Sulfated Hetero-Polysaccharides Protect SH-SY5Y Cells from H2O2-Induced Apoptosis by Affecting the PI3K/Akt Signaling Pathway
Abstract
:1. Introduction
2. Results
2.1. Chemical Analysis
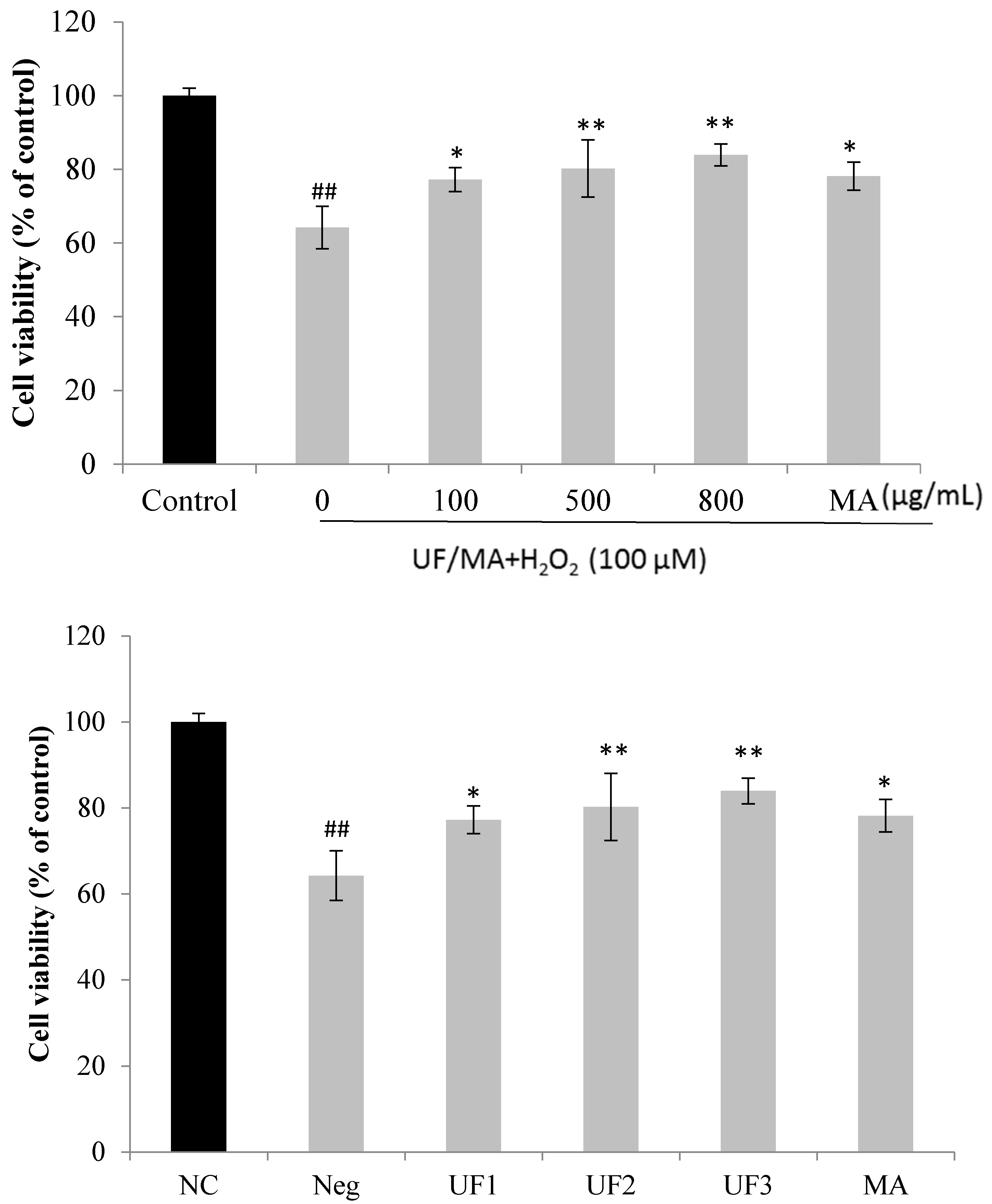
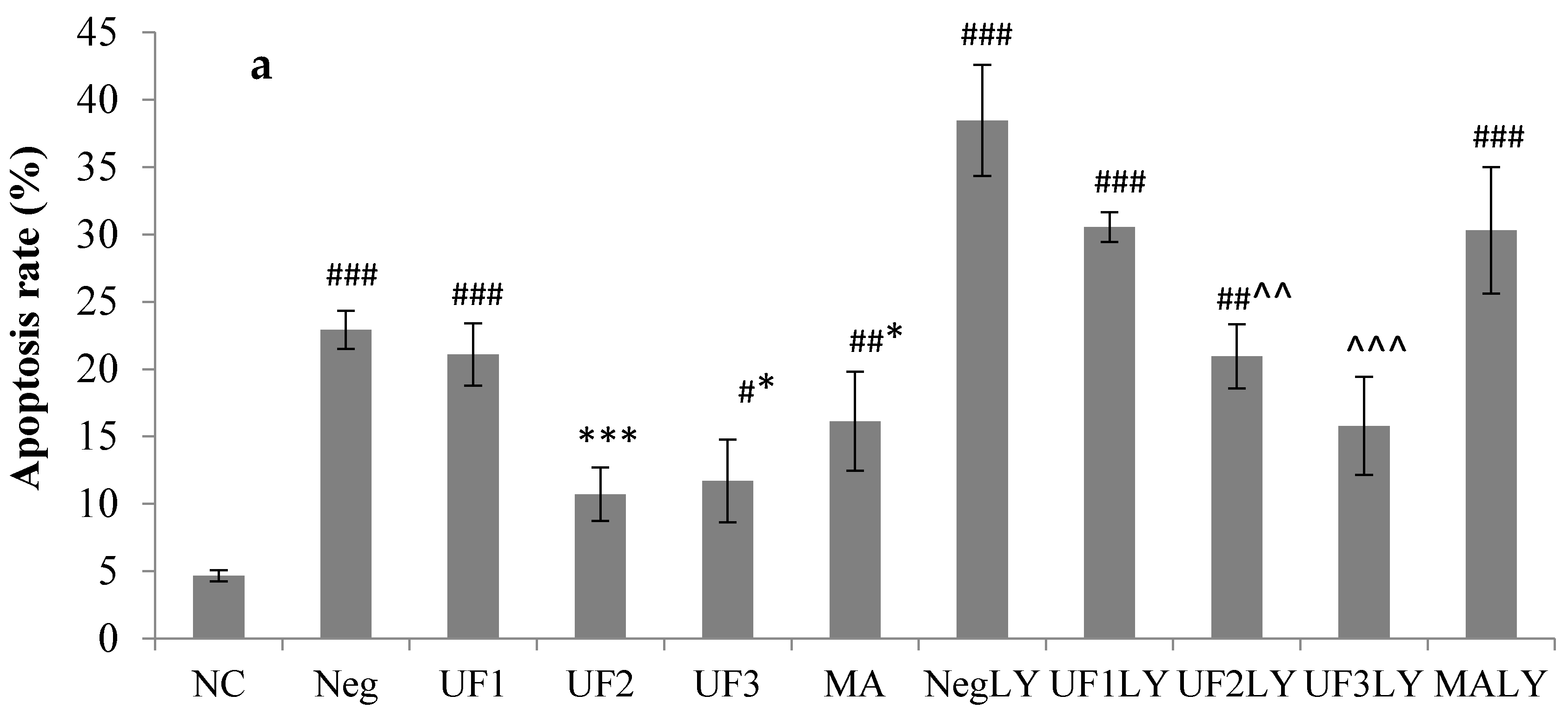
2.2. Protective Effect of UF on H2O2-Induced Neurotoxicity in SH-SY5Y Cells
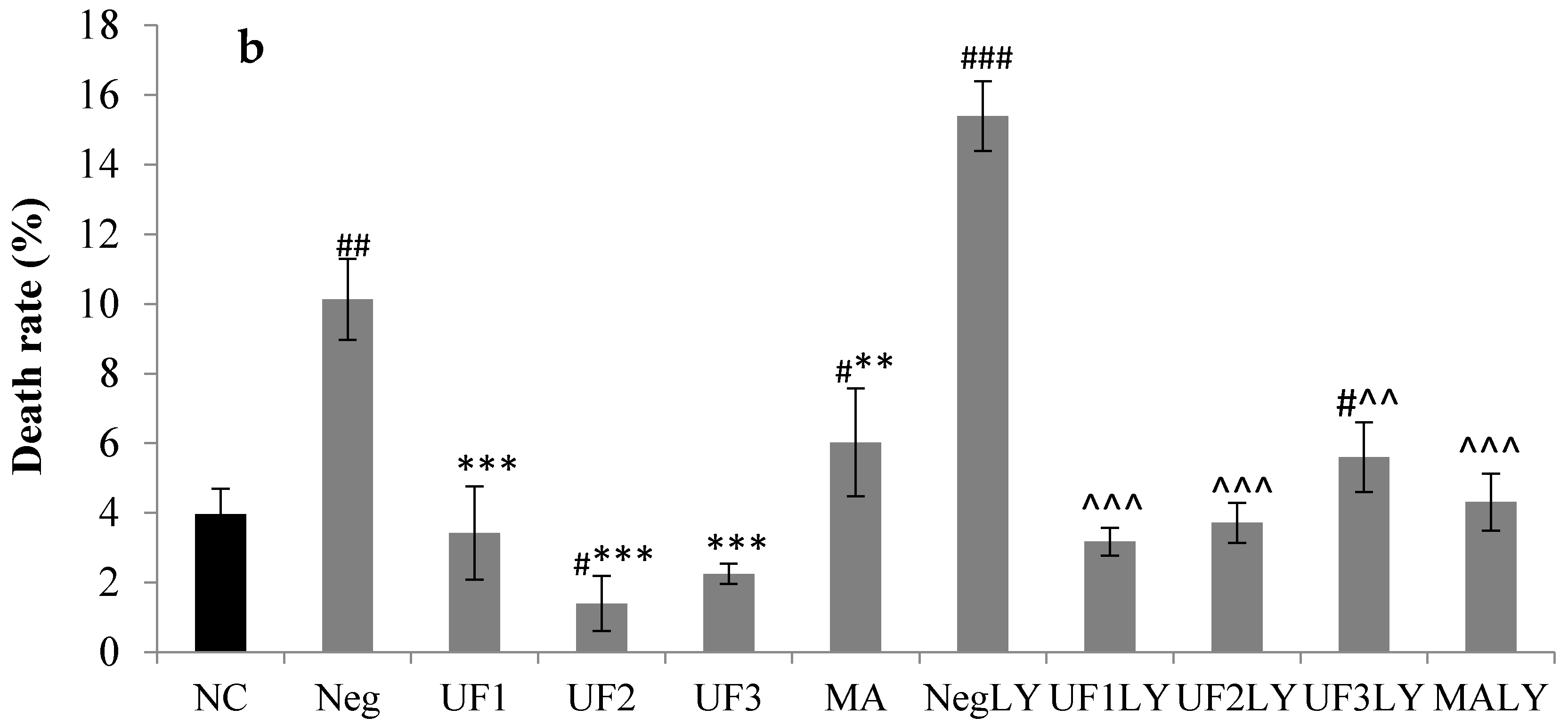
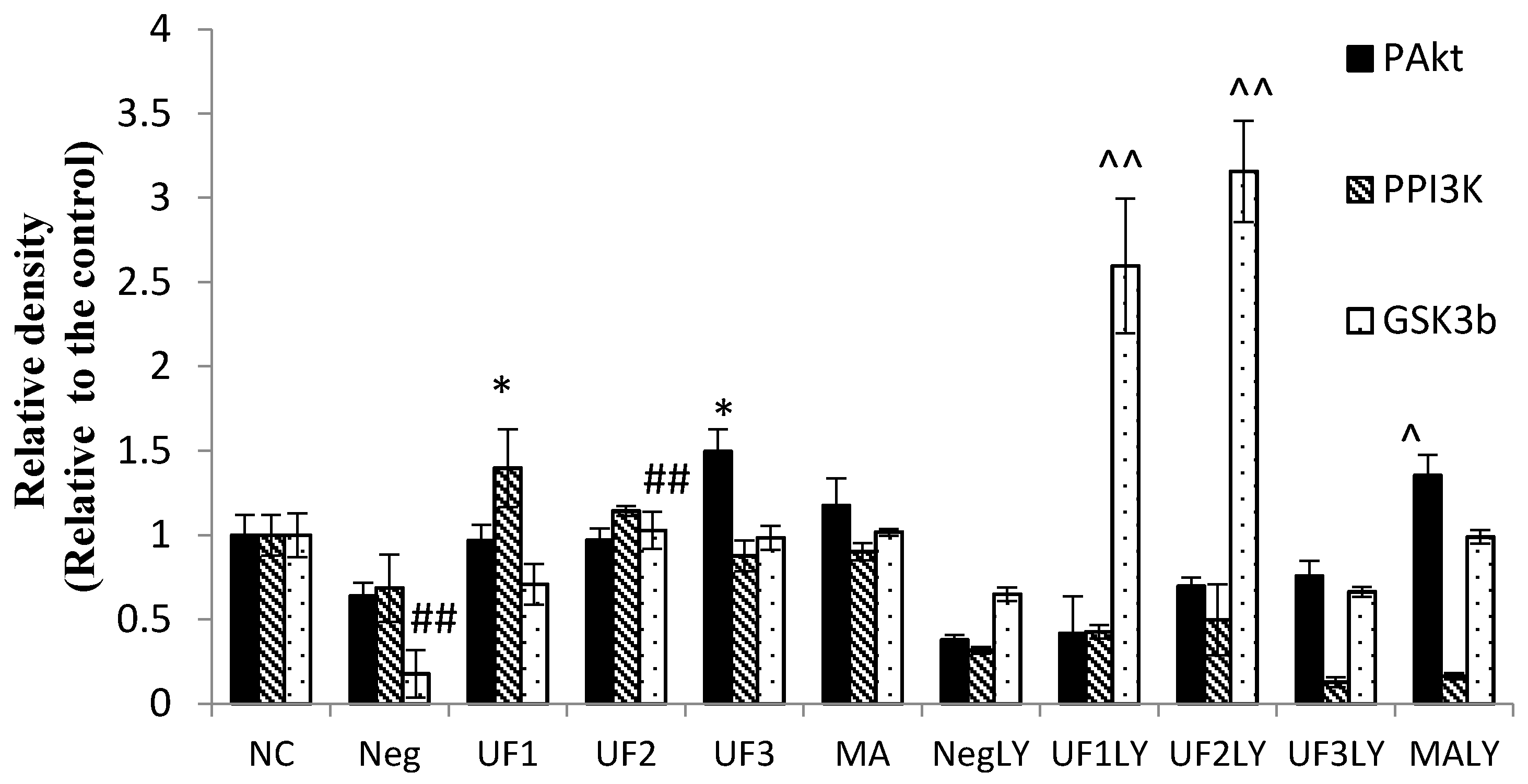
2.3. Immunocytochemistry
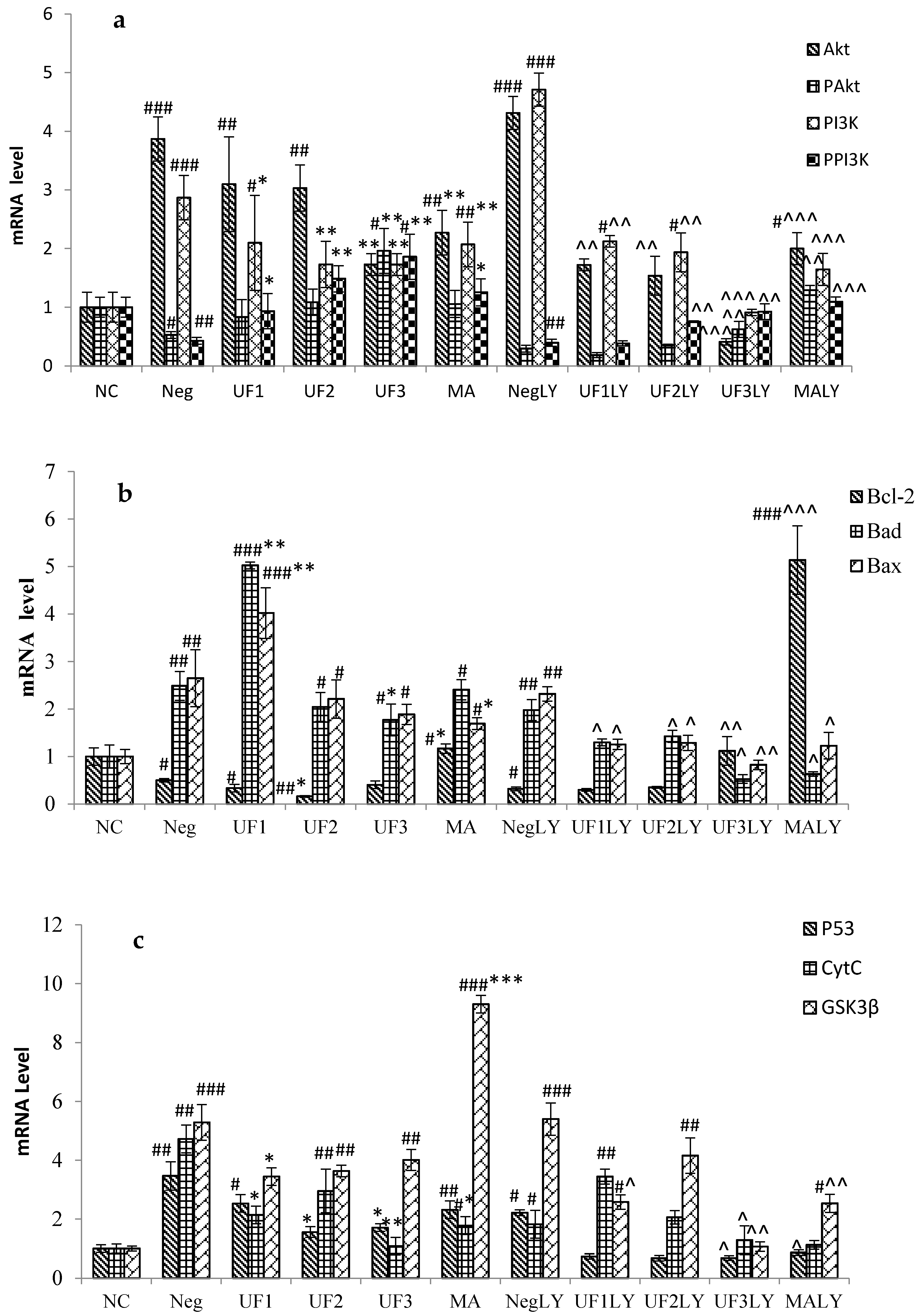
2.4. Real-Time PCR
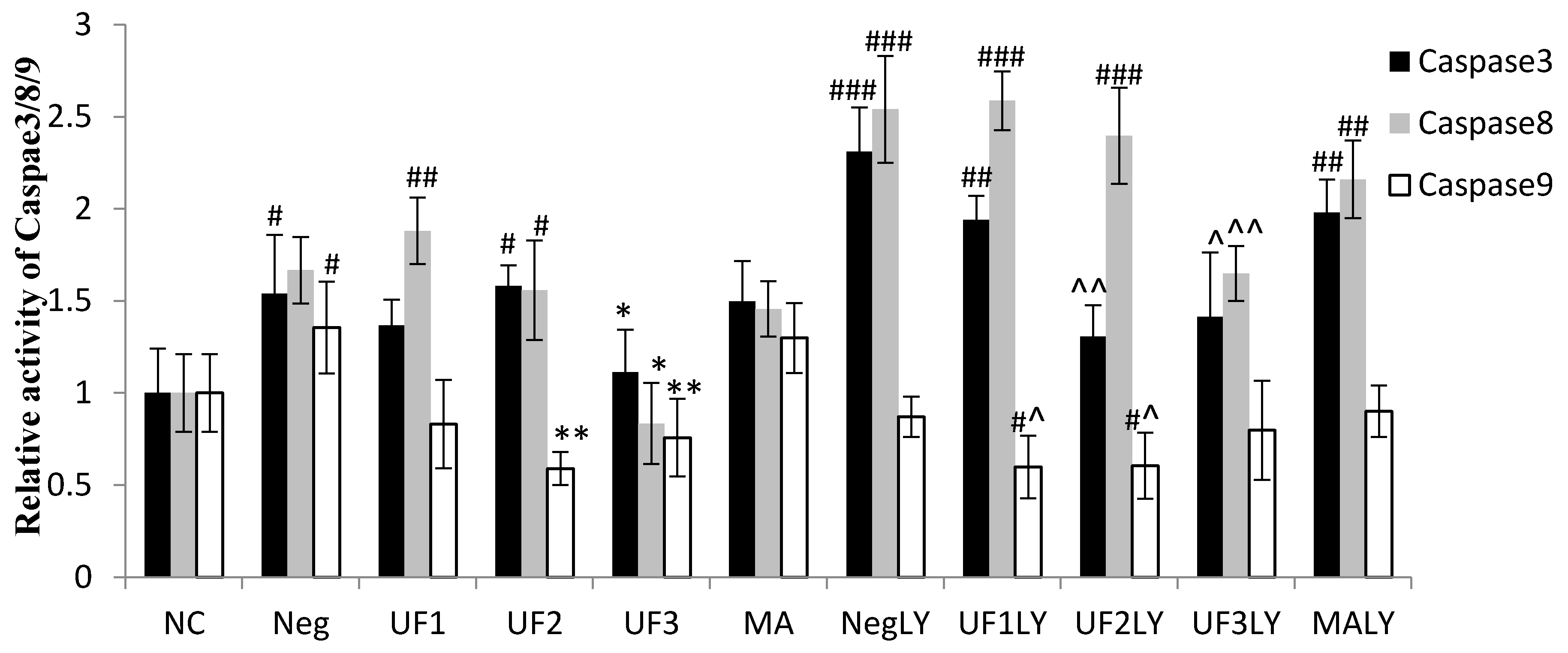
2.5. Caspase-3, Caspase-8 and Caspase-9 Activity
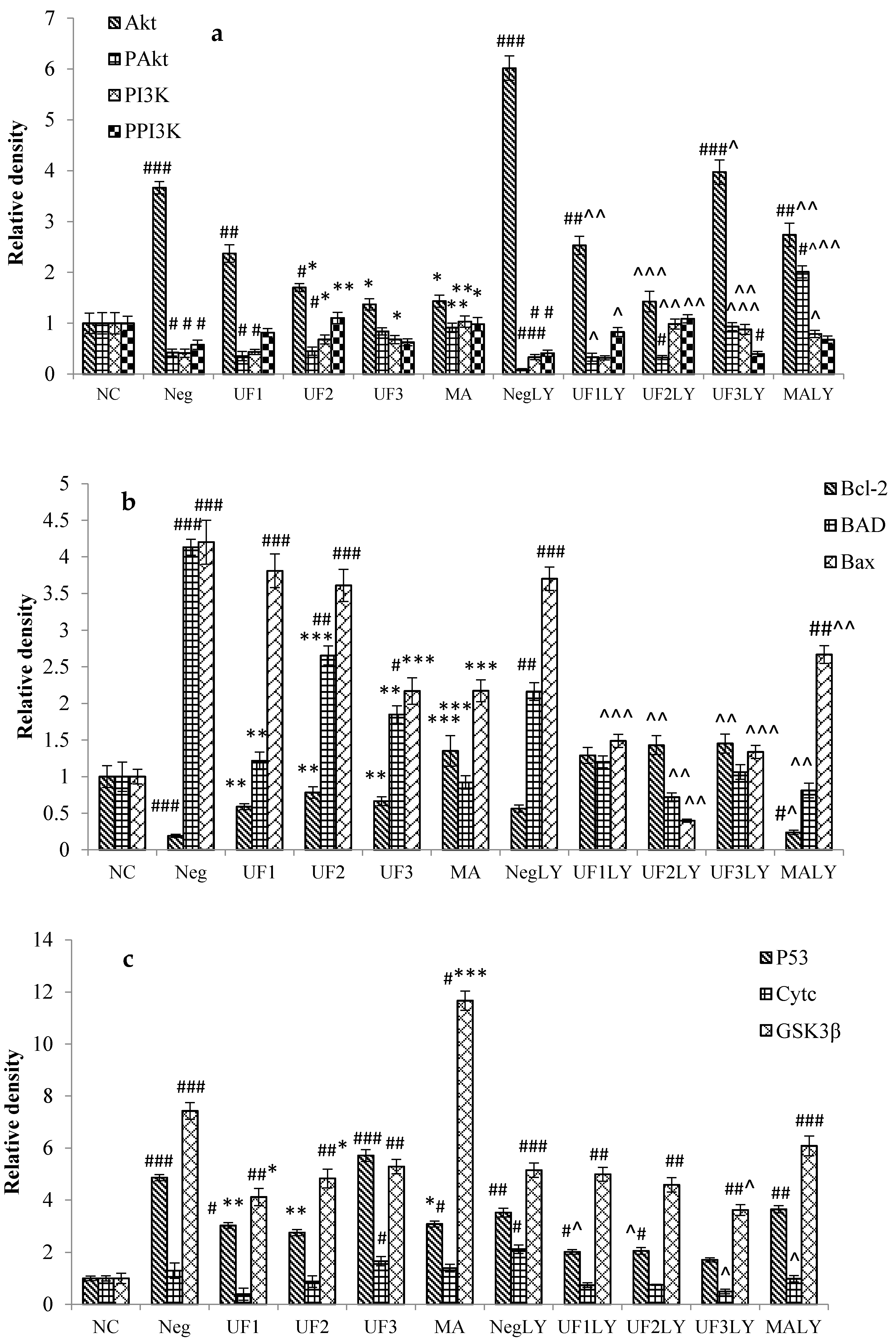
2.6. Western Blotting
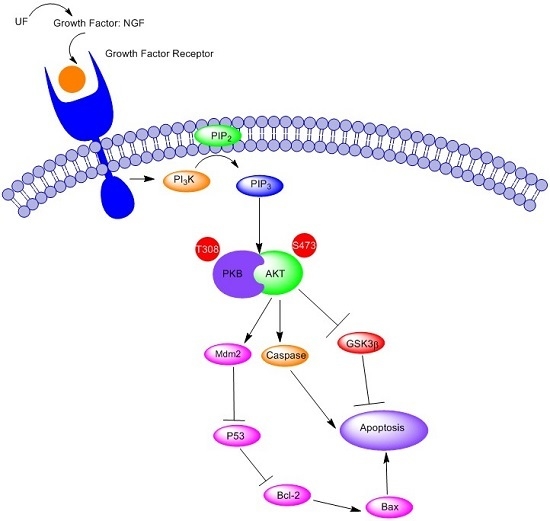
3. Discussion
4. Materials and Methods
4.1. Preparation of Sulfated Polysaccharides
4.2. Analytical Methods
4.3. Cell Culture and Treatments
4.4. Measurement of Cell Viability by MTT
4.5. Observation of Morphological Changes
4.6. Immunocytochemistry
4.7. Real-Time PCR
4.8. Caspase-3, Caspase-8 and Caspase-9 Activities
4.9. Western Blotting
4.10. Statistical Analysis of Data
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kuter, K.; Kratochwil, M.; Berghauzen-Maciejewska, K.; Glowacka, U.; Sugawa, M.D.; Ossowska, K.; Dencher, N.A. Adaptation within mitochondrial oxidative phosphorylation supercomplexes and membrane viscosity during degeneration of dopaminergic neurons in an animal model of early Parkinson’s disease. Biochim. Biophys. Acta Mol. Basis Dis. 2016, 1862, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Je, G.; Kim, Y.S. Transcriptional mutagenesis by 8-oxodG in alpha-synuclein aggregation and the pathogenesis of Parkinson’s disease. Exp. Mol. Med. 2015, 47–54. [Google Scholar] [CrossRef]
- Song, I.U.; Lee, K.S.; Park, J.W.; Kim, J.S. Role of microglia-mediated neuroinflammation in the aging process and pathogenesis of Parkinson’s disease. Mov. Disord. 2014, 29, S78. [Google Scholar]
- Bir, A.; Sen, O.; Anand, S.; Khemka, V.K.; Banerjee, P.; Cappai, R.; Sahoo, A.; Chakrabarti, S. alpha-synuclein-induced mitochondrial dysfunction in isolated preparation and intact cells: Implications in the pathogenesis of Parkinson’s disease. J. Neurochem. 2014, 131, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.W.; Gehrke, S.; Wu, Z.H. RNA metabolism in the pathogenesis of Parkinson’s disease. Brain Res. 2014, 1584, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, A.; Vassallo, N. The Centrality of Mitochondria in the Pathogenesis and Treatment of Parkinson’s Disease. CNS Neurosci. Ther. 2014, 20, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.Q.; Kong, X.C.; Kong, L.B.; Wu, L.Y.; Sun, H.; Zhang, D. Squamosamide derivative FLZ protected dopaminergic neuron by activating Akt signaling pathway in 6-OHDA-induced in vivo and in vitro Parkinson’s disease models. Brain Res. 2014, 1547, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.Q.; Kong, X.C.; Qian, C.; Zhang, D. Flz protects dopaminergic neuron through activiting protein kinaseb/mammalian target of rapamycin pathway and inhibiting Rtp801 expression in Parkinson’s disease modeles. Neuroscience 2012, 202, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Tatton, W.G.; Chalmers-Redman, R.; Brown, D.; Tatton, N. Apoptosis in Parkinson’s disease: Signals for neuronal degradation. Ann. Neurol. 2003, 53, S61–S70. [Google Scholar] [CrossRef] [PubMed]
- Perier, C.; Bove, J.; Vila, M. Mitochondria and Programmed Cell Death in Parkinson’s Disease: Apoptosis and Beyond. Antioxid. Redox Signal. 2012, 16, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.H.; Dar, K.B.; Anees, S.; Zargar, M.A.; Masood, A.; Sofi, M.A.; Ganie, S.A. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed. Pharmacother. 2015, 74, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Levy, O.A.; Malagelada, C.; Greene, L.A. Cell death pathways in Parkinson’s disease: Proximal triggers, distal effectors, and final steps. Apoptosis 2009, 14, 478–500. [Google Scholar] [CrossRef] [PubMed]
- Cheung, E.C.C.; Melanson-Drapeau, L.; Cregan, S.P.; Vanderluit, J.L.; Ferguson, K.L.; McIntosh, W.C.; Park, D.S.; Bennett, S.A.L.; Slack, R.S. Apoptosis-inducing factor is a key factor in neuronal cell death propagated by BAX-dependent and BAX-independent mechanisms. J. Neurosci. 2005, 25, 1324–1334. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.Z.; Zhang, Q.B.; Wang, H.M.; Cui, Y.Q.; Sun, Z.L.; Yang, J.; Zheng, Y.; Jia, J.; Yu, F.; Wang, X.; et al. Fucoidan protects against dopaminergic neuron death in vivo and in vitro. Eur. J. Pharmacol. 2009, 617, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Q.B.; Zhang, Z.S.; Li, Z. Antioxidant activity of sulfated polysaccharide fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2008, 42, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, H.D.; Jin, W.H.; Zhang, H.; Zhang, Q.B. Structure-activity relationship of sulfated hetero/galactofucan polysaccharides on dopaminergic neuron. Int. J. Biol. Macromol. 2016, 82, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Przedborski, S.; Ischiropoulos, H. Reactive oxygen and nitrogen species: Weapons of neuronal destruction in models of Parkinson’s disease. Antioxid. Redox Signal. 2005, 7, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Simone, F.; Adrienne, M.; Gorman, B.; Osamu, H.; Samali, A. Cellular Stress Responses: Cell Survival and Cell Death. Int. J. Cell Biol. 2010, 23. [Google Scholar] [CrossRef]
- Wall, N.R.; Mohammad, R.M.; Al-Katib, A.M. Bax: Bcl-2 ratio modulation by bryostatin 1 and novel antitubulin agents is important for susceptibility to drug induced apoptosis in the human early pre-B acute lymphoblastic leukemia cell line, Reh. Leuk. Res. 1999, 23, 881–888. [Google Scholar] [CrossRef]
- Mabeau, S.; Kloareg, B.; Joseleau, J.P. Fractionation and Analysis of Fucans from Brown-Algae. Phytochemistry 1990, 29, 2441–2445. [Google Scholar] [CrossRef]
- Jin, W.; Wang, J.; Ren, S.; Song, N.; Zhang, Q.B. Structural Analysis of a Heteropolysaccharide from Saccharina japonica by Electrospray Mass Spectrometry in Tandem with Collision-Induced Dissociation Tandem Mass Spectrometry (ESI-CID-MS/MS). Mar. Drugs 2012, 10, 2138–2152. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.J.; Cooper, G.M. Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth-factor. Science 1995, 267, 2003–2006. [Google Scholar] [CrossRef] [PubMed]
- Hetman, M.; Kanning, K.; Cavanaugh, J.E.; Xia, Z.G. Neuroprotection by brain-derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol 3-kinase. J. Biol. Chem. 1999, 274, 22569–22580. [Google Scholar] [CrossRef] [PubMed]
- Franke, T.F.; Kaplan, D.R.; Cantley, L.C. PI3K: Downstream AKTion blocks apoptosis. Cell 1997, 88, 435–437. [Google Scholar] [CrossRef]
- Jaisin, Y.; Thampithak, A.; Suksamrarn, A.; Phumala-Morales, N.; Govitrapong, P.; Sanvarinda, Y. Protective effect of curcumin analogue (compound 005) against 6-OHDA induced apoptosis in SH-SY5Y cells. J. Neurochem. 2010, 115, 54. [Google Scholar]
- Rodriguez-Blanco, J.; Martin, V.; Herrera, F.; Garcia-Santos, G.; Antolin, I.; Rodriguez, C. Intracellular signaling pathways involved in post-mitotic dopaminergic PC12 cell death induced by 6-hydroxydopamine. J. Neurochem. 2008, 107, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Q.; Zhang, J.C.; Flechner, L.; Hyun, T.; Yam, A.; Franke, T.F.; Pierce, J.H. Protein kinase C-alpha overexpression stimulates Akt activity and suppresses apoptosis induced by interleukin 3 withdrawal. Oncogene 1999, 18, 6564–6572. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Pan, J.; Chen, S.D. Kinases and kinase signaling pathways: Potential therapeutic targets in Parkinson’s disease. Prog. Neurobiol. 2012, 98, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Signore, A.P.; Weng, Z.F.; Hastings, T.; Van Laar, A.D.; Liang, Q.H.; Lee, Y.J.; Chen, J. Erythropoietin protects against 6-hydroxydopamine-induced dopaminergic cell death. J. Neurochem. 2006, 96, 428–443. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.X.; Yang, X.; Sreejayan, N.; Ren, J. Acetaldehyde promotes rapamycin-dependent activation of p70(S6K) and glucose uptake despite inhibition of Akt and mTOR in dopaminergic SH-SY5Y human neuroblastoma cells. Exp. Neurol. 2007, 203, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, F.; Zhang, Q.; Zhang, Z.; Shi, X.; Li, P. Synthesized different derivatives of low molecular fucoidan extracted from Laminaria japonica and their potential antioxidant activity in vitro. Int. J. Biol. Macromol. 2009, 44, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substabces. Anal. Chem. 1956, 28, 350–357. [Google Scholar] [CrossRef]
- Kawai, Y.; Seno, N.; Anno, K. A modified method for chondrosulfatase assay. Anal. Chem. 1969, 32, 314–321. [Google Scholar] [CrossRef]
- Bitter, T.; Muir, H.M. A modified uronic acid carbazole reaction. Anal. Chem. 1962, 4, 330–334. [Google Scholar] [CrossRef]
- Honda, S.; Akao, E.; Suzuki, S.; Okuda, M.; Kakehi, K.; Nakamura, J. High-performance liquid chromatography of reducing carbohydrates as strongly ultraviolet-absorbing and electrochemically sensitive 1-phenyl-3-methyl5-pyrazolone derivatives. Anal. Biochem. 1989, 180, 351–357. [Google Scholar] [CrossRef]
- Abarikwu, S.O.; Farombi, E.O.; Pant, A.B. Biflavanone-kolaviron protects human dopaminergic SH-SY5Y cells against atrazine induced toxic insult. Toxicol. In Vitro 2011, 25, 848–858. [Google Scholar] [CrossRef] [PubMed]


| Sample | Fucose | Uronic Acid | Sulfate | Molecular Weight (Da) | Neutral Sugar (Molar Ratio) a Fuc Gal Man Glc Rha Xyl | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| DF | 28.7 | 3.65 | 30.1 | 9544 | 1.000 | 0.579 | 0.038 | 0.159 | 0.054 | 0.033 |
| UF | 19.12 | 14.25 | 21.21 | 6500 | 1.000 | 0.713 | 0.112 | 0.257 | 0.113 | 0.087 |
| Target Genes | Primer Sequences | Amplicon (bp) | |
|---|---|---|---|
| Akt | FP | GCCGCTACTACGCCATGAAGA | 214 |
| RP | CCCGTTGGCATACTCCATCAC | ||
| Bad | FP | AGGCCATCAGCAACAACATAAGT | 158 |
| RP | GACAGCTTTGTGCTGGATCTGTG | ||
| Bax | FP | GGCGAATTGGACATGAAC | 182 |
| RP | CCGAAGTAGGAGAGGAGG | ||
| Bcl-2 | FP | CCCCAGAAGAAACTGAACC | 195 |
| RP | GCATCTCCTTGTCTACGC | ||
| CytC | FP | TGATGAGGAGATGGCTTG | 181 |
| RP | TCTGTTTCTTTGCGTGGA | ||
| GSK3β | FP | ATTCCCTCAAATTAAGGCACCTCC | 142 |
| RP | ATACTCCAGCAGACGGCTACACAG | ||
| p53 | FP | GGCGAATTGGAGATGAAC | 156 |
| RP | CCGAAGTAGGAGAGGAGG | ||
| PAkt | FP | TCTACAACCAGGACCATGAGAA | 117 |
| RP | GAGTAGGAGAACTGGGGAAGT | ||
| NGF | FP | TCCAGGTGCATAGCGTAATG | 195 |
| RP | CTCCGGTGAGTCCTGTTGAA | ||
| TrkA | FP | ATGAGACCAGCTGTATCT | 167 |
| RP | CATTCTCAAGTGGGAGC | ||
| GAPDH | FP | TTCACCACCATGGAGAAGGC | 247 |
| RP | GGCATGGACTGTGGTCATGA | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Liu, H.; Zhang, X.; Li, X.; Geng, L.; Zhang, H.; Zhang, Q. Sulfated Hetero-Polysaccharides Protect SH-SY5Y Cells from H2O2-Induced Apoptosis by Affecting the PI3K/Akt Signaling Pathway. Mar. Drugs 2017, 15, 110. https://doi.org/10.3390/md15040110
Wang J, Liu H, Zhang X, Li X, Geng L, Zhang H, Zhang Q. Sulfated Hetero-Polysaccharides Protect SH-SY5Y Cells from H2O2-Induced Apoptosis by Affecting the PI3K/Akt Signaling Pathway. Marine Drugs. 2017; 15(4):110. https://doi.org/10.3390/md15040110
Chicago/Turabian StyleWang, Jing, Huaide Liu, Xuan Zhang, Xinpeng Li, Lihua Geng, Hong Zhang, and Quanbin Zhang. 2017. "Sulfated Hetero-Polysaccharides Protect SH-SY5Y Cells from H2O2-Induced Apoptosis by Affecting the PI3K/Akt Signaling Pathway" Marine Drugs 15, no. 4: 110. https://doi.org/10.3390/md15040110
APA StyleWang, J., Liu, H., Zhang, X., Li, X., Geng, L., Zhang, H., & Zhang, Q. (2017). Sulfated Hetero-Polysaccharides Protect SH-SY5Y Cells from H2O2-Induced Apoptosis by Affecting the PI3K/Akt Signaling Pathway. Marine Drugs, 15(4), 110. https://doi.org/10.3390/md15040110