Isoaaptamine Induces T-47D Cells Apoptosis and Autophagy via Oxidative Stress
Abstract
:1. Introduction
2. Results
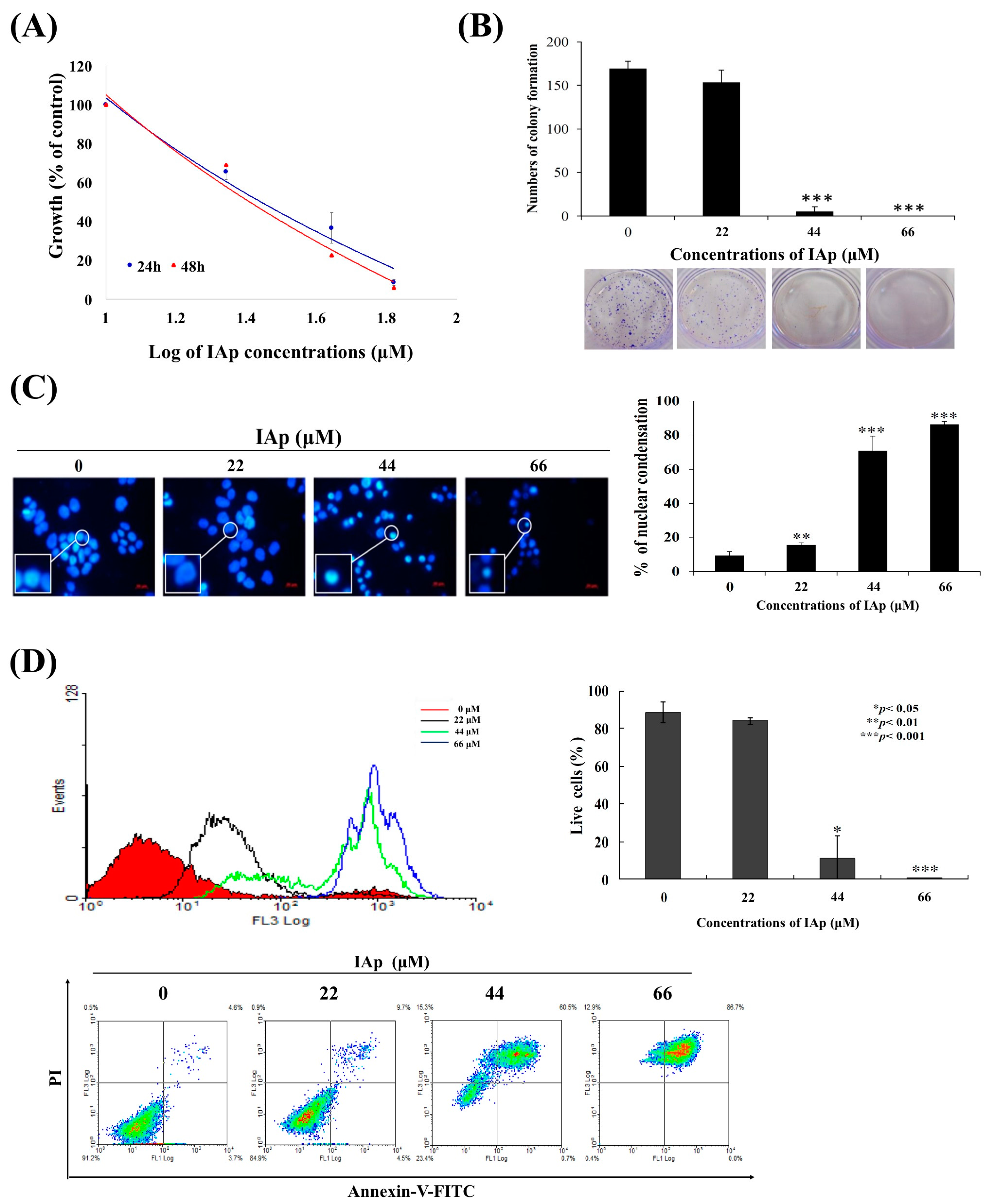
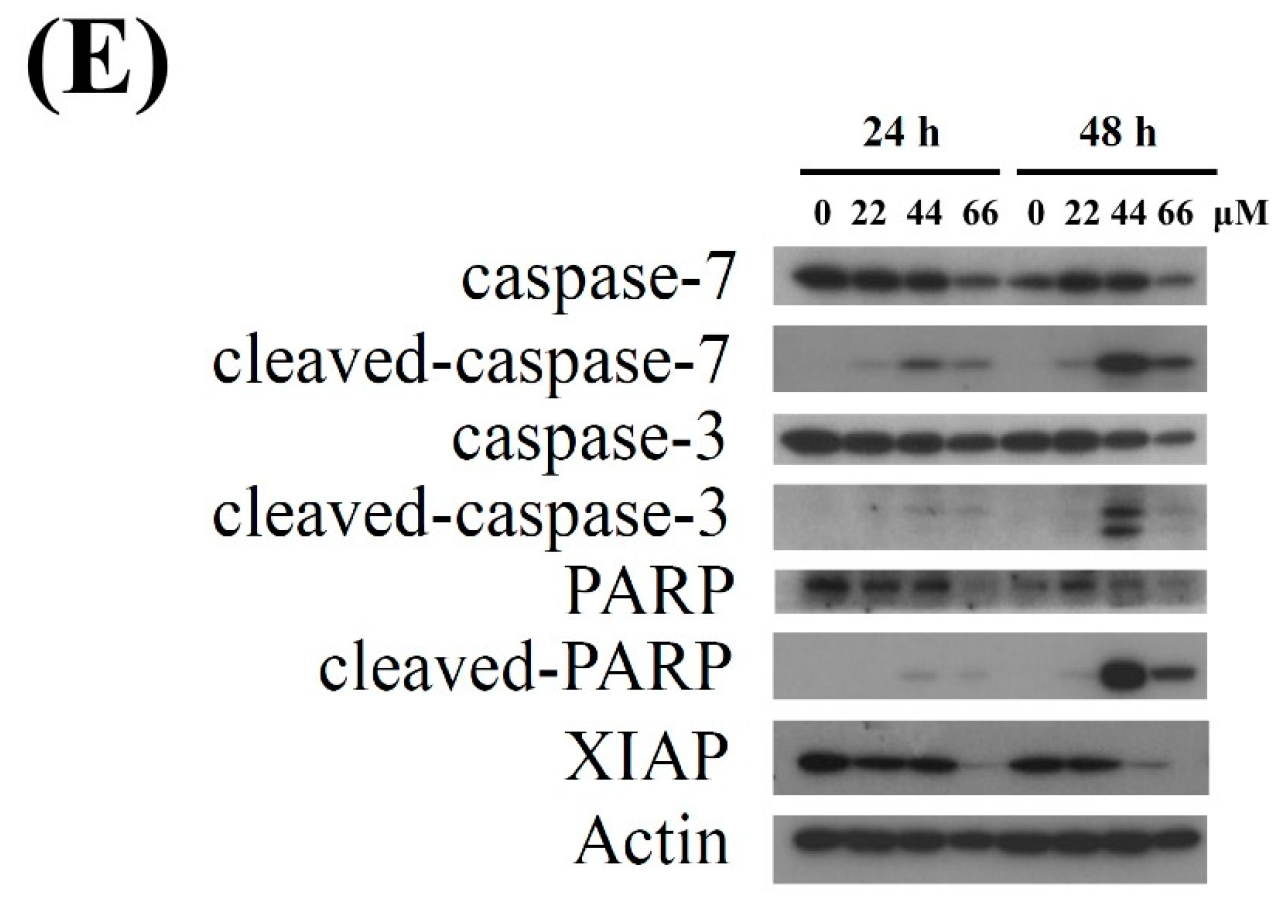
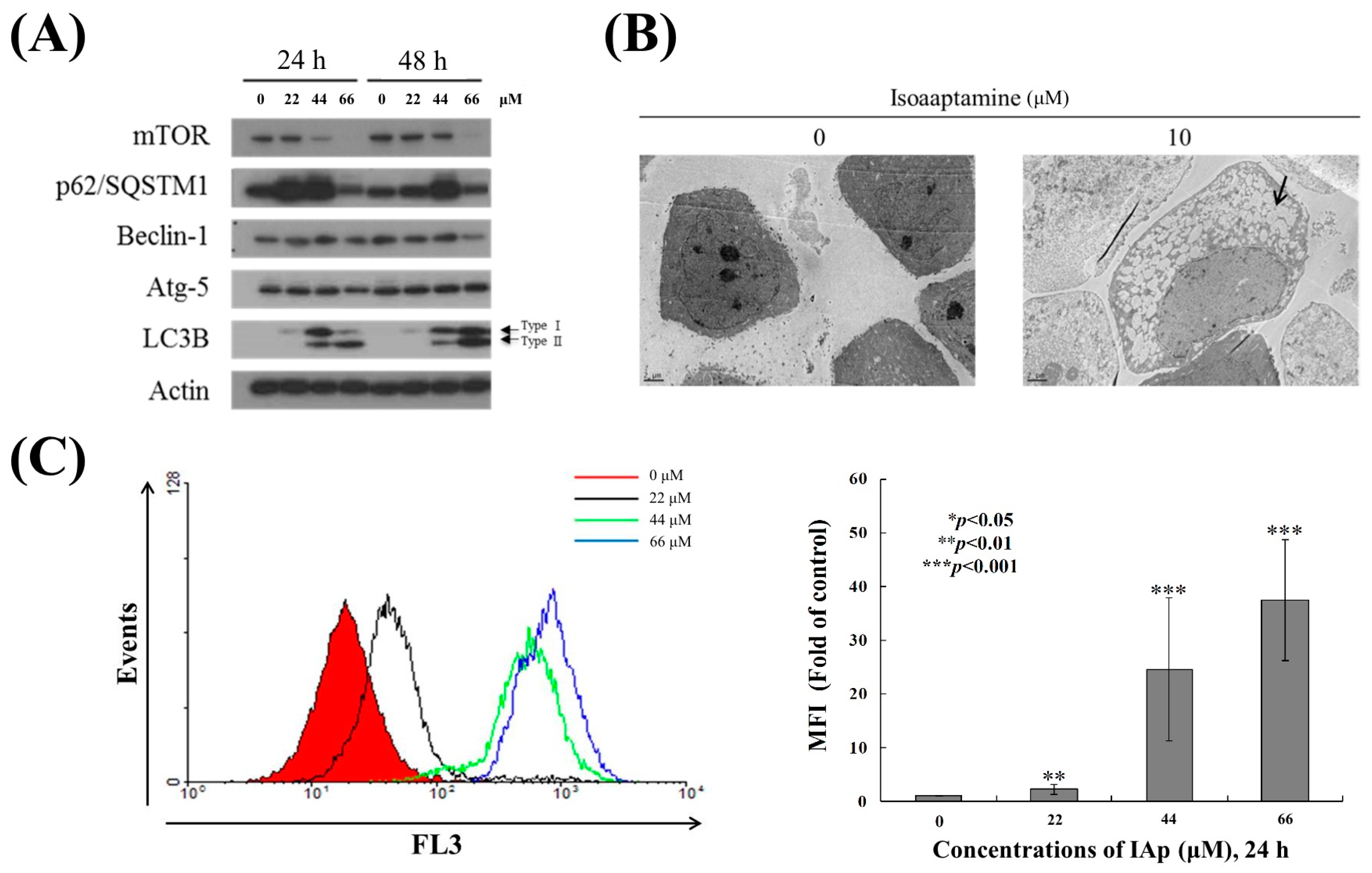
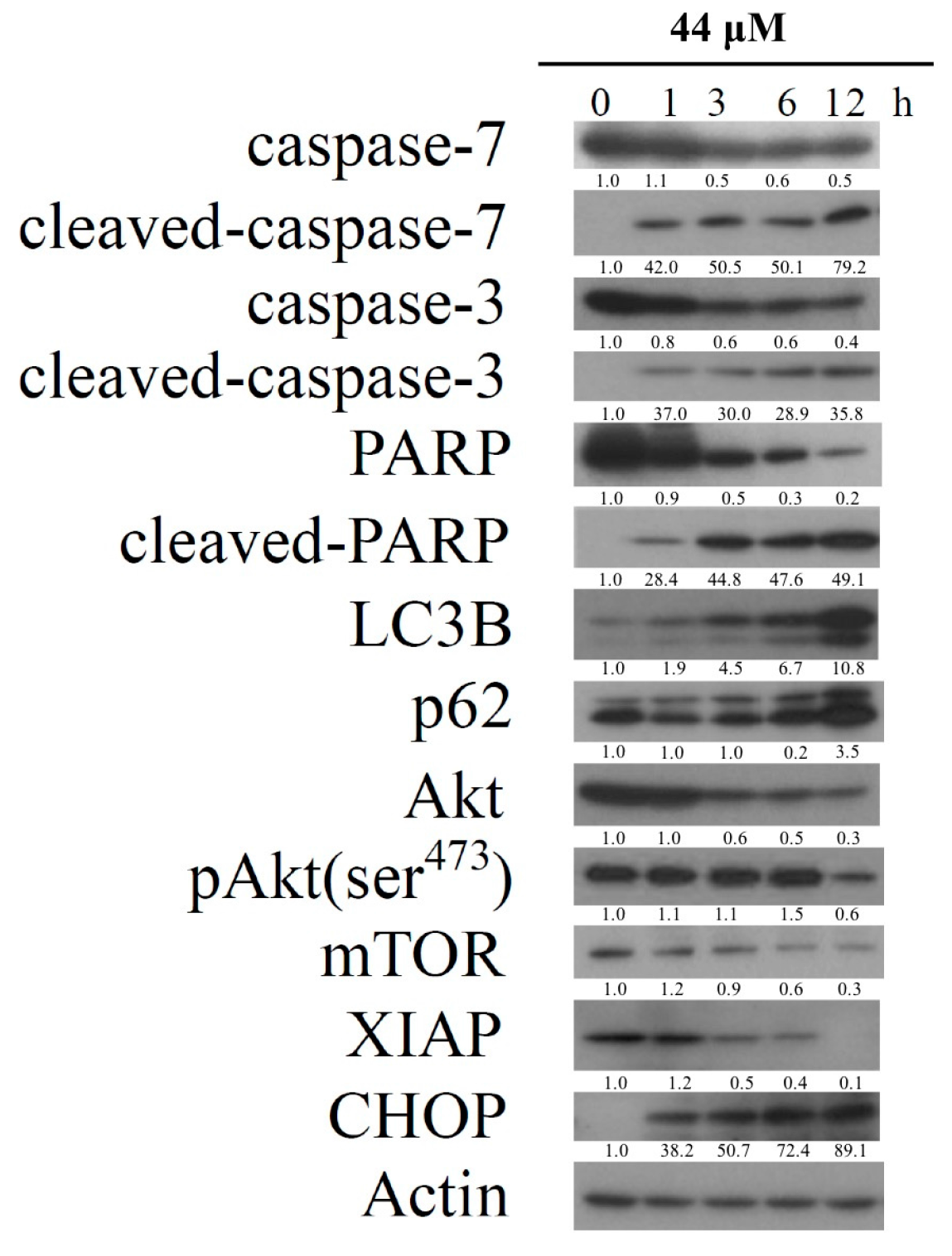
2.1. Isoaaptamine Isolated from Sponge Aaptos sp. Induces Apoptosis and Autophagy in Breast T-47D Cancer Cells
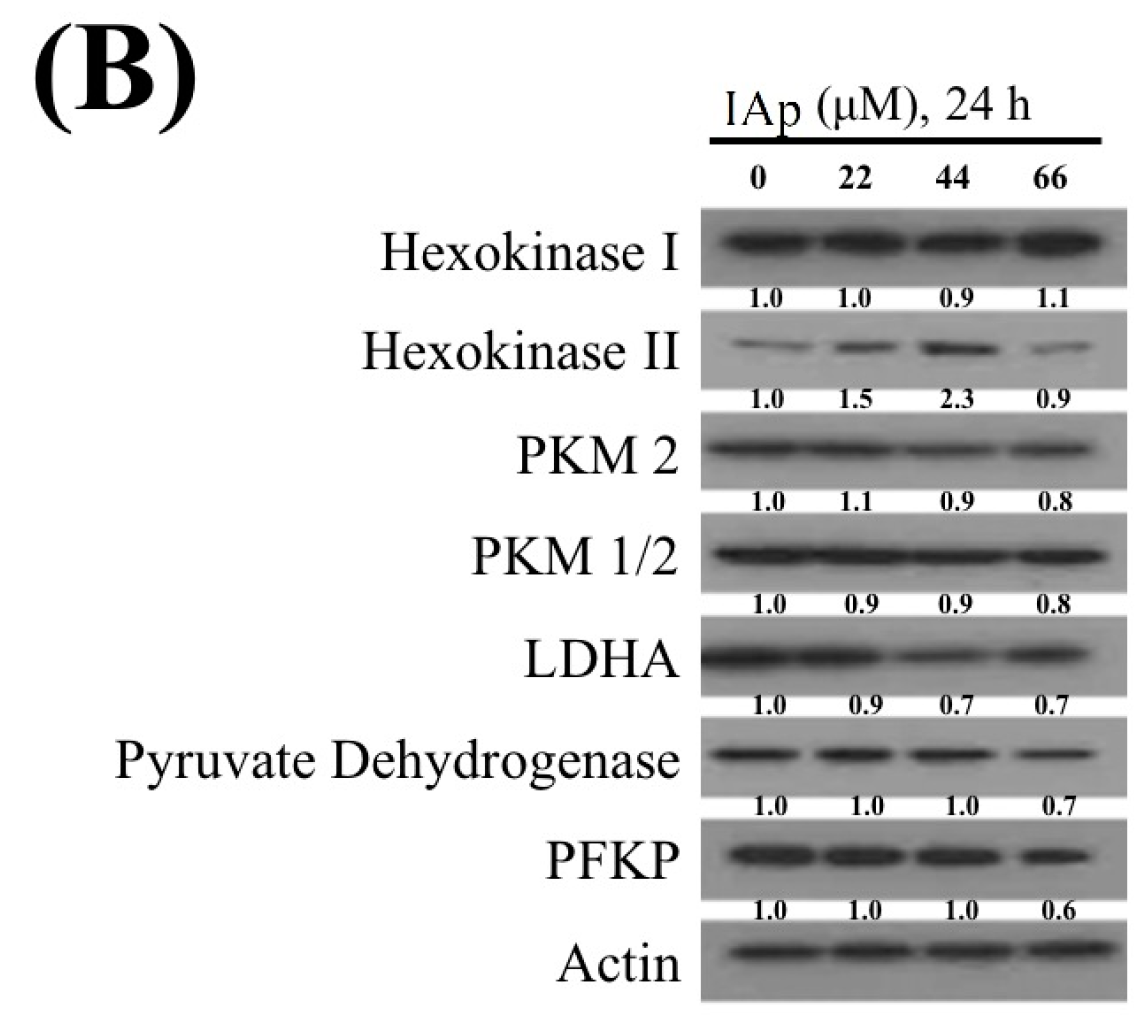
2.2. Effect of IAp on the Disruption of Mitochondrial Membrane Potential and the Expression of Mitochondrial Glycolysis-Related Proteins in Breast T-47D Cancer Cells
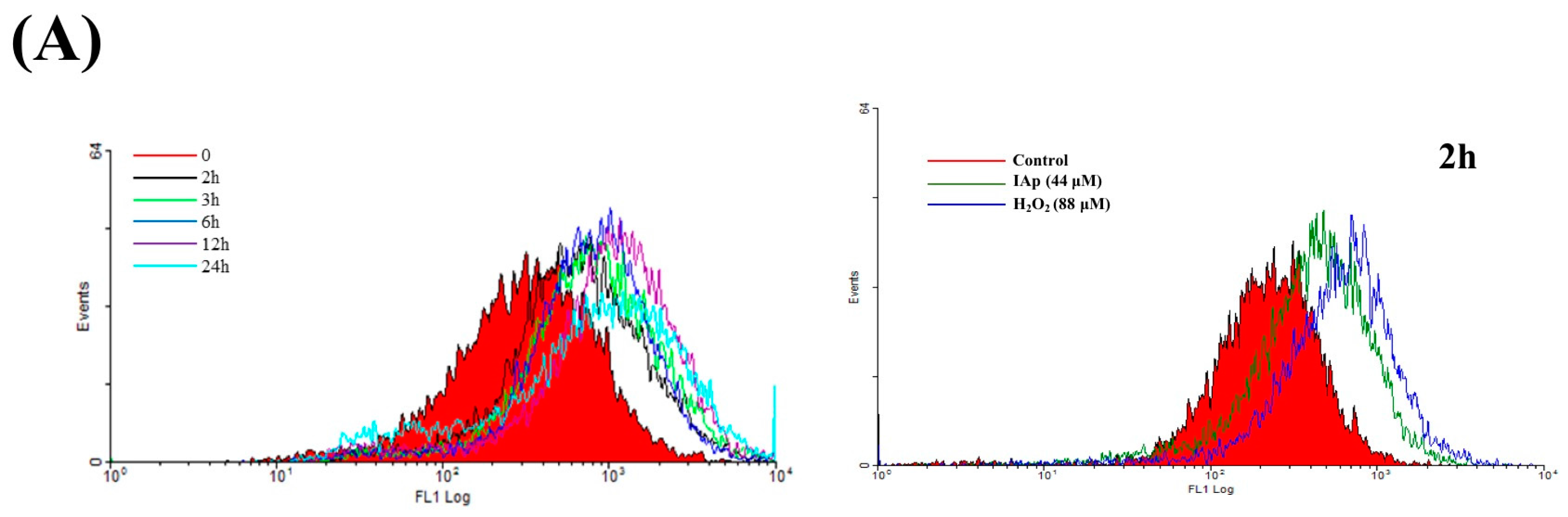
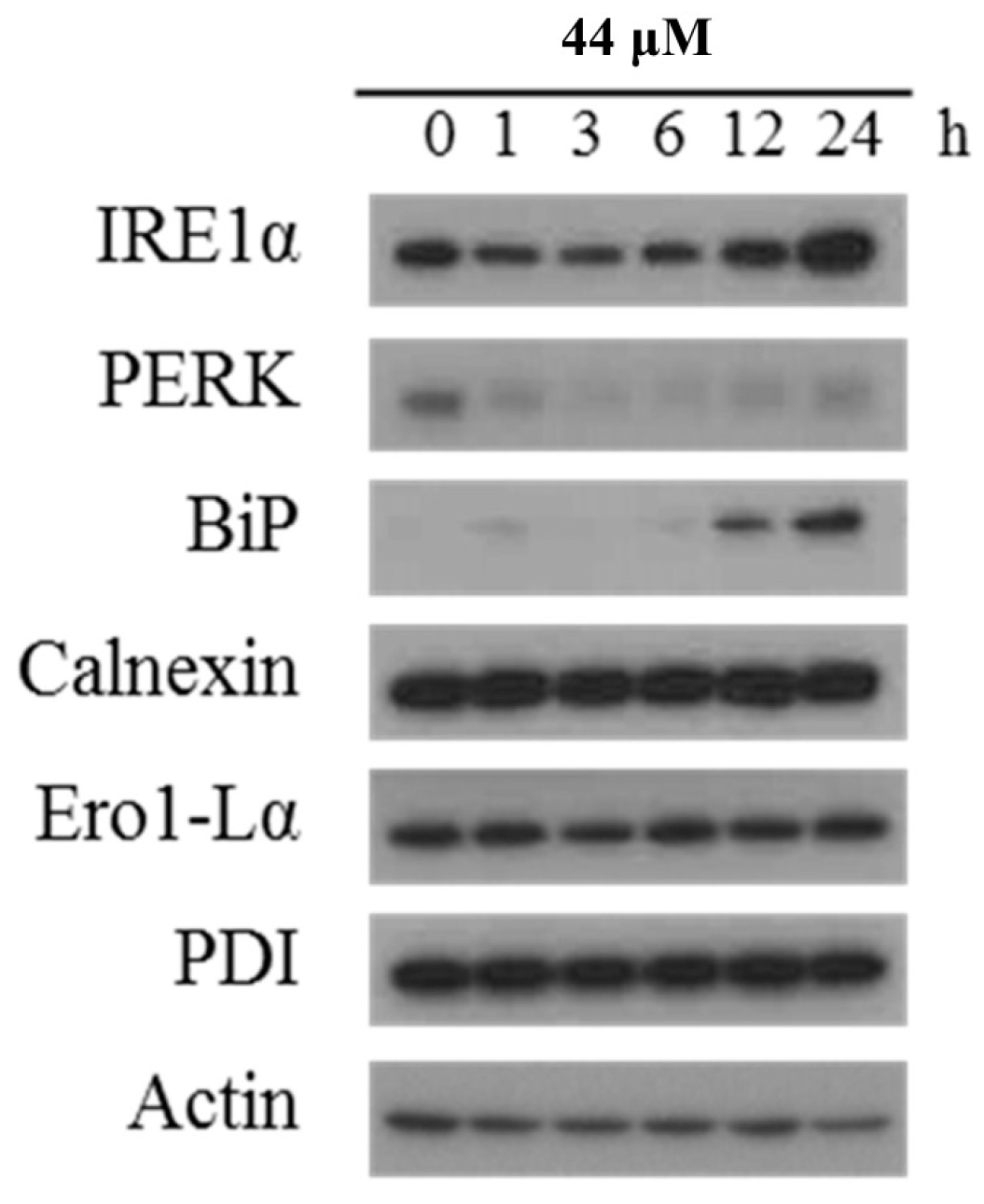
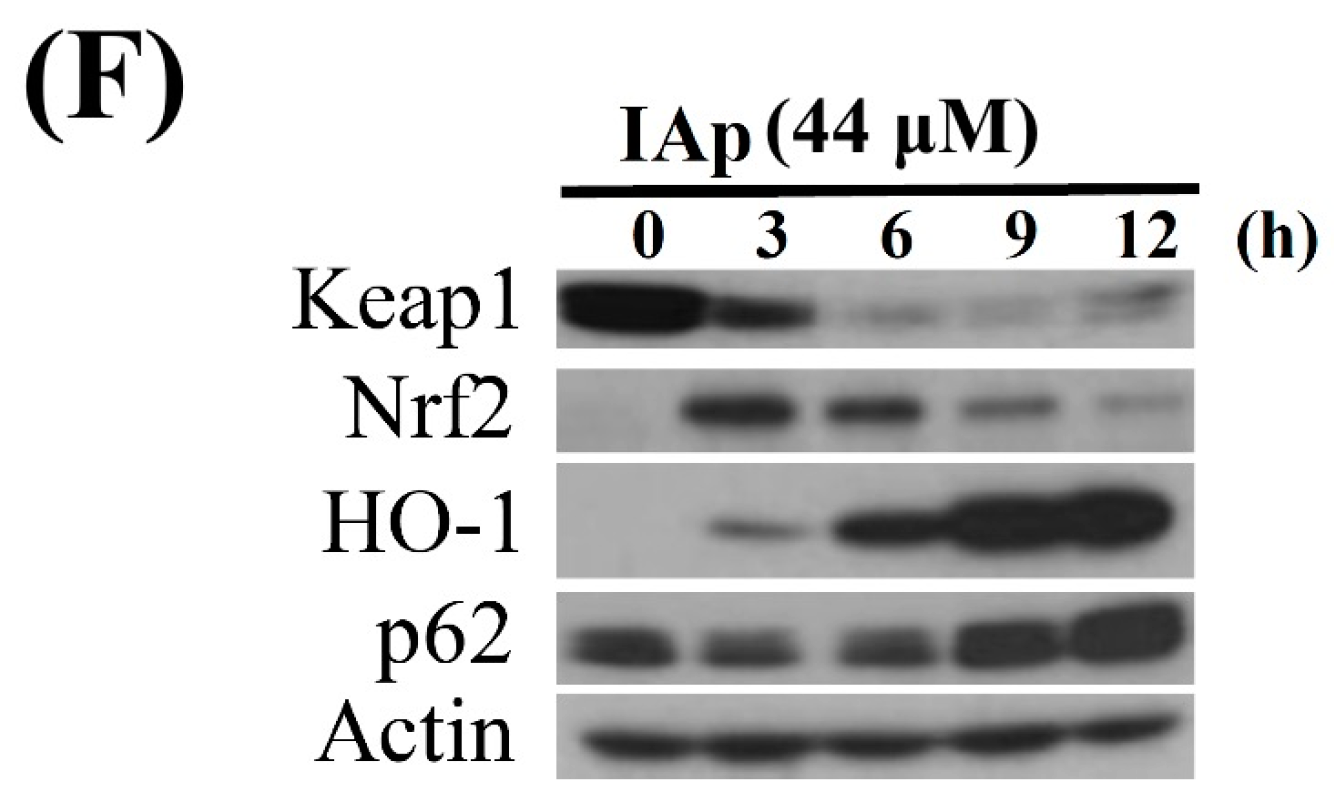
2.3. Effect of IAp on ROS Generation and the Expression of Endoplasmic Reticulum (ER) Stress-Related Proteins in Breast T-47D Cancer Cells
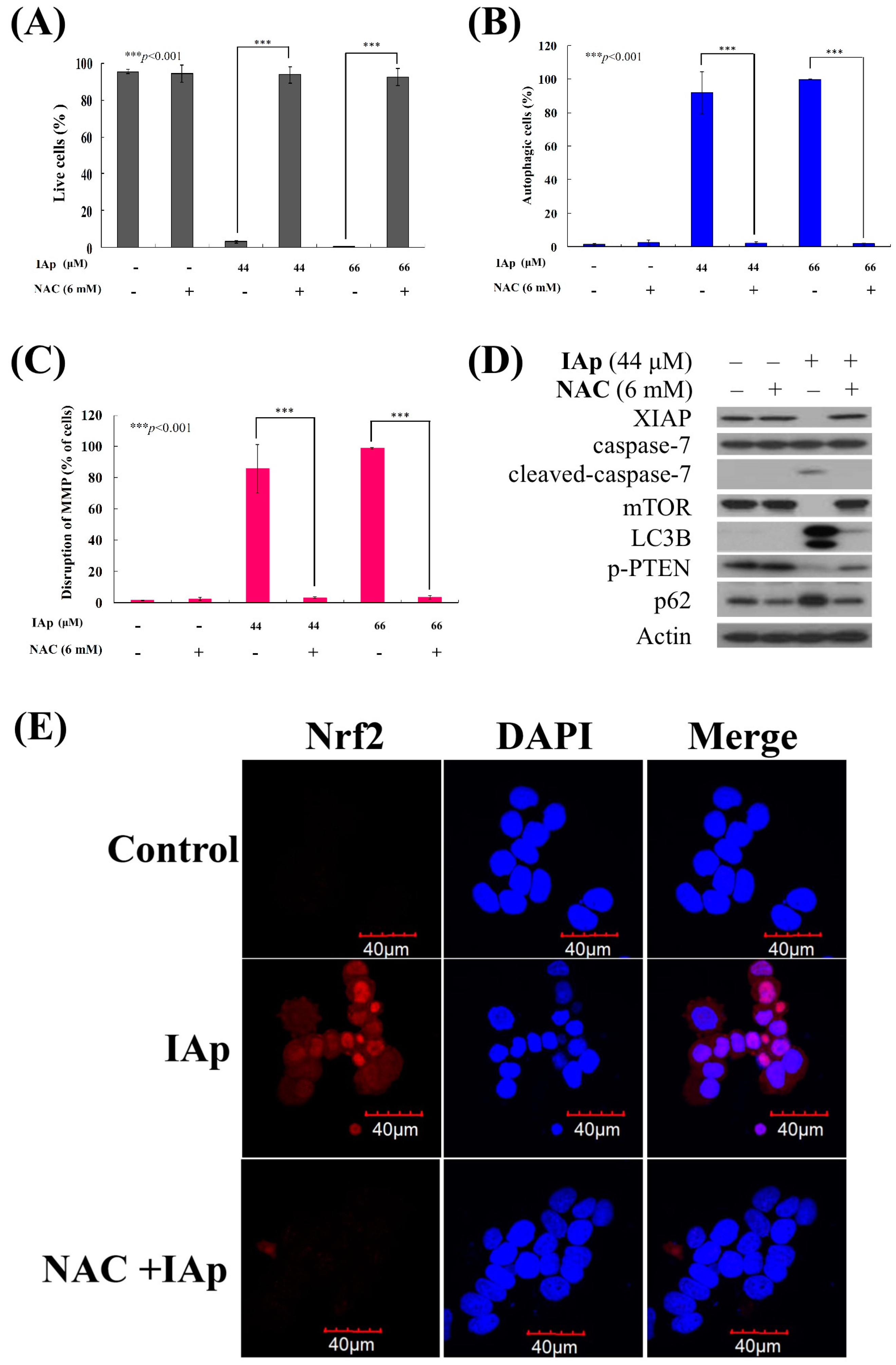
2.4. Apoptosis and Autophagy Induced by IAp Is Mediated by Excessive ROS Generation
3. Discussion
4. Experimental Section
4.1. Bioassay Materials
4.2. Preparation of the Marine Alkaloid Stock Solution
4.3. MTT Proliferation Assay
4.4. Annexin V/PI Apoptotic Assay
4.5. Determination of ROS Generation and MMP Disruption
4.6. Immunofluorescence Analysis
4.7. Statistics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Palanisamy, S.K.; Rajendran, N.M.; Marino, A. Natural products diversity of marine ascidians (tunicates; ascidiacea) and successful drugs in clinical development. Nat. Prod. Bioprospect. 2017, 7, 1–111. [Google Scholar] [CrossRef] [PubMed]
- Routh, S.; Nandagopal, K. Patent survey of resveratrol, taxol, podophyllotoxin, withanolides and their derivatives used in anticancer therapy. Recent Pat. Biotechnol. 2017, 11, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Cheki, M.; Mihandoost, E.; Shirazi, A.; Mahmoudzadeh, A. Prophylactic role of some plants and phytochemicals against radio-genotoxicity in human lymphocytes. J. Cancer Res. Ther. 2016, 12, 1234–1242. [Google Scholar] [PubMed]
- Ngo, L.T.; Okogun, J.I.; Folk, W.R. 21st century natural product research and drug development and traditional medicines. Nat. Prod. Rep. 2013, 30, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Basmadjian, C.; Zhao, Q.; Bentouhami, E.; Djehal, A.; Nebigil, C.G.; Johnson, R.A.; Serova, M.; de Gramont, A.; Faivre, S.; Raymond, E.; et al. Cancer wars: Natural products strike back. Front. Chem. 2014, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Habli, Z.; Toumieh, G.; Fatfat, M.; Rahal, O.N.; Gali-Muhtasib, H. Emerging cytotoxic alkaloids in the battle against cancer: Overview of molecular mechanisms. Molecules 2017, 22, 250. [Google Scholar] [CrossRef] [PubMed]
- Debatin, K.M. Apoptosis pathways in cancer and cancer therapy. Cancer Immunol. Immunother. 2004, 53, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2013, 30, 237–323. [Google Scholar] [CrossRef] [PubMed]
- Mioso, R.; Marante, F.J.; Bezerra, R.S.; Borges, F.V.; Santos, B.V.; Laguna, I.H. Cytotoxic compounds derived from marine sponges. A review (2010–2012). Molecules 2017, 22, 208. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.H.; Liu, Y.C.; Su, J.H.; El-Shazly, M.; Wu, C.F.; Du, Y.C.; Hsu, Y.M.; Yang, J.C.; Weng, M.K.; Chou, C.H.; et al. Antileukemic scalarane sesterterpenoids and meroditerpenoid from Carteriospongia (Phyllospongia) sp., induce apoptosis via dual inhibitory effects on topoisomerase ii and Hsp90. Sci. Rep. 2016, 6, 36170. [Google Scholar] [CrossRef] [PubMed]
- Shih, S.P.; Lee, M.G.; El-Shazly, M.; Juan, Y.S.; Wen, Z.H.; Du, Y.C.; Su, J.H.; Sung, P.J.; Chen, Y.C.; Yang, J.C.; et al. Tackling the cytotoxic effect of a marine polycyclic quinone-type metabolite: Halenaquinone induces molt 4 cells apoptosis via oxidative stress combined with the inhibition of HDAC and topoisomerase activities. Mar. Drugs 2015, 13, 3132–3153. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.B.; Yang, F.; Sun, F.; Li, J.; Jiao, W.H.; Gan, J.H.; Hu, W.Z.; Lin, H.W. Aaptamine derivatives with antifungal and anti-HIV-1 activities from the south china sea sponge Aaptos aaptos. Mar. Drugs 2014, 12, 6003–6013. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Woo, S.W.; Kim, M.S.; Park, J.E.; Hwang, J.K. Anti-photoaging effect of aaptamine in UVB-irradiated human dermal fibroblasts and epidermal keratinocytes. J. Asian Nat. Prod. Res. 2014, 16, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.L.; Zhang, P.P.; Wang, P.Q.; Yu, H.B.; Sun, F.; Hu, W.Z.; Wu, W.H.; Zhang, X.; Chen, F.; Chu, Z.Y.; et al. The cytotoxic and mechanistic effects of aaptamine on hepatocellular carcinoma. Anticancer Agents Med. Chem. 2015, 15, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Shaari, K.; Ling, K.C.; Rashid, Z.M.; Jean, T.P.; Abas, F.; Raof, S.M.; Zainal, Z.; Lajis, N.H.; Mohamad, H.; Ali, A.M. Cytotoxic aaptamines from Malaysian Aaptos aaptos. Mar. Drugs 2009, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Diers, J.A.; Bowling, J.J.; Duke, S.O.; Wahyuono, S.; Kelly, M.; Hamann, M.T. Zebra mussel antifouling activity of the marine natural product aaptamine and analogs. Mar. Biotechnol. 2006, 8, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Diers, J.A.; Ivey, K.D.; El-Alfy, A.; Shaikh, J.; Wang, J.; Kochanowska, A.J.; Stoker, J.F.; Hamann, M.T.; Matsumoto, R.R. Identification of antidepressant drug leads through the evaluation of marine natural products with neuropsychiatric pharmacophores. Pharmacol. Biochem. Behav. 2008, 89, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Bowling, J.J.; Pennaka, H.K.; Ivey, K.; Wahyuono, S.; Kelly, M.; Schinazi, R.F.; Valeriote, F.A.; Graves, D.E.; Hamann, M.T. Antiviral and anticancer optimization studies of the DNA-binding marine natural product aaptamine. Chem. Biol. Drug Des. 2008, 71, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Dyshlovoy, S.A.; Venz, S.; Shubina, L.K.; Fedorov, S.N.; Walther, R.; Jacobsen, C.; Stonik, V.A.; Bokemeyer, C.; Balabanov, S.; Honecker, F. Activity of aaptamine and two derivatives, demethyloxyaaptamine and isoaaptamine, in cisplatin-resistant germ cell cancer. J. Proteom. 2014, 96, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Dyshlovoy, S.A.; Fedorov, S.N.; Shubina, L.K.; Kuzmich, A.S.; Bokemeyer, C.; Keller-von Amsberg, G.; Honecker, F. Aaptamines from the marine sponge Aaptos sp. Display anticancer activities in human cancer cell lines and modulate AP-1-, NF-kappaB-, and p53-dependent transcriptional activity in mouse JB6 Cl41 cells. BioMed Res. Int. 2014, 2014, 469309. [Google Scholar] [CrossRef] [PubMed]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef] [PubMed]
- Zepeda, A.B.; Pessoa, A., Jr.; Castillo, R.L.; Figueroa, C.A.; Pulgar, V.M.; Farias, J.G. Cellular and molecular mechanisms in the hypoxic tissue: Role of HIF-1 and ROS. Cell Biochem. Funct. 2013, 31, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.C. Mitochondrial reactive oxygen species are required for hypoxic HIF alpha stabilization. Adv. Exp. Med. Biol. 2006, 588, 165–170. [Google Scholar] [PubMed]
- Behrend, L.; Henderson, G.; Zwacka, R.M. Reactive oxygen species in oncogenic transformation. Biochem. Soc. Trans. 2003, 31, 1441–1444. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, D.G.; Budd, S.L. Mitochondria and neuronal survival. Physiol. Rev. 2000, 80, 315–360. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Korfhagen, T.R.; Whitsett, J.A. Surfactant protein D regulates NF-kappa B and matrix metalloproteinase production in alveolar macrophages via oxidant-sensitive pathways. J. Immunol. 2001, 166, 7514–7519. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, H.; Tada-Oikawa, S.; Hiraku, Y.; Kojima, M.; Kawanishi, S. Mechanism of apoptosis induced by doxorubicin through the generation of hydrogen peroxide. Life Sci. 2005, 76, 1439–1453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Zhao, W.; Venkataraman, S.; Robbins, M.E.; Buettner, G.R.; Kregel, K.C.; Oberley, L.W. Activation of matrix metalloproteinase-2 by overexpression of manganese superoxide dismutase in human breast cancer MCF-7 cells involves reactive oxygen species. J. Biol. Chem. 2002, 277, 20919–20926. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; McMahon, M. Nrf2 and Keap1 mutations: Permanent activation of an adaptive response in cancer. Trends Biochem. Sci. 2009, 34, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/small Maf heterodimer mediates the induction of phase ii detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, I.G.; Choi, B.H.; Kwak, M.K. Activation of Nrf2 by p62 and proteasome reduction in sphere-forming breast carcinoma cells. Oncotarget 2015, 6, 8167–8184. [Google Scholar] [CrossRef] [PubMed]
- Katsuragi, Y.; Ichimura, Y.; Komatsu, M. P62/SQSTM1 functions as a signaling hub and an autophagy adaptor. FEBS J. 2015, 282, 4672–4678. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, M.C.; Zhang, D.D. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Gene Dev. 2013, 27, 2179–2191. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.B.; Kwon, K.R.; Lee, S.H.; Lee, S.H. Lannea coromandelica (Houtt.) Merr. induces heme oxygenase 1 (HO-1) expression and reduces oxidative stress via the p38/c-Jun N-terminal kinase-nuclear factor erythroid 2-related factor 2 (p38/JNK-Nrf2)-mediated antioxidant pathway. Int. J. Mol. Sci. 2017, 18, 266. [Google Scholar] [CrossRef] [PubMed]
- Hynes, N.E.; MacDonald, G. ErbB receptors and signaling pathways in cancer. Curr. Opin. Cell Biol. 2009, 21, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Franke, T.F.; Yang, S.I.; Chan, T.O.; Datta, K.; Kazlauskas, A.; Morrison, D.K.; Kaplan, D.R.; Tsichlis, P.N. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell 1995, 81, 727–736. [Google Scholar] [CrossRef]
- Sun, W.L. Ambra1 in autophagy and apoptosis: Implications for cell survival and chemotherapy resistance. Oncol. Lett. 2016, 12, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Xu, Y.; Wang, X.; Han, W.; Pan, H.; Xiao, M. Metformin: A novel but controversial drug in cancer prevention and treatment. Mol. Pharm. 2015, 12, 3783–3791. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.X.; Zhang, Q.F.; Pan, F.; Huang, J.L.; Li, B.L.; Hu, M.; Li, M.Q.; Chen, C. Hydroxycamptothecin shows antitumor efficacy on HeLa cells via autophagy activation mediated apoptosis in cervical cancer. Eur. J. Gynaecol. Oncol. 2016, 37, 238–243. [Google Scholar] [PubMed]
- Kumar, A.; Singh, B.; Sharma, P.R.; Bharate, S.B.; Saxena, A.K.; Mondhe, D.M. A novel microtubule depolymerizing colchicine analogue triggers apoptosis and autophagy in HCT-116 colon cancer cells. Cell Biochem. Funct. 2016, 34, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.Y.; Kim, S.W.; Park, K.C.; Yun, M. The bifunctional autophagic flux by 2-deoxyglucose to control survival or growth of prostate cancer cells. BMC Cancer 2015, 15, 623. [Google Scholar] [CrossRef] [PubMed]
- Asati, V.; Mahapatra, D.K.; Bharti, S.K. PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways inhibitors as anticancer agents: Structural and pharmacological perspectives. Eur. J. Med. Chem. 2016, 109, 314–341. [Google Scholar] [CrossRef] [PubMed]
- Itakura, E.; Mizushima, N. P62 targeting to the autophagosome formation site requires self-oligomerization but not LC3 binding. J. Cell Biol. 2011, 192, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Sharma, A.; Mir, M.C.; Drazba, J.A.; Heston, W.D.; Magi-Galluzzi, C.; Hansel, D.; Rubin, B.P.; Klein, E.A.; Almasan, A. Autophagic flux determines cell death and survival in response to Apo2L/TRAIL (dulanermin). Mol. Cancer 2014, 13, 70. [Google Scholar] [CrossRef] [PubMed]
- Halicka, H.D.; Garcia, J.; Li, J.; Zhao, H.; Darzynkiewicz, Z. Synergy of 2-deoxy-d-glucose combined with berberine in inducing the lysosome/autophagy and transglutaminase activation-facilitated apoptosis. Apoptosis 2017, 22, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nartiss, Y.; Steipe, B.; McQuibban, G.A.; Kim, P.K. Ros-induced mitochondrial depolarization initiates PARK2/PARKIN-dependent mitochondrial degradation by autophagy. Autophagy 2012, 8, 1462–1476. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhao, S.; Zhang, Y.; Wu, J.; Peng, H.; Fan, J.; Liao, J. Reactive oxygen species-mediated endoplasmic reticulum stress and mitochondrial dysfunction contribute to polydatin-induced apoptosis in human nasopharyngeal carcinoma CNE cells. J. Cell. Biochem. 2011, 112, 3695–3703. [Google Scholar] [CrossRef] [PubMed]
- Abbiati, G.; Doda, A.; Dell’Acqua, M.; Pirovano, V.; Facoetti, D.; Rizzato, S.; Rossi, E. Synthesis of two unnatural oxygenated aaptaminoids. J. Org. Chem. 2012, 77, 10461–10467. [Google Scholar] [CrossRef] [PubMed]
- Freitag, A.; Wessler, I.; Racke, K. Phosphodiesterase inhibitors suppress alpha2-adrenoceptor-mediated 5-hydroxytryptamine release from tracheae of newborn rabbits. Eur. J. Pharm. 1998, 354, 67–71. [Google Scholar] [CrossRef]
- Hsieh, M.J.; Chien, S.Y.; Lin, J.T.; Yang, S.F.; Chen, M.K. Polyphyllin G induces apoptosis and autophagy cell death in human oral cancer cells. Phytomedicine 2016, 23, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.H.; Lai, H.Y.; Chen, Y.C.; Li, C.F.; Huang, H.S.; Liu, H.S.; Tsai, Y.S.; Wang, J.M. Metformin promotes apoptosis in hepatocellular carcinoma through the CEBPD-induced autophagy pathway. Oncotarget 2017, 8, 13832–13845. [Google Scholar] [CrossRef] [PubMed]
- Granato, M.; Rizzello, C.; Gilardini Montani, M.S.; Cuomo, L.; Vitillo, M.; Santarelli, R.; Gonnella, R.; D’Orazi, G.; Faggioni, A.; Cirone, M. Quercetin induces apoptosis and autophagy in primary effusion lymphoma cells by inhibiting PI3K/AKT/mTOR and STAT3 signaling pathways. J. Nutr. Biochem. 2017, 41, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Lewinska, A.; Adamczyk-Grochala, J.; Kwasniewicz, E.; Deregowska, A.; Wnuk, M. Diosmin-induced senescence, apoptosis and autophagy in breast cancer cells of different p53 status and ERK activity. Toxicol. Lett. 2017, 265, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Cai-McRae, X.; Zhong, H.; Karantza, V. Sequestosome 1/p62 facilitates HER2-induced mammary tumorigenesis through multiple signaling pathways. Oncogene 2015, 34, 2968–2977. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Dinkova-Kostova, A.T. The cytoprotective role of the Keap1-Nrf2 pathway. Arch. Toxicol. 2011, 85, 241–272. [Google Scholar] [CrossRef] [PubMed]
- Georgakopoulos, N.D.; Frison, M.; Alvarez, M.S.; Bertrand, H.; Wells, G.; Campanella, M. Reversible Keap1 inhibitors are preferential pharmacological tools to modulate cellular mitophagy. Sci. Rep. 2017, 7, 10303. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Lamark, T.; Sjottem, E.; Larsen, K.B.; Awuh, J.A.; Overvatn, A.; McMahon, M.; Hayes, J.D.; Johansen, T. P62/SQSTM1 is a target gene for transcription factor Nrf2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J. Biol. Chem. 2010, 285, 22576–22591. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Karin, M. Signaling pathways leading to nuclear factor-kappa B activation. Methods Enzymol. 2000, 319, 273–279. [Google Scholar] [PubMed]
- Robbins, D.J.; Nybakken, K.E.; Kobayashi, R.; Sisson, J.C.; Bishop, J.M.; Therond, P.P. Hedgehog elicits signal transduction by means of a large complex containing the kinesin-related protein costal2. Cell 1997, 90, 225–234. [Google Scholar] [CrossRef]
- Bertrand, H.C.; Schaap, M.; Baird, L.; Georgakopoulos, N.D.; Fowkes, A.; Thiollier, C.; Kachi, H.; Dinkova-Kostova, A.T.; Wells, G. Design, synthesis, and evaluation of triazole derivatives that induce Nrf2 dependent gene products and inhibit the Keap1-Nrf2 protein-protein interaction. J. Med. Chem. 2015, 58, 7186–7194. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Kim, M.K.; Lee, K.; Lee, K.M.; Choi, Y.K.; Shin, Y.C.; Cho, S.G.; Ko, S.G. SH003 represses tumor angiogenesis by blocking VEGF binding to VEGFR2. Oncotarget 2016, 7, 32969–32979. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Dong, Y.; Yin, S.; Zhao, C.; Huo, Y.; Fan, L.; Hu, H. Patulin induces pro-survival functions via autophagy inhibition and p62 accumulation. Cell Death Dis. 2013, 4, e822. [Google Scholar] [CrossRef] [PubMed]
- Linares, J.F.; Amanchy, R.; Greis, K.; Diaz-Meco, M.T.; Moscat, J. Phosphorylation of p62 by cdk1 controls the timely transit of cells through mitosis and tumor cell proliferation. Mol. Cell. Biol. 2011, 31, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.K.; Cho, S.G.; Choi, Y.J.; Yun, Y.J.; Lee, K.M.; Lee, K.; Yoo, H.H.; Shin, Y.C.; Ko, S.G. SH003 suppresses breast cancer growth by accumulating p62 in autolysosomes. Oncotarget 2017, 8, 88386–88400. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Yamanokuchi, R.; Yoshitomi, M.; Sato, K.; Ikeda, T.; Rotinsulu, H.; Mangindaan, R.E.; de Voogd, N.J.; van Soest, R.W.; Yokosawa, H. Aaptamine, an alkaloid from the sponge Aaptos suberitoides, functions as a proteasome inhibitor. Bioorg. Med. Chem. Lett. 2010, 20, 3341–3343. [Google Scholar] [CrossRef] [PubMed]
- Su, J.H.; Chen, Y.C.; El-Shazly, M.; Du, Y.C.; Su, C.W.; Tsao, C.W.; Liu, L.L.; Chou, Y.; Chang, W.B.; Su, Y.D.; et al. Towards the small and the beautiful: A small dibromotyrosine derivative from Pseudoceratina sp. Sponge exhibits potent apoptotic effect through targeting IKK/NFkappaB signaling pathway. Mar. Drugs 2013, 11, 3168–3185. [Google Scholar] [CrossRef] [PubMed]
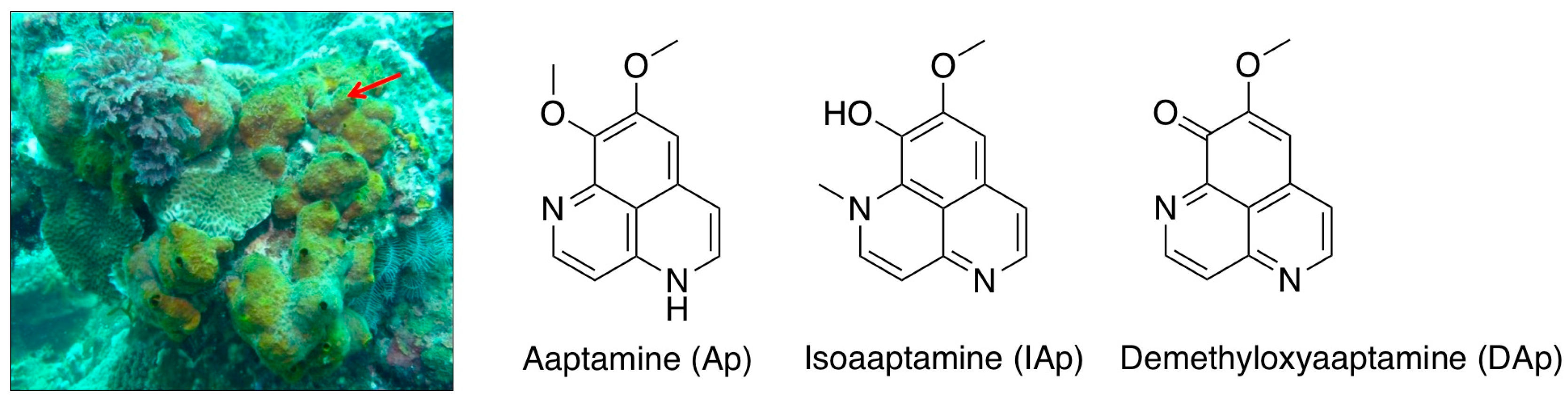


| T-47D | MCF-7 | MDA-MB-231 | |
|---|---|---|---|
| Aaptamine (Ap) | NA a | NA a | NA a |
| Isoaaptamine (IAp) | 30.13 ± 3.07 | 49.12 ± 12.28 | 49.56 ± 2.19 |
| Demethyloxyaaptamine (DAp) | 33.02 ± 8.49 | 23.11 ± 2.36 | 19.34 ± 3.77 |
| Staurosporine b | 0.45 ± 0.01 | 0.11 ± 0.01 | 0.45 ± 0.01 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-F.; Lee, M.-G.; El-Shazly, M.; Lai, K.-H.; Ke, S.-C.; Su, C.-W.; Shih, S.-P.; Sung, P.-J.; Hong, M.-C.; Wen, Z.-H.; et al. Isoaaptamine Induces T-47D Cells Apoptosis and Autophagy via Oxidative Stress. Mar. Drugs 2018, 16, 18. https://doi.org/10.3390/md16010018
Wu C-F, Lee M-G, El-Shazly M, Lai K-H, Ke S-C, Su C-W, Shih S-P, Sung P-J, Hong M-C, Wen Z-H, et al. Isoaaptamine Induces T-47D Cells Apoptosis and Autophagy via Oxidative Stress. Marine Drugs. 2018; 16(1):18. https://doi.org/10.3390/md16010018
Chicago/Turabian StyleWu, Chih-Fung, Man-Gang Lee, Mohamed El-Shazly, Kuei-Hung Lai, Seng-Chung Ke, Chiang-Wen Su, Shou-Ping Shih, Ping-Jyun Sung, Ming-Chang Hong, Zhi-Hong Wen, and et al. 2018. "Isoaaptamine Induces T-47D Cells Apoptosis and Autophagy via Oxidative Stress" Marine Drugs 16, no. 1: 18. https://doi.org/10.3390/md16010018
APA StyleWu, C. -F., Lee, M. -G., El-Shazly, M., Lai, K. -H., Ke, S. -C., Su, C. -W., Shih, S. -P., Sung, P. -J., Hong, M. -C., Wen, Z. -H., & Lu, M. -C. (2018). Isoaaptamine Induces T-47D Cells Apoptosis and Autophagy via Oxidative Stress. Marine Drugs, 16(1), 18. https://doi.org/10.3390/md16010018







