Marine Antifreeze Proteins: Structure, Function, and Application to Cryopreservation as a Potential Cryoprotectant
Abstract
:1. Introduction
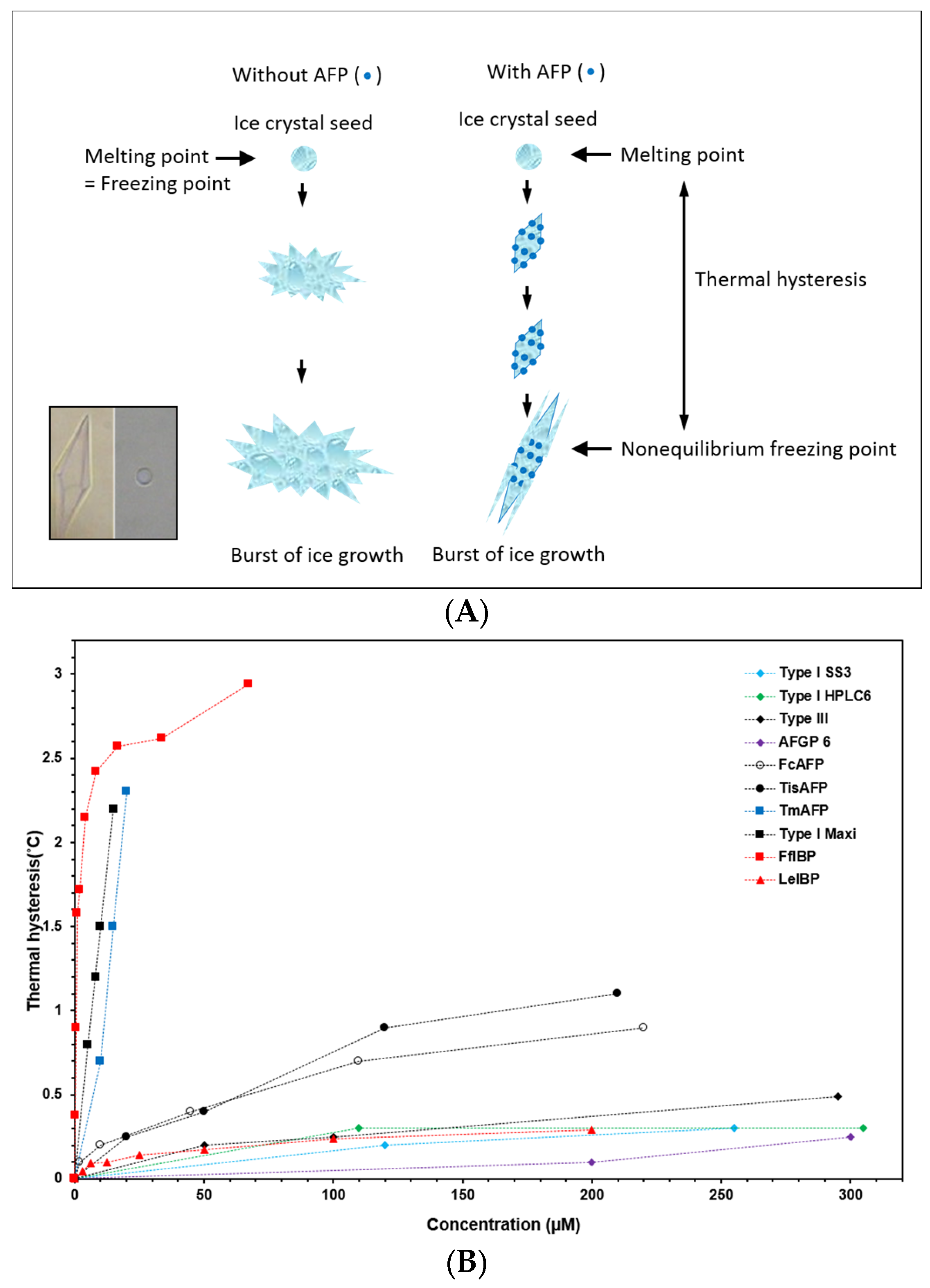
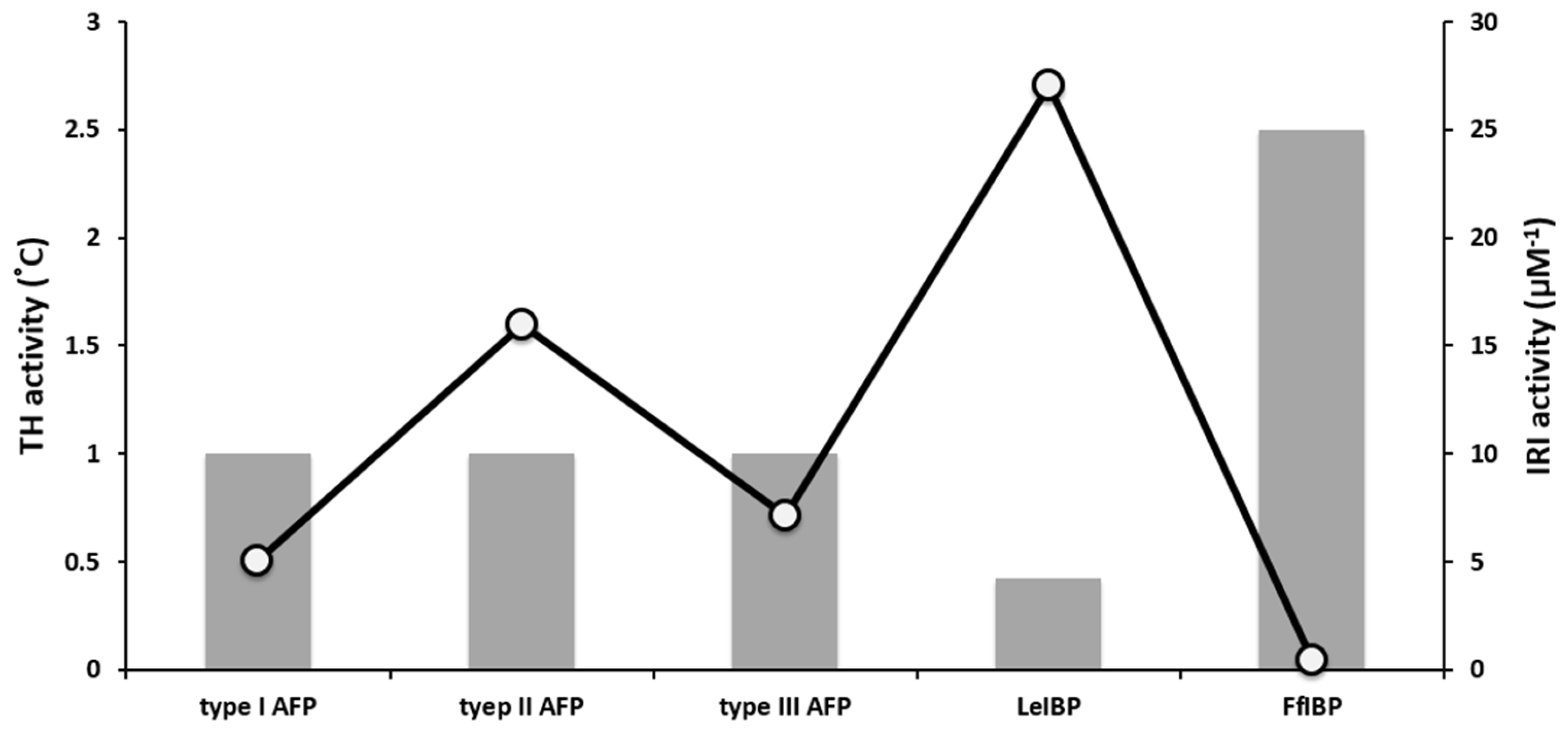
2. AFP Properties: Thermal Hysteresis (TH), Ice Recrystallization Inhibition (IRI), and Interaction with Biological Membranes
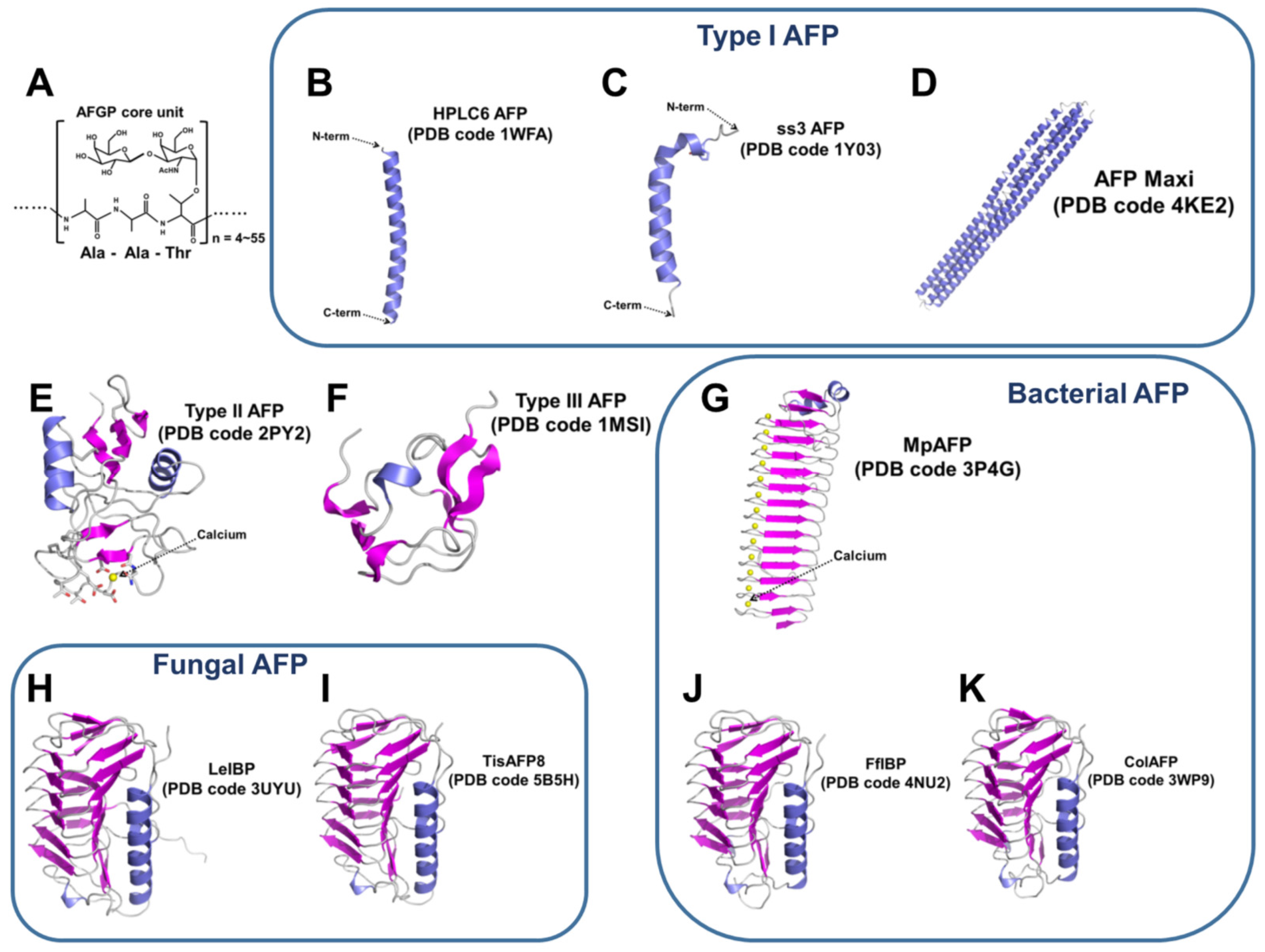
3. Marine-Derived AFPs
3.1. Fish AFPs
3.1.1. Type I AFPs
3.1.2. Type II AFPs
3.1.3. Type III AFPs
3.2. Fungal AFPs
3.3. Diatom AFPs
3.4. Bacterial AFPs
4. Cryopreservation Using AFPs as a Potential Cryoprotectants (CPAs)
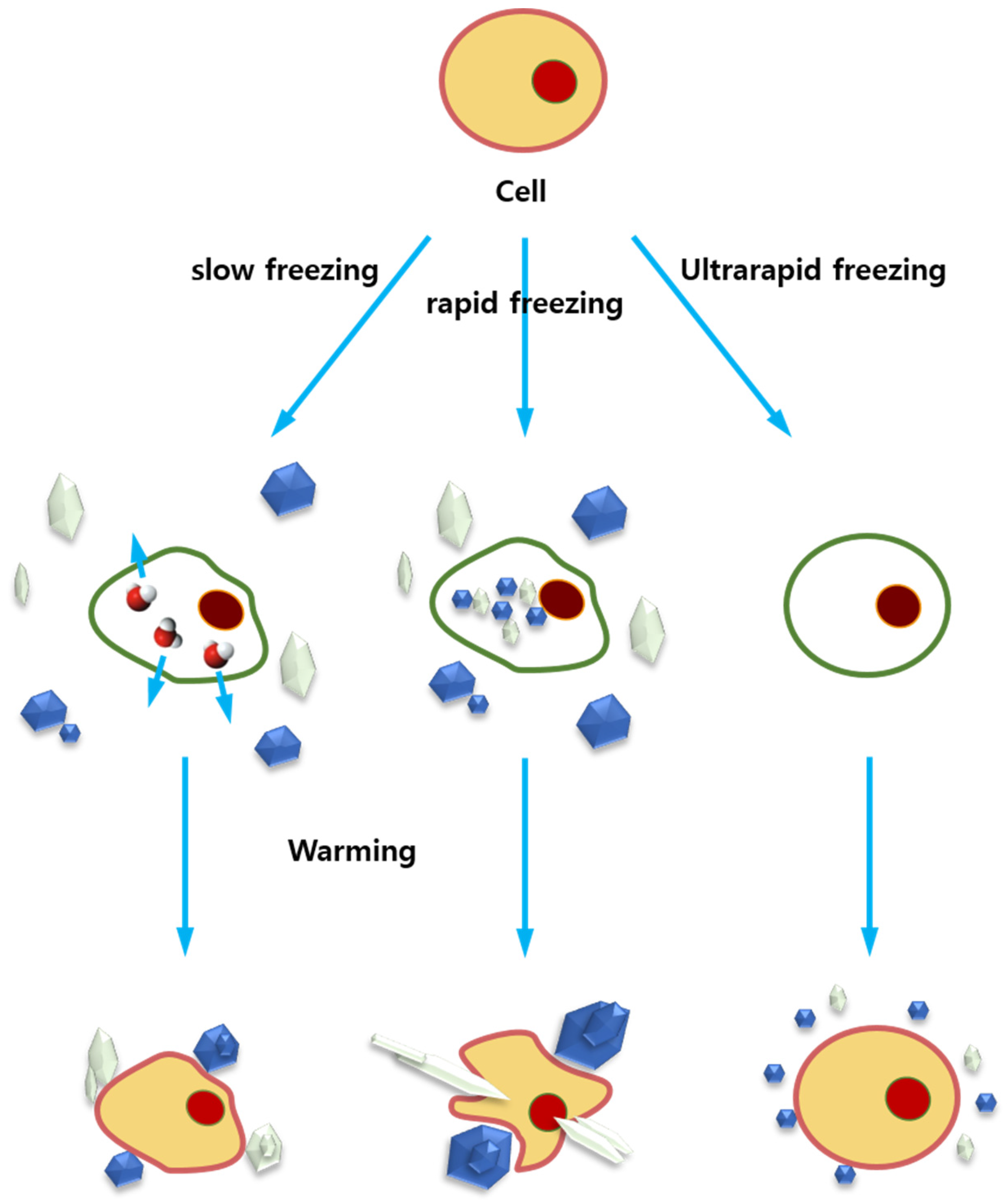
4.1. Cryopreservation and Ice Recrystallization
4.2. AFPs in Cryopreservation
5. Conclusions and Perspective
Acknowledgments
Conflicts of Interest
References
- DeVries, A.L.; Wohlschlag, D.E. Freezing resistance in some Antarctic fishes. Science 1969, 163, 1073–1075. [Google Scholar] [CrossRef] [PubMed]
- DeVries, A.L.; Komatsu, S.K.; Feeney, R.E. Chemical and physical properties of freezing point-depressing glycoproteins from Antarctic fishes. J. Biol. Chem. 1970, 245, 2901–2908. [Google Scholar] [PubMed]
- DeVries, A.L. Glycoproteins as biological antifreeze agents in antarctic fishes. Science 1971, 172, 1152–1155. [Google Scholar] [CrossRef] [PubMed]
- DeVries, A.L. Freezing resistance in fishes of the Antarctic penninsula. Antarct. J. US 1969, 4, 104–105. [Google Scholar]
- Davies, P.L. Ice-binding proteins: A remarkable diversity of structures for stopping and starting ice growth. Trends Biochem. Sci. 2014, 39, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A.; Davies, P.L.; Laybourn-Parry, J. A hyperactive, Ca2+-dependent antifreeze protein in an Antarctic bacterium. FEMS Microbiol. Lett. 2005, 245, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Do, H.; Kim, S.J.; Kim, H.J.; Lee, J.H. Structure-based characterization and antifreeze properties of a hyperactive ice-binding protein from the Antarctic bacterium Flavobacterium frigoris PS1. Acta Crystallogr. D Biol. Crystallogr. 2014, 70, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Raymond, J.A.; Fritsen, C.; Shen, K. An ice-binding protein from an Antarctic sea ice bacterium. FEMS Microbiol. Ecol. 2007, 61, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Hanada, Y.; Singh, S.M.; Tsuda, S. Antifreeze protein activity in Arctic cryoconite bacteria. FEMS Microbiol. Lett. 2014, 351, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Park, K.S.; Park, S.; Park, H.; Song, Y.H.; Kang, S.H.; Kim, H.J. An extracellular ice-binding glycoprotein from an Arctic psychrophilic yeast. Cryobiology 2010, 60, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Boo, S.Y.; Wong, C.M.V.L.; Rodrigues, K.F.; Najimudin, N.; Murad, A.M.A.; Mahadi, N.M. Thermal stress responses in Antarctic yeast, Glaciozyma antarctica PI12, characterized by real-time quantitative PCR. Polar Biol. 2013, 36, 381–389. [Google Scholar] [CrossRef]
- Hashim, N.H.; Bharudin, I.; Nguong, D.L.; Higa, S.; Bakar, F.D.; Nathan, S.; Rabu, A.; Kawahara, H.; Illias, R.M.; Najimudin, N.; et al. Characterization of Afp1, an antifreeze protein from the psychrophilic yeast Glaciozyma antarctica PI12. Extremophiles 2013, 17, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Kiko, R. Acquisition of freeze protection in a sea-ice crustacean through horizontal gene transfer? Polar Biol. 2010, 33, 543–556. [Google Scholar] [CrossRef]
- Jung, W.; Gwak, Y.; Davies, P.L.; Kim, H.J.; Jin, E. Isolation and characterization of antifreeze proteins from the antarctic marine microalga Pyramimonas gelidicola. Mar. Biotechnol. 2014, 16, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Gwak, I.G.; Jung, W.; Kim, H.J.; Kang, S.H.; Jin, E. Antifreeze protein in Antarctic marine diatom, Chaetoceros neogracile. Mar. Biotechnol. 2009, 12, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Janech, M.; Krell, A.; Mock, T.; Kang, J.-S.; Raymond, J. Ice-binding proteins from sea ice diatoms (bacillariophyceae). J. Phycol. 2006, 42, 410–416. [Google Scholar] [CrossRef] [Green Version]
- Krell, A.; Beszteri, B.; Dieckmann, G.; Glöckner, G.; Valentin, K.; Mock, T. A new class of ice-binding proteins discovered in a salt-stress-induced cDNA library of the psychrophilic diatom Fragilariopsis cylindrus (Bacillariophyceae). Eur. J. Phycol. 2008, 43, 423–433. [Google Scholar] [CrossRef]
- Kang, J.S.; Raymond, J.A. Reduction of freeze-thaw-induced hemolysis of red blood cells by an algal ice-binding protein. Cryo Lett. 2004, 25, 307–310. [Google Scholar]
- Raymond, J.A.; Janech, M.; Fritsen, C. Novel ice-binding proteins from a psychrophilic antarctic alga (Chlamydomonadaceae, chlorophyceae). J. Phycol. 2009, 45, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.L.; Hew, C.L. Biochemistry of fish antifreeze proteins. FASEB J. 1990, 4, 2460–2468. [Google Scholar] [PubMed]
- Hanada, Y.; Nishimiya, Y.; Miura, A.; Tsuda, S.; Kondo, H. Hyperactive antifreeze protein from an Antarctic sea ice bacterium Colwellia sp. has a compound ice-binding site without repetitive sequences. FEBS J. 2014, 281, 3576–3590. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.H.H.; Kar, R.K.; Asmawi, A.A.; Rahman, M.B.A.; Murad, A.M.A.; Mahadi, N.M.; Basri, M.; Rahman, R.N.Z.A.; Salleh, A.B.; Chatterjee, S.; et al. Solution structures, dynamics, and ice growth inhibitory activity of peptide fragments derived from an antarctic yeast protein. PLoS ONE 2012, 7, e49788. [Google Scholar] [CrossRef] [PubMed]
- Bayer-Giraldi, M.; Weikusat, I.; Besir, H.; Dieckmann, G. Characterization of an antifreeze protein from the polar diatom Fragilariopsis cylindrus and its relevance in sea ice. Cryobiology 2011, 63, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, C.; Kabisch, J.; Palm, G.J.; Valentin, K.; Schweder, T.; Krell, A. Heterologous expression, refolding and functional characterization of two antifreeze proteins from Fragilariopsis cylindrus (Bacillariophyceae). Cryobiology 2011, 63, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Hew, C.L.; Davies, P.L.; Fletcher, G. Antifreeze protein gene transfer in Atlantic salmon. Mol. Mar. Biol. Biotechnol. 1992, 1, 309–317. [Google Scholar] [PubMed]
- Wohrmann, A.P. Antifreeze glycopeptides of the high-Antarctic silverfish Pleuragramma antarcticum (Notothenioidei). Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1995, 111, 121–129. [Google Scholar] [CrossRef]
- Barrett, J. Thermal hysteresis proteins. Int. J. Biochem. Cell Biol. 2001, 33, 105–117. [Google Scholar] [CrossRef]
- Ben, R.N. Antifreeze glycoproteins-preventing the growth of ice. Chembiochem 2001, 2, 161–166. [Google Scholar] [CrossRef]
- Bouvet, V.; Ben, R.N. Antifreeze glycoproteins. Cell Biochem. Biophys. 2003, 39, 133–144. [Google Scholar] [CrossRef]
- Harding, M.M.; Anderberg, P.I.; Haymet, A.D. “Antifreeze” glycoproteins from polar fish. Eur. J. Biochem. 2003, 270, 1381–1392. [Google Scholar] [CrossRef] [PubMed]
- Fuller, B.J. Cryoprotectants: The essential antifreezes to protect life in the frozen state. Cryo Lett. 2004, 25, 375–388. [Google Scholar]
- Kristiansen, E.; Zachariassen, K.E. The mechanism by which fish antifreeze proteins cause thermal hysteresis. Cryobiology 2005, 51, 262–280. [Google Scholar] [CrossRef] [PubMed]
- Bar Dolev, M.; Braslavsky, I.; Davies, P.L. Ice-binding proteins and their function. Annu. Rev. Biochem. 2016, 85, 515–542. [Google Scholar] [CrossRef] [PubMed]
- Raymond, J.A.; DeVries, A.L. Freezing behavior of fish blood glycoproteins with antifreeze properties. Cryobiology 1972, 9, 541–547. [Google Scholar] [CrossRef]
- Raymond, J.A.; DeVries, A.L. Adsorption inhibition as a mechanism of freezing resistance in polar fishes. Proc. Natl. Acad. Sci. USA 1977, 74, 2589–2593. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.W.; Beaglehole, D.; Devries, A.L. Antifreeze glycopeptide adsorption on single crystal ice surfaces using ellipsometry. Biophys. J. 1993, 64, 1878–1884. [Google Scholar] [CrossRef]
- Wilson, P.W. Explaining thermal hysteresis by the Kelvin effect. Cryo Lett. 1993, 14, 31–36. [Google Scholar]
- Wilson, P.W.; Leader, J.P. Stabilization of supercooled fluids by thermal hysteresis proteins. Biophys. J. 1995, 68, 2098–2107. [Google Scholar] [CrossRef]
- Celik, Y.; Drori, R.; Pertaya-Braun, N.; Altan, A.; Barton, T.; Bar-Dolev, M.; Groisman, A.; Davies, P.L.; Braslavsky, I. Microfluidic experiments reveal that antifreeze proteins bound to ice crystals suffice to prevent their growth. Proc. Natl. Acad. Sci. USA 2013, 110, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Knight, C.A.; Cheng, C.C.; DeVries, A.L. Adsorption of alpha-helical antifreeze peptides on specific ice crystal surface planes. Biophys. J. 1991, 59, 409–418. [Google Scholar] [CrossRef]
- Do, H.; Lee, J.H.; Lee, S.G.; Kim, H.J. Crystallization and preliminary X-ray crystallographic analysis of an ice-binding protein (FfIBP) from Flavobacterium frigoris PS1. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2012, 68, 806–809. [Google Scholar] [CrossRef] [PubMed]
- Drori, R.; Celik, Y.; Davies, P.L.; Braslavsky, I. Ice-binding proteins that accumulate on different ice crystal planes produce distinct thermal hysteresis dynamics. J. R. Soc. Interface 2014, 11, 2014526. [Google Scholar] [CrossRef] [PubMed]
- Pertaya, N.; Marshall, C.B.; Celik, Y.; Davies, P.L.; Braslavsky, I. Direct visualization of spruce budworm antifreeze protein interacting with ice crystals: Basal plane affinity confers hyperactivity. Biophys. J. 2008, 95, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Do, H.; Lee, J.H.; Park, S.I.; Kim, E.J.; Kim, S.J.; Kang, S.H.; Kim, H.J. Characterization of the ice-binding protein from Arctic yeast Leucosporidium sp. AY30. Cryobiology 2012, 64, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, G.L.; Hew, C.L.; Davies, P.L. Antifreeze proteins of teleost fishes. Annu. Rev. Physiol. 2001, 63, 359–390. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.; Campbell, R.L.; Gwak, Y.; Kim, J.I.; Davies, P.L.; Jin, E. New cysteine-rich ice-binding protein secreted from Antarctic microalga, Chloromonas sp. PLoS ONE 2016, 11, e0154056. [Google Scholar] [CrossRef] [PubMed]
- Knight, C.A.; DeVries, A.L.; Oolman, L.D. Fish antifreeze protein and the freezing and recrystallization of ice. Nature 1984, 308, 295–296. [Google Scholar] [CrossRef] [PubMed]
- Knight, C.A.; Wen, D.; Laursen, R.A. Nonequilibrium antifreeze peptides and the recrystallization of ice. Cryobiology 1995, 32, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Raymond, J.A.; Fritsen, C.H. Semipurification and ice recrystallization inhibition activity of ice-active substances associated with Antarctic photosynthetic organisms. Cryobiology 2001, 43, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Raymond, J.A.; Knight, C.A. Ice binding, recrystallization inhibition, and cryoprotective properties of ice-active substances associated with Antarctic sea ice diatoms. Cryobiology 2003, 46, 174–181. [Google Scholar] [CrossRef]
- Jia, Z.; Davies, P.L. Antifreeze proteins: An unusual receptor-ligand interaction. Trends Biochem. Sci. 2002, 27, 101–106. [Google Scholar] [CrossRef]
- Knight, C.A.; Duman, J.G. Inhibition of recrystallization of ice by insect thermal hysteresis proteins: A possible cryoprotective role. Cryobiology 1986, 23, 256–262. [Google Scholar] [CrossRef]
- Worrall, D.; Elias, L.; Ashford, D.; Smallwood, M.; Sidebottom, C.; Lillford, P.; Telford, J.; Holt, C.; Bowles, D. A carrot leucine-rich-repeat protein that inhibits ice recrystallization. Science 1998, 282, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Smallwood, M.; Worrall, D.; Byass, L.; Elias, L.; Ashford, D.; Doucet, C.J.; Holt, C.; Telford, J.; Lillford, P.; Bowles, D.J. Isolation and characterization of a novel antifreeze protein from carrot (Daucus carota). Biochem. J. 1999, 340, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Sidebottom, C.; Buckley, S.; Pudney, P.; Twigg, S.; Jarman, C.; Holt, C.; Telford, J.; McArthur, A.; Worrall, D.; Hubbard, R.; et al. Heat-stable antifreeze protein from grass. Nature 2000, 406, 256. [Google Scholar] [CrossRef] [PubMed]
- Hew, C.L.; Yang, D.S. Protein interaction with ice. Eur. J. Biochem. 1992, 203, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Wohrmann, A. Antifreeze glycoproteins in fishes: Structure, mode of action and possible applications. Tierarztl. Prax. 1996, 24, 1–9. [Google Scholar] [PubMed]
- Knight, C.A.; Hallett, J.; DeVries, A.L. Solute effects on ice recrystallization: An assessment technique. Cryobiology 1988, 25, 55–60. [Google Scholar] [CrossRef]
- Tomczak, M.M.; Marshall, C.B.; Gilbert, J.A.; Davies, P.L. A facile method for determining ice recrystallization inhibition by antifreeze proteins. Biochem. Biophys. Res. Commun. 2003, 311, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Jackman, J.; Noestheden, M.; Moffat, D.; Pezacki, J.P.; Findlay, S.; Ben, R.N. Assessing antifreeze activity of AFGP 8 using domain recognition software. Biochem. Biophys. Res. Commun. 2007, 354, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Olijve, L.L.C.; Oude Vrielink, A.S.; Voets, I.K. A simple and quantitative method to evaluate ice recrystallization kinetics using the circle Hough Transform algorithm. Cryst. Growth Des. 2016, 16, 4190–4195. [Google Scholar] [CrossRef]
- Olijve, L.L.C.; Meister, K.; DeVries, A.L.; Duman, J.G.; Guo, S.; Bakker, H.J.; Voets, I.K. Blocking rapid ice crystal growth through nonbasal plane adsorption of antifreeze proteins. Proc. Natl. Acad. Sci. USA 2016, 113, 3740–3745. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.O.; Brown, A.; Middleton, A.J.; Tomczak, M.M.; Walker, V.K.; Davies, P.L. Ice restructuring inhibition activities in antifreeze proteins with distinct differences in thermal hysteresis. Cryobiology 2010, 61, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, A.K.; Do, H.; Park, K.S.; Moh, S.H.; Chi, Y.M.; Kim, H.J. Structural basis for the antifreeze activity of an ice-binding protein from an Arctic yeast. J. Biol. Chem. 2012, 287, 11460–11468. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, S.K.; Youm, H.W.; Kim, H.J.; Lee, J.R.; Suh, C.S.; Kim, S.H. Effects of three different types of antifreeze proteins on mouse ovarian tissue cryopreservation and transplantation. PLoS ONE 2015, 10, e0126252. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Lee, H.J.; Kim, H.J.; Lee, J.H.; Ko, Y.; Kim, S.M.; Lee, J.R.; Suh, C.S.; Kim, S.H. Effects of antifreeze proteins on the vitrification of mouse oocytes: Comparison of three different antifreeze proteins. Hum. Reprod. 2015, 30, 2110–2119. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.R.; Youm, H.W.; Lee, H.J.; Jee, B.C.; Suh, C.S.; Kim, S.H. Effect of antifreeze protein on mouse ovarian tissue cryopreservation and transplantation. Yonsei Med. J. 2015, 56, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.F.; Hansen, T.N. Antifreeze protein modulates cell survival during cryopreservation: Mediation through influence on ice crystal growth. Proc. Natl. Acad. Sci. USA 1992, 89, 8953–8957. [Google Scholar] [CrossRef] [PubMed]
- Amir, G.; Rubinsky, B.; Kassif, Y.; Horowitz, L.; Smolinsky, A.K.; Lavee, J. Preservation of myocyte structure and mitochondrial integrity in subzero cryopreservation of mammalian hearts for transplantation using antifreeze proteins-an electron microscopy study. Eur. J. Cardiothorac. Surg. 2003, 24, 292–297. [Google Scholar] [CrossRef]
- Rubinsky, B.; Arav, A.; Fletcher, G.L. Hypothermic protection-a fundamental property of “antifreeze” proteins. Biochem. Biophys. Res. Commun. 1991, 180, 566–571. [Google Scholar] [CrossRef]
- Quinn, P.J. A lipid-phase separation model of low-temperature damage to biological membranes. Cryobiology 1985, 22, 128–146. [Google Scholar] [CrossRef]
- Hays, L.M.; Feeney, R.E.; Crowe, L.M.; Crowe, J.H.; Oliver, A.E. Antifreeze glycoproteins inhibit leakage from liposomes during thermotropic phase transitions. Proc. Natl. Acad. Sci. USA 1996, 93, 6835–6840. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, M.M.; Hincha, D.K.; Estrada, S.D.; Wolkers, W.F.; Crowe, L.M.; Feeney, R.E.; Tablin, F.; Crowe, J.H. A mechanism for stabilization of membranes at low temperatures by an antifreeze protein. Biophys. J. 2002, 82, 874–881. [Google Scholar] [CrossRef]
- Tomczak, M.M.; Vigh, L.; Meyer, J.D.; Manning, M.C.; Hincha, D.K.; Crowe, J.H. Lipid unsaturation determines the interaction of AFP type I with model membranes during thermotropic phase transitions. Cryobiology 2002, 45, 135–142. [Google Scholar] [CrossRef]
- Tablin, F.; Oliver, A.E.; Walker, N.J.; Crowe, L.M.; Crowe, J.H. Membrane phase transition of intact human platelets: Correlation with cold-induced activation. J. Cell. Physiol. 1996, 168, 305–313. [Google Scholar] [CrossRef]
- Crowe, J.H.; Crowe, L.M. Water and carbohydrate interactions with membranes: Studies with infrared spectroscopy and differential scanning calorimetry methods. Methods Enzymol. 1986, 127, 696–703. [Google Scholar] [PubMed]
- Hays, L.M.; Crowe, J.H.; Wolkers, W.; Rudenko, S. Factors affecting leakage of trapped solutes from phospholipid vesicles during thermotropic phase transitions. Cryobiology 2001, 42, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, M.M.; Hincha, D.K.; Estrada, S.D.; Feeney, R.E.; Crowe, J.H. Antifreeze proteins differentially affect model membranes during freezing. Biochim. Biophys. Acta 2001, 1511, 255–263. [Google Scholar] [CrossRef]
- Kun, H.; Byk, G.; Mastai, Y. Effects of antifreeze protein fragments on the properties of model membranes. Adv. Exp. Med. Biol. 2009, 611, 85–86. [Google Scholar] [PubMed]
- Wu, Y.; Fletcher, G.L. Efficacy of antifreeze protein types in protecting liposome membrane integrity depends on phospholipid class. Biochim. Biophys. Acta 2001, 1524, 11–16. [Google Scholar] [CrossRef]
- Kun, H.; Mastai, Y. Isothermal calorimetry study of the interactions of type I antifreeze proteins with a lipid model membrane. Protein Pept. Lett. 2009, 17, 739–743. [Google Scholar] [CrossRef]
- Tomczak, M.M.; Hincha, D.K.; Crowe, J.H.; Harding, M.M.; Haymet, A.D. The effect of hydrophobic analogues of the type I winter flounder antifreeze protein on lipid bilayers. FEBS Lett. 2003, 551, 13–19. [Google Scholar] [CrossRef]
- Rubinsky, B.; Arav, A.; Mattioli, M.; Devries, A.L. The effect of antifreeze glycopeptides on membrane potential changes at hypothermic temperatures. Biochem. Biophys. Res. Commun. 1990, 173, 1369–1374. [Google Scholar] [CrossRef]
- Negulescu, P.A.; Rubinsky, B.; Fletcher, G.L.; Machen, T.E. Fish antifreeze proteins block Ca entry into rabbit parietal cells. Am. J. Physiol. 1992, 263, C1310–C1313. [Google Scholar] [PubMed]
- Arav, A.; Rubinsky, B.; Seren, E.; Roche, J.F.; Boland, M.P. The role of thermal hysteresis proteins during cryopreservation of oocytes and embryos. Theriogenology 1994, 41, 107–112. [Google Scholar] [CrossRef]
- Rubinsky, B.; Mattioli, M.; Arav, A.; Barboni, B.; Fletcher, G.L. Inhibition of Ca2+ and K+ currents by “antifreeze” proteins. Am. J. Physiol. 1992, 262, R542–R545. [Google Scholar] [PubMed]
- Wang, J.H.; Bian, H.W.; Huang, C.N.; Ge, J.G. Studies on the application of antifreeze proteins in cryopreservation of rice suspension cells. Shi Yan Sheng Wu Xue Bao 1999, 32, 271–276. [Google Scholar] [PubMed]
- Wang, L.H.; Wusteman, M.C.; Smallwood, M.; Pegg, D.E. The stability during low-temperature storage of an antifreeze protein isolated from the roots of cold-acclimated carrots. Cryobiology 2002, 44, 307–310. [Google Scholar] [CrossRef]
- Ishiguro, H.; Rubinsky, B. Influence of fish antifreeze proteins on the freezing of cell suspensions with cryoprotectant penetrating cells. Int. J. Heat Mass Transf. 1998, 41, 1907–1915. [Google Scholar] [CrossRef]
- Payne, S.R.; Oliver, J.E.; Upreti, G.C. Effect of antifreeze proteins on the motility of ram spermatozoa. Cryobiology 1994, 31, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Lagneaux, D.; Huhtinen, M.; Koskinen, E.; Palmer, E. Effect of anti-freeze protein (AFP) on the cooling and freezing of equine embryos as measured by DAPI-staining. Equine Vet. J. Suppl. 1997, 25, 85–87. [Google Scholar] [CrossRef]
- Shaw, J.M.; Ward, C.; Trounson, A.O. Evaluation of propanediol, ethylene glycol, sucrose and antifreeze proteins on the survival of slow-cooled mouse pronuclear and 4-cell embryos. Hum. Reprod. 1995, 10, 396–402. [Google Scholar] [PubMed]
- Mezhevikina, L.M.; Karanova, M.V. The use of antifreeze glycoproteins in the freezing in liquid nitrogen of early mouse embryos. Izv. Akad. Nauk. Seriia Biol. Akad. Nauk. 1994, 2, 172–177. [Google Scholar]
- Soltys, K.A.; Batta, A.K.; Koneru, B. Successful nonfreezing, subzero preservation of rat liver with 2,3-butanediol and type I antifreeze protein. J. Surg. Res. 2001, 96, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Zhang, L.; Wang, B.; Yan, B.; Wang, Q. Cryopreservation of marine diatom algae by encapsulation-vitrification. Cryo Lett. 2009, 30, 224–231. [Google Scholar]
- Larese, A.; Acker, J.; Muldrew, K.; Yang, H.Y.; McGann, L. Antifreeze proteins induce intracellular nucleation. Cryoletters 1996, 17, 175–182. [Google Scholar]
- Scholander, P.F.; Van Dam, L.; Kanwisher, J.W.; Hammel, H.T.; Gordon, M.S. Supercooling and osmoregulation in Arctic fish. J. Cell. Physiol. 1957, 49, 5–24. [Google Scholar] [CrossRef]
- Komatsu, S.K.; DeVries, A.L.; Feeney, R.E. Studies of the structure of freezing point-depressing glycoproteins from an Antarctic fish. J. Biol. Chem. 1970, 245, 2909–2913. [Google Scholar] [PubMed]
- Duman, J.G.; Devries, A.L. Freezing resistance in winter flounder Pseudopleuronectes americanus. Nature 1974, 247, 237–238. [Google Scholar] [CrossRef]
- Slaughter, D.; Fletcher, G.L.; Ananthanarayanan, V.S.; Hew, C.L. Antifreeze proteins from the sea raven, Hemitripterus americanus. Further evidence for diversity among fish polypeptide antifreezes. J. Biol. Chem. 1981, 256, 2022–2026. [Google Scholar] [PubMed]
- Feeney, R.E.; Yeh, Y. Antifreeze proteins from fish bloods. Adv. Protein Chem. 1978, 32, 191–282. [Google Scholar] [PubMed]
- Burcham, T.S.; Osuga, D.T.; Chino, H.; Feeney, R.E. Analysis of antifreeze glycoproteins in fish serum. Anal. Biochem. 1984, 139, 197–204. [Google Scholar] [CrossRef]
- Kao, M.H.; Fletcher, G.L.; Wang, N.C.; Hew, C.L. The relationship between molecular weight and antifreeze polypeptide activity in marine fish. Can. J. Zool. 1986, 64, 578–582. [Google Scholar] [CrossRef]
- Ahlgren, J.A.; Cheng, C.C.; Schrag, J.D.; DeVries, A.L. Freezing avoidance and the distribution of antifreeze glycopeptides in body fluids and tissues of Antarctic fish. J. Exp. Biol. 1988, 137, 549–563. [Google Scholar] [PubMed]
- Hew, C.L.; Wang, N.C.; Yan, S.; Cai, H.; Sclater, A.; Fletcher, G.L. Biosynthesis of antifreeze polypeptides in the winter flounder. Eur. J. Biochem. 1986, 160, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.; Houston, M.E., Jr.; Hodges, R.S.; Kay, C.M.; Sykes, B.D.; Loewen, M.C.; Davies, P.L.; Sönnichsen, F.D. A diminished role for hydrogen bonds in antifreeze protein binding to ice. Biochemistry 1997, 36, 14652–14660. [Google Scholar] [CrossRef] [PubMed]
- Haymet, A.D.; Ward, L.G.; Harding, M.M.; Knight, C.A. Valine substituted winter flounder “antifreeze”: Preservation of ice growth hysteresis. FEBS Lett. 1998, 430, 301–306. [Google Scholar] [CrossRef]
- Loewen, M.C.; Chao, H.; Houston, M.E., Jr.; Baardsnes, J.; Hodges, R.S.; Kay, C.M.; Sykes, B.D.; Sönnichsen, F.D.; Davies, P.L. Alternative roles for putative ice-binding residues in type I antifreeze protein. Biochemistry 1999, 38, 4743–4749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Laursen, R.A. Structure-function relationships in a type I antifreeze polypeptide. The role of threonine methyl and hydroxyl groups in antifreeze activity. J. Biol. Chem. 1998, 273, 34806–34812. [Google Scholar] [CrossRef] [PubMed]
- Vasina, E.N.; Paszek, E.; Nicolau, D., Jr.; Nicolau, D.V. The BAD project: Data mining, database and prediction of protein adsorption on surfaces. Lab Chip 2009, 9, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Baardsnes, J.; Kondejewski, L.H.; Hodges, R.S.; Chao, H.; Kay, C.; Davies, P.L. New ice-binding face for type I antifreeze protein. FEBS Lett. 1999, 463, 87–91. [Google Scholar] [CrossRef]
- Ewart, K.V.; Yang, D.S.; Ananthanarayanan, V.S.; Fletcher, G.L.; Hew, C.L. Ca2+-dependent antifreeze proteins. Modulation of conformation and activity by divalent metal ions. J. Biol. Chem. 1996, 271, 16627–16632. [Google Scholar] [PubMed]
- Gronwald, W.; Loewen, M.C.; Lix, B.; Daugulis, A.J.; Sönnichsen, F.D.; Davies, P.L.; Sykes, B.D. The solution structure of type II antifreeze protein reveals a new member of the lectin family. Biochemistry 1998, 37, 4712–4721. [Google Scholar] [CrossRef] [PubMed]
- Drickamer, K. C-type lectin-like domains. Curr. Opin. Struct. Biol. 1999, 9, 585–590. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Lin, Q.; Kosinski, J.; Seetharaman, J.; Bujnicki, J.M.; Sivaraman, J.; Hew, C.L. Structure and evolutionary origin of Ca2+-dependent herring type II antifreeze protein. PLoS ONE 2007, 2, e548. [Google Scholar] [CrossRef] [PubMed]
- Nishimiya, Y.; Kondo, H.; Takamichi, M.; Sugimoto, H.; Suzuki, M.; Miura, A.; Tsuda, S. Crystal structure and mutational analysis of Ca2+-independent type II antifreeze protein from longsnout poacher, Brachyopsis rostratus. J. Mol. Biol. 2008, 382, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Ewart, K.V.; Lin, Q.; Hew, C.L. Structure, function and evolution of antifreeze proteins. Cell. Mol. Life Sci. 1999, 55, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Miura, R.; Takemoto, Y.; Tsuda, S.; Kawahara, H.; Obata, H. Type II antifreeze protein from a mid-latitude freshwater fish, Japanese smelt (Hypomesus nipponensis). Biosci. Biotechnol. Biochem. 2003, 67, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.; Feeney, R.E. Antifreeze proteins: Structures and mechanisms of function. Chem. Rev. 1996, 96, 601–618. [Google Scholar] [CrossRef] [PubMed]
- Antson, A.A.; Smith, D.J.; Roper, D.I.; Lewis, S.; Caves, L.S.; Verma, C.S.; Buckley, S.L.; Lillford, P.J.; Hubbard, R.E. Understanding the mechanism of ice binding by type III antifreeze proteins. J. Mol. Biol. 2001, 305, 875–889. [Google Scholar] [CrossRef] [PubMed]
- Hew, C.L.; Wang, N.C.; Joshi, S.; Fletcher, G.L.; Scott, G.K.; Hayes, P.H.; Buettner, B.; Davies, P.L. Multiple genes provide the basis for antifreeze protein diversity and dosage in the ocean pout, Macrozoarces americanus. J. Biol. Chem. 1988, 263, 12049–12055. [Google Scholar] [PubMed]
- Nishimiya, Y.; Sato, R.; Takamichi, M.; Miura, A.; Tsuda, S. Co-operative effect of the isoforms of type III antifreeze protein expressed in Notched-fin eelpout, Zoarces elongatus Kner. FEBS J. 2005, 272, 482–492. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, C.I.; Chao, H.; Sönnichsen, F.D.; Sykes, B.D.; Davies, P.L. Effect of type III antifreeze protein dilution and mutation on the growth inhibition of ice. Biophys. J. 1996, 71, 2346–2355. [Google Scholar] [CrossRef]
- Baardsnes, J.; Davies, P.L. Contribution of hydrophobic residues to ice binding by fish type III antifreeze protein. Biochim. Biophys. Acta 2002, 1601, 49–54. [Google Scholar] [CrossRef]
- Chao, H.; Sönnichsen, F.D.; DeLuca, C.I.; Sykes, B.D.; Davies, P.L. Structure-function relationship in the globular type III antifreeze protein: Identification of a cluster of surface residues required for binding to ice. Protein Sci. 1994, 3, 1760–1769. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; DeLuca, C.I.; Chao, H.; Davies, P.L. Structural basis for the binding of a globular antifreeze protein to ice. Nature 1996, 384, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Sönnichsen, F.D.; DeLuca, C.I.; Davies, P.L.; Sykes, B.D.; Sönnichsen, F.D.; DeLuca, C.I.; Davies, P.L.; Sykes, B.D. Refined solution structure of type III antifreeze protein: Hydrophobic groups may be involved in the energetics of the protein–ice interaction. Structure 1996, 4, 1325–1337. [Google Scholar] [CrossRef]
- Leiter, A.; Rau, S.; Winger, S.; Muhle-Goll, C.; Luy, B.; Gaukel, V. Influence of heating temperature, pressure and pH on recrystallization inhibition activity of antifreeze protein type III. J. Food Eng. 2016, 187, 53–61. [Google Scholar] [CrossRef]
- Hamada, T.; Ito, Y.; Abe, T.; Hayashi, F.; Guntert, P.; Inoue, M.; Kigawa, T.; Terada, T.; Shirouzu, M.; Yoshida, M.; et al. Solution structure of the antifreeze-like domain of human sialic acid synthase. Protein Sci. 2006, 15, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-G.; Park, C.-J.; Kim, H.-E.; Seo, Y.-J.; Lee, A.-R.; Choi, S.-R.; Lee, S.S.; Lee, J.-H. Comparison of backbone dynamics of the type III antifreeze protein and antifreeze-like domain of human sialic acid synthase. J. Biomol. NMR 2015, 61, 137–150. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, C.I.; Davies, P.L.; Ye, Q.; Jia, Z. The effects of steric mutations on the structure of type III antifreeze protein and its interaction with ice. J. Mol. Biol. 1998, 275, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Graether, S.P.; DeLuca, C.I.; Baardsnes, J.; Hill, G.A.; Davies, P.L.; Jia, Z. Quantitative and qualitative analysis of type III antifreeze protein structure and function. J. Biol. Chem. 1999, 274, 11842–11847. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Jia, Z. Ice-binding surface of fish type III antifreeze. Biophys. J. 1999, 77, 1602–1608. [Google Scholar] [CrossRef]
- Garnham, C.P.; Natarajan, A.; Middleton, A.J.; Kuiper, M.J.; Braslavsky, I.; Davies, P.L. Compound ice-binding site of an antifreeze protein revealed by mutagenesis and fluorescent tagging. Biochemistry 2010, 49, 9063–9071. [Google Scholar] [CrossRef] [PubMed]
- Garnham, C.P.; Nishimiya, Y.; Tsuda, S.; Davies, P.L. Engineering a naturally inactive isoform of type III antifreeze protein into one that can stop the growth of ice. FEBS Lett. 2012, 586, 3876–3881. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Seo, Y.; Kim, M.; Eo, Y.; Ahn, H.; Lee, A.; Park, C.; Ryu, K.; Cheong, H.; Lee, S.S. NMR study of the antifreeze activities of active and inactive isoforms of a type III antifreeze protein. FEBS Lett. 2016, 590, 4202–4212. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; DeVries, A.L.; Cheng, C.H. Antifreeze peptide heterogeneity in an antarctic eel pout includes an unusually large major variant comprised of two 7 kDa type III AFPs linked in tandem. Biochim. Biophys. Acta 1995, 1247, 163–172. [Google Scholar] [CrossRef]
- Miura, K.; Ohgiya, S.; Hoshino, T.; Nemoto, N.; Suetake, T.; Miura, A.; Spyracopoulos, L.; Kondo, H.; Tsuda, S. NMR analysis of type III antifreeze protein intramolecular dimer. Structural basis for enhanced activity. J. Biol. Chem. 2001, 276, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Ohgiya, S.; Hoshino, T.; Nemoto, N.; Odaira, M.; Nitta, K.; Tsuda, S. Determination of the solution structure of the N-domain plus linker of Antarctic eel pout antifreeze protein RD3. J. Biochem. 1999, 126, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Raymond, J.A.; Christner, B.C.; Schuster, S.C. A bacterial ice-binding protein from the Vostok ice core. Extremophiles 2008, 12, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.; Suzuki, K.; Nishimiya, Y.; Kondo, H.; Miura, A.; Tsuda, S.; Hoshino, T. Comparison of functional properties of two fungal antifreeze proteins from Antarctomyces psychrotrophicus and Typhula ishikariensis. FEBS J. 2010, 277, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, S.G.; Do, H.; Park, J.C.; Kim, E.; Choe, Y.H.; Han, S.J.; Kim, H.J. Optimization of the pilot-scale production of an ice-binding protein by fed-batch culture of Pichia pastoris. Appl. Microbiol. Biotechnol. 2013, 97, 3383–3393. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.Y.; Lee, J.H.; Han, S.J.; Park, H.; Lee, S.G. Effect of the antifreeze protein from the Arctic yeast Leucosporidium sp. AY30 on cryopreservation of the marine diatom Phaeodactylum tricornutum. Appl. Biochem. Biotechnol. 2015, 175, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Koh, H.Y.; Lee, J.H.; Kang, S.H.; Kim, H.J. Cryopreservative effects of the recombinant ice-binding protein from the arctic yeast Leucosporidium sp. on red blood cells. Appl. Biochem. Biotechnol. 2012, 167, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Shim, H.E.; Lee, J.H.; Kang, Y.-C.; Hur, Y.B. Ice-binding protein derived from Glaciozyma can improve the viability of cryopreserved mammalian cells. J. Microbiol. Biotechnol. 2015, 25, 1989–1996. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Hanada, Y.; Sugimoto, H.; Hoshino, T.; Garnham, C.P.; Davies, P.L.; Tsuda, S. Ice-binding site of snow mold fungus antifreeze protein deviates from structural regularity and high conservation. Proc. Natl. Acad. Sci. USA 2012, 109, 9360–9365. [Google Scholar] [CrossRef] [PubMed]
- Hashim, N.H.F.; Sulaiman, S.; Bakar, F.D.A.; Illias, R.M.; Kawahara, H.; Najimudin, N.; Mahadi, N.M.; Murad, A.M.A. Molecular cloning, expression and characterisation of Afp4, an antifreeze protein from Glaciozyma antarctica. Polar Biol. 2014, 37, 1495–1505. [Google Scholar] [CrossRef]
- Bayer-Giraldi, M.; Uhlig, C.; John, U.; Mock, T.; Valentin, K.; Bayer-Giraldi, M.; Uhlig, C.; John, U.; Mock, T.; Valentin, K. Antifreeze proteins in polar sea ice diatoms: Diversity and gene expression in the genus Fragilariopsis. Environ. Microbiol. 2010, 12, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A.; Hill, P.J.; Dodd, C.E.; Laybourn-Parry, J. Demonstration of antifreeze protein activity in Antarctic lake bacteria. Microbiology 2004, 150, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Garnham, C.P.; Gilbert, J.A.; Hartman, C.P.; Campbell, R.L.; Laybourn-Parry, J.; Davies, P.L. A Ca2+-dependent bacterial antifreeze protein domain has a novel beta-helical ice-binding fold. Biochem. J. 2008, 411, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Raymond, J.A.; Kim, H.J. Possible role of horizontal gene transfer in the colonization of sea ice by algae. PLoS ONE 2012, 7, e35968. [Google Scholar] [CrossRef] [PubMed]
- Gogarten, J.P.; Doolittle, W.F.; Lawrence, J.G. Prokaryotic evolution in light of gene transfer. Mol. Biol. Evol. 2002, 19, 2226–2238. [Google Scholar] [CrossRef] [PubMed]
- Anesio, A.M.; Mindl, B.; Laybourn-Parry, J.; Hodson, A.J.; Sattler, B. Viral dynamics in cryoconite holes on a high Arctic glacier (Svalbard). J. Geophys. Res. Biogeosci. 2007, 112, G04S31. [Google Scholar] [CrossRef]
- Pucciarelli, S.; Chiappori, F.; Devaraj, R.R.; Yang, G.; Yu, T.; Ballarini, P.; Miceli, C. Identification and analysis of two sequences encoding ice-binding proteins obtained from a putative bacterial symbiont of the psychrophilic Antarctic ciliate Euplotes focardii. Antarct. Sci. 2014, 26, 491–501. [Google Scholar] [CrossRef]
- Mangiagalli, M.; Bar-Dolev, M.; Tedesco, P.; Natalello, A.; Kaleda, A.; Brocca, S.; Pascale, D.; Pucciarelli, S.; Miceli, C.; Bravslavsky, I. Cryo-protective effect of an ice-binding protein derived from Antarctic bacteria. FEBS J. 2016. [Google Scholar] [CrossRef] [PubMed]
- Mazur, P. Freezing of living cells: Mechanisms and implications. Am. J. Physiol. 1984, 247, C125–C142. [Google Scholar] [PubMed]
- Naaldijk, Y.; Staude, M.; Fedorova, V.; Stolzing, A. Effect of different freezing rates during cryopreservation of rat mesenchymal stem cells using combinations of hydroxyethyl starch and dimethylsulfoxide. BMC Biotechnol. 2012, 12, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fowler, A.; Toner, M. Cryo-injury and biopreservation. Ann. N. Y. Acad. Sci. 2006, 1066, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Chaytor, J.L.; Tokarew, J.M.; Wu, L.K.; Leclre, M.; Tam, R.Y.; Capicciotti, C.J.; Guolla, L.; Von Moos, E.; Findlay, C.S.; Allan, D.S.; et al. Inhibiting ice recrystallization and optimization of cell viability after cryopreservation. Glycobiology 2012, 22, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Fahy, G.M.; MacFarlane, D.R.; Angell, C.A.; Meryman, H.T. Vitrification as an approach to cryopreservation. Cryobiology 1984, 21, 407–426. [Google Scholar] [CrossRef]
- Wowk, B.; Leitl, E.; Rasch, C.M.; Mesbah-Karimi, N.; Harris, S.B.; Fahy, G.M. Vitrification enhancement by synthetic ice blocking agents. Cryobiology 2000, 40, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Mugnano, J.A.; Wang, T.; Layne, J.R., Jr.; DeVries, A.L.; Lee, R.E., Jr. Antifreeze glycoproteins promote intracellular freezing of rat cardiomyocytes at high subzero temperatures. Am. J. Physiol. 1995, 269, R474–R479. [Google Scholar] [PubMed]
- Hansen, T.N.; Smith, K.M.; Brockbank, K.G. Type I antifreeze protein attenuates cell recoveries following cryopreservation. Transpl. Proc. 1993, 25, 3182–3184. [Google Scholar]
- Wang, T.; Zhu, Q.; Yang, X.; Layne, J.R., Jr.; Devries, A.L. Antifreeze glycoproteins from antarctic notothenioid fishes fail to protect the rat cardiac explant during hypothermic and freezing preservation. Cryobiology 1994, 31, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Zilli, L.; Beirão, J.; Schiavone, R.; Herraez, M.P.; Gnoni, A.; Vilella, S. Comparative proteome analysis of cryopreserved flagella and head plasma membrane proteins from sea bream spermatozoa: Effect of antifreeze proteins. PLoS ONE 2014, 9, e99992. [Google Scholar] [CrossRef] [PubMed]
- Robles, V.; Cabrita, E.; Anel, L.; Herraez, M.P. Microinjection of the antifreeze protein type III (AFPIII) in turbot (Scophthalmus maximus) embryos: Toxicity and protein distribution. Aquaculture 2006, 261, 1299–1306. [Google Scholar] [CrossRef]
- Beirão, J.; Zilli, L.; Vilella, S.; Cabrita, E.; Schiavone, R.; Herraez, M.P. Improving sperm cryopreservation with antifreeze proteins: Effect on gilthead seabream (Sparus aurata) plasma membrane lipids. Biol. Reprod. 2012, 86, 59. [Google Scholar] [CrossRef] [PubMed]
- Prathalingam, N.S.; Holt, W.V.; Revell, S.G.; Mirczuk, S.; Fleck, R.A.; Watson, P.F. Impact of antifreeze proteins and antifreeze glycoproteins on bovine sperm during freeze-thaw. Theriogenology 2006, 66, 1894–1900. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Yoon, J.H.; Park, G.H.; Bae, S.H.; Kim, H.J.; Kim, M.S.; Hwang, Y.J.; Kim, D.Y. Influence of antifreeze proteins on boar sperm DNA damaging during cryopreservation. Dev. Biol. 2011, 356, 195. [Google Scholar] [CrossRef]
- Qadeer, S.; Khan, M.A.; Ansari, M.S.; Rakha, B.A.; Ejaz, R.; Iqbal, R.; Younis, M.; Ullah, N.; DeVries, A.L.; Akhter, S. Efficiency of antifreeze glycoproteins for cryopreservation of Nili-Ravi (Bubalus bubalis) buffalo bull sperm. Anim. Reprod. Sci. 2015, 157, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Qadeer, S.; Khan, M.A.; Ansari, M.S.; Rakha, B.A.; Ejaz, R.; Husna, A.U.; Ashiq, M.; Iqbal, R.; Ullah, N.; Akhter, S. Evaluation of antifreeze protein III for cryopreservation of Nili-Ravi (Bubalus bubalis) buffalo bull sperm. Anim. Reprod. Sci. 2014, 148, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Ideta, A.; Aoyagi, Y.; Tsuchiya, K.; Nakamura, Y.; Hayama, K.; Shirasawa, A.; Sakaguchi, K.; Tominaga, N.; Nishimiya, Y.; Tsuda, S. Prolonging hypothermic storage (4 °C) of bovine embryos with fish antifreeze protein. J. Reprod. Dev. 2015, 61, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hirano, Y.; Nishimiya, Y.; Matsumoto, S.; Matsushita, M.; Todo, S.; Miura, A.; Komatsu, Y.; Tsuda, S. Hypothermic preservation effect on mammalian cells of type III antifreeze proteins from notched-fin eelpout. Cryobiology 2008, 57, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Kamijima, T.; Sakashita, M.; Miura, A.; Nishimiya, Y.; Tsuda, S. Antifreeze protein prolongs the life-time of insulinoma cells during hypothermic preservation. PLoS ONE 2013, 8, e73643. [Google Scholar] [CrossRef] [PubMed]
- Baguisi, A.; Arav, A.; Crosby, T.F.; Roche, J.F.; Boland, M.P. Hypothermic storage of sheep embryos with antifreeze proteins: Development in vitro and in vivo. Theriogenology 1997, 48, 1017–1024. [Google Scholar] [CrossRef]
- Nishijima, K.; Tanaka, M.; Sakai, Y.; Koshimoto, C.; Morimoto, M.; Watanabe, T.; Fan, J.; Kitajima, S. Effects of type III antifreeze protein on sperm and embryo cryopreservation in rabbit. Cryobiology 2014, 69, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.W.; Jee, B.C.; Lee, J.R.; Suh, C.S. Effect of antifreeze protein supplementation in vitrification medium on mouse oocyte developmental competence. Fertil. Steril. 2011, 96, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Karanova, M.V.; Pronina, N.D.; Tsvetkova, L.I. The effect of antifreeze glycoproteins on survival and quality of fish spermatozoa under the conditions of long-term storage at +4 degree C. Izv. Akad. Nauk. Ser. Biol. 2002, 1, 88–92. [Google Scholar]
- Halwani, D.O.; Brockbank, K.G.; Duman, J.G.; Campbell, L.H. Recombinant Dendroides canadensis antifreeze proteins as potential ingredients in cryopreservation solutions. Cryobiology 2014, 68, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Karanova, M.V.; Mezhevikina, L.M.; Petropavlov, N.N. Study of cryoprotective properties of antifreeze glycoproteins from the white sea cod Gadus morhua on low temperature freezing of mouse embryos. Biofizika 1994, 40, 1341–1347. [Google Scholar]
- Wen, Y.; Zhao, S.; Chao, L.; Yu, H.; Song, C.; Shen, Y.; Chen, H.; Deng, X. The protective role of antifreeze protein 3 on the structure and function of mature mouse oocytes in vitrification. Cryobiology 2014, 69, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Rubinsky, L.; Raichman, N.; Lavee, J.; Frenk, H.; Ben-Jacob, E.; Bickler, P.E. Antifreeze protein suppresses spontaneous neural activity and protects neurons from hypothermia/re-warming injury. Neurosci. Res. 2010, 67, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Rubinsky, B.; Arav, A.; Devries, A.L. The cryoprotective effect of antifreeze glycopeptides from antarctic fishes. Cryobiology 1992, 29, 69–79. [Google Scholar] [CrossRef]
- Amir, G.; Rubinsky, B.; Smolinsky, A.K.; Lavee, J. Successful use of ocean pout thermal hysteresis protein (antifreeze protein III) in cryopreservation of transplanted mammalian heart at subzero temperature. J. Hear Lung Transplant. 2002, 21, 137. [Google Scholar] [CrossRef]
- Jo, J.W.; Jee, B.C.; Suh, C.S.; Kim, S.H. The Beneficial Effects of antifreeze proteins in the vitrification of immature mouse oocytes. PLoS ONE 2012, 7, e37043. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Páramo, S.; Pérez-Cerezales, S.; Robles, V.; Anel, L.; Herraez, M.P. Incorporation of antifreeze proteins into zebrafish embryos by a non-invasive method. Cryobiology 2008, 56, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Páramo, S.; Barbosa, V.; Pérez-Cerezales, S.; Robles, V.; Herraez, M.P. Cryoprotective effects of antifreeze proteins delivered into zebrafish embryos. Cryobiology 2009, 58, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Tursman, D.; Duman, J.G. Cryoprotective effects of thermal hysteresis protein on survivorship of frozen gut cells from the freeze-tolerant centipede Lithobius forficatus. J. Exp. Zool. 1995, 272, 249–257. [Google Scholar] [CrossRef]
- Amir, G.; Rubinsky, B.; Horowitz, L.; Miller, L.; Leor, J.; Kassif, Y.; Mishaly, D.; Smolinsky, A.K.; Lavee, J. Prolonged 24-hour subzero preservation of heterotopically transplanted rat hearts using antifreeze proteins derived from arctic fish. Ann. Thorac. Surg. 2004, 77, 1648–1655. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, L.; Paynter, S.J.; Fuller, B.J.; Shaw, R.W.; DeVries, A.L. Vitrification of mature mouse oocytes in a 6 M Me2SO solution supplemented with antifreeze glycoproteins: The effect of temperature. Cryobiology 1998, 37, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.M.; Ward, C.; Trounson, A.O. Survival of mouse blastocysts slow cooled in propanediol or ethylene glycol is influenced by the thawing procedure, sucrose and antifreeze proteins. Theriogenology 1995, 43, 1289–1300. [Google Scholar] [CrossRef]
- Koshimoto, C.; Mazur, P. Effects of warming rate, temperature, and antifreeze proteins on the survival of mouse spermatozoa frozen at an optimal rate. Cryobiology 2002, 45, 49–59. [Google Scholar] [CrossRef]
- Matsumoto, S.; Matsusita, M.; Morita, T.; Kamachi, H.; Tsukiyama, S.; Furukawa, Y.; Koshida, S.; Tachibana, Y.; Nishimura, S.; Todo, S. Effects of synthetic antifreeze glycoprotein analogue on islet cell survival and function during cryopreservation. Cryobiology 2006, 52, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Arav, A.; Rubinsky, B.; Fletcher, G.; Seren, E. Cryogenic protection of oocytes with antifreeze proteins. Mol. Reprod. Dev. 1993, 36, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Younis, A.I.; Rooks, B.; Khan, S.; Gould, K.G. The effects of antifreeze peptide III (AFP) and insulin transferrin selenium (ITS) on cryopreservation of chimpanzee (Pan troglodytes) spermatozoa. J. Androl. 1998, 19, 207–214. [Google Scholar] [PubMed]
- Robles, V.; Barbosa, V.; Herraez, M.P.; Martinez-Paramo, S.; Cancela, M.L. The antifreeze protein type I (AFP I) increases seabream (Sparus aurata) embryos tolerance to low temperatures. Theriogenology 2007, 68, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.; Davies, P.L.; Carpenter, J.F. Effects of antifreeze proteins on red blood cell survival during cryopreservation. J. Exp. Biol. 1996, 199, 2071–2076. [Google Scholar] [PubMed]
- Chaves, D.F.; Campelo, I.S.; Silva, M.M.A.S.; Bhat, M.H.; Teixeira, D.I.A.; Melo, L.M.; Souza-Fabjan, J.M.G.; Mermillod, P.; Freitas, V.J.F. The use of antifreeze protein type III for vitrification of in vitro matured bovine oocytes. Cryobiology 2016, 73, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Bouvet, V.R.; Lorello, G.R.; Ben, R.N. Aggregation of antifreeze glycoprotein fraction 8 and its effect on antifreeze activity. Biomacromolecules 2006, 7, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Qadeer, S.; Khan, M.A.; Shahzad, Q.; Azam, A.; Ansari, M.S.; Rakha, B.A.; Ejaz, R.; Husna, A.U.; Duman, J.G.; Akhter, S. Efficiency of beetle (Dendroides canadensis) recombinant antifreeze protein for buffalo semen freezability and fertility. Theriogenology 2016, 86, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, W.; Moos, E.; Jackman, J.; Mealing, G.; Monette, R.; Ben, R.N. In vitro studies of antifreeze glycoprotein (AFGP) and a C-linked AFGP analogue. Biomacromolecules 2007, 8, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Paramo, S.; Perez-Cerezales, S.; Barbosa, V.; Robles, V.; Herraez, M.P. Advances on fish embryo cryopreservation using antifreeze proteins. Biol. Reprod. 2008, 78, 152. [Google Scholar]
- Ideta, A.; Aoyagi, Y.; Tsuchiya, K.; Kamijima, T.; Nishimiya, Y.; Tsuda, S. A simple medium enables bovine embryos to be held for seven days at 4 °C. Sci. Rep. 2013, 3, 1173. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Rubinsky, B.; Fletcher, G.L. Hypothermic preservation of whole mammalian organs with antifreeze proteins. Cryo-Letters 1992, 13, 59–66. [Google Scholar]
- Amir, G.; Horowitz, L.; Rubinsky, B.; Yousif, B.S.; Lavee, J.; Smolinsky, A.K. Subzero nonfreezing cryopresevation of rat hearts using antifreeze protein I and antifreeze protein III. Cryobiology 2004, 48, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Rubinsky, B.; Arav, A.; Hong, J.S.; Lee, C.Y. Freezing of mammalian livers with glycerol and antifreeze proteins. Biochem. Biophys. Res. Commun. 1994, 200, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.L.; Sykes, B.D. Antifreeze proteins. Curr. Opin. Struct. Biol. 1997, 7, 828–834. [Google Scholar] [CrossRef]
- Arakawa, T.; Kita, Y.; Timasheff, S.N. Protein precipitation and denaturation by dimethyl sulfoxide. Biophys. Chem. 2007, 131, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Oliver, E.E.; Christner, B.C.; Luo, B.-H. Functional Analysis of a bacterial antifreeze protein indicates a cooperative effect between its two ice-binding domains. Biochemistry 2016, 55, 3975–3983. [Google Scholar] [CrossRef] [PubMed]
- Nishimiya, Y.; Ohgiya, S.; Tsuda, S. Artificial multimers of the type III antifreeze protein. Effects on thermal hysteresis and ice crystal morphology. J. Biol. Chem. 2003, 278, 32307–32312. [Google Scholar] [CrossRef] [PubMed]
- Baardsnes, J.; Kuiper, M.J.; Davies, P.L. Antifreeze protein dimer: When two ice-binding faces are better than one. J. Biol. Chem. 2003, 278, 38942–38947. [Google Scholar] [CrossRef] [PubMed]
- Can, O.; Holland, N.B. Utilizing avidity to improve antifreeze protein activity: A type III antifreeze protein trimer exhibits increased thermal hysteresis activity. Biochemistry 2013, 52, 8745–8752. [Google Scholar] [CrossRef] [PubMed]
- Stevens, C.A.; Drori, R.; Zalis, S.; Braslavsky, I.; Davies, P.L. Dendrimer-linked antifreeze proteins have superior activity and thermal recovery. Bioconjug. Chem. 2015, 26, 1908–1915. [Google Scholar] [CrossRef] [PubMed]
- Phippen, S.W.; Stevens, C.A.; Vance, T.D.R.; King, N.P.; Baker, D.; Davies, P.L. Multivalent display of antifreeze proteins by fusion to self-assembling protein cages enhances ice-binding activities. Biochemistry 2016, 55, 6811–6820. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.K.; Lee, J.H.; Murugan, R.N.; Lee, S.G.; Do, H.; Koh, H.Y.; Shim, H.E.; Kim, H.C.; Kim, H.J. Antifreeze peptides and glycopeptides, and their derivatives: Potential uses in biotechnology. Mar. Drugs 2013, 11, 2013–2041. [Google Scholar] [CrossRef] [PubMed]
- Balcerzak, A.K.; Capicciotti, C.J.; Briard, J.G.; Ben, R.N. Designing ice recrystallization inhibitors: From antifreeze (glyco) proteins to small molecules. RSC Adv. 2014, 4, 42682–42696. [Google Scholar] [CrossRef]
- Liu, S.; Ben, R.N. C-linked galactosyl serine AFGP analogues as potent recrystallization inhibitors. Org. Lett. 2005, 7, 2385–2388. [Google Scholar] [CrossRef] [PubMed]
- Garner, J.; Harding, M.M. Design and synthesis of antifreeze glycoproteins and mimics. Chembiochem 2010, 11, 2489–2498. [Google Scholar] [CrossRef] [PubMed]
- Hachisu, M.; Hinou, H.; Takamichi, M.; Tsuda, S.; Koshida, S.; Nishimura, S. One-pot synthesis of cyclic antifreeze glycopeptides. Chem. Commun. 2009. [Google Scholar] [CrossRef] [PubMed]
- Garner, J.; Harding, M.M. Design and synthesis of alpha-helical peptides and mimetics. Org. Biomol. Chem. 2007, 5, 3577–3585. [Google Scholar] [CrossRef] [PubMed]
- Can, O.; Holland, N.B. Conjugation of type I antifreeze protein to polyallylamine increases thermal hysteresis activity. Bioconjug. Chem. 2011, 22, 2166–2171. [Google Scholar] [CrossRef] [PubMed]

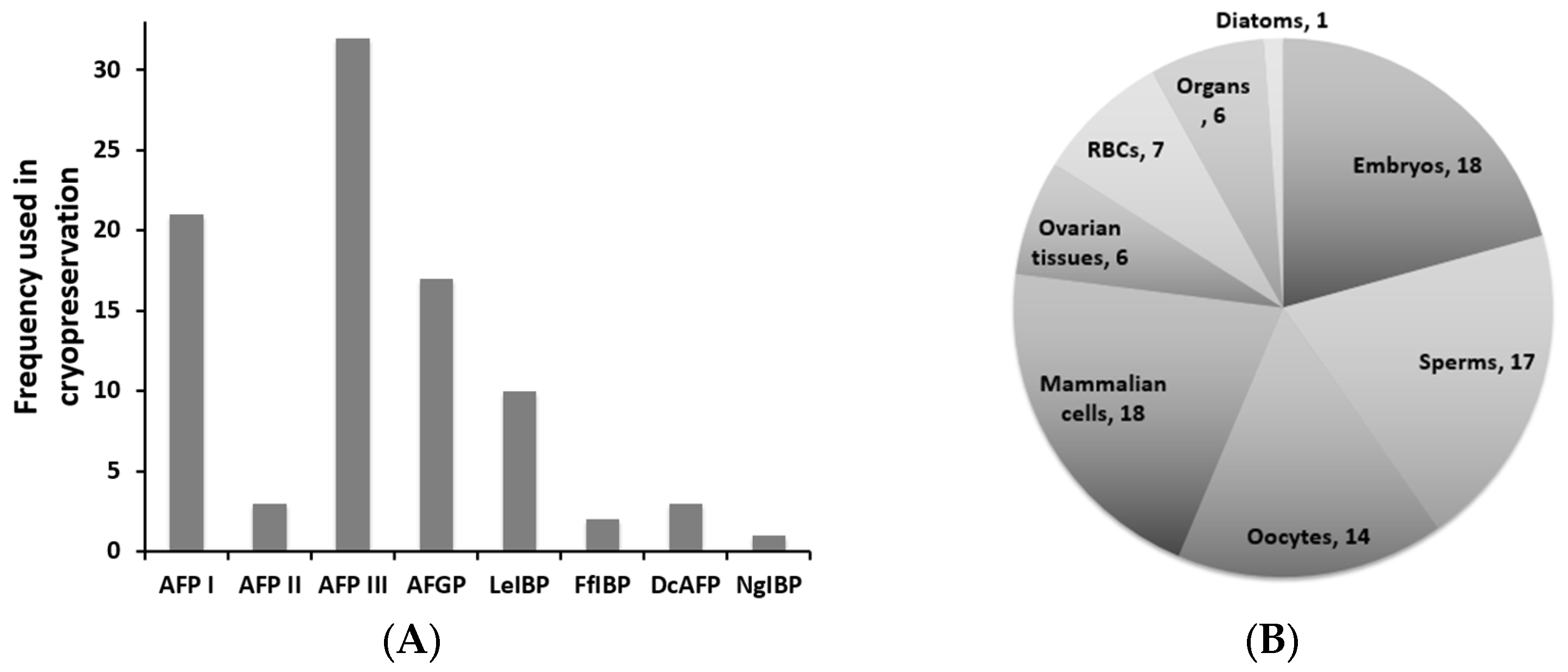
| AFPs | Origin Species of AFPs | Cryopreserved Biological Samples | AFP Quantities Used | Freezing Methods | References | |
|---|---|---|---|---|---|---|
| Organisms | Sample Types | |||||
| III | Fish | Turbot (Scophthalmus maximus) | Embryos | 20 nL (final conc. 0.77 mg/mL) of 10 mg/mL type III AFP injected in yolk sac 0.23914 mm3) | Vitrification | [166] |
| I/III | Fish | Gilthead seabream (Sparus aurata) | Sperm | 1 μg/mL | Vitrification | [167] |
| I/III/AFGP | Fish | Bull | Sperm | 0.1, 1, 10, and 100 μg/mL | Cryopreservation | [168] |
| LeIBP | Yeast | Boar | Sperm | 0.01, 0.1, and 1 mg/mL | Cryopreservation | [169] |
| III | Fish | Mouse | Ovarian tissue | 0.1, 1, and 10 mg/mL | Vitrification | [65] |
| FfIBP | Bacteria | |||||
| LeIBP | Yeast | |||||
| AFGP | Fish | Buffalo | Sperm | 0.1, 1, and 10 μg/mL | Vitrification | [170] |
| III | Fish | Buffalo | Sperm | 0.01, 0.1, 1, and 10 mg/mL | Cryopreservation | [171] |
| III | Fish | Bovine | Embryos | 10 mg/mL | Hypothermic | [172] |
| AFGP | Fish | Pig | Oocyte | 40 mg/mL | Hypothermic | [83] |
| I | Winter flounder | Bovine | Oocyte | 20 mg/mL | Hypothermic | [70] |
| II | Sea raven | |||||
| III | Eel pout | |||||
| III | Notched-fin eelpout | Human | HepG2 | 2–10 mg/mL | Hypothermic | [173] |
| I/III/AFGP | Fish | Rat | RIN-5F cells (insulinoma) | 10 mg/mL | Hypothermic | [174] |
| I | Winter flounder | Sheep | Embryo | 1 or 10 mg/mL | Hypothermic | [175] |
| III | Ocean pout | |||||
| III | Fish | Rabbit | Sperm | 0.1, 1, 10, and 100 μg/mL | Vitrification | [176] |
| Embryo | 100, 500, and 1000 μg/mL | |||||
| III | Fish | Mouse | Oocyte | 500 ng/mL | Vitrification | [177] |
| LeIBP | Yeast | Diatom | Diatom | 0.1 mg/mL | Cryopreservation | [143] |
| AFGP | Fish | Carp | Spermatozoa | 2–10 mg/mL | Hypothermic | [178] |
| I | Fish | Mouse | Pronuclear embryos, 4-cell embryos | 0.1 and 1.0 mg/mL | Vitrification | [92] |
| III | 0.1 mg/mL | |||||
| I/III | Fish | Sea bream | Spermatozoa | 0.1, 1, and 10 μg/mL | Cryopreservation | [165] |
| AFGP | Fish | Equine | Embryos | 20 mg/mL | Hypothermic/Cryopreservation | [91] |
| DcAFP | Insect | Mouse | A10 smooth muscle cell | 1 μg/mL | Cryopreservation | [179] |
| AFGP | Gadus morhua | Mouse | Embryos | 20 mg/mL | Vitrification | [180] |
| III | Fish | Mouse | Ovarian tissue | 0, 5, and 20 mg/mL | Vitrification | [67] |
| III | Fish | Mouse | Mature oocyte | 2.5 mg/mL | Vitrification | [181] |
| I | Fish | Rat | Hippocampal slice cultures | 10 mg/mL | Hypothermic | [182] |
| AFGP | Fish | Pig | Oocyte | 40 mg/mL | Vitrification | [183] |
| AFGP | Fish | Mouse | Embryos | 20 mg/mL | Vitrification | [183] |
| LeIBP | Yeast | Human | Red blood cells | 0.4–0.8 mg/mL | Cryopreservation | [144] |
| III | Fish | Rat | Heart | 3, 5, and 15 mg/mL | Hypothermic | [184] |
| AFGP | Fish | Rat | Cardiomyoctes | 0.5–10 mg/mL | Hypothermic (−4 °C) | [162] |
| III | Fish | Mouse | Oocytes | 500 ng/mL | Cryopreservation | [185] |
| AFGP | Fish | Rat | Cardiac | 10 μg/mL, 10 and 15 mg/mL | Hypothermic | [164] |
| I/III | Fish | Zebra fish | Embryo | 40 μg/mL | Hypothermic | [186] |
| NgIBP | Diatom | Human | Red blood cells | 25, 50, and 77 μg/mL | Cryopreservation | [18] |
| I/III | Fish | Zebra fish | Embryo | 40 μg/mL | Vitrification/Cryopreservation | [187] |
| DcAFP | Insect | Centipede | Gut cells | 0.02 mg/mL | Cryopreservation | [188] |
| III | Fish | Rat | Hearts | 15 mg/mL | Hypothermic | [189] |
| AFGP | Fish | Mouse | Oocytes | 1 mg/mL | Cryopreservation | [190] |
| I/III | Fish | Mouse | Blastocysts | 0.1, 1.0 mg/mL | Cryopreservation | [191] |
| I/III/AFGP | Fish | Mouse | Spermatozoa | 1–100 μg/mL | Cryopreservation | [192] |
| AFGP | Synthetic | Rat | Islet cell | 500 μg/mL | Cryopreservation | [193] |
| I/II/III/AFGP | Fish | Mouse | Oocytes | 20 mg/mL | Vitrification | [194] |
| I | Fish | Human | Myelogenous leukemia cells | 0~1000 μg/mL | Cryopreservation | [163] |
| III | Ocean pout | Chimpanzee (Pan troglodytes) | Spermatozoa | 1, 10, and 100 μg/mL | Cryopreservation | [195] |
| I | Fish | Seabream | Embryos | 20 nL of 10 mg/mL type I AFP injected | Vitrification | [196] |
| III | Fish | Turbot | Embryos | 10 mg/mL | Hypothermic | [166] |
| I | Fish | Rat | Liver | 1 mg/mL | Hypothermic | [94] |
| I/III | Fish | Rat | Hearts | 10, 15, and 20 mg/mL | Hypothermic | [69] |
| I/II/III | Fish | Human | Red blood cells | 0–1.54 mg/mL | Cryopreservation | [197] |
| I | Fish | Human | Red blood cells | 5–160 μg/mL | Cryopreservation | [68] |
| III | Eel pout | Mouse | Oocyte | 0.1 mg/mL | Vitrification | [66] |
| FfIBP | Bacteria | 0.05 mg/mL | ||||
| LeIBP | Yeast | 0.1 mg/mL | ||||
| III | Eel pout | Bovine | Oocyte | 0.5–1 μg/mL | Vitrification | [198] |
| AFGP8 | Fish | Bovine | Oocyte | 1 mM (2.6 mg/mL) | Vitrification | [199] |
| DcAFP | Beetle Dendroides canadensis | Buffalo | Semen | 0.1, 1.0, and 10 μg/mL | Cryopreservation | [200] |
| LeIBP | Yeast | Human | Cell lines | 0.1 mg/mL | Cryopreservation | [145] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.J.; Lee, J.H.; Hur, Y.B.; Lee, C.W.; Park, S.-H.; Koo, B.-W. Marine Antifreeze Proteins: Structure, Function, and Application to Cryopreservation as a Potential Cryoprotectant. Mar. Drugs 2017, 15, 27. https://doi.org/10.3390/md15020027
Kim HJ, Lee JH, Hur YB, Lee CW, Park S-H, Koo B-W. Marine Antifreeze Proteins: Structure, Function, and Application to Cryopreservation as a Potential Cryoprotectant. Marine Drugs. 2017; 15(2):27. https://doi.org/10.3390/md15020027
Chicago/Turabian StyleKim, Hak Jun, Jun Hyuck Lee, Young Baek Hur, Chang Woo Lee, Sun-Ha Park, and Bon-Won Koo. 2017. "Marine Antifreeze Proteins: Structure, Function, and Application to Cryopreservation as a Potential Cryoprotectant" Marine Drugs 15, no. 2: 27. https://doi.org/10.3390/md15020027






