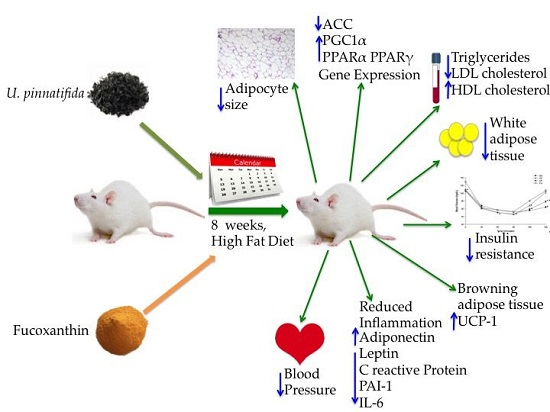
Undaria pinnatifida and Fucoxanthin Ameliorate Lipogenesis and Markers of Both Inflammation and Cardiovascular Dysfunction in an Animal Model of Diet-Induced Obesity
Abstract
:1. Introduction
2. Results
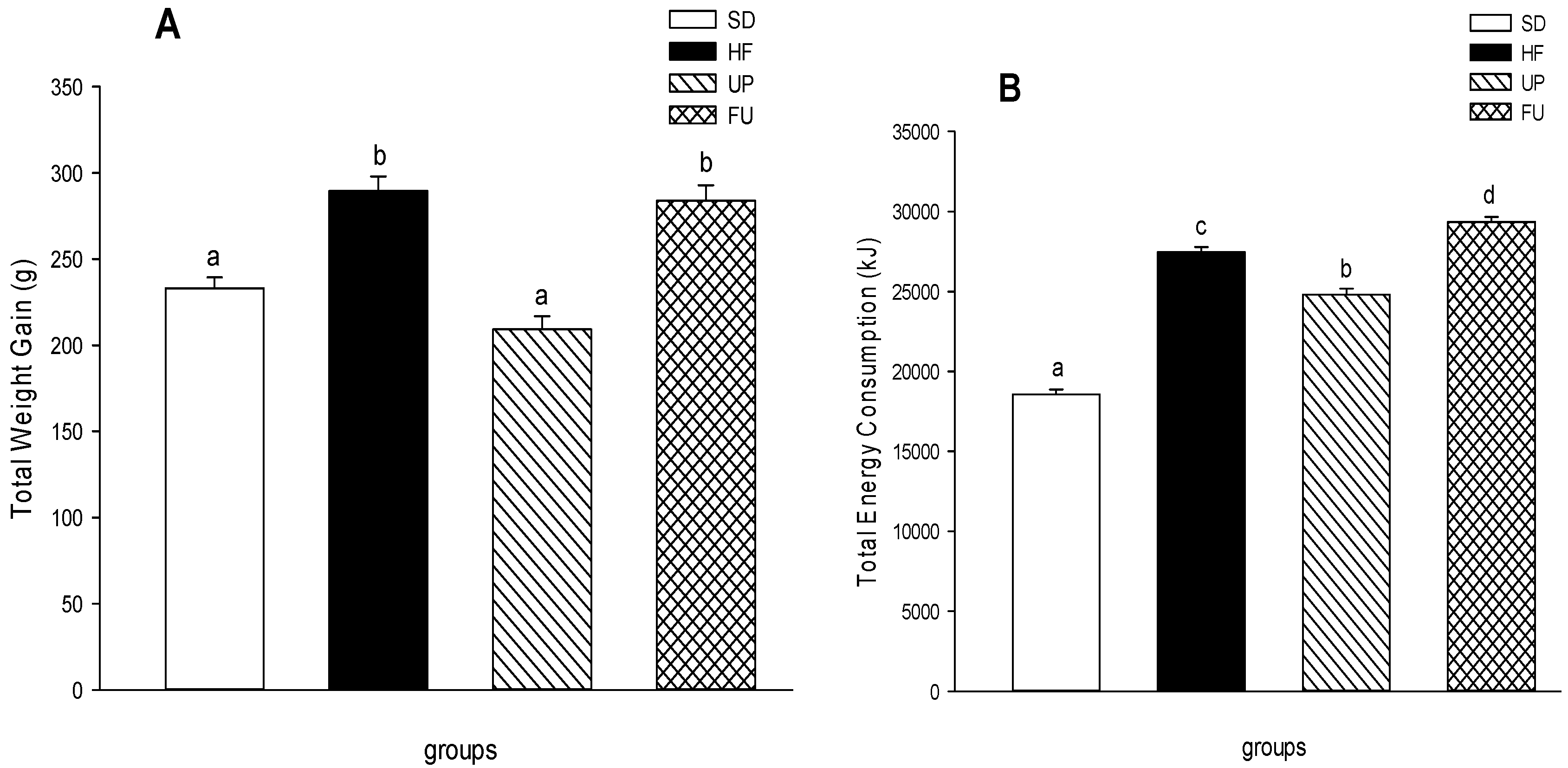
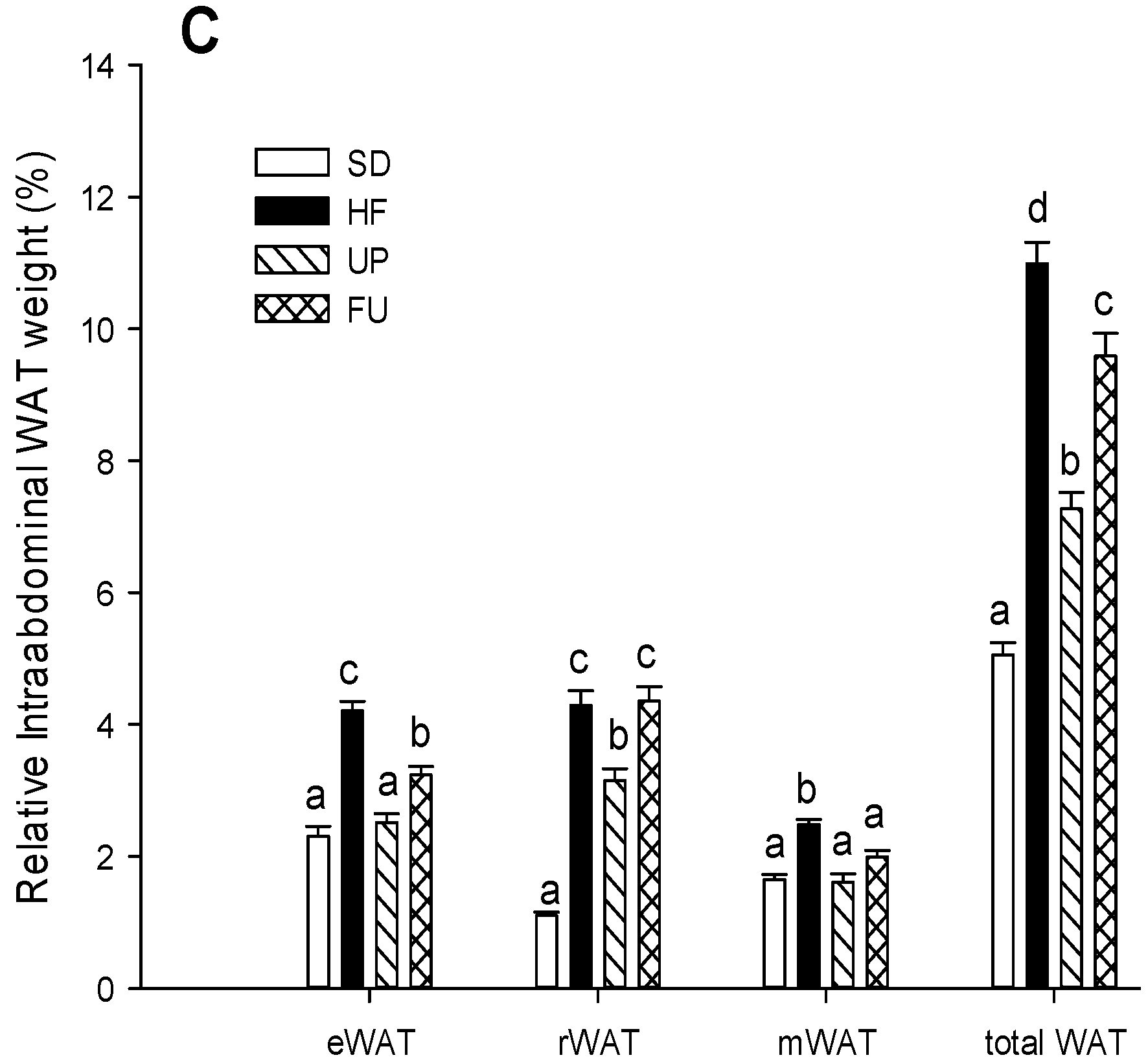
2.1. Effect of Undaria pinnatifida and Fucoxanthin on Body Weight Gain, Energy Intake, and Body Fat Mass
2.2. Effect of Undaria pinnatifida and Fucoxanthin on Serum Lipid Profile
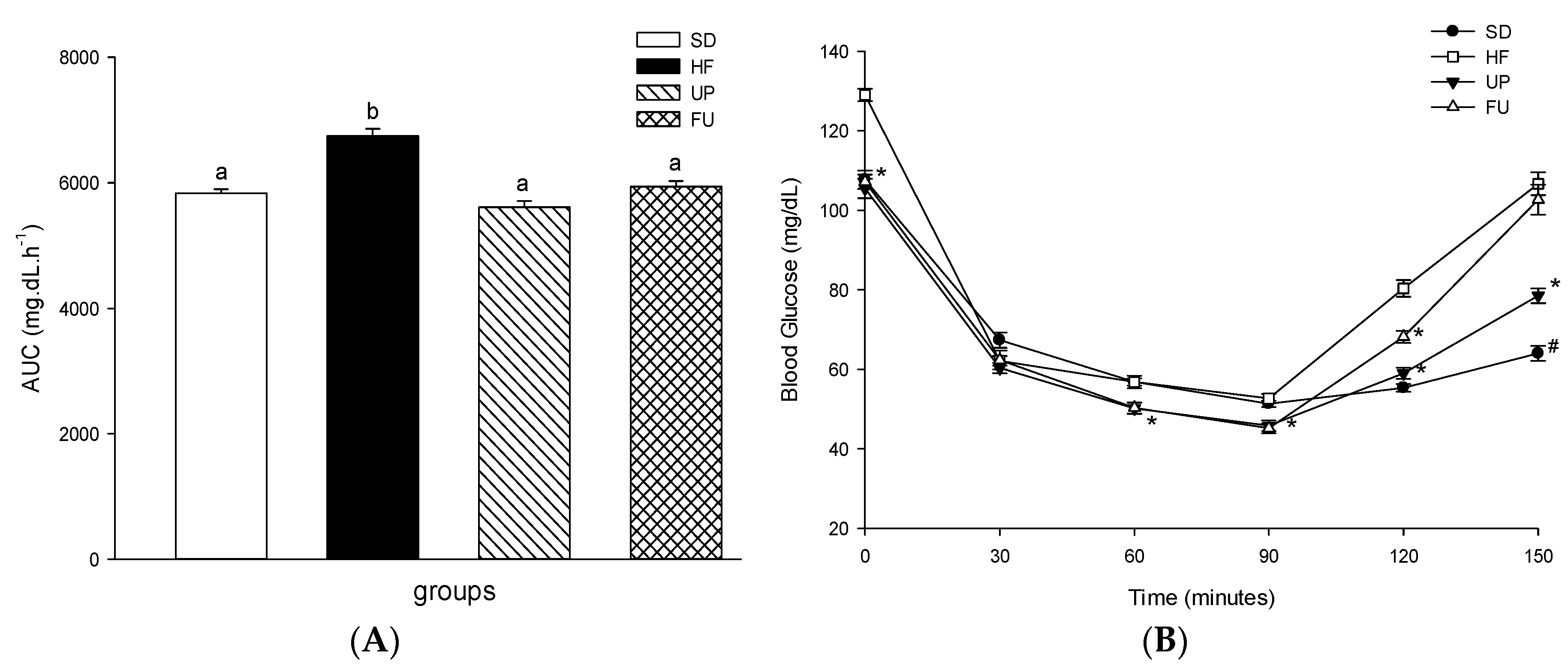
2.3. Effect of Undaria pinnatifida and Fucoxanthin on Glucose Homeostasis and Insulin Resistance
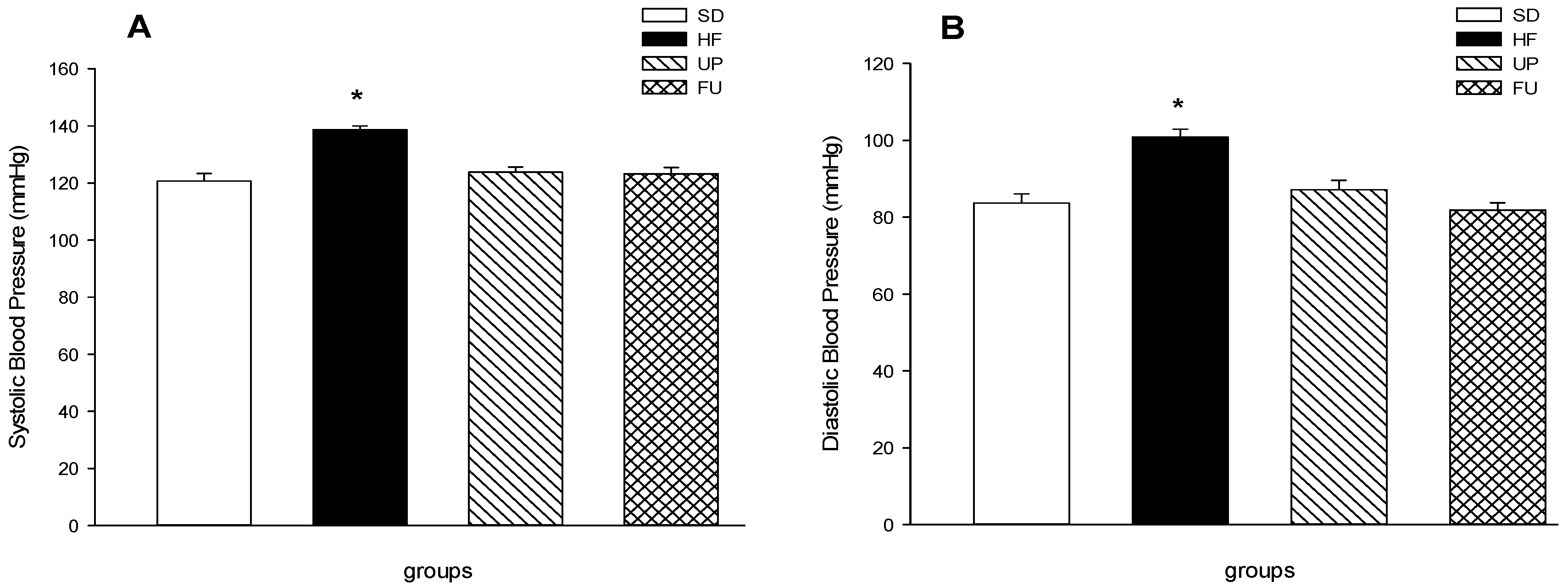
2.4. Effect of Undaria pinnatifida and Fucoxanthin on Blood Pressure
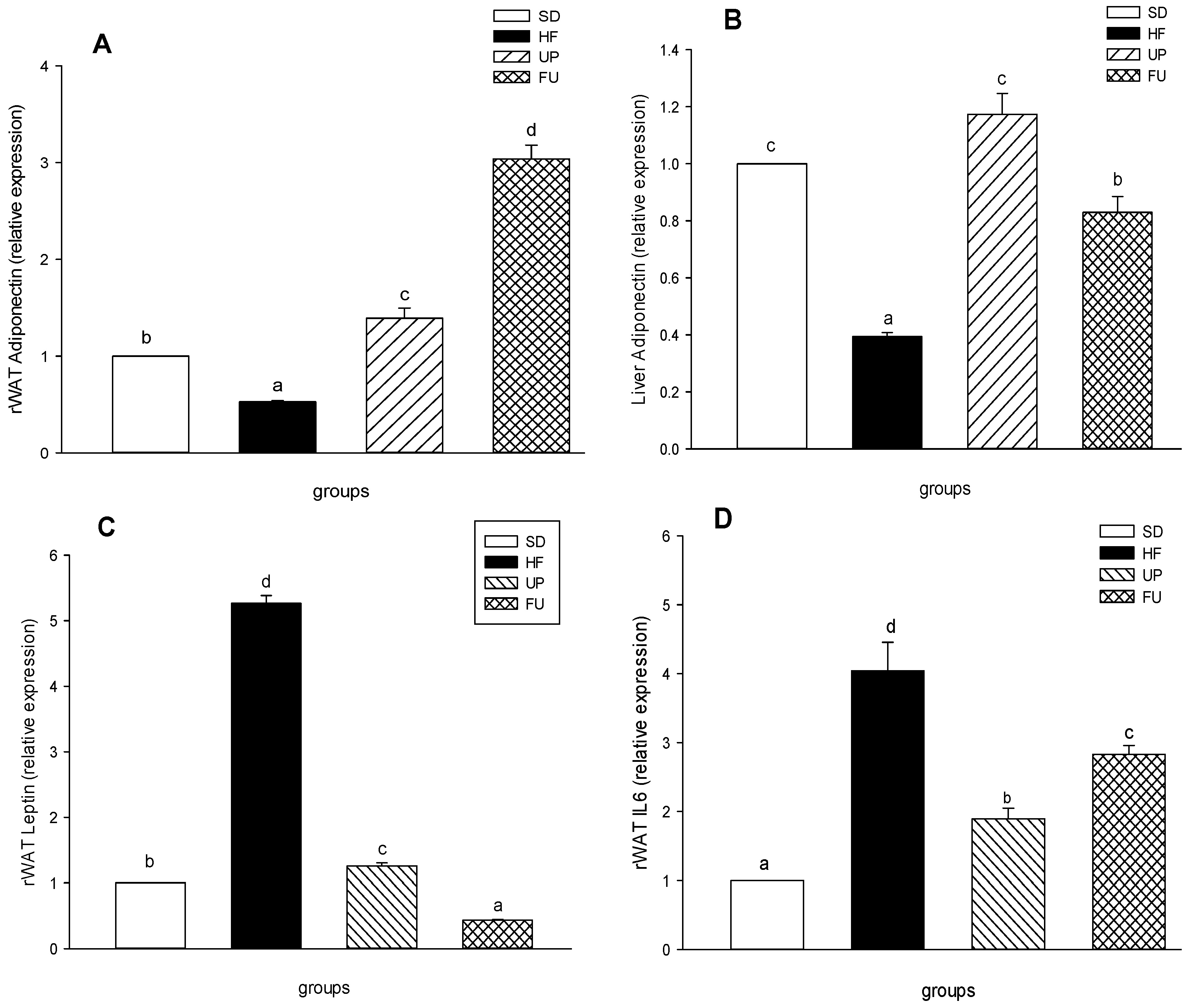
2.5. Effect of Undaria pinnatifida and Fucoxanthin on Cytokines in Blood Serum
2.6. Effect of Undaria pinnatifida and Fucoxanthin on Gene Expression of Inflammatory Markers
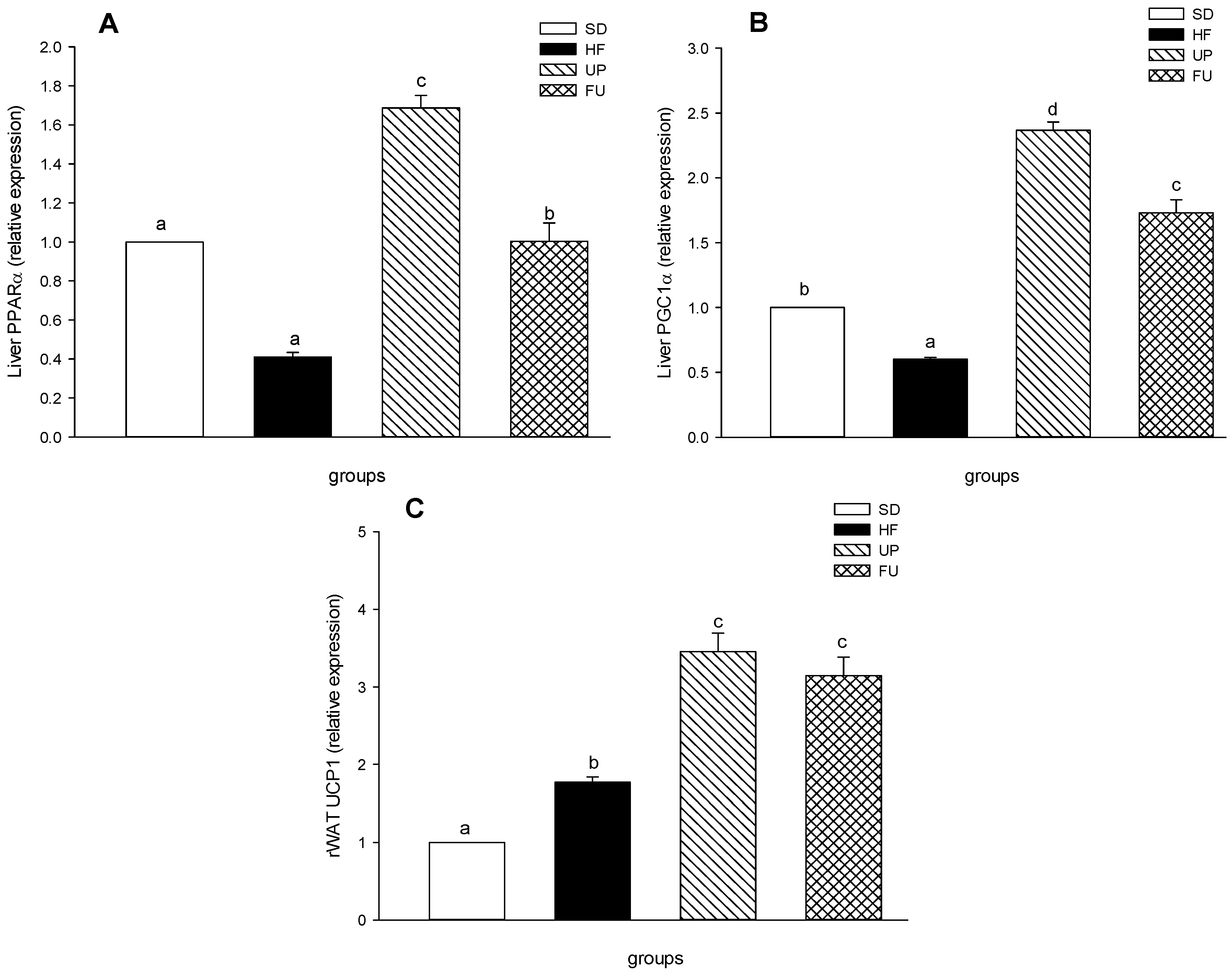
2.7. Effects of Undaria pinnatifida and Fucoxanthin on PPARα, PGC1α, and UCP1 Gene Expression
2.8. Effect of Undaria pinnatifida and Fucoxanthin on PPARγ and ACC Gene Expression
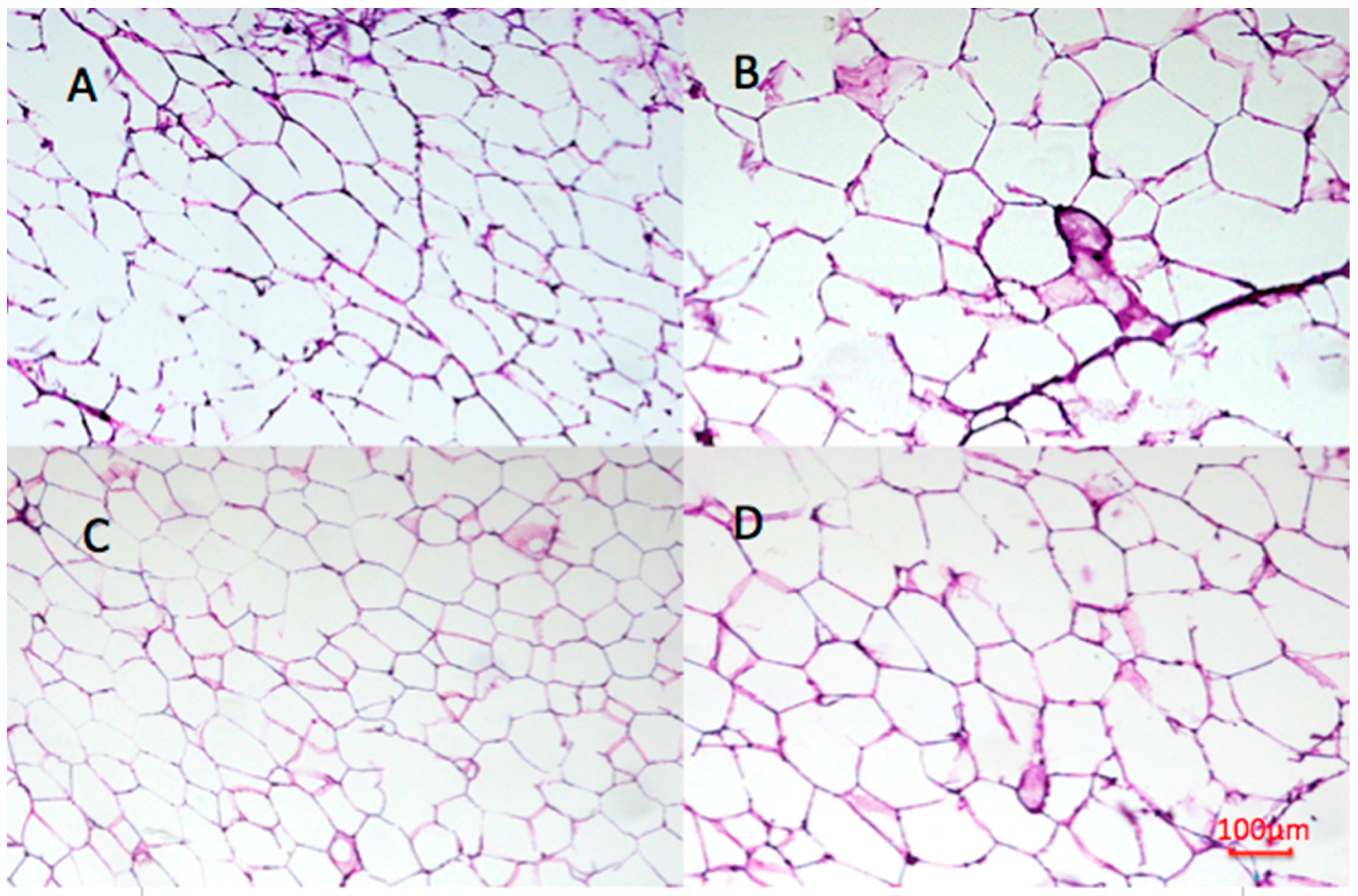
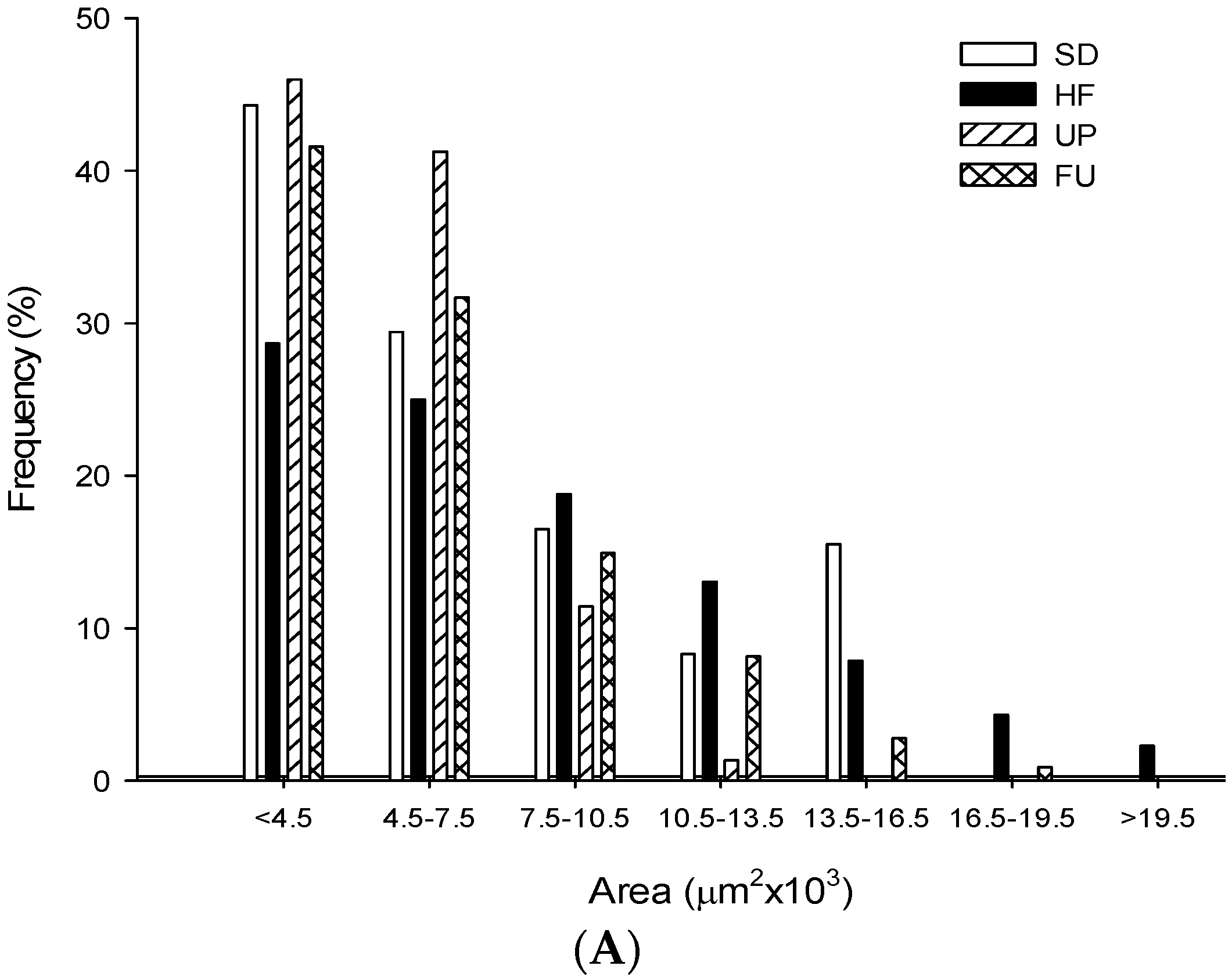
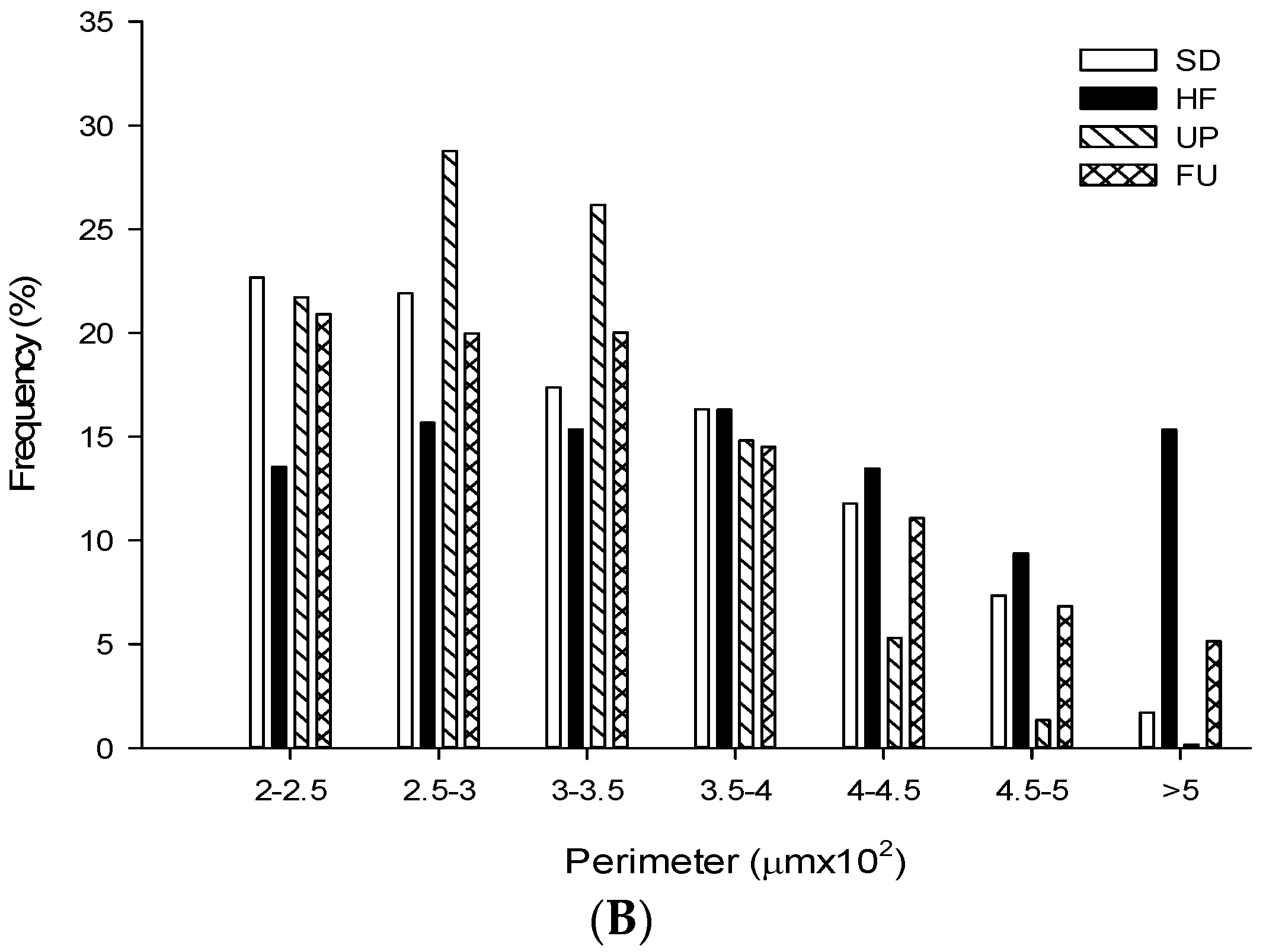
2.9. Effect of Undaria pinnatifida and Fucoxanthin on Adipocyte Size and Cellular Density
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Rat Model
4.3. Biochemical Analysis
4.4. Measurement of Insulin Sensitivity and Insulin Serum Levels
4.5. Blood Pressure Measurement
4.6. Cytokine Levels in Blood Serum
4.7. RNA Extraction
4.8. Real Time PCR
4.9. Histological Analysis of WAT
4.10. Image Analysis
4.11. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ACC | Acetyl Co-A carboxylase |
| CPT1 | Carnitine palmitoyltransferase 1 |
| CYP7A1 | Cholesterol 7 alpha-hydroxylase |
| EPA | Eicosapentaenoic acid |
| HDL | High density lipoprotein |
| IL-6 | Interleukin 6 |
| IRS | Insulin receptor substrate |
| LDL | Low density lipoprotein |
| MAPK | Mitogen activated protein kinase |
| PGC1α | PPARγ coactivator 1α |
| PPARα | Peroxisome-proliferator-activated receptor α |
| PPARγ | Peroxisome-proliferator-activated receptor γ |
| SREBP-1c | Sterol regulatory element-binding protein 1c |
| TG | Triglycerides |
| UCP-1 | Uncoupling protein 1 |
| VLDL | Very low density lipoprotein |
| WAT | White Adipose Tissue |
References
- World Health Organization. Media Center. Obesity and Overweight. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed on 18 April 2016).
- Wofford, L.G. Systematic Review of Childhood Obesity Prevention. Pediatr. Nurs. 2008, 23, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Champagne, C.M. Beyond Energy Balance: There is More to Obesity than Kilocalories. J. Am. Diet Assoc. 2005, 105, 27–23. [Google Scholar] [CrossRef] [PubMed]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal Chemodiversity and Bioactivity: Sources of Natural Variability and Implications for Commercial Application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Min, K.H.; Han, J.S.; Lee, D.H.; Park, D.B.; Jung, W.K.; Park, P.J.; Jeon, B.T.; Kim, S.K.; Jeon, Y.J. Effects of Brown Algae, Ecklonia cava on Glucose and Lipid Metabolism in C57BL/KsJ-db/db Mice, a Model of Type 2 Diabetes mellitus. Food Chem. Toxicol. 2012, 50, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Mizuno, Y.; Sibayama, S.; Hosokawa, M.; Miyashita, K. Antiobesity Effects of Undaria Lipid Capsules Prepared with Scallop Phospholipids. J. Food Sci. 2011, 76, H2–H6. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Ko, D.O.; Kim, B.S.; Ham, K.S.; Kang, S.G. Beneficial Effect of Seaweed on High-fat Diet-induced Oxidative Stress and Insulin Resistance in Rats. Food Sci. Biothecnol. 2015, 24, 2185–2191. [Google Scholar] [CrossRef]
- Fung, A.; Hamid, N.; Lu, J. Fucoxanthin Content and Antioxidant Properties of Undaria pinnatifida. Food Chem. 2013, 136, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Yuan, J.P.; Wu, C.F.; Wang, J.H. Fucoxanthin, a Marine Carotenoid Present in Brown Seaweeds and Diatoms: Metabolism and Bioactivities Relevant to Human Health. Mar. Drugs 2011, 9, 2806–1828. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, M.; Miyashita, T.; Nishikawa, S.; Emi, S.; Tsukui, T.; Beppu, F.; Okada, T.; Miyashita, K. Fucoxanthin Regulates Adipocytokine mRNA Expression in White Adipose Tissue of Diabetic/obese KK-Ay Mice. Arch. Biochem. Biophys. 2010, 504, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, Y.; Li, C.; Fu, Y.; Cai, F.; Chen, Q.; Li, D. Combination of Fucoxanthin and Conjugated Linoleic Acid Attenuates Body Weight Gain and Improves Lipid Metabolism in High-fat Diet-induced Obese Rats. Arch. Biochem. Biophys. 2012, 519, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.N.; Jeon, S.M.; Kim, H.J.; Lee, M.K.; Shin, S.K.; Shin, Y.C.; Park, Y.B.; Choi, M.S. Fucoxanthin Supplementation Improves Plasma and Hepatic Lipid Metabolism and Blood Glucose Concentration in High-fat Fed C57BL/6N Mice. Chem. Biol. Interact. 2010, 186, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, K.; Nishikawa, S.; Hosokawa, M. Therapeutic Effect of Fucoxanthin on Metabolic Syndrome and Type 2 Diabetes. In Nutritional and Therapeutic Interventions for Diabetes and Metabolic Syndrome; Bagchi, D., Sreejayan, N., Eds.; Academic Press: London, UK, 2012; Volume 1, pp. 367–379. [Google Scholar]
- Muradian, K.; Vaiserman, A.; Min, K.J.; Fraifeld, V.E. Fucoxanthin and Lipid Metabolism: A Minireview. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Bonet, M.L.; Oliver, P.; Palou, A. Pharmacological and Nutritional Agents Promoting Browning of White Adipose Tissue. Biochim. Biophys. Acta 2013, 1831, 969–985. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Murakami-Funayama, K.; Miyashita, K. Anti-obesity and Anti-diabetic Effects of Fucoxanthin on Diet-induced Obesity Conditions in a Murine Model. Mol. Med. Rep. 2009, 2, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Beppu, F.; Hosokawa, M.; Yim, M.J.; Shinoda, T.; Miyashita, K. Down-Regulation of Hepatic Stearoyl-CoA Desaturase-1 Expression by Fucoxanthin via Leptin Signaling in Diabetic/Obese KK-Ay Mice. Lipids 2013, 48, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Funayama, K.; Miyashita, K. Fucoxanthin from Edible Seaweed, Undaria pinnatifida, Shows Antiobesity Effect through UCP1 Expression in White Adipose Tissues. Biochem. Biophys. Res. Commun. 2005, 332, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.T.; Chou, H.N.; Huang, C.J. Dietary Fucoxanthin Increases Metabolic Rate and Upregulated mRNA Expression of the PGC-1alpha Network, Mitochondrial Biogenesis and Fusion Genes in White Adipose Tissues of Mice. Mar. Drugs 2014, 12, 964–982. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Lee, M.K.; Park, Y.B.; Shin, Y.C.; Choi, M.S. Beneficial Effects of Undaria pinnatifida Ethanol Extract o Diet-induced-insulin Resistance in C57BL/6J Mice. Food Chem. Toxicol. 2011, 49, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Pouyal, H.; Panchal, S.K.; Ward, L.C.; Brown, L. Effects of ALA, EPA, DHA in High-Carboydrate, High-Fat Diet-Induced Metabolic Syndrome in Rats. J. Nutr. Biochem. 2013, 24, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, J.Y.; Choi, W.H.; Lee, S.S. Effects of Seaweed Supplementation on Blood Glucose Concentration, Lipid Profile, and Antioxidant Enzyme Activities in Patients with Type 2 Diabetes mellitus. Nutr. Res. Pract. 2008, 2, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Ha, A.W.; Kim, W.K. The Effect of Fucoxanthin Rich Power on the Lipid Metabolism in Rats with a High Fat Diet. Nutr. Res. Pract. 2013, 7, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.M.; Kim, H.J.; Woo, M.N.; Lee, M.K.; Shin, Y.C.; Park, Y.B.; Choi, M.S. Fucoxanthin-rich Seaweed Extract Suppresses Body Weight Gain and Improves Lipid Metabolism in High-fat-fed C57BL/6J Mice. Biotechnol. J. 2010, 5, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.I.; Shin, H.S.; Kim, H.M.; Yoon, S.A.; Kang, S.W.; Kim, J.H.; Ko, H.C.; Kim, S.J. Petalonia binghamiae Extract and Its Constituent Fucoxanthin Ameliorate High-Fat Diet-Induced Obesity by Activating AMP-Activated Protein Kinase. J. Agric. Food Chem. 2012, 60, 3389–3395. [Google Scholar] [CrossRef] [PubMed]
- Awazawa, M.; Ueki, K.; Inabe, K.; Yamauchi, T.; Kubota, N.; Kaneko, K.; Kobayashi, M.; Iwane, A.; Sasako, T.; Okazaki, Y.; et al. Adiponectin Enhances Insulin Sensitivity by Increasing Hepatic IRS-2 Expression via a Macrophage-Derived Il-6-Dependant Pathway. Cell Metab. 2011, 13, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Jin, Y.B.; Lee, H.; Cha, M.; Sohn, E.; Moon, J.; Park, C.; Chun, S.; Jung, E.S.; Hong, J.S.; et al. Brown alga Ecklonia cava Attenuates Type 1 Diabetes by Activating AMPK and Akt Signaling pathways. Food Chem. Toxicol. 2010, 48, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Billakanti, J.M.; Catchpole, O.J.; Fenton, T.A.; Mitchell, K.A.; MacKenzie, A.D. Enzyme-assited Extraction of Fucoxanthin and Lipids Containing Polyunsaturated Fatty Acids from Undaria pinnatifida Using Dimethyl Ether and Ethanol. Process Biochem. 2013, 48, 1999–2008. [Google Scholar] [CrossRef]
- Khan, M.N.A.; Cho, J.Y.; Lee, M.C.; Kang, J.Y.; Park, N.G.; Fujii, H.; Hong, Y.K. Isolation of Two Anti-inflammatory and One Pro-inflammatory Polyunsaturated Fatty Acids from the Brown Seaweed Undaria pinnatifida. J. Agric. Food Chem. 2007, 55, 6984–6988. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Higuera, A.; Quevedo-Corona, L.; Paniagua-Castro, N.; Chamorro-Ceballos, G.; Milliar-García, A.; Jaramillo-Flores, M.E. Antioxidant Enzymes Gene Expression and Antihypertensive Effects of Seaweeds Ulva linza and Lessonia trabeculata in Rats Fed a High-fat and High-sucrose Diet. J. Appl. Phycol. 2014, 26, 597–605. [Google Scholar] [CrossRef]
- Jung, H.A.; Hyun, S.K.; Kim, H.R.; Choi, J.S. Angiotensin-converting Enzyme I Inhibitory Activity of Phlorotannins from Ecklonia stolonifera. Fish. Sci. 2006, 72, 1292–1299. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and Bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed]
- Gammone, M.A.; D’Orazio, N. Anti-Obesity Activity of the Marine Carotenoid Fucoxanthin. Mar. Drugs 2015, 13, 2196–2214. [Google Scholar] [CrossRef] [PubMed]
- Yudkin, J.S.; Stehouwer, C.D.A.; Emeis, J.J.; Coppack, S.W. C-Reactive Protein in Healthy Subjects: Associations with Obesity, Insulin Resistance, and Endothelial Dysfunction. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Ahima, R.; Osei, S. Leptin Signaling. Physiol. Behav. 2004, 81, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.; Godlewski, G.; Earley, B.; Zhou, L.; Jourdan, T.; Szanda, G.; Cinar, R.; Kunos, G. Role of Adiponectin in the Metabolic Effects of Cannabinoid Type 1 Receptor Blockade in Mice with Diet-induced Obesity. Am. J. Physiol. Endocrinol. Metab. 2014, 306, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.N.; Jeon, S.M.; Shin, Y.C.; Lee, M.K.; Kang, M.A.; Choi, M.S. Anti-obese Property of Fucoxanthin is Partly Mediated by Altering Lipid-regulating Enzymes and Uncoupling Proteins of Visceral Adipose Tissue in Mice. Mol. Nutr. Food Res. 2009, 53, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Hiuge, A.; Hiuge, A.; Tenenhaum, A.; Maeda, N.; Benderly, M.; Kumada, M.; Eisman, E.; Tanne, Z.; Matas, D.; Hibuse, T.; et al. Effects of Peroxisome Proliferator Activated Receptor Ligands Bezafibrate and Fenofibrate on Adiponectin Level. Arterioscelr. Thromb. Vasc. Biol. 2007, 27, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Nakai, M.; Maeda, H.; Hosokawa, M.; Sashima, T.; Miyashita, K. Suppresive Effect of Neoxanthin on the Differentiation of 3T3-L1 Adipose Cells. J. Oleo Sci. 2008, 57, 345–351. [Google Scholar] [PubMed]
- Medina-Gomez, G.; Gray, S.L.; Yetukuri, L.; Shimomura, K.; Virtue, S.; Campbell, M.; Curtis, R.K.; Jimenez-Linan, M.; Blount, M.; Yeo, G.S.H.; et al. PPAR gamma 2 Prevents Lipotoxicity by Controlling Adipose Tissue Expandability and Peripheral Lipid Metabolism. PLoS Genet. 2007, 3, e64. [Google Scholar] [CrossRef] [PubMed]
- Leonardini, A.; Laviola, L.; Perrini, S.; Natalicchio, A.; Giorgino, F. Cross-Talk between PPARγ and Insulin Signaling and Modulation of Insulin Sensitivity. PPAR Res. 2009, 2009, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Morán-Salvador, E.; Lopez-Parra, M.; García-Alonso, V.; Titos, E.; Martínez-Clemente, M.; González-Périz, A.; López-Vicario, C.; Barak, Y.; Arroyo, V.; Clària, J. Role for PPARγ in Obesity-induced Hepatic Steatosis as Determined by Hepatocyte-and Macrophage-specific Conditional Knockouts. FASEB J. 2011, 25, 2538–2550. [Google Scholar] [CrossRef] [PubMed]
- Gavriola, O.; Haluzik, M.; Matsusue, K.; Cutson, J.J.; Johnson, L.; Dietz, K.R.; Nicol, C.J.; Vinson, C.; Gonzalez, F.J.; Reitman, M.L. Liver Peroxisome Proliferator-activated Receptor γ Contributes to Hepatic Steatosis, Triglyceride Clearance, and Regulation of Body Fat Mass. J. Biol. Chem. 2003, 36, 34268–34276. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.M.; Toubro, S.; Astrup, A. PPARgamma Agonists int he Treatment of Type II diabetes: Is Increased Fatness Commensurate with Long-Term Efficacy? Int. J. Obes. 2003, 27, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Mendiola, J.A.; Marin, F.R.; Hernandez, S.F.; Arredondo, F.J.; Señorans, E.; Ibañez, E.; Reglero, G. Characterization via liquid chromatography coupled to diode array detector and tandem mass spectrometry of supercritical fluid antioxidant extracts of Spirulina platensis microalga. J. Sep. Sci. 2005, 28, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, C.; Racotta, R.; Quevedo, L. Glucosamine Attenuates Increases of Intraabdominal Fat, Serum Leptin Levels, and Insulin Resistance Induced by a High-fat Diet in Rats. Nutr. Res. 2010, 30, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Galarraga, M.; Campión, J.; Muñoz-Barrutia, A.; Boqué, N.; Moreno, H.; Martínez, J.A.; Milagro, F.; Ortiz-de-Solórzano, C. Adiposoft: Automated Software for the Analysis of White Adipose Tissue Cellularity in Histological Sections. J. Lipid Res. 2012, 53, 2791–2796. [Google Scholar] [CrossRef] [PubMed]


| Weekly Food Consumption (g) | ||||
|---|---|---|---|---|
| Week | SD | HF | UP | FU |
| 1 | 198.12 ± 5.92 a | 204.17 ± 6.99 a | 183.73 ± 4.14 a | 231.77 ± 3.48 b |
| 2 | 184.18 ± 5.84 b | 180.30 ± 6.95 b | 153.73 ± 3.67 a | 207.20 ± 3.75 c |
| 3 | 188.48 ± 4.33 b | 183.76 ± 5.34 b | 152.07 ± 3.34 a | 190.57 ± 5.57 b |
| 4 | 182.70 ± 4.69 ab | 183.16 ±4.57 b | 157.48 ± 3.89 a | 170.93 ± 4.05 ab |
| 5 | 177.45 ± 4.31 ab | 174.47 ± 5.08 ab | 167.27 ± 3.91 a | 189.08 ± 5.73 b |
| 6 | 176.80 ± 5.47 a | 176.48 ± 3.87 a | 173.85 ± 4.53 a | 195.12 ± 5.17a |
| 7 | 166.88 ± 2.23 a | 168.28 ± 4.06 ab | 162.62 ± 4.25 a | 188.77 ± 5.43 b |
| 8 | 164.22 ± 5.24 ab | 188.15 ± 4.77 b | 166.88 ± 3.73 a | 185.08 ± 5.32 ab |
| Average | 179.85 ± 3.92 ab | 182.35 ± 3.81 ab | 164.70 ± 3.76 a | 194.81 ± 6.37 b |
| Biochemical Parameters | SD | HF | UP | FU |
|---|---|---|---|---|
| Triacylglycerides (mg/dL) | 80.38 ± 2.05 b | 153.76 ± 3.41 d | 69.50 ± 1.97 a | 89.12 ± 2.09 c |
| Cholesterol (mg/dL) | 54.67 ± 2.04 a | 90.74 ± 1.85 c | 53.93 ± 0.95 a | 61.69 ± 1.29 b |
| HDL-Cholesterol (mg/dL) | 37.58 ± 0.25 b | 28.73 ± 0.73 a | 36.46 ± 0.39 b | 40.51 ± 0.59 c |
| LDL/VLDL-Cholesterol (mg/dL) | 9.35 ± 0.34 a | 55.36 ± 0.84 d | 13.64 ± 0.46 b | 16.20 ± 0.20 c |
| Glucose (mg/dL) | 83.83 ± 2.01 a | 139.5 ± 2.08 c | 86 ± 3.17 a | 99.5 ± 3.15 b |
| Insulin (ng/mL) | 3.49 ± 0.13 b | 5.21 ± 0.20 d | 2.93 ± 0.07 a | 3.99 ± 0.15 c |
| HOMA | 20.79 ± 0.69 a | 51.59 ± 1.39 c | 18.04±1.09 a | 28.23±1.31 b |
| Groups | Area (μm2 ×103) | Perimeter (μm ×102) | Ferret (μm ×102) | Cellular Density (# cells/mm2) |
|---|---|---|---|---|
| SD | 5.79 ± 2.96 b | 3.25 ± 0.84 b | 1.19 ± 0.33 b | 163.79 ± 9.89 b |
| HF | 8.02 ± 4.67 c | 3.74 ± 1.11 d | 1.31 ± 0.38 c | 111.78 ± 9.53 a |
| UP | 5.05 ± 2.00 a | 3.01 ± 0.61 a | 1.08 ± 0.23 a | 198.94 ± 17.92 c |
| FU | 6.01 ± 3.37 b | 3.33 ± 0.92 c | 1.21 ± 0.34 b | 160.65 ± 15.09 b |
| Component | Composition (g/kg diet) |
|---|---|
| Sucrose | 340.0 |
| Milk fat | 210.0 |
| Casein | 195.0 |
| Corn Starch | 140.5 |
| Cellulose | 50.0 |
| Mineral Mix 1 | 43.0 |
| Vitamin Mix 2 | 15.0 |
| DL Methionine | 3.0 |
| Choline | 2.0 |
| Cholesterol | 1.5 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grasa-López, A.; Miliar-García, Á.; Quevedo-Corona, L.; Paniagua-Castro, N.; Escalona-Cardoso, G.; Reyes-Maldonado, E.; Jaramillo-Flores, M.-E. Undaria pinnatifida and Fucoxanthin Ameliorate Lipogenesis and Markers of Both Inflammation and Cardiovascular Dysfunction in an Animal Model of Diet-Induced Obesity. Mar. Drugs 2016, 14, 148. https://doi.org/10.3390/md14080148
Grasa-López A, Miliar-García Á, Quevedo-Corona L, Paniagua-Castro N, Escalona-Cardoso G, Reyes-Maldonado E, Jaramillo-Flores M-E. Undaria pinnatifida and Fucoxanthin Ameliorate Lipogenesis and Markers of Both Inflammation and Cardiovascular Dysfunction in an Animal Model of Diet-Induced Obesity. Marine Drugs. 2016; 14(8):148. https://doi.org/10.3390/md14080148
Chicago/Turabian StyleGrasa-López, Ameyalli, Ángel Miliar-García, Lucía Quevedo-Corona, Norma Paniagua-Castro, Gerardo Escalona-Cardoso, Elba Reyes-Maldonado, and María-Eugenia Jaramillo-Flores. 2016. "Undaria pinnatifida and Fucoxanthin Ameliorate Lipogenesis and Markers of Both Inflammation and Cardiovascular Dysfunction in an Animal Model of Diet-Induced Obesity" Marine Drugs 14, no. 8: 148. https://doi.org/10.3390/md14080148