Chytridiomycosis of Marine Diatoms—The Role of Stress Physiology and Resistance in Parasite-Host Recognition and Accumulation of Defense Molecules
Abstract
:1. Introduction
2. Results
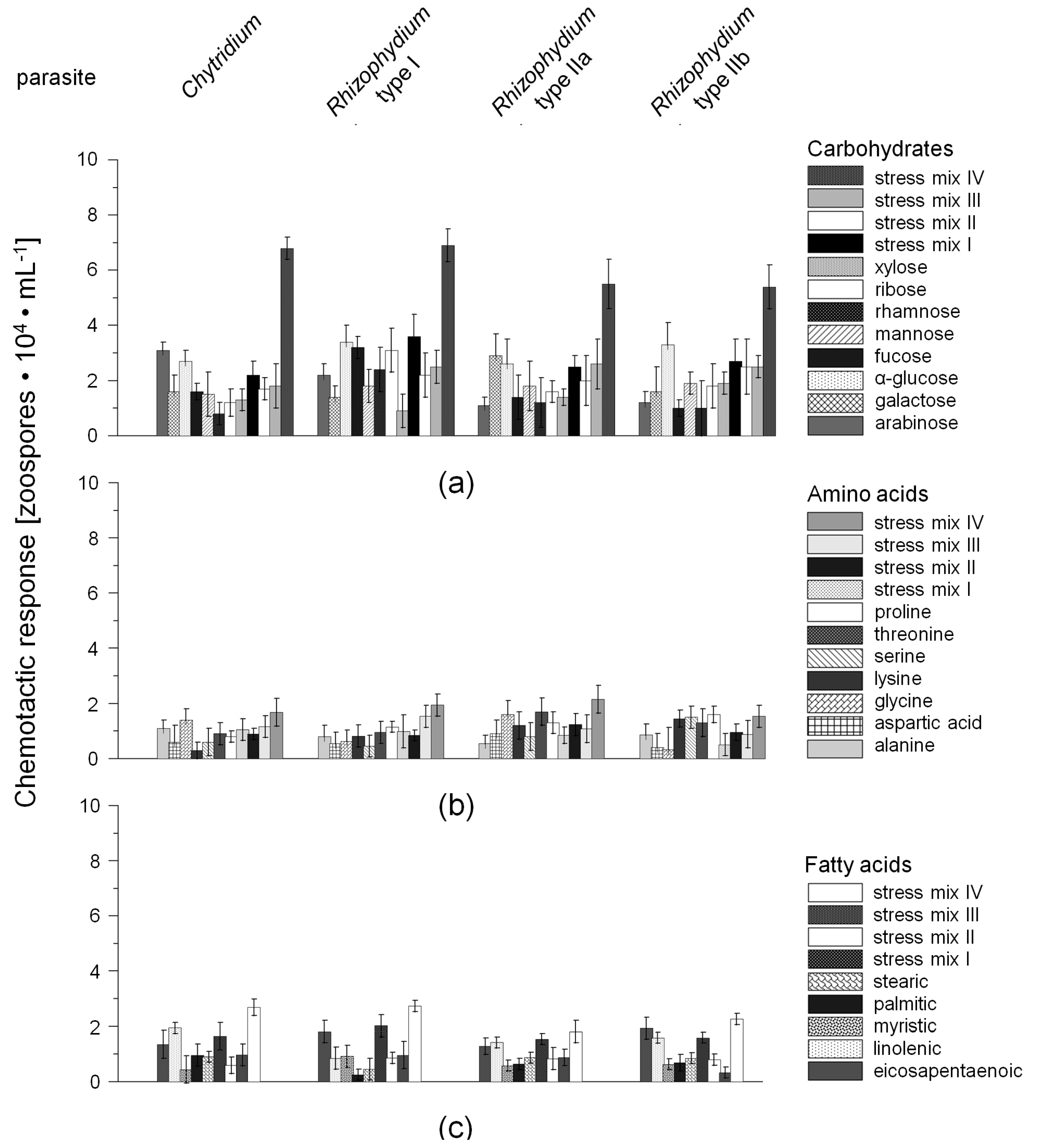
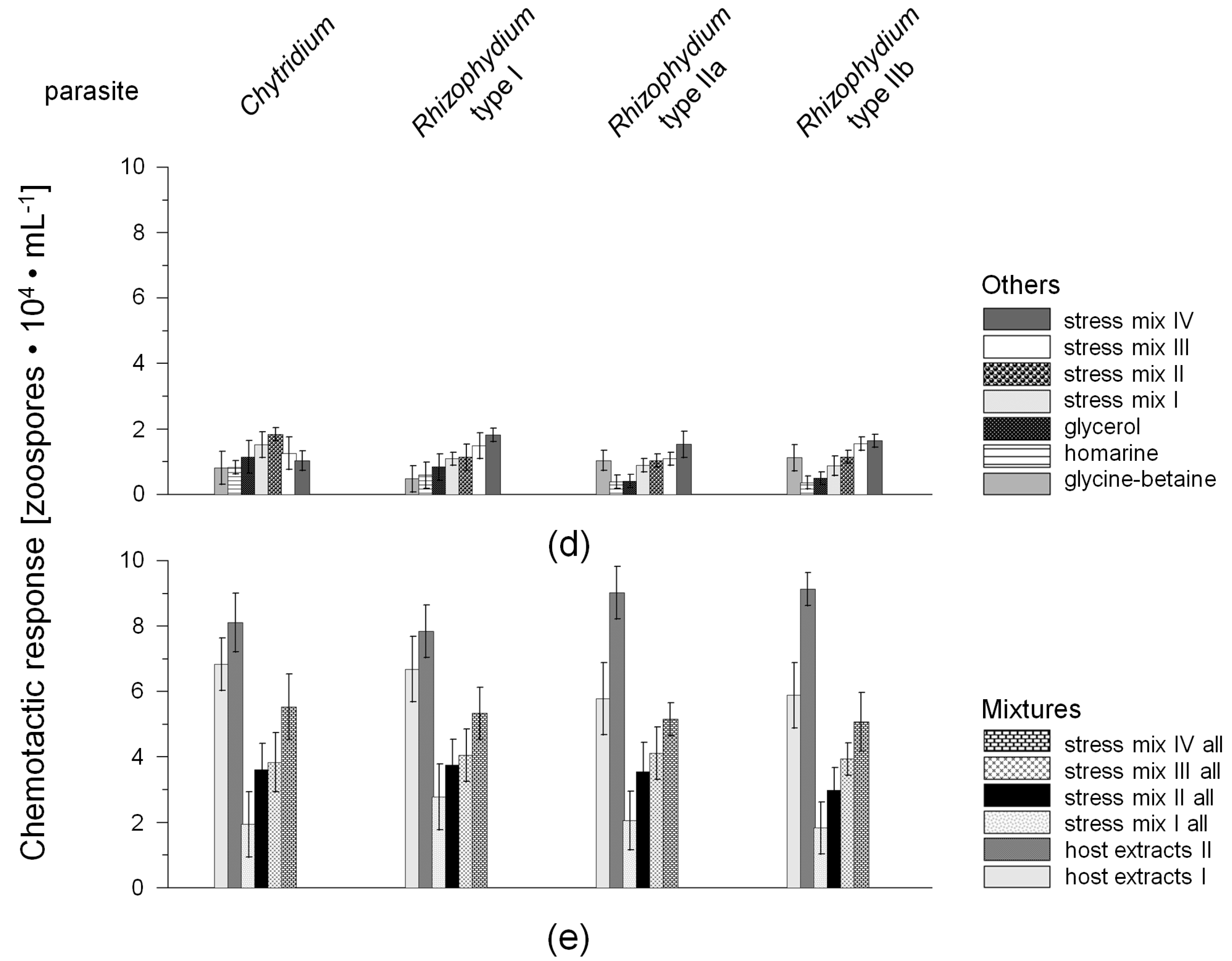
2.1. Chemotactic Responses (CR) of Zoospores
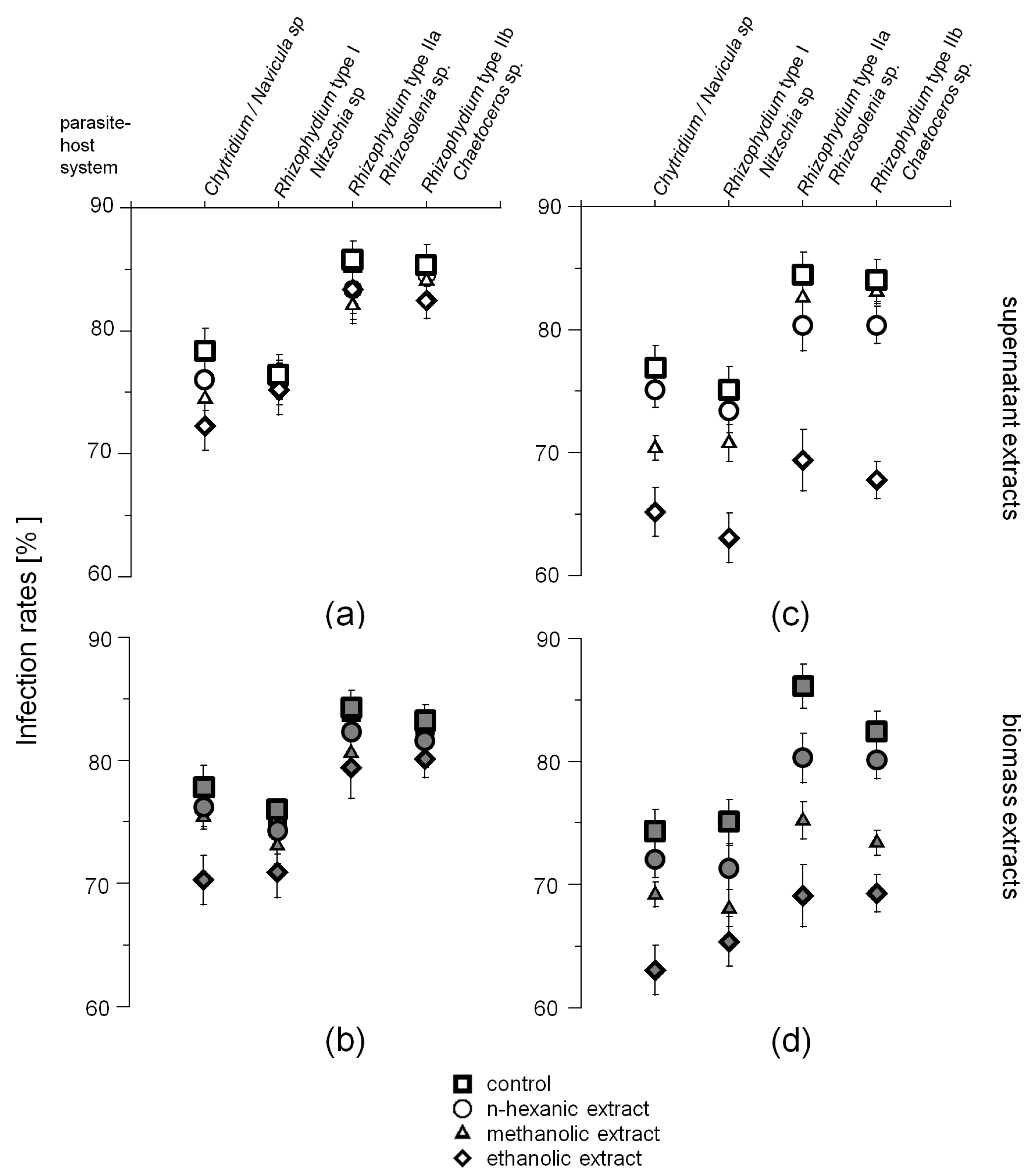
2.2. Phytochemical Screening
2.3. Zoospore Activity Screening
3. Discussion
3.1. Critical Remarks
3.2. Chemotaxis of Chytrid Zoospores
3.2.1. Role of Environmental Factors
3.2.2. Chemotaxis versus Other Factors or Attractants
3.3. Potential Host-Defense Molecules and Infection Prevalence
4. Materials and Methods
4.1. Chemicals
4.2. Organisms
4.2.1. Isolation and Maintenance Prior to Experiments
4.2.2. Species Identification
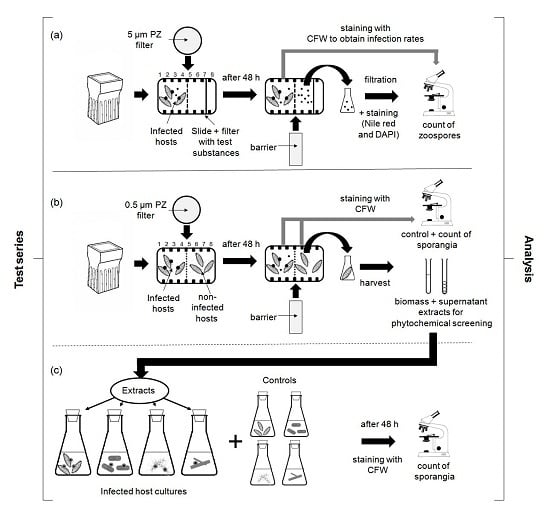
4.3. Experimental Designs
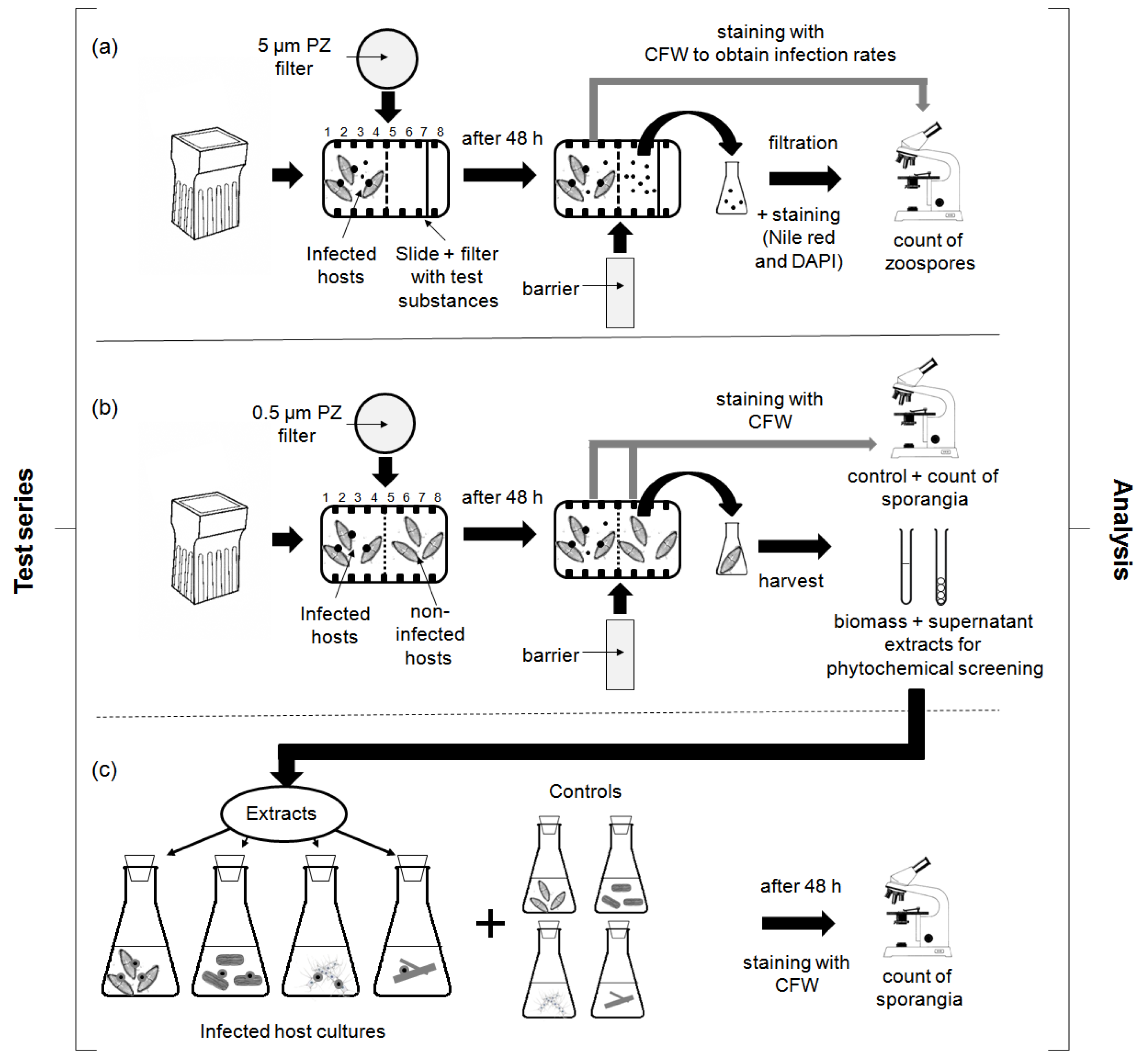
4.3.1. Chemotaxis Experiments
4.3.2. Screening for Defense Molecules (Non-Contact-Co-Culturing Approach)
4.3.3. Zoospore Activity Screening
4.4. Analysis
4.4.1. Microscopy
4.4.1.1. Number of Zoospores
4.4.1.2. Infection Prevalence
4.4.2. Phytochemical Screening
4.4.3. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schmid-Hempel, P. Evolutionary Parasitology: The Integrated Study of Infections, Immunology, Ecology, and Genetics; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Lafferty, K.D.; Dobson, A.P.; Kuris, A.M. Parasites dominate food web links. Proc. Natl. Acad. Sci. USA 2006, 103, 11211–11216. [Google Scholar] [CrossRef] [PubMed]
- Sime-Ngando, T. Phytoplankton chytridiomycosis: Fungal parasites of phytoplankton and their imprints on the food web dynamics. Front. Microbiol. 2012, 3, 361. [Google Scholar] [CrossRef] [PubMed]
- Kagami, M.; de Bruin, A.; Ibelings, B.W.; van Donk, E. Parasitic chytrids: Their effects on phytoplankton communities and food-web dynamics. Hydrobiologia 2007, 578, 113–129. [Google Scholar] [CrossRef]
- Scholz, B.; Küpper, F.C.; Vyverman, W.; Karsten, U. Eukaryotic pathogens (Chytridiomycota and Oomycota) infecting marine microphytobenthic diatoms—A methodological comparison. J. Phycol. 2014, 50, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Scholz, B.; Küpper, F.C.; Vyverman, W.; Karsten, U. Effects of eukaryotic pathogens (Chytridiomycota and Oomycota) on marine microphytobenthic diatom community compositions in the Solthörn tidal flat (southern North Sea, Germany). Eur. J. Phycol. 2016, 5, 253–269. [Google Scholar] [CrossRef]
- Scholz, B.; Guillou, L.; Marano, A.V.; Neuhauser, S.; Sullivan, B.K.; Karsten, U.; Küpper, F.C.; Gleason, F.H. Zoosporic parasites infecting marine diatoms—A black box that needs to be opened. Fungal Ecol. 2016, 19, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, M.H.; Jara, A.M.; Pantoja, S. Fungal parasites infect marine diatoms in the upwelling ecosystem of the Humboldt current system off central Chile. Environ. Microbiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- De Vargas, C.; Audic, S.; Henry, N.; Decelle, J.; Mahé, F.; Logares, R.; Lara, E.; Berney, C.; Le Bescot, N.; Probert, I.; et al. Eukaryotic plankton diversity in the sunlit global ocean. Science 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richards, T.A.; Leonard, G.; Mahé, F.; del Campo, J.; Romac, S.; Jones, M.D.; Maguire, F.; Dunthorn, M.; De Vargas, C.; Massana, R.; et al. Molecular diversity and distribution of marine fungi across 130 European environmental samples. Proc. R. Soc. B 2015, 282, 20152243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassett, B.T.; Ducluzeau, A.-L.L.; Collins, R.E.; Gradinger, R. Spatial distribution of aquatic marine fungi across the western Arctic and sub-Arctic. Environ. Microbiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Hassett, B.T.; Gradinger, R. Chytrids dominate arctic marine fungal communities. Environ. Microbiol. 2016, 18, 2001–2009. [Google Scholar] [CrossRef] [PubMed]
- James, T.Y.; Kauff, F.; Schoch, C.L.; Matheny, P.B.; Hofstetter, V.; Cox, C.J.; Celio, G.; Gueidan, C.; Fraker, E.; Miadlikowska, J.; et al. Reconstructing the early evolution of fungi using a six-gene phylogeny. Nature 2006, 443, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Canter, H.M. Studies on British chytrids. XII. Fungal parasites of the phytoplankton. Ann. Bot. 1951, 15, 129–156. [Google Scholar]
- Beakes, G.W.; Canter, H.M.; Jaworski, G.H.M. Comparative ultrastructural ontogeny of zoosporangia of Zygorhizidium affluens and Z. planktonicum, chytrid parasites of the diatom Asterionella formosa. Mycol. Res. 1992, 96, 1047–1059. [Google Scholar] [CrossRef]
- Sparrow, F.K. Aquatic Phycomycetes; University of Michigan Press: Ann Arbor, MI, USA, 1960. [Google Scholar]
- Piotrowski, J.S. Physiology, Enzyme Production, and Zoospore Behavior of Balrachochytrium dendrobatidis, a Chytrid Pathogenic to Amphibians. Master’s Thesis, University of Maine, Orono, ME, USA, 2002. [Google Scholar]
- Carlile, M.J. The zoospore and its problems. Br. Mycol. Soc. 1986, 11, 105–118. [Google Scholar]
- Gleason, F.K.; Lilje, O. Structure and function of fungal zoospores: Ecological implications. Fungal Ecol. 2009, 2, 53–59. [Google Scholar] [CrossRef]
- Von Broembsen, S.L.; Deacon, J.W. Effects of calcium on germination and further zoospore release from zoospore cysts of Phytophthora parasitica. Mycol. Res. 1996, 100, 1498–1504. [Google Scholar] [CrossRef]
- Bruning, K. Infection of the diatom Asterionella by a chytrid. 2. Effects of light on survival and epidemic development of the parasite. J. Plank Res. 1991, 13, 119–129. [Google Scholar] [CrossRef]
- Muehlstein, L.K.; Amon, J.P.; Leffler, D.L. Chemotaxis in the marine fungus Rhizophydium littoreum. Appl. Environ. Microbiol. 1988, 54, 1668–1672. [Google Scholar] [PubMed]
- Pohnert, G. Diatom/Copepod interactions in plankton: The indirect chemical defense of unicellular algae. ChemBioChem 2005, 6, 946–959. [Google Scholar] [CrossRef] [PubMed]
- Pohnert, G.; Adolph, S.; Wichard, T. Short synthesis of labeled and unlabeled 6Z,9Z,12Z,15-hexadecatetraenoic acid as metabolic probes for biosynthetic studies on diatoms. Chem. Phys. Lipids 2004, 131, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Pohnert, G. Wound-activated chemical defense in unicellular planktonic algae. Angew. Chem. Int. Ed. 2000, 39, 4352–4354. [Google Scholar] [CrossRef]
- Pohnert, G.; Steinke, M.; Tollrian, R. Chemical Cues, Defense Metabolites and the Shaping Of Pelagic Interspecific Interactions. Trends Ecol. Evol. 2007, 22, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Round, F.E.; Crawford, R.M.; Mann, D.G. The Diatoms. Biology and Morphology of the Genera; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Armbrust, E.V. The life of diatoms in the world’s oceans. Nature 2009, 459, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Armbrust, E.V.; Berges, J.; Bowler, C.; Green, B.R.; Martinez, D.; Putnam, N.H.; Zhou, S.; Allen, A.E.; Apt, K.E.; Bechner, M.; et al. The genome of the diatom Thalassiosira pseudonana: Ecology, evolution, and metabolism. Science 2004, 306, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, C.; Büchel, C.; Fisahn, J.; Goss, R.; Jakob, T.; LaRoche, J.; Lavaud, J.; Lohr, M.; Riebesell, U.; Stehfest, K.; et al. The regulation of carbon and nutrient assimilation in diatoms is significantly different from green algae. Protist 2006, 157, 91–124. [Google Scholar] [CrossRef] [PubMed]
- Plettner, I. Stressphysiologie bei Antarktischen Diatomeen—Ökophysiologische Untersuchungen zur Bedeutung von Prolin bei der Anpassung an hohe Salinitäten und tiefe Temperaturen. Ph.D. Thesis, University of Bremen, Bremen, Germany, 2002. [Google Scholar]
- Krell, A.; Funck, D.; Plettner, I.; John, U.; Dieckmann, G. Regulation of proline metabolism under salt stress in the psychrophilic diatom Fragilariopsis cylindrus (Bacillariophyceae). J. Phycol. 2007, 43, 753–762. [Google Scholar] [CrossRef]
- Scholz, B.; Liebezeit, G. Compatible solutes in three marine intertidal microphytobenthic Wadden sea diatoms exposed to different salinities. Eur. J. Phycol. 2012, 47, 393–407. [Google Scholar] [CrossRef]
- Scholz, B.; Liebezeit, G. Compatible solutes and fatty acid compositions of five marine intertidal microphytobenthic Wadden sea diatoms exposed to different temperatures (southern North Sea). Diatom Res. 2013. [Google Scholar] [CrossRef]
- Scholz, B.; Rúa, A.; Liebezeit, G. Effects of UV radiation on five marine microphytobenthic Wadden sea diatoms, isolated from the Solthörn tidal flat (Lower Saxony, southern North Sea)—Part II: Carbohydrate, amino- and fatty acid compositions. Eur. J. Phycol. 2014, 49, 97–114. [Google Scholar] [CrossRef]
- Hare, P.D.; Cress, W.A. Metabolic implications of stress-induced accumulation in plants. Plant Growth Regul. 1997, 21, 79–102. [Google Scholar] [CrossRef]
- Bohnert, H.J.; Ayoubi, P.; Borchert, C.; Bressan, R.A.; Burnap, R.L.; Cushman, J.C.; Cushman, M.A.; Deyholos, M.; Fischer, R.; Galbraith, D.W. A genomics approach towards salt stress tolerance. Plant Physiol. Biochem. 2001, 39, 295–311. [Google Scholar] [CrossRef]
- Brown, A.D.; Simpson, J.R. Water relations of sugar-tolerant yeast: The role of intracellular polyols. J. Gen. Microbiol. 1972, 72, 589–591. [Google Scholar] [CrossRef] [PubMed]
- Kirst, G.O.; Wiencke, C. Ecophysiology of polar algae. J. Phycol. 1995, 31, 181–199. [Google Scholar] [CrossRef]
- DasSarma, S.; Arora, P. Halophiles. In Encyclopedia of Life Sciences; John Wiley & Sons: Chichester, UK, 2001. [Google Scholar]
- Chen, T.; Murata, N. Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr. Opin. Plant Biol. 2002, 5, 250–257. [Google Scholar] [CrossRef]
- Bisson, M.A.; Kirst, G.O. Osmotic acclimation and turgor pressure regulation in algae. Naturwissenschaften 1995, 82, 461–471. [Google Scholar] [CrossRef]
- Erdmann, N.; Hagemann, M. Salt acclimation of algae and cyanobacteria: A comparison. In Algal Adaptation to Environmental Stresses; Rai, L.C., Gaur, J.P., Eds.; Springer: Heidelberg, Germany, 2001; pp. 323–397. [Google Scholar]
- Kirst, G.O. Salinity tolerance of eukaryotic marine algae. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1990, 40, 21–53. [Google Scholar] [CrossRef]
- Van Bergeijk, S.A.; Van der Zee, C.; Stal, L.J. Uptake and excretion of dimethylsuphoniopropionate is driven by salinity changes in the marine benthic diatom Cylindrotheca closterium. Eur. J. Phycol. 2003, 38, 341–349. [Google Scholar] [CrossRef]
- Johnson, T.W., Jr.; Sparrow, F.K., Jr. Fungi in Oceans and Estuaries; Hafner Publishing Co.: Darien, CT, USA, 1961. [Google Scholar]
- Letcher, P.M.; Powell, M.J. A Taxonomic Summary and Revision of Rhizophydium (Rhizophydiales, Chytridiomycota); The University of Alabama: Tuscaloosa, AL, USA, 2012. [Google Scholar]
- Gaur, R.; Mishra, L.; Gupta, S.K. Diffusion and Transport of Molecules in Living Cells. In Modelling and Simulation of Diffusive Processes; Springer International Publishing: Basel, Switzerland, 2014; pp. 27–49. [Google Scholar]
- Brodin, B.; Steffansen, B.; Nielsen, C.U. Passive diffusion of drug substances: The concepts of flux and permeability. In Molecular Biopharmaceutics; Steffansen, B., Brodin, B., Nielsen, C.U., Eds.; Pharmaceutical Press: London, UK, 2010; pp. 135–152. [Google Scholar]
- Ormonde, P.; Horstedt, P.; O’Toole, R.; Milton, D.L. Role of motility in adherence to and invasion of a fish cell line by Vibrio anguillarum. J. Bacteriol. 2000, 182, 2326–2328. [Google Scholar] [CrossRef] [PubMed]
- Koch, J.W. Studies of the motile cells of chytrids. V. Flagellar retraction in posteriorly uniflagellate fungi. Am. J. Bot. 1968, 55, 841–859. [Google Scholar] [CrossRef]
- Lux, R.; Miller, J.N.; Park, N.; Shi, W. Motility and chemotaxis in tissue penetration of oral epithelial cell layers by Treponema denticola. Infect. Immun. 2001, 69, 6276–6283. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.G.; Küpper, F.C.; Küpper, H. Infection experiments reveal broad host ranges of Eurychasma dicksonii (Oomycota) and Chytridium polysiphoniae (Chytridiomycota), two eukaryotic parasites in marine brown algae (Phaeophyceae). Phycol. Res. 1999, 47, 217–223. [Google Scholar] [CrossRef]
- Gachon, C.M.M.; Küpper, H.; Küpper, F.C.; Šetlík, I. Witnessing effects of a pathogen on photosynthesis of its host at the cellular level: Chlorophyll fluorescence kinetic microscopy of Pylaiella littoralis (Phaeophyceae) infected by Chytridium polysiphoniae (Chytridiomycota). Eur. J. Phycol. 2006, 41, 395–403. [Google Scholar] [CrossRef]
- Lawrence, P.O. The biochemical and physiological-effects of insect host on the development and ecology of their insect parasites—An overview. Arch. Insect Biochem. Physiol. 1990, 13, 217–228. [Google Scholar] [CrossRef]
- Dickson, D.M.J.; Kirst, G.O. Osmotic adjustment in marine eukaryotic algae: The role of inorganic ions, quaternary ammonium, tertiary sulfonium and carbohydrate solutes: I. Diatoms and a rhodophyte. New Phytol. 1987, 106, 645–655. [Google Scholar] [CrossRef]
- Nothnagel, J. The Effects of Salinity and Light Intensity on the Osmolyte Concentrations, Cell Volumes and Growth Rates of the Antarctic Sea-Ice Diatoms Chaetoceros sp. and Navicula sp. with Emphasis on the Amino Acid Proline. Ph.D. Thesis, University of Bremen, Bremen, Germany, 1995. [Google Scholar]
- Fujii, S.; Nishimoto, N.; Notoya, N.; Hellebust, J.A. Growth and osmoregulation of Chaetoceros muelleri in relation to salinity. Plant Cell Physiol. 1995, 36, 759–764. [Google Scholar]
- Garza-Sánchez, F.; Chapman, D.J.; Cooper, J.B. Nitzschia ovalis (Bacillariophyceae) mono lake strain accumulates 1,4/2,5 cyclohexanetetrol in response to increased salinity. J. Phycol. 2009, 45, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Paul, J.S. Osmoregulation in the marine diatom Cylindrotheca fusiformis. J. Phycol. 1979, 15, 280–284. [Google Scholar] [CrossRef]
- Canter, H.M.; Jaworski, G.H.M. Some general observations on zoospores of the chytrid Rhizophydium planktonicum Canter emend. New Phytol. 1980, 84, 515–531. [Google Scholar] [CrossRef]
- Van West, P.; Morris, B.M.; Reid, B.; Appiah, A.A.; Osborne, M.C.; Campbell, T.A.; Shepherd, S.J. Oomycete plant pathogens use electric fields to target roots. Mol. Plant Microbe Interact. 2002, 15, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Savory, A.I.M.; Grenville-Briggs, L.J.; Wawra, S.; van West, P.; Davidson, F.A. Auto-aggregation in zoospores of Phytophthora infestans: The cooperative roles of bioconvection and chemotaxis. J. R. Soc. Interface 2014, 11, 20140017. [Google Scholar] [CrossRef] [PubMed]
- Gow, N.A.R. Nonchemical signals used for host location and invasion by fungal pathogens. Trends Microbiol. 1993, 1, 45–50. [Google Scholar] [CrossRef]
- Cushnie, T.P.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Scholz, B.; Küpper, F.C.; Vyverman, W.; Ólafsson, H.G.; Karsten, U. Effects of environmental parameters on chytrid infection prevalence of four marine diatoms—A laboratory case study. Bot. Mar. 2017. submitted. [Google Scholar]
- Guillard, R.R.L.; Ryther, J.H. Studies of marine diatoms. I. Cyclotella nana Hustedt and Detonula confervacea Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Schrader, H.-J. Proposal of a standardized method for cleaning deep-sea and land-exposed marine sediments. Nova Hedwigia Beih 1973, 45, 403–409. [Google Scholar]
- Scholz, B.; Einarsson, H. Microphytobenthic community composition of two sub-Arctic intertidal flats in Huna Bay (Northern Iceland). Eur. J. Phycol. 2015, 50, 182–206. [Google Scholar] [CrossRef]
- Turner, B.B. The chemical composition of Oscillaria rolifica. J. Am. Chem. Soc. 1916, 38, 1402–1417. [Google Scholar] [CrossRef]
- Scholz, B.; Liebezeit, G. Chemical screening for bioactive substances in culture media of microalgae and cyanobacteria from marine and brackish water habitats: First results. Pharm. Biol. 2006, 44, 544–549. [Google Scholar] [CrossRef]
- Tiwari, P.; Kumar, B.; Kaur, M.; Kaur, G.; Kaur, H. Phytochemical screening and extraction: A Review. Int. Pharm. Sci. 2011, 1, 98–106. [Google Scholar]
- Wilson, R.; Sargent, J.R. High-resolution separation of polyunsaturated fatty acids by argentation thin-layer chromatography. J. Chromatogr. 1992, 623, 403–407. [Google Scholar] [CrossRef]
- Usunobun, U.; Okolie, N.P.; Anyanwu, O.G.; Adegbegi, A.J.; Egharevba, M.E. Phytochemical screening and proximate composition of Annona muricata leaves. Eur. J. Bot. Plant Sci. Phytol. 2015, 2, 18–28. [Google Scholar]
- LeBlanc, B.W.; Davis, O.K.; Boue, S.; DeLucca, A.; Deeby, T. Antioxidant activity of sonoran desert bee pollen. Food Chem. 2009, 115, 1299–1305. [Google Scholar] [CrossRef]
- Holfeld, H. Infection of the single-celled diatom Stephanodiscus alpinus by the chytrid Zygorhizidium: Parasite distribution within host population, changes in host cell size, and host-parasite size relationship. Limnol. Oceanogr. 2000, 45, 1440–1444. [Google Scholar] [CrossRef]
- Harborne, J.B. Phytochemical Methods—A Guide to Modern Techniques of Plant Analysis; Chapman and Hall: London, UK, 1998; p. 278. [Google Scholar]

| Host Species (Author) | Parasite | Description of the Parasites |
|---|---|---|
| Navicula Bory NavHrú1542S NavHrú1523R | Chytridium type I | Sporangium on a stalk (extrametrical, up to 15.8 μm long), ovate or ellipsoid (also spherical was observed), around 16 μm long by 10–16 μm wide. Similarities with Chytridium sp. |
| Nitzschia Hassall NitzIsa15101S NitzIsa15106R | Rhizophydium type I | Sporangium: sessile, ovoid or spherical (globose), 17–35 μm high, 17–40 μm diameter; wall thin and smooth, colorless, double-contoured, 1–1.5 μm thick, with a broad apical or sub apical papilla. Similarities with Rhizophydium Schenk |
| Rhizosolenia Brightwell RhizSka1503S RhizSka1512R Chaetoceros Ehrenberg ChaeSka1517S ChaeSka1511R | Rhizophydium type II | Sporangium: sessile, ovoid, 5–11 μm high by 5–9 μm in diameter, wall smooth, colorless. Similarities with Rhizophydium Schenk |
| Compounds (g·L−1) | Concentrations | ||||
|---|---|---|---|---|---|
| Individual | Mix I | Mix II | Mix III | Mix IV | |
| Carbohydrates | |||||
| arabinose | 1.1 × 10−2 | 1.3 × 10−2 | 0.9 × 10−2 | 0.8 × 10−2 | 1.5 × 10−2 |
| galactose | 1.4 × 10−2 | 1.2 × 10−2 | 1.5 × 10−2 | 2.0 × 10−2 | 4.5 × 10−2 |
| α-glucose | 5.5 × 10−1 | 4.3 × 10−1 | 4.6 × 10−1 | 3.9 × 10−1 | 3.7 × 10−1 |
| fucose | 2.6 × 10−2 | 2.3 × 10−2 | 2.1 × 10−2 | 1.5 × 10−2 | 1.8 × 10−2 |
| mannose | 3.3 × 10−2 | 4.3 × 10−2 | 4.6 × 10−1 | 2.5 × 10−1 | 3.5 × 10−1 |
| rhamnose | 1.5 × 10−1 | 2.6 × 10−1 | 2.4 × 10−1 | 2.9 × 10−1 | 2.8 × 10−1 |
| ribose | 2.8 × 10−1 | 2.5 × 10−1 | 2.7 × 10−1 | 3.4 × 10−1 | 2.4 × 10−1 |
| xylose | 1.9 × 10−2 | 1.3 × 10−2 | 1.5 × 10−2 | 2.0 × 10−2 | 3.5 × 10−2 |
| Amino acids | |||||
| alanine | 3.5 × 10−2 | 8.5 × 10−2 | 1.9 × 10−2 | 0.7 × 10−3 | 0.5 × 10−3 |
| aspartic acid | 8.2 × 10−1 | 4.3 × 10−1 | 1.2 × 10−2 | 8.2 × 10−2 | 1.5 × 10−1 |
| glycine | 6.5 × 10−3 | 8.4 × 10−3 | 2.3 × 10−3 | 1.1 × 10−3 | 1.0 × 10−3 |
| lysine | 4.2 × 10−3 | 2.7 × 10−2 | 0.5 × 10−2 | 0.5 × 10−2 | 3.4 × 10−3 |
| serine | 5.3 × 10−2 | 8.1 × 10−2 | 8.3 × 10−2 | 1.2 × 10−2 | 0.6 × 10−2 |
| threonine | 3.5 × 10−2 | 6.4 × 10−3 | 3.9 × 10−3 | 8.8 × 10−2 | 8.2 × 10−2 |
| proline | 4.2 × 10−3 | 50.1 × 10−1 | 20.9 × 10−1 | 15.3 × 10−1 | 56.0 × 10−1 |
| Fatty acids | |||||
| eicosapentaenoic (20:5) | 3.8 × 10−2 | 4.5 × 10−2 | 4.9 × 10−1 | 6.8 × 10−1 | 8.3 × 10−2 |
| linolenic (18:2) | 1.1 × 10−3 | 9.8 × 10−2 | 9.3 × 10−2 | 6.4 × 10−2 | 8.1 × 10−2 |
| myristic (14:0) | 5.2 × 10−4 | 5.3 × 10−3 | 5.5 × 10−4 | 4.9 × 10−4 | 2.3 × 10−4 |
| palmitic (16:0) | 5.1 × 10−4 | 5.6 × 10−3 | 5.0 × 10−4 | 6.7 × 10−4 | 1.5 × 10−4 |
| stearic (18:0) | 5.9 × 10−4 | 6.2 × 10−3 | 6.3 × 10−3 | 3.4 × 10−4 | 3.2 × 10−4 |
| Others 1 | |||||
| glycine-betaine | 1.1 × 10−4 | 1.0 × 10−2 | 3.9 × 10−3 | 1.1 × 10−2 | 4.3 × 10−2 |
| homarine | 0.8 × 10−4 | 1.7 × 10−2 | 2.3 × 10−3 | 3.0 × 10−2 | 3.3 × 10−2 |
| glycerol | 1.2 × 10−4 | 2.7 × 10−2 | 1.6 × 10−3 | 4.1 × 10−2 | 5.1 × 10−2 |
| Chytrid-Diatom Pair | Number of Zoospores | ||
|---|---|---|---|
| Control 1 | Control 2 | Control 3 | |
| Chytridium sp./Navicula sp. | 0.052 | 0.041 | 0.049 |
| Rhizophydium type I/Nitzschia sp. | 0.083 | 0.086 | 0.088 |
| Rhizophydium type IIa/Rhizosolenia sp. | 0.147 | 0.129 | 0.141 |
| Rhizophydium type IIb/Chaetoceros sp. | 0.135 | 0.123 | 0.139 |
| Compound Group | Extraction Solvent | Calibration Standard | Method | Reference |
|---|---|---|---|---|
| Aldehydes | MeOH (80%) | Formaldehyde CH2O | Schiff's and Fehling‘s tests * | [70] |
| Alkaloids | EtOH (99%) | Piperine C17H19NO3 | Mayer’s and Wagner’s reagent * | [71] |
| Carbohydrates | EtOH (99%) | D-glucose C6H12O6 | Fehling’s Test | [72] |
| PUFA | n-Hexane | Stearidonic acid C18H28O2 | Argentation thin layer chromatography 1 | [73] |
| Flavonoids | EtOH (99%) | Quercetin C15H10O7 | Alkaline Reagent Test | [72] |
| Glycosides | EtOH (99%) | Oleandrin C32H48O9 | Keller-Killiani Test | [74] |
| Phenols | EtOH (99%) | Hydroquinone C6H6O2 | Folin-Ciocalteu reagent/FeCl3 | [75] |
| Phytosterols | MeOH (80%) | Ergosterol C28H44O | Liebermann-Burchardt test | [72] |
| Saponins | EtOH (99%) | Saponin S4521 | Frothing test | [71] |
| Tannins | EtOH (99%) | Tannic acid C76H52O46 | Gelatine-Saltblock test | [71] |
| Triterpenoides | EtOH (99%) | 18β-Oleanane C30H52 | Salkowski’s Test | [75] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scholz, B.; Küpper, F.C.; Vyverman, W.; Ólafsson, H.G.; Karsten, U. Chytridiomycosis of Marine Diatoms—The Role of Stress Physiology and Resistance in Parasite-Host Recognition and Accumulation of Defense Molecules. Mar. Drugs 2017, 15, 26. https://doi.org/10.3390/md15020026
Scholz B, Küpper FC, Vyverman W, Ólafsson HG, Karsten U. Chytridiomycosis of Marine Diatoms—The Role of Stress Physiology and Resistance in Parasite-Host Recognition and Accumulation of Defense Molecules. Marine Drugs. 2017; 15(2):26. https://doi.org/10.3390/md15020026
Chicago/Turabian StyleScholz, Bettina, Frithjof C. Küpper, Wim Vyverman, Halldór G. Ólafsson, and Ulf Karsten. 2017. "Chytridiomycosis of Marine Diatoms—The Role of Stress Physiology and Resistance in Parasite-Host Recognition and Accumulation of Defense Molecules" Marine Drugs 15, no. 2: 26. https://doi.org/10.3390/md15020026
APA StyleScholz, B., Küpper, F. C., Vyverman, W., Ólafsson, H. G., & Karsten, U. (2017). Chytridiomycosis of Marine Diatoms—The Role of Stress Physiology and Resistance in Parasite-Host Recognition and Accumulation of Defense Molecules. Marine Drugs, 15(2), 26. https://doi.org/10.3390/md15020026