scholar] [CrossRef]
- Vishu Kumar, A.B.; Varadaraj, M.C.; Gowda, L.R.; Tharanathan, R.N. Characterization of chito-oligosaccharides prepared by chitosanolysis with the aid of papain and Pronase, and their bactericidal action against Bacillus cereus and Escherichia coli. Biochem. J. 2005, 391, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Lauzardo, A.N.; Bautista-Baños, S.; Velázquez-Del Valle, M.G.; Méndez-Montealvo, M.G.; Sánchez-Rivera, M.M.; Bello-Pérez, L.A. Antifungal effects of chitosan with different molecular weights on in vitro development of Rhizopus stolonifer (Ehrenb.:Fr.) Vuill. Carbohydr. Polym. 2008, 73, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.-C.; Su, Y.-P.; Chen, C.-C.; Jia, G.; Wang, H.-L.; Wu, J.C.G.; Lin, J.-G. Relationship between antibacterial activity of chitosan and surface characteristics of cell wall. Acta Pharmacol. Sin. 2004, 25, 932–936. [Google Scholar] [PubMed]
- Zhong, Z.; Xing, R.; Liu, S.; Wang, L.; Cai, S.; Li, P. Synthesis of acyl thiourea derivatives of chitosan and their antimicrobial activities in vitro. Carbohydr. Res. 2008, 343, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Rhoades, J.; Roller, S. Antimicrobial Actions of Degraded and Native Chitosan against Spoilage Organisms in Laboratory Media and Foods. Appl. Environ. Microbiol. 2000, 66, 80–86. [Google Scholar] [CrossRef] [PubMed]
- No, H. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef]
- Helander, I.; Nurmiaho-Lassila, E.-L.; Ahvenainen, R.; Rhoades, J.; Roller, S. Chitosan disrupts the barrier properties of the outer membrane of Gram-negative bacteria. Int. J. Food Microbiol. 2001, 71, 235–244. [Google Scholar] [CrossRef]
- Raafat, D.; Von Bargen, K.; Haas, A.; Sahl, H.G. Insights into the mode of action of chitosan as an antibacterial compound. Appl. Environ. Microbiol. 2008, 74, 3764–3773. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewska, A.; Boorsma, A.; Brul, S.; Klaas, J.; Klis, F.M.; Hellingwerf, K.J. Transcriptional Response of Saccharomyces cerevisiae to the Plasma Membrane-Perturbing Compound Chitosan Transcriptional Response of Saccharomyces cerevisiae to the Plasma Membrane-Perturbing Compound Chitosan. Eukaryot. Cell 2005, 4, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Sudarshan, N.R.; Hoover, D.G.; Knorr, D. Antibacterial action of chitosan. Food Biotechnol. 1992, 6, 257–272. [Google Scholar] [CrossRef]
- Sebti, I.; Martial-Gros, A.; Carnet-Pantiez, A.; Grelier, S.; Coma, V. Chitosan Polymer as Bioactive Coating and Film against Aspergillus niger Contamination. J. Food Sci. 2005, 70, 100–104. [Google Scholar] [CrossRef]
- Tokura, S.; Ueno, K.; Miyazaki, S.; Nishi, N. Molecular weight dependent antimicrobial activity by chitosan. In New Macromolecular Architecture and Functions; Kamachi, M., Nakamura, A., Eds.; Springer Berlin Heidelberg: Berlin, Heidelberg, 1996. [Google Scholar]
- Chung, Y. Effect of abiotic factors on the antibacterial activity of chitosan against waterborne pathogens. Bioresour. Technol. 2003, 88, 179–184. [Google Scholar] [CrossRef]
- Burkatovskaya, M.; Castano, A.P.; Demidova-Rice, T.N.; Tegos, G.P.; Hamblin, M.R. Effect of chitosan acetate bandage on wound healing in infected and noninfected wounds in mice. Wound Repair Regen. 2008, 16, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Heo, D.N.; Moon, J.-H.; Park, H.N.; Ko, W.-K.; Bae, M.S.; Lee, J.B.; Park, S.W.; Kim, E.-C.; Lee, C.H.; et al. Chitosan/Polyurethane Blended Fiber Sheets Containing Silver Sulfadiazine for Use as an Antimicrobial Wound Dressing. J. Nanosci. Nanotechnol. 2014, 14, 7488–7494. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.B.; Wang, F.Y.; Ding, W.; Zhang, Y.; Ding, J.; Cai, D.X.; Yu, K.F.; Yang, J.; Yang, L.; Xu, Y.Q. Fabrication and Evaluation of Porous Keratin/chitosan (KCS) Scaffolds for Effectively Accelerating Wound Healing. Biomed. Environ. Sci. 2015, 28, 178–189. [Google Scholar] [PubMed]
- Miguel, S.P.; Ribeiro, M.P.; Brancal, H.; Coutinho, P.; Correia, I.J. Thermoresponsive chitosan-agarose hydrogel for skin regeneration. Carbohydr. Polym. 2014, 111, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Anisha, B.S.; Biswas, R.; Chennazhi, K.P.; Jayakumar, R. Chitosan-hyaluronic acid/nano silver composite sponges for drug resistant bacteria infected diabetic wounds. Int. J. Biol. Macromol. 2013, 62, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.A.; Cabral, J.D.; Brooks, H.J.L.; Moratti, S.C.; Hanton, L.R. Antimicrobial properties of a chitosan dextran-based hydrogel for surgical use. Antimicrob. Agents Chemother. 2012, 56, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Du, W.-L.; Niu, S.-S.; Xu, Y.-L.; Xu, Z.-R.; Fan, C.-L. Antibacterial activity of chitosan tripolyphosphate nanoparticles loaded with various metal ions. Carbohydr. Polym. 2009, 75, 385–389. [Google Scholar] [CrossRef]
- Qi, L.; Xu, Z.; Jiang, X.; Hu, C.; Zou, X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Neoh, K.G.; Kang, E.T.; Wang, W. Antibacterial and mechanical properties of bone cement impregnated with chitosan nanoparticles. Biomaterials 2006, 27, 2440–2449. [Google Scholar] [CrossRef] [PubMed]
- Alt, V.; Bechert, T.; Steinrücke, P.; Wagener, M.; Seidel, P.; Dingeldein, E.; Domann, E.; Schnettler, R. An in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticulate silver bone cement. Biomaterials 2004, 25, 4383–4891. [Google Scholar] [CrossRef] [PubMed]
- Barreras, U.S.; Méndez, F.T.; Martínez, R.E.M.; Valencia, C.S.; Rodríguez, P.R.M.; Rodríguez, J.P.L. Chitosan nanoparticles enhance the antibacterial activity of chlorhexidine in collagen membranes used for periapical guided tissue regeneration. Mater. Sci. Eng. C. Mater. Biol. Appl. 2016, 58, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Aider, M. Chitosan application for active bio-based films production and potential in the food industry: Review. LWT-Food Sci. Technol. 2010, 43, 837–842. [Google Scholar] [CrossRef]
- Agulló, E.; Rodríguez, M.S.; Ramos, V.; Albertengo, L. Present and Future Role of Chitin and Chitosan in Food. Macromol. Biosci. 2003, 3, 521–530. [Google Scholar] [CrossRef]
- Tahiri, I.; Desbiens, M.; Lacroix, C.; Kheadr, E.; Fliss, I. Growth of Carnobacterium divergens M35 and production of Divergicin M35 in snow crab by-product, a natural-grade medium. LWT-Food Sci. Technol. 2009, 42, 624–632. [Google Scholar] [CrossRef]
- Devlieghere, F.; Vermeulen, A.; Debevere, J. Chitosan: Antimicrobial activity, interactions with food components and applicability as a coating on fruit and vegetables. Food Microbiol. 2004, 21, 703–714. [Google Scholar] [CrossRef]
- Dutta, P.K.; Tripathi, S.; Mehrotra, G.K.; Dutta, J. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 2009, 114, 1173–1182. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Abdou, E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng. C. Mater. Biol. Appl. 2013, 33, 1819–1841. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, E.-R.; Worley, S.D.; Broughton, R. The chemistry and applications of antimicrobial polymers: A state-of-the-art review. Biomacromolecules 2007, 8, 1359–1384. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Haile, A. Multifunctional properties of cotton fabric treated with chitosan and carboxymethyl chitosan. Carbohydr. Polym. 2007, 69, 164–171. [Google Scholar] [CrossRef]
- Ye, W.; Leung, M.F.; Xin, J.; Kwong, T.L.; Lee, D.K.L.; Li, P. Novel core-shell particles with poly(n-butyl acrylate) cores and chitosan shells as an antibacterial coating for textiles. Polymer (Guildf). 2005, 46, 10538–10543. [Google Scholar] [CrossRef]
- Illum, L. Chitosan and its use as a pharmaceutical excipient. Pharm. Res. 1998, 15, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Felt, O.; Buri, P.; Gurny, R. Chitosan: A unique polysaccharide for drug delivery. Drug Dev. Ind. Pharm. 1998, 24, 979–993. [Google Scholar] [CrossRef] [PubMed]
- Rajalakshmi, R.; Indira Muzib, Y.; Aruna, U.; Vinesha, V.; Rupangada, V.; Krishna moorthy, S.B. Chitosan Nanoparticles—An Emerging Trend In Nanotechnology. Int. J. Drug Deliv. 2014, 6, 204–229. [Google Scholar]
- Shukla, S.K.; Mishra, A.K.; Arotiba, O.A.; Mamba, B.B. Chitosan-based nanomaterials: A state-of-the-art review. Int. J. Biol. Macromol. 2013, 59, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Sinha, V.R.; Singla, A.K.; Wadhawan, S.; Kaushik, R.; Kumria, R.; Bansal, K.; Dhawan, S. Chitosan microspheres as a potential carrier for drugs. Int. J. Pharm. 2004, 274, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.; Ng, T.; Wong, J.; Chan, W. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. drugs 2015, 13, 5156–5186. [Google Scholar]
- Bawarski, W.E.; Chidlowsky, E.; Bharali, D.J.; Mousa, S.A. Emerging nanopharmaceuticals. Nanomed. Nanotech. Biol. Med. 2008, 4, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Kohane, D.S. Microparticles and nanoparticles for drug delivery. Biotechnol. Bioeng. 2007, 96, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Brun-Graeppi, A.K.A.S.; Richard, C.; Bessodes, M.; Scherman, D.; Merten, O.-W. Cell microcarriers and microcapsules of stimuli-responsive polymers. J. Control. Release 2011, 149, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Anton, N.; Benoit, J.-P.; Saulnier, P. Design and production of nanoparticles formulated from nano-emulsion templates—A review. J. Control. Release 2008, 128, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Pinto Reis, C.; Neufeld, R.J.; Ribeiro, A.J.; Veiga, F. Nanoencapsulation I. Methods for preparation of drug-loaded polymeric nanoparticles. Nanomedicine 2006, 2, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Mizrahy, S.; Peer, D. Polysaccharides as building blocks for nanotherapeutics. Chem. Soc. Rev. 2012, 41, 2623. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Sheu, D.-C.; Li, S.-Y.; Duan, K.-J.; Chen, C.W. Production of galactooligosaccharides by β-galactosidase immobilized on glutaraldehyde-treated chitosan beads. Biotechnol. Tech. 1998, 12, 273–276. [Google Scholar] [CrossRef]
- Du, Y.-Z.; Ying, X.-Y.; Wang, L.; Zhai, Y.; Yuan, H.; Yu, R.-S.; Hu, F.-Q. Sustained release of ATP encapsulated in chitosan oligosaccharide nanoparticles. Int. J. Pharm. 2010, 392, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ma, L.; Liu, Y.; Xu, F.; Nie, J.; Ma, G. Design and characterization of antitumor drug paclitaxel-loaded chitosan nanoparticles by W/O emulsions. Int. J. Biol. Macromol. 2012, 50, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, O.A.C.; Airoldi, C. Some studies of crosslinking chitosan-glutaraldehyde interaction in a homogeneous system. Int. J. Biol. Macromol. 1999, 26, 119–128. [Google Scholar] [CrossRef]
- Leung, H.W. Ecotoxicology of glutaraldehyde: Review of environmental fate and effects studies. Ecotoxicol. Environ. Saf. 2001, 49, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Wanchoo, R.K.; Hardeep, S.K.S. Chitosan Hydrogel Beads: A Comparative Study with Glutaraldehyde, Epichlorohydrin and Genipin as Crosslinkers. J. Polym. Mater. 2013, 31, 211–223. [Google Scholar]
- Khurma, J.R.; Rohindra, D.R.; Nand, A.V. Swelling and Thermal Characteristics of Genipin Crosslinked Chitosan and Poly(vinyl pyrrolidone) Hydrogels. Polym. Bull. 2005, 54, 195–204. [Google Scholar] [CrossRef]
- Moura, M.J.; Figueiredo, M.M.; Gil, M.H. Rheological study of genipin cross-linked chitosan hydrogels. Biomacromolecules 2007, 8, 3823–3829. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.A. Genipin-crosslinked chitosan hydrogels as biomedical and pharmaceutical aids. Carbohydr. Polym. 2009, 77, 1–9. [Google Scholar] [CrossRef]
- Karnchanajindanun, J.; Srisa-ard, M.; Baimark, Y. Genipin-cross-linked chitosan microspheres prepared by a water-in-oil emulsion solvent diffusion method for protein delivery. Carbohydr. Polym. 2011, 85, 674–680. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, W.; Kim, H.-I. pH-responsive release behavior of genipin-crosslinked chitosan/poly(ethylene glycol) hydrogels. J. Appl. Polym. Sci. 2012, 125, 290–298. [Google Scholar] [CrossRef]
- Arteche Pujana, M.; Pérez-Álvarez, L.; Cesteros Iturbe, L.C.; Katime, I. Biodegradable chitosan nanogels crosslinked with genipin. Carbohydr. Polym. 2013, 94, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Hurst, G.A.; Novakovic, K. A facile in situ morphological characterization of smart genipin-crosslinked chitosan–poly(vinyl pyrrolidone) hydrogels. J. Mater. Res. 2013, 28, 2401–2408. [Google Scholar] [CrossRef]
- Nwosu, C.J.; Hurst, G.A.; Novakovic, K. Hydrogels: Influence of Composition and Postsynthesis Treatment on pH Responsive Behaviour. Adv. Mater. Sci. Eng. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Lai, J.Y. Biocompatibility of chemically cross-linked gelatin hydrogels for ophthalmic use. J. Mater. Sci. Mater. Med. 2010, 21, 1899–1911. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, M.; Hartmann, J.F.; Borbely, J. Preparation and characterization of chitosan-based nanoparticles. Biomacromolecules 2005, 6, 2521–2527. [Google Scholar] [CrossRef] [PubMed]
- Prata, A.S.; Grosso, C.R.F. Production of microparticles with gelatin and chitosan. Carbohydr. Polym. 2015, 116, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Frohbergh, M.E.; Katsman, A.; Botta, G.P.; Lazarovici, P.; Schauer, C.L.; Wegst, U.G.K.; Lelkes, P.I. Electrospun hydroxyapatite-containing chitosan nanofibers crosslinked with genipin for bone tissue engineering. Biomaterials 2012, 33, 9167–9178. [Google Scholar] [CrossRef] [PubMed]
- Chaiyasan, W.; Srinivas, S.P.; Tiyaboonchai, W. Crosslinked chitosan-dextran sulfate nanoparticle for improved topical ocular drug delivery. Mol. Vis. 2015, 21, 1224–1234. [Google Scholar] [PubMed]
- Liu, Z.; Lv, D.; Liu, S.; Gong, J.; Wang, D.; Xiong, M.; Chen, X.; Xiang, R.; Tan, X. Alginic acid-coated chitosan nanoparticles loaded with legumain DNA vaccine: Effect against breast cancer in mice. PLoS ONE 2013, 8, e60190. [Google Scholar] [CrossRef] [PubMed]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Mater. Sci. Eng. R Reports 2015, 93, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Bodmeier, R.; Chen, H.; Paeratakul, O. A novel approach to the oral delivery of micro- or nanoparticles. Pharm. Res 1989, 6, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Remunan-Lopez, C. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J. Appl. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Liu, H.; Gao, C. Preparation and properties of ionically cross-linked chitosan nanoparticles. Polym. Adv. Technol. 2009, 20, 613–619. [Google Scholar] [CrossRef]
- Xu, Y.; Du, Y. Effect of molecular structure of chitosan on protein delivery properties of chitosan nanoparticles. Int. J. Pharm. 2003, 250, 215–226. [Google Scholar] [CrossRef]
- Fernández-Urrusuno, R.; Calvo, P.; Remuñán-López, C.; Vila-Jato, J.L.; Alonso, M.J. Enhancement of nasal absorption of insulin using chitosan nanoparticles. Pharm. Res. 1999, 16, 1576–1581. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Li, Y.; Zhao, H.; Zheng, J.; Xu, H.; Wei, G.; Hao, J.; Cui, F. Bioadhesive polysaccharide in protein delivery system: Chitosan nanoparticles improve the intestinal absorption of insulin in vivo. Int. J. Pharm. 2002, 249, 139–147. [Google Scholar] [CrossRef]
- da Silva, S.B.; Ferreira, D.; Pintado, M.; Sarmento, B. Chitosan-based nanoparticles for rosmarinic acid ocular delivery—In vitro tests. Int. J. Biol. Macromol. 2015, 84, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Thakur, K.; Sharma, G.; Kush, P.; Jain, U.K. Fabrication, characterization and cytotoxicity studies of ionically cross-linked docetaxel loaded chitosan nanoparticles. Carbohydr. Polym. 2016, 137, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Sipoli, C.C.; Radaic, A.; Santana, N.; de Jesus, M.B.; de la Torre, L.G. Chitosan nanoparticles produced with the gradual temperature decrease technique for sustained gene delivery. Biochem. Eng. J. 2015, 103, 114–121. [Google Scholar] [CrossRef]
- Gan, Q.; Wang, T.; Cochrane, C.; McCarron, P. Modulation of surface charge, particle size and morphological properties of chitosan-TPP nanoparticles intended for gene delivery. Colloids Surf. B. Biointerfaces 2005, 44, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Nasti, A.; Zaki, N.M.; de Leonardis, P.; Ungphaiboon, S.; Sansongsak, P.; Rimoli, M.G.; Tirelli, N. Chitosan/TPP and Chitosan/TPP-hyaluronic Acid Nanoparticles: Systematic Optimisation of the Preparative Process and Preliminary Biological Evaluation. Pharm. Res. 2009, 26, 1918–1930. [Google Scholar] [CrossRef] [PubMed]
- des Rieux, A.; Fievez, V.; Garinot, M.; Schneider, Y.-J.; Préat, V. Nanoparticles as potential oral delivery systems of proteins and vaccines: A mechanistic approach. J. Control. Release 2006, 116, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, K.; Ganguly, K.; Nadagouda, M.N.; Aminabhavi, T.M. Polymeric hydrogels for oral insulin delivery. J. Control. Release 2013, 165, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Grenha, A.; Seijo, B.; Remuñán-López, C. Microencapsulated chitosan nanoparticles for lung protein delivery. Eur. J. Pharm. Sci. 2005, 25, 427–437. [Google Scholar] [CrossRef] [PubMed]
- De Campos, A.M.; Sánchez, A.; Alonso, M.J. Chitosan nanoparticles: A new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to cyclosporin A. Int. J. Pharm. 2001, 224, 159–168. [Google Scholar] [CrossRef]
- Peniche, C.; Argüelles-Monal, W.; Peniche, H.; Acosta, N. Chitosan: An Attractive Biocompatible Polymer for Microencapsulation. Macromol. Biosci. 2003, 3, 511–520. [Google Scholar] [CrossRef]
- Kizilay, E.; Kayitmazer, A.B.; Dubin, P.L. Complexation and coacervation of polyelectrolytes with oppositely charged colloids. Adv. Colloid Interface Sci. 2011, 167, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-andrews, H.; Ba, J.G.; Cruz-sosa, F.; Vernon-carter, E.J. Gum Arabic—Chitosan Complex Coacervation. Biomacromolecules 2007, 8, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Sadek, R.F.H. Mutual interaction of polyelectrolytes. Science 1949, 110, 552–554. [Google Scholar]
- Rahaiee, S.; Shojaosadati, S.A.; Hashemi, M.; Moini, S.; Razavi, S.H. Improvement of crocin stability by biodegradeble nanoparticles of chitosan-alginate. Int. J. Biol. Macromol. 2015, 79, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Siddalingappa, B.; Chan, P.H.H.; Benson, H.A.E. Development of a chitosan-based nanoparticle formulation for delivery of a hydrophilic hexapeptide, dalargin. Biopolym. Pept. Sci. Sect. 2008, 90, 663–670. [Google Scholar] [CrossRef] [PubMed]
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan—A review. J. Control. Release 2006, 114, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.Q.; Roy, K.; Troung-Le, V.L.; Janes, K.A.; Lin, K.Y.; Wang, Y.; August, J.T.; Leong, K.W. Chitosan-DNA nanoparticles as gene carriers: Synthesis, characterization and transfection efficiency. J. Control. Release 2001, 70, 399–421. [Google Scholar] [CrossRef]
- Deacon, J.; Abdelghany, S.M.; Quinn, D.J.; Schmid, D.; Megaw, J.; Donnelly, R.F.; Jones, D.S.; Kissenpfennig, A.; Elborn, J.S.; Gilmore, B.F.; et al. Antimicrobial efficacy of tobramycin polymeric nanoparticles for Pseudomonas aeruginosa infections in cystic fibrosis: Formulation, characterisation and functionalisation with dornase alfa (DNase). J. Control. Release 2015, 198, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Reist, M.; Mayer, J.; Felt, O.; Gurny, R. Structure and interactions in chitosan hydrogels formed by complexation or aggregation for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 35–52. [Google Scholar] [CrossRef]
- Sarmento, B.; Martins, S.; Ribeiro, A.; Veiga, F.; Neufeld, R.; Ferreira, D. Development and Comparison of Different Nanoparticulate Polyelectrolyte Complexes as Insulin Carriers. Int. J. Pept. Res. Ther. 2006, 12, 131–138. [Google Scholar] [CrossRef]
- Sarmento, B.; Ribeiro, A.; Veiga, F.; Sampaio, P.; Neufeld, R.; Ferreira, D. Alginate/Chitosan Nanoparticles are Effective for Oral Insulin Delivery. Pharm. Res. 2007, 24, 2198–2206. [Google Scholar] [CrossRef] [PubMed]
- Rajaonarivony, M.; Vauthier, C.; Couarraze, G.; Puisieux, F.; Couvreur, P. Development of a new drug carrier made from alginate. J. Pharm. Sci. 1993, 82, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Loquercio, A.; Castell-Perez, E.; Gomes, C.; Moreira, R.G. Preparation of Chitosan-Alginate Nanoparticles for Trans-cinnamaldehyde Entrapment. J. Food Sci. 2015, 80, 2305–2315. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Dai, Y.; Zhang, J.; Wang, A.; Wei, Q. Chitosan-Alginate Nanoparticles as a Novel Drug Delivery System for Nifedipine. Int. J. Biomed. Sci. 2008, 4, 221–228. [Google Scholar] [PubMed]
- Sarmento, B.; Ribeiro, A.J.; Veiga, F.; Ferreira, D.C.; Neufeld, R.J. Insulin-Loaded Nanoparticles are Prepared by Alginate Ionotropic Pre-Gelation Followed by Chitosan Polyelectrolyte Complexation. J. Nanosci. Nanotechnol. 2007, 7, 2833–2841. [Google Scholar] [CrossRef] [PubMed]
- Oyarzun-Ampuero, F.A.; Brea, J.; Loza, M.I.; Torres, D.; Alonso, M.J. Chitosan-hyaluronic acid nanoparticles loaded with heparin for the treatment of asthma. Int. J. Pharm. 2009, 381, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Duceppe, N.; Tabrizian, M. Factors influencing the transfection efficiency of ultra low molecular weight chitosan/hyaluronic acid nanoparticles. Biomaterials 2009, 30, 2625–2631. [Google Scholar] [CrossRef] [PubMed]
- Aruffo, A.; Stamenkovic, I.; Melnick, M.; Underhill, C.B.; Seed, B. CD44 is the principal cell surface receptor for hyaluronate. Cell 1990, 61, 1303–1313. [Google Scholar] [CrossRef]
- Li, C.; Hein, S.; Wang, K. Chitosan-Carrageenan Polyelectrolyte Complex for the Delivery of Protein Drugs. ISRN Biomater. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Sarmento, B.; Ribeiro, A.; Veiga, F.; Ferreira, D. Development and characterization of new insulin containing polysaccharide nanoparticles. Colloids Surf. B. Biointerfaces 2006, 53, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Mounica Reddy, M.; Shanmugam, V.; Kaza, R. Design and Characterization of Insulin Nanoparticles for Oral Delivery. Int. J. Innov. Pharm. Res. 2012, 3, 238–243. [Google Scholar]
- Avadi, M.R.; Sadeghi, A.M.M.; Mohammadpour, N.; Abedin, S.; Atyabi, F.; Dinarvand, R.; Rafiee-Tehrani, M. Preparation and characterization of insulin nanoparticles using chitosan and Arabic gum with ionic gelation method. Nanomedicine 2010, 6, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Percival, S.S.; Cheng, Q.; Li, Z.; Rowe, C.A.; Gu, L. Preparation, characterization, and induction of cell apoptosis of cocoa procyanidins-gelatin-chitosan nanoparticles. Eur. J. Pharm. Biopharm. 2012, 82, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Birch, N.P.; Schiffman, J.D. Characterization of self-assembled polyelectrolyte complex nanoparticles formed from chitosan and pectin. Langmuir 2014, 30, 3441–3447. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, Q. Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery. Int. J. Biol. Macromol. 2014, 64, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Davidenko, N.; Blanco, M.D.; Peniche, C.; Becherán, L.; Guerrero, S.; Teijón, J.M. Effects of different parameters on the characteristics of chitosan-poly(acrylic acid) nanoparticles obtained by the method of coacervation. J. Appl. Polym. Sci. 2009, 111, 2362–2371. [Google Scholar] [CrossRef]
- Hu, Y.; Jiang, X.; Ding, Y.; Ge, H.; Yuan, Y.; Yang, C. Synthesis and characterization of chitosan–poly(acrylic acid) nanoparticles. Biomaterials 2002, 23, 3193–3201. [Google Scholar] [CrossRef]
- Cooper, C.L.; Dubin, P.L.; Kayitmazer, A.B.; Turksen, S. Polyelectrolyte–protein complexes. Curr. Opin. Colloid Interface Sci. 2005, 10, 52–78. [Google Scholar] [CrossRef]
- Boddohi, S.; Moore, N.; Johnson, P.A.; Kipper, M.J. Polysaccharide-based polyelectrolyte complex nanoparticles from chitosan, heparin, and hyaluronan. Biomacromolecules 2009, 10, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Ting, Y.; Zeng, X.; Huang, Q. Bioactive peptides/chitosan nanoparticles enhance cellular antioxidant activity of (−)-epigallocatechin-3-gallate. J. Agric. Food Chem. 2013, 61, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.W.; Mao, H.-Q.; Truong-Le, V.L.; Roy, K.; Walsh, S.M.; August, J.T. DNA-polycation nanospheres as non-viral gene delivery vehicles. J. Control. Release 1998, 53, 183–193. [Google Scholar] [CrossRef]
- Richardson, S. Potential of low molecular mass chitosan as a DNA delivery system: Biocompatibility, body distribution and ability to complex and protect DNA. Int. J. Pharm. 1999, 178, 231–243. [Google Scholar] [CrossRef]
- Lavertu, M.; Méthot, S.; Tran-Khanh, N.; Buschmann, M.D. High efficiency gene transfer using chitosan/DNA nanoparticles with specific combinations of molecular weight and degree of deacetylation. Biomaterials 2006, 27, 4815–4824. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, M.D.; Merzouki, A.; Lavertu, M.; Thibault, M.; Jean, M.; Darras, V. Chitosans for delivery of nucleic acids. Adv. Drug Deliv. Rev. 2013, 65, 1234–1270. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.B.; Li, H.; Yuan, Y.B. Preparation of cholesterol-modified chitosan self-aggregated nanoparticles for delivery of drugs to ocular surface. Carbohydr. Polym. 2006, 65, 337–345. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Liu, L.-R.; Jiang, Q.; Zhang, Q.-Q. Self-aggregated nanoparticles of cholesterol-modified chitosan conjugate as a novel carrier of epirubicin. Eur. Polym. J. 2007, 43, 43–51. [Google Scholar] [CrossRef]
- Lee, K.Y.; Jo, W.H.; Kwon, I.C.; Kim, Y.; Jeong, S.Y. Structural Determination and Interior Polarity of Self-Aggregates Prepared from Deoxycholic Acid-Modified Chitosan in Water. Macromolecules 1998, 31, 378–383. [Google Scholar] [CrossRef]
- Chen, X.G.; Lee, C.M.; Park, H.J. O/W emulsification for the self-aggregation and nanoparticle formation of linoleic acid-modified chitosan in the aqueous system. J. Agric. Food Chem. 2003, 51, 3135–3139. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.-Z.; Wang, L.; Yuan, H.; Wei, X.-H.; Hu, F.-Q. Preparation and characteristics of linoleic acid-grafted chitosan oligosaccharide micelles as a carrier for doxorubicin. Colloids Surf. B. Biointerfaces 2009, 69, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Rampino, A.; Borgogna, M.; Blasi, P.; Bellich, B.; Cesàro, A. Chitosan nanoparticles: Preparation, size evolution and stability. Int. J. Pharm. 2013, 455, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Fàbregas, A.; Miñarro, M.; García-Montoya, E.; Pérez-Lozano, P.; Carrillo, C.; Sarrate, R.; Sánchez, N.; Ticó, J.R.; Suñé-Negre, J.M. Impact of physical parameters on particle size and reaction yield when using the ionic gelation method to obtain cationic polymeric chitosan-tripolyphosphate nanoparticles. Int. J. Pharm. 2013, 446, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Mathematical modeling of drug delivery. Int. J. Pharm. 2008, 364, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.Z.; Zhu, K.J. A novel approach to prepare tripolyphosphate/chitosan complex beads for controlled release drug delivery. Int. J. Pharm. 2000, 201, 51–58. [Google Scholar] [CrossRef]
- Casettari, L.; Illum, L. Chitosan in nasal delivery systems for therapeutic drugs. J. Control. Release 2014, 190, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Castro, S.P.M.; Paulín, E.G.L. Is Chitosan a New Panacea? Areas of Application. In The Complex World of Polysacchrides; Karunaratne, D.N., Ed.; InTech Publisher: Rijeka, Croatia, 2012; pp. 3–46. [Google Scholar]








| DS | Mw | Mn | Mw/Mn | [η] | Rh | Rg | Recovery | |
|---|---|---|---|---|---|---|---|---|
| kg/mol | kg/mol | dL/g | nm | nm | % | |||
| VLMW-chit | - | 30 | 18 | 1.7 | 0.7 | 6.4 | - | 94 |
| LMW-chit | - | 128 | 56 | 2.3 | 2.3 | 15.3 | - | 82 |
| TM-chit | 0.71 | 109 | 46 | 2.3 | 0.7 | 9.7 | - | 80 |
| Gal-chit | 0.50 | 473 | 102 | 5.1 | 1.9 | 21.3 | 50.8 | 99 |
| Glc-chit | 0.42 | 554 | 96 | 6.4 | 2.4 | 23.8 | 61.7 | 93 |
| Structural Features Change | Case-Polymers | Solution Behavior |
|---|---|---|
| chain linkage | cellulose | stiff and insoluble |
| pullulan | flexible and soluble | |
| side chains | curdlan | linear, insoluble |
| scleroglucan | branched, soluble | |
| non-sugar substituents | deacetylated gellan | gel with Ca, Mg |
| native gellan (acetylated) | soluble with Ca, Mg | |
| ionic groups (carboxyl) | Chitin | neutral, insoluble |
| pH > 6 | hyaluronan | ionic, soluble, pH-depend. |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesàro, A. “The Good, the Bad and the Ugly” of Chitosans. Mar. Drugs 2016, 14, 99. https://doi.org/10.3390/md14050099
Bellich B, D’Agostino I, Semeraro S, Gamini A, Cesàro A. “The Good, the Bad and the Ugly” of Chitosans. Marine Drugs. 2016; 14(5):99. https://doi.org/10.3390/md14050099
Chicago/Turabian StyleBellich, Barbara, Ilenia D’Agostino, Sabrina Semeraro, Amelia Gamini, and Attilio Cesàro. 2016. "“The Good, the Bad and the Ugly” of Chitosans" Marine Drugs 14, no. 5: 99. https://doi.org/10.3390/md14050099