Sponges: A Reservoir of Genes Implicated in Human Cancer
Abstract
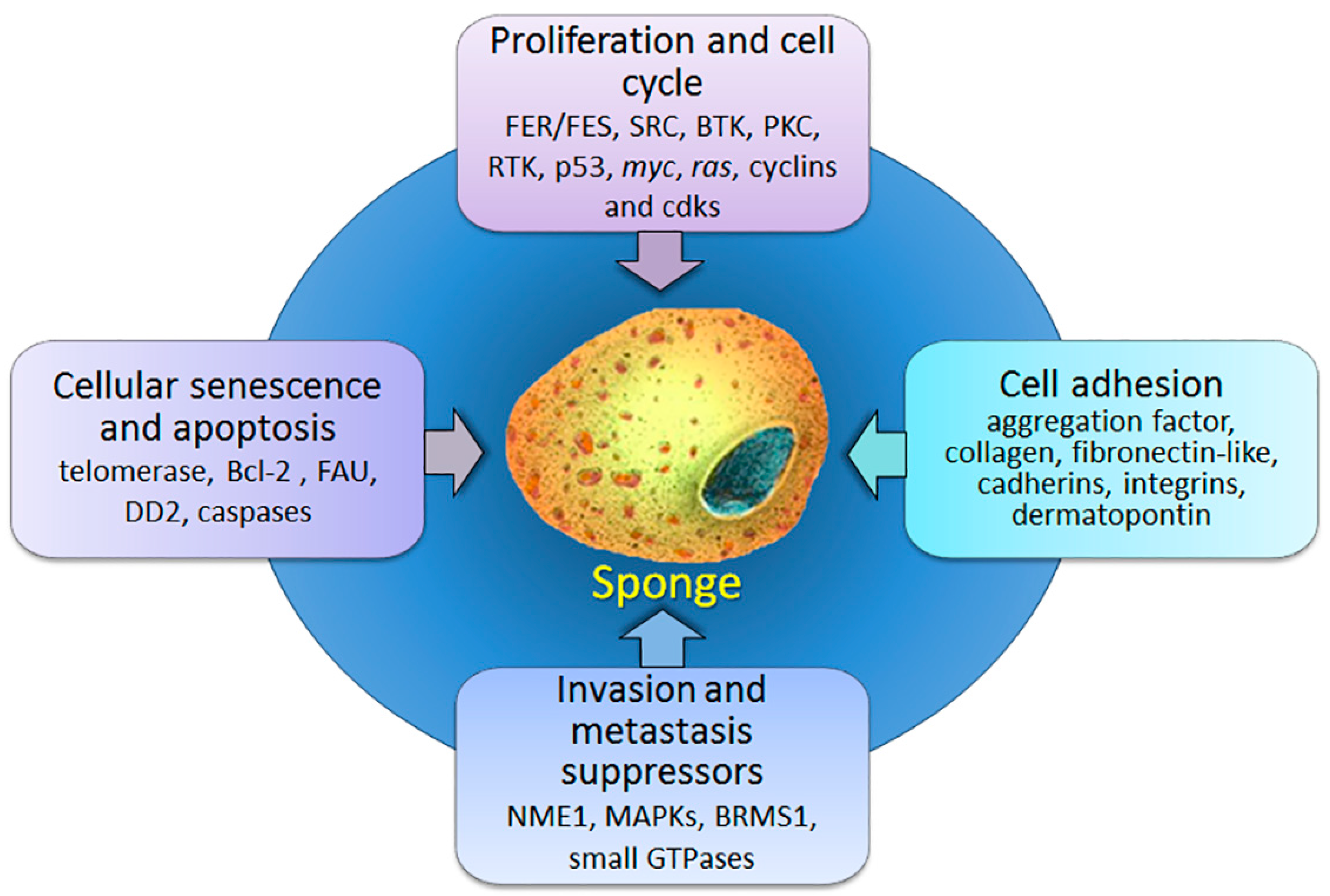
:1. Introduction
2. Proliferation and Cell Cycle
2.1. Protein Kinases
2.2. Protein Tyrosine Kinases
3. Players in Cellular Senescence and Apoptosis
4. Cell Anchoring Molecules
5. Metastasis Suppressor Genes
6. Cancer Associated Genes in Marine Sponges-Final Remarks and Future Challenges
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Aktipis, C.A.; Boddy, A.M.; Jansen, G.; Hibner, U.; Hochberg, M.E.; Maley, C.C.; Wilkinson, G.S. Cancer across the tree of life: Cooperation and cheating in multicellularity. Philos. Trans. R. Soc. B 2015, 370, 20140219. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.C.W.; Lineweaver, C.H. Cancer tumors as metazoa 1.0: Tapping genes of ancient ancestors. Phys. Biol. 2011, 8, 015001. [Google Scholar] [CrossRef] [PubMed]
- Domazet-Lošo, T.; Klimovich, A.; Anokhin, B.; Anton-Erxleben, F.; Hamm, M.J.; Lange, C.; Bosch, T.C.G. Naturally occurring tumours in the basal metazoan hydra. Nat. Commun. 2014, 5, 4222. [Google Scholar] [CrossRef] [PubMed]
- Domazet-Lošo, T.; Tautz, D. An ancient evolutionary origin of genes associated with human genetic diseases. Mol. Biol. Evol. 2008, 25, 2699–2707. [Google Scholar] [CrossRef] [PubMed]
- Domazet-Lošo, T.; Tautz, D. Phylostratigraphic tracking of cancer genes suggests a link to the emergence of multicellularity in metazoa. BMC Biol. 2010, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Love, G.D.; Grosjean, E.; Stalvies, C.; Fike, D.A.; Grotzinger, J.P.; Bradley, A.S.; Kelly, A.E.; Bhatia, M.; Meredith, W.; Snape, C.E.; et al. Fossil steroids record the appearance of demospongiae during the cryogenian period. Nature 2009, 457, 718–721. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.; Simakov, O.; Chapman, J.; Fahey, B.; Gauthier, M.E.; Mitros, T.; Richards, G.S.; Conaco, C.; Dacre, M.; Hellsten, U.; et al. The Amphimedon queenslandica genome and the evolution of animal complexity. Nature 2010, 466, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Harcet, M.; Roller, M.; Ćetković, H.; Perina, D.; Wiens, M.; Müller, W.E.; Vlahovicek, K. Demosponge EST sequencing reveals a complex genetic toolkit of the simplest metazoans. Mol. Biol. Evol. 2010, 27, 2747–2756. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Hunter, T. A 1001 protein-kinases. Cell 1987, 50, 823–829. [Google Scholar] [CrossRef]
- Garg, R.; Benedetti, L.G.; Abera, M.B.; Wang, H.; Abba, M.; Kazanietz, M.G. Protein kinase c and cancer: What we know and what we do not. Oncogene 2014, 33, 5225–5237. [Google Scholar] [CrossRef] [PubMed]
- Seack, J.; Kruse, M.; Muller, I.M.; Müller, W.E. Promoter and exon-intron structure of the protein kinase C gene from the marine sponge Geodia cydonium: Evolutionary considerations and promoter activity. Biochim. Biophys. Acta 1999, 1444, 241–253. [Google Scholar] [CrossRef]
- Müller, W.E.; Rottmann, M.; Diehl-Seifert, B.; Kurelec, B.; Uhlenbruck, G.; Schröder, H.C. Role of the aggregation factor in the regulation of phosphoinositide metabolism in sponges—Possible consequences on calcium efflux and on mitogenesis. J. Biol. Chem. 1987, 262, 9850–9858. [Google Scholar] [PubMed]
- Rottmann, M.; Schröder, H.C.; Gramzow, M.; Renneisen, K.; Kurelec, B.; Dorn, A.; Friese, U.; Müller, W.E. Specific phosphorylation of proteins in pore complex-laminae from the sponge Geodia cydonium by the homologous aggregation factor and phorbol ester. Role of protein kinase C in the phosphorylation of DNA topoisomerase II. EMBO J. 1987, 6, 3939–3944. [Google Scholar] [PubMed]
- Kruse, M.; Gamulin, V.; Cetkovic, H.; Pancer, Z.; Müller, I.M.; Müller, W.E.G. Molecular evolution of the metazoan protein kinase C multigene family. J. Mol. Evol. 1996, 43, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Cetkovic, H.; Grebenjuk, V.A.; Müller, W.E.G.; Gamulin, V. Src proteins/src genes: From sponges to mammals. Gene 2004, 342, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Cetkovic, H.; Müller, W.E.G.; Gamulin, V. Bruton tyrosine kinase-like protein, BtkSD, is present in the marine sponge Suberites domuncula. Genomics 2004, 83, 743–745. [Google Scholar] [CrossRef] [PubMed]
- Cetkovic, H.; Müller, I.M.; Müller, W.E.; Gamulin, V. Characterization and phylogenetic analysis of a cDNA encoding the Fes/FER related, non-receptor protein-tyrosine kinase in the marine sponge Sycon raphanus. Gene 1998, 216, 77–84. [Google Scholar] [CrossRef]
- Suga, H.; Katoh, K.; Miyata, T. Sponge homologs of vertebrate protein tyrosine kinases and frequent domain shufflings in the early evolution of animals before the parazoan-eumetazoan split. Gene 2001, 280, 195–201. [Google Scholar] [CrossRef]
- Suga, H.; Koyanagi, M.; Hoshiyama, D.; Ono, K.; Iwabe, N.; Kuma, K.; Miyata, T. Extensive gene duplication in the early evolution of animals before the parazoan-eumetazoan split demonstrated by G proteins and protein tyrosine kinases from sponge and hydra. J. Mol. Evol. 1999, 48, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Perovic-Ottstadt, S.; Cetkovic, H.; Gamulin, V.; Schröder, H.C.; Kropf, K.; Moss, C.; Korzhev, M.; Diehl-Seifert, B.; Müller, I.M.; Müller, W.E. Molecular markers for germ cell differentiation in the demosponge Suberites domuncula. Int. J. Dev. Biol. 2004, 48, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Kruse, M.; Müller, I.M.; Müller, W.E. Early evolution of metazoan serine/threonine and tyrosine kinases: Identification of selected kinases in marine sponges. Mol. Biol. Evol. 1997, 14, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Gamulin, V.; Skorokhod, A.; Kavsan, V.; Müller, I.M.; Müller, W.E. Experimental indication in favor of the introns-late theory: The receptor tyrosine kinase gene from the sponge Geodia cydonium. J. Mol. Evol. 1997, 44, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.; Schacke, H. Characterization of the receptor protein-tyrosine kinase gene from the marine sponge Geodia cydonium. Prog. Mol. Subcell. Biol. 1996, 17, 183–208. [Google Scholar] [PubMed]
- Fischman, K.; Edman, J.C.; Shackleford, G.M.; Turner, J.A.; Rutter, W.J.; Nir, U. A murine fer testis-specific transcript (ferT) encodes a truncated Fer protein. Mol. Cell. Biol. 1990, 10, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.V. A new method by which sponges may be artificially reared. Science 1907, 25, 912–915. [Google Scholar] [CrossRef] [PubMed]
- Hackenmiller, R.; Kim, J.; Feldman, R.A.; Simon, M.C. Abnormal stat activation, hematopoietic homeostasis, and innate immunity in c-fes-/- mice. Immunity 2000, 13, 397–407. [Google Scholar] [CrossRef]
- Ahn, J.; Truesdell, P.; Meens, J.; Kadish, C.; Yang, X.; Boag, A.H.; Craig, A.W. Fer protein-tyrosine kinase promotes lung adenocarcinoma cell invasion and tumor metastasis. Mol. Cancer Res. 2013, 11, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Oneyama, C.; Yoshikawa, Y.; Ninomiya, Y.; Iino, T.; Tsukita, S.; Okada, M. Fer tyrosine kinase oligomer mediates and amplifies Src-induced tumor progression. Oncogene 2016, 35, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Biscardi, J.S.; Ishizawar, R.C.; Silva, C.M.; Parsons, S.J. Tyrosine kinase signalling in breast cancer—Epidermal growth factor receptor and c-Src interactions in breast cancer. Breast Cancer Res. 2000, 2, 203–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawlings, D.J.; Saffran, D.C.; Tsukada, S.; Largaespada, D.A.; Grimaldi, J.C.; Cohen, L.; Mohr, R.N.; Bazan, J.F.; Howard, M.; Copeland, N.G.; et al. Mutation of unique region of bruton’s tyrosine kinase in immunodeficient xid mice. Science 1993, 261, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Conley, M.E.; Dobbs, A.K.; Farmer, D.M.; Kilic, S.; Paris, K.; Grigoriadou, S.; Coustan-Smith, E.; Howard, V.; Campana, D. Primary B cell immunodeficiencies: Comparisons and contrasts. Annu. Rev. Immunol. 2009, 27, 199–227. [Google Scholar] [CrossRef] [PubMed]
- Perina, D.; Mikoc, A.; Harcet, M.; Imesek, M.; Sladojevic, D.; Brcko, A.; Cetkovic, H. Characterization of bruton’s tyrosine kinase gene and protein from marine sponge Suberites domuncula. Croat. Chem. Acta 2012, 85, 223–229. [Google Scholar] [CrossRef]
- Finkelstein, L.D.; Schwartzberg, P.L. Tec kinases: Shaping T-cell activation through actin. Trends Cell Biol. 2004, 14, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Horwood, N.J.; Mahon, T.; McDaid, J.P.; Campbell, J.; Mano, H.; Brennan, F.M.; Webster, D.; Foxwell, B.M.J. Bruton’s tyrosine kinase is required for lipopolysaccharide-induced tumor necrosis factor alpha production. J. Exp. Med. 2003, 197, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Böhm, M.; Hentschel, U.; Friedrich, A.B.; Fieseler, L.; Steffen, R.; Gamulin, V.; Müller, I.M.; Müller, W.E.G. Molecular response of the sponge Suberites domuncula to bacterial infection. Mar. Biol. 2001, 139, 1037–1045. [Google Scholar]
- Sharma, P.S.; Sharma, R.; Tyagi, R. Inhibitors of cyclin dependent kinases: Useful targets for cancer treatment. Curr. Cancer Drug Target 2008, 8, 53–75. [Google Scholar] [CrossRef]
- Cao, L.; Chen, F.; Yang, X.; Xu, W.; Xie, J.; Yu, L. Phylogenetic analysis of CDK and cyclin proteins in premetazoan lineages. BMC Evol. Biol. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Cetkovic, H.; Mikoc, A.; Müller, W.E.G.; Gamulin, V. Ras-like small GTpases form a large family of proteins in the marine sponge Suberites domuncula. J. Mol. Evol. 2007, 64, 332–341. [Google Scholar] [CrossRef] [PubMed]
- King, N.; Westbrook, M.J.; Young, S.L.; Kuo, A.; Abedin, M.; Chapman, J.; Fairclough, S.; Hellsten, U.; Isogai, Y.; Letunic, I.; et al. The genome of the choanoflagellate Monosiga brevicollis and the origin of metazoans. Nature 2008, 451, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Young, S.L.; Diolaiti, D.; Conacci-Sorrell, M.; Ruiz-Trillo, I.; Eisenman, R.N.; King, N. Premetazoan ancestry of the Myc-Max network. Mol. Biol. Evol. 2011, 28, 2961–2971. [Google Scholar] [CrossRef] [PubMed]
- Belyi, V.A.; Ak, P.; Markert, E.; Wang, H.; Hu, W.; Puzio-Kuter, A.; Levine, A.J. The origins and evolution of the p53 family of genes. Cold Spring Harb. Perspect. Biol. 2010, 2, a001198. [Google Scholar] [CrossRef] [PubMed]
- Morin, G.B. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell 1989, 59, 521–529. [Google Scholar] [CrossRef]
- Sakai, M.; Okumura, S.I.; Onuma, K.; Senbokuya, H.; Yamamori, K. Identification of a telomere sequence type in three sponge species (Porifera) by fluorescence in situ hybridization analysis. Fish. Sci. 2007, 73, 77–80. [Google Scholar] [CrossRef]
- Koziol, C.; Borojevic, R.; Steffen, R.; Muller, W.E.G. Sponges (Porifera) model systems to study the shift from immortal to senescent somatic cells: The telomerase activity in somatic cells. Mech. Ageing Dev. 1998, 100, 107–120. [Google Scholar] [CrossRef]
- Hiyama, E.; Hiyama, K. Telomere and telomerase in stem cells. Br. J. Cancer 2007, 96, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- Gramzow, M.; Schröder, H.C.; Fritsche, U.; Kurelec, B.; Robitzki, A.; Zimmermann, H.; Friese, K.; Kreuter, M.H.; Müller, W.E. Role of phospholipase a2 in the stimulation of sponge cell proliferation by homologous lectin. Cell 1989, 59, 939–948. [Google Scholar] [CrossRef]
- Wiens, M.; Müller, W.E.G. Cell death in Porifera: Molecular players in the game of apoptotic cell death in living fossils. Can. J. Zool. 2006, 84, 307–321. [Google Scholar] [CrossRef]
- Wiens, M.; Krasko, A.; Perovic, S.; Müller, W.E.G. Caspase-mediated apoptosis in sponges: Cloning and function of the phylogenetic oldest apoptotic proteases from Metazoa. Biochim. Biophys. Acta 2003, 1593, 179–189. [Google Scholar] [CrossRef]
- Wiens, M.; Krasko, A.; Müller, C.I.; Müller, W.E.G. Molecular evolution of apoptotic pathways: Cloning of key domains from sponges (Bcl-2 homology domains and death domains) and their phylogenetic relationships. J. Mol. Evol. 2000, 50, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Wiens, M.; Krasko, A.; Blumbach, B.; Müller, I.M.; Müller, W.E.G. Increased expression of the potential proapoptotic molecule DD2 and increased synthesis of leukotriene B-4 during allograft rejection in a marine sponge. Cell Death Differ. 2000, 7, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Caria, S.; Hinds, M.G.; Kvansakul, M. Structural insight into an evolutionarily ancient programmed cell death regulator—The crystal structure of marine sponge BHP2 bound to LB-Bak-2. Cell Death Dis. 2017, 8, e2543. [Google Scholar] [CrossRef] [PubMed]
- Pickard, M.R.; Mourtada-Maarabouni, M.; Williams, G.T. Candidate tumour suppressor Fau regulates apoptosis in human cells: An essential role for Bcl-G. Biochim. Biophys. Acta 2011, 1812, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Pickard, M.R.; Green, A.R.; Ellis, I.O.; Caldas, C.; Hedge, V.L.; Mourtada-Maarabouni, M.; Williams, G.T. Dysregulated expression of Fau and MELK is associated with poor prognosis in breast cancer. Breast Cancer Res. 2009, 11, R60. [Google Scholar] [CrossRef] [PubMed]
- Pickard, M.R.; Edwards, S.E.; Cooper, C.S.; Williams, G.T. Apoptosis regulators Fau and Bcl-G are down-regulated in prostate cancer. Prostate 2010, 70, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Moss, E.L.; Mourtada-Maarabouni, M.; Pickard, M.R.; Redman, C.W.; Williams, G.T. FAU regulates carboplatin resistance in ovarian cancer. Genes Chromosomes Cancer 2010, 49, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Perina, D.; Korolija, M.; Hadzija, M.P.; Grbesa, I.; Beluzic, R.; Imesek, M.; Morrow, C.; Marjanovic, M.P.; Bakran-Petricioli, T.; Mikoc, A.; et al. Functional and structural characterization of FAU gene/protein from marine sponge Suberites domuncula. Mar. Drugs 2015, 13, 4179–4196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Liao, W.J.; Liao, J.M.; Liao, P.; Lu, H. Ribosomal proteins: Functions beyond the ribosome. J. Mol. Cell Biol. 2015, 7, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Perina, D.; Korolija, M.; Mikoc, A.; Roller, M.; Plese, B.; Imesek, M.; Morrow, C.; Batel, R.; Cetkovic, H. Structural and functional characterization of ribosomal protein gene introns in sponges. PLoS ONE 2012, 7, e42523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perina, D.; Cetkovic, H.; Harcet, M.; Premzl, M.; Lukic-Bilela, L.; Müller, W.E.; Gamulin, V. The complete set of ribosomal proteins from the marine sponge Suberites domuncula. Gene 2006, 366, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Lock, J.G.; Wehrle-Haller, B.; Stromblad, S. Cell-matrix adhesion complexes: Master control machinery of cell migration. Semin. Cancer Biol. 2008, 18, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Cohen, O.; Feinstein, E.; Kimchi, A. Dap-kinase is a Ca2+ calmodulin-dependent, cytoskeletal-associated protein kinase, with cell death-inducing functions that depend on its catalytic activity. EMBO J. 1997, 16, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, U.; Christofori, G. Cell adhesion in tumor invasion and metastasis: Loss of the glue is not enough. Biochim. Biophys. Acta 2001, 1552, 39–45. [Google Scholar] [CrossRef]
- Müller, W.E.G. Cell-membranes in sponges. Int. Rev. Cytol. 1982, 77, 129–181. [Google Scholar]
- Müller, W.E.G.; Zahn, R.K. Purification and characterization of a species-specific aggregation factor in sponges. Exp. Cell Res. 1973, 80, 95–104. [Google Scholar] [CrossRef]
- Henkart, P.; Humphreys, S.; Humphreys, T. Characterization of sponge aggregation factor. A unique proteoglycan complex. Biochemistry 1973, 12, 3045–3050. [Google Scholar] [CrossRef] [PubMed]
- Schröder, H.C.; Kuchino, Y.; Gramzow, M.; Kurelec, B.; Friese, U.; Uhlenbruck, G.; Müller, W.E. Induction of Ras gene expression by homologous aggregation factor in cells from the sponge Geodia cydonium. J. Biol. Chem. 1988, 263, 16334–16340. [Google Scholar] [PubMed]
- Müller, W.E.; Kurelec, B.; Zahn, R.K.; Muller, I.; Vaith, P.; Uhlenbruck, G. Aggregation of sponge cells. Function of a lectin in its homologous biological system. J. Biol. Chem. 1979, 254, 7479–7481. [Google Scholar] [PubMed]
- Hanisch, F.G.; Saur, A.; Müller, W.E.G.; Conrad, J.; Uhlenbruck, G. Further characterization of a lectin and its invivo receptor from Geodia cydonium. Biochim. Biophys. Acta 1984, 801, 388–395. [Google Scholar] [CrossRef]
- Pfeifer, K.; Frank, W.; Schröder, H.C.; Gamulin, V.; Rinkevich, B.; Batel, R.; Müller, I.M.; Müller, W.E. Cloning of the polyubiquitin cDNA from the marine sponge Geodia cydonium and its preferential expression during reaggregation of cells. J. Cell Sci. 1993, 106, 545–553. [Google Scholar] [PubMed]
- Pancer, Z.; Kruse, M.; Müller, I.; Müller, W.E. On the origin of metazoan adhesion receptors: Cloning of integrin alpha subunit from the sponge Geodia cydonium. Mol. Biol. Evol. 1997, 14, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, W.; Perovic, S.; Kruse, M.; Schröder, H.C.; Krasko, A.; Batel, R.; Müller, W.E. Origin of the integrin-mediated signal transduction. Functional studies with cell cultures from the sponge Suberites domuncula. Eur. J. Biochem. 1999, 260, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E. Molecular phylogeny of Metazoa (animals): Monophyletic origin. Naturwissenschaften 1995, 82, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Schutze, J.; Skorokhod, A.; Müller, I.M.; Müller, W.E. Molecular evolution of the metazoan extracellular matrix: Cloning and expression of structural proteins from the demosponges Suberites domuncula and Geodia cydonium. J. Mol. Evol. 2001, 53, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Hulpiau, P.; Van Roy, F. Molecular evolution of the cadherin superfamily. Int. J. Biochem. Cell Biol. 2009, 41, 349–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nichols, S.A.; Roberts, B.W.; Richter, D.J.; Fairclough, S.R.; King, N. Origin of metazoan cadherin diversity and the antiquity of the classical cadherin/beta-catenin complex. Proc. Natl. Acad. Sci. USA 2012, 109, 13046–13051. [Google Scholar] [CrossRef] [PubMed]
- Bokel, C.; Brown, N.H. Integrins in development: Moving on, responding to, and sticking to the extracellular matrix. Dev. Cell 2002, 3, 311–321. [Google Scholar] [CrossRef]
- Johnson, M.S.; Lu, N.; Denessiouk, K.; Heino, J.; Gullberg, D. Integrins during evolution: Evolutionary trees and model organisms. Biochim. Biophys. Acta 2009, 1788, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Brower, D.L.; Brower, S.M.; Hayward, D.C.; Ball, E.E. Molecular evolution of integrins: Genes encoding integrin beta subunits from a coral and a sponge. Proc. Natl. Acad. Sci. USA 1997, 94, 9182–9187. [Google Scholar] [CrossRef] [PubMed]
- Stephan, F. Spontaneous tumors in the planarian Dugesia tigrina. Comptes Rendus des Seances de la Societe de Biologie et de Ses Filiales 1962, 156, 920–922. [Google Scholar] [PubMed]
- Steeg, P.S.; Bevilacqua, G.; Kopper, L.; Thorgeirsson, U.P.; Talmadge, J.E.; Liotta, L.A.; Sobel, M.E. Evidence for a novel gene associated with low tumor metastatic potential. J. Natl. Cancer Inst. 1988, 80, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Bilitou, A.; Watson, J.; Gartner, A.; Ohnuma, S. The NM23 family in development. Mol. Cell. Biochem. 2009, 329, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Hartsough, M.T.; Morrison, D.K.; Salerno, M.; Palmieri, D.; Ouatas, T.; Mair, M.; Patrick, J.; Steeg, P.S. Nm23-H1 metastasis suppressor phosphorylation of kinase suppressor of Ras via a histidine protein kinase pathway. J. Biol. Chem. 2002, 277, 32389–32399. [Google Scholar] [CrossRef] [PubMed]
- Postel, E.H. Multiple biochemical activities of NM23/NDP kinase in gene regulation. J. Bioenerg. Biomembr. 2003, 35, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Harcet, M.; Lukic-Bilela, L.; Cetkovic, H.; Müller, W.E.G.; Gamulin, V. Identification and analysis of cDNAs encoding two nucleoside diphosphate kinases (NDPK/NM23) from the marine sponge Suberites domuncula. Croat. Chem Acta 2005, 78, 343–348. [Google Scholar]
- Perina, D.; Bosnar, M.H.; Bago, R.; Mikoc, A.; Harcet, M.; Dezeljin, M.; Cetkovic, H. Sponge non-metastatic group I Nme gene/protein—Structure and function is conserved from sponges to humans. BMC Evol. Biol. 2011, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Cetkovic, H.; Perina, D.; Harcet, M.; Mikoc, A.; Bosnar, M.H. Nme family of proteins—Clues from simple animals. Naunyn Schmiedebergs Arch. Pharmacol. 2015, 388, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Perina, D.; Bosnar, M.H.; Mikoc, A.; Müller, W.E.G.; Cetkovic, H. Characterization of Nme6-like gene/protein from marine sponge Suberites domuncula. Naunyn Schmiedebergs Arch. Pharmacol. 2011, 384, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, K.; Rossman, K.L.; Der, C.J. The Ras superfamily at a glance. J. Cell Sci. 2005, 118, 843–846. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.M.; Fuentes, G.; Rausell, A.; Valencia, A. The Ras protein superfamily: Evolutionary tree and role of conserved amino acids. J. Cell Biol. 2012, 196, 189–201. [Google Scholar] [CrossRef]
- Schaeffer, D.J. Planarians as a model system for in vivo tumorigenesis studies. Ecotoxicol. Environ. Saf. 1993, 25, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarsky, L.T. Coral disease dynamics in the central Philippines. Dis. Aquat. Organ. 2006, 69, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Peters, E.C.; Halas, J.C.; Mccarty, H.B. Calicoblastic neoplasms in acropora-palmata, with a review of reports on anomalies of growth and form in corals. J. Natl. Cancer Inst. 1986, 76, 895–912. [Google Scholar] [PubMed]
- Andersen, R.J. Sponging off nature for new drug leads. Biochem. Pharmacol. 2017, 139, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Torres, V.; Encinar, J.A.; Herranz-Lopez, M.; Perez-Sanchez, A.; Galiano, V.; Barrajon-Catalan, E.; Micol, V. An updated review on marine anticancer compounds: The use of virtual screening for the discovery of small-molecule cancer drugs. Molecules 2017, 22, 1037. [Google Scholar] [CrossRef] [PubMed]
- Halim, H.; Chunhacha, P.; Suwanborirux, K.; Chanvorachote, P. Anticancer and antimetastatic activities of renieramycin M, a marine tetrahydroisoquinoline alkaloid, in human non-small cell lung cancer cells. Anticancer Res. 2011, 31, 193–201. [Google Scholar] [PubMed]
- Sharma, S.; Guru, S.K.; Manda, S.; Kumar, A.; Mintoo, M.J.; Prasad, V.D.; Sharma, P.R.; Mondhe, D.M.; Bharate, S.B.; Bhushan, S. A marine sponge alkaloid derivative 4-chloro fascaplysin inhibits tumor growth and VEGF mediated angiogenesis by disrupting PI3K/Akt/mTOR signaling cascade. Chem. Biol. Interact. 2017, 275, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.J.; Zhou, F.; Al-Kareef, A.M.Q.; Wang, H. Anticancer agents from marine sponges. J. Asian Nat. Prod. Res. 2015, 17, 64–88. [Google Scholar] [CrossRef] [PubMed]
- Anjum, K.; Abbas, S.Q.; Shah, S.A.A.; Akhter, N.; Batool, S.; Ul Hassan, S.S. Marine sponges as a drug treasure. Biomol. Ther. 2016, 24, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Skropeta, D.; Pastro, N.; Zivanovic, A. Kinase inhibitors from marine sponges. Mar. Drugs 2011, 9, 2131–2154. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Peters, B.M.; Kurz, L.; Schatzman, R.C.; Mccarley, D.; Lou, L.; Crews, P. An alkaloid protein-kinase-C inhibitor, xestocyclamine-A, from the marine sponge Xestospongia sp. J. Am. Chem. Soc. 1993, 115, 10436–10437. [Google Scholar] [CrossRef]
- Patil, A.D.; Freyer, A.J.; Killmer, L.; Hofmann, G.; Randall, K. Z-axinohydantoin and debromo-Z-axinohydantoin from the sponge Stylotella aurantium: Inhibitors of protein kinase C. Nat. Prod. Lett. 1997, 9, 201–207. [Google Scholar] [CrossRef]
- Patil, A.D.; Freyer, A.J.; Killmer, L.; Offen, P.; Carte, B.; Jurewicz, A.J.; Johnson, R.K. Frondosins, five new sesquiterpene hydroquinone derivatives with novel skeletons from the sponge Dysidea frondosa: Inhibitors of interleukin-8 receptors. Tetrahedron 1997, 53, 5047–5060. [Google Scholar] [CrossRef]
- Willis, R.H.; DeVries, D.J. BRS1, A C30 BIS-Amino, BIS-Hydroxy polyunsaturated lipid from an Australian calcareous sponge that inhibits protein kinase C. Toxicon 1997, 35, 1125–1129. [Google Scholar] [CrossRef]
- Shigemori, H.; Madono, T.; Sasaki, T.; Mikami, Y.; Kobayashi, J. Nakijiquinone-A and nakijiquinone-B, new antifungal sesquiterpenoid quinones with an amino-acid residue from an Okinawan marine sponge. Tetrahedron 1994, 50, 8347–8354. [Google Scholar] [CrossRef]
- Kobayashi, J.; Madono, T.; Shigemori, H. Nakijiquinone-C and nakijiquinone-D, new sesquiterpenoid quinones with a hydroxy amino-acid residue from a marine sponge inhibiting c-erbB-2 kinase. Tetrahedron 1995, 51, 10867–10874. [Google Scholar] [CrossRef]
- He, H.Y.; Kulanthaivel, P.; Baker, B.J. New cytotoxic sesterterpenes from the marine sponge Spongia sp. Tetrahedron Lett. 1994, 35, 7189–7192. [Google Scholar] [CrossRef]
- Longley, R.E.; Harmody, D. A rapid colorimetric microassay to detect agonists antagonists of protein-kinase-C based on adherence of EL-4.IL-2 cells. J. Antibiot. 1991, 44, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Horton, P.A.; Koehn, F.E.; Longley, R.E.; Mcconnell, O.J. Lasonolide-A, a new cytotoxic macrolide from the marine sponge Forcepia sp. J. Am. Chem. Soc. 1994, 116, 6015–6016. [Google Scholar] [CrossRef]
- Isbrucker, R.A.; Guzman, E.A.; Pitts, T.P.; Wright, A.E. Early effects of lasonolide A on pancreatic cancer cells. J. Pharmacol. Exp. Ther. 2009, 331, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Alvi, K.A.; Jaspars, M.; Crews, P.; Strulovici, B.; Oto, E. Penazetidine-A, an alkaloid inhibitor of protein-kinase-C. Bioorg. Med. Chem. Lett. 1994, 4, 2447–2450. [Google Scholar] [CrossRef]
- Cimino, G.; Derosa, S.; Destefano, S.; Mazzarella, L.; Puliti, R.; Sodano, G. Isolation and X-ray crystal-structure of a novel bromo-compound from 2 marine sponges. Tetrahedron Lett. 1982, 23, 767–768. [Google Scholar] [CrossRef]
- Meijer, L.; Thunnissen, A.M.W.H.; White, A.W.; Garnier, M.; Nikolic, M.; Tsai, L.H.; Walter, J.; Cleverley, K.E.; Salinas, P.C.; Wu, Y.Z.; et al. Inhibition of cyclin-dependent kinases, GSK-3 beta and CK1 by hymenialdisine, a marine sponge constituent. Chem. Biol. 2000, 7, 51–63. [Google Scholar] [CrossRef]
- Killday, K.B.; Yarwood, D.; Sills, M.A.; Murphy, P.T.; Hooper, J.N.; Wright, A.E. Microxine, a new cdc2 kinase inhibitor from the Australian marine sponge Microxina species. J. Nat. Prod. 2001, 64, 525–526. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.R.; Carter, E.J.; Huff, B.C.; Morris, J.C. Variolins and related alkaloids. Chem. Rev. 2009, 109, 3080–3098. [Google Scholar] [CrossRef] [PubMed]
- Soni, R.; Muller, L.; Furet, P.; Schoepfer, J.; Stephan, C.; Zumstein-Mecker, S.; Fretz, H.; Chaudhuri, B. Inhibition of cyclin-dependent kinase 4 (Cdk4) by fascaplysin, a marine natural product. Biochem. Biophys. Res. Commun. 2000, 275, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J.; Suzuki, M.; Tsuda, M. Konbu’acidin a, a new bromopyrrole alkaloid with cdk4 inhibitory activity from Hymeniacidon sponge. Tetrahedron 1997, 53, 15681–15684. [Google Scholar] [CrossRef]
- Bifulco, G.; Bruno, I.; Minale, L.; Riccio, R.; Debitus, C.; Bourdy, G.; Vassas, A. Bioactive prenylhydroquinone sulfates and a novel c-31 furanoterpene alcohol sulfate from the marine sponge, Ircinia sp. J. Nat. Prod. 1995, 58, 1444–1449. [Google Scholar] [CrossRef]
- Alvi, K.A.; Diaz, M.C.; Crews, P.; Slate, D.L.; Lee, R.H.; Moretti, R. Evaluation of new sesquiterpene quinones from 2 Dysidea sponge species as inhibitors of protein tyrosine kinase. J. Org. Chem. 1992, 57, 6604–6607. [Google Scholar] [CrossRef]
- Lee, R.H.; Slate, D.L.; Moretti, R.; Alvi, K.A.; Crews, P. Marine sponge polyketide inhibitors of protein tyrosine kinase. Biochem. Biophys. Res. Commun. 1992, 184, 765–772. [Google Scholar] [CrossRef]
- Kreuter, M.H.; Leake, R.E.; Rinaldi, F.; Mullerklieser, W.; Maidhof, A.; Müller, W.E.G.; Schröder, H.C. Inhibition of intrinsic protein tyrosine kinase-activity of EGF-receptor kinase complex from human breast-cancer cells by the marine sponge metabolite (+)-aeroplysinin-1. Comp. Biochem. Phys. B 1990, 97, 151–158. [Google Scholar] [CrossRef]
- Hertiani, T.; Edrada-Ebel, R.A.; Kubbutat, M.H.G.; van Soest, R.W.M.; Proksch, P. Protein kinase inhibitors from Indonesian sponge Axynissa sp. Indones. J. Pharm. 2008, 19, 78–85. [Google Scholar]
- Calcabrini, C.; Catanzaro, E.; Bishayee, A.; Turrini, E.; Fimognari, C. Marine sponge natural products with anticancer potential: An updated review. Mar. Drugs 2017, 15, 310. [Google Scholar] [CrossRef] [PubMed]
- Gordon, E.M.; Sankhala, K.K.; Chawla, N.; Chawla, S.P. Trabectedin for soft tissue sarcoma: Current status and future perspectives. Adv. Ther. 2016, 33, 1055–1071. [Google Scholar] [CrossRef] [PubMed]
- Dyshlovoy, S.A.; Fedorov, S.N.; Shubina, L.K.; Kuzmich, A.S.; Bokemeyer, C.; Keller-von Amsberg, G.; Honecker, F. Aaptamines from the marine sponge Aaptos sp. display anticancer activities in human cancer cell lines and modulate AP-1-, NF-kappa b-, and p53-dependent transcriptional activity in mouse JB6 CL41 cells. BioMed Res. Int. 2014, 2014, 469309. [Google Scholar] [CrossRef] [PubMed]
- Berry, E.; Hardt, J.L.; Clardy, J.; Lurain, J.R.; Kim, J.J. Induction of apoptosis in endometrial cancer cells by psammaplysene a involves foxo1. Gynecol. Oncol. 2009, 112, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Zhou, Q.X.; Zhang, L.; Zhong, Y.X.; Fan, G.W.; Zhang, Z.; Wang, R.; Jin, M.H.; Qiu, Y.L.; Kong, D.X. Stellettin B induces apoptosis in human chronic myeloid leukemia cells via targeting PI3K and Stat5. Oncotarget 2017, 8, 28906–28921. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, Q.; Peng, X.; Zhou, C.; Zhong, Y.X.; Chen, X.; Qiu, Y.L.; Jin, M.H.; Gong, M.; Kong, D.X. Stellettin B induces G1 arrest, apoptosis and autophagy in human non-small cell lung cancer A549 cells via blocking PI3K/Akt/mTOR pathway. Sci. Rep. 2016, 6, 27071. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.A.; Zhou, Q.X.; Guo, W.Z.; Qiu, Y.L.; Wang, R.; Jin, M.H.; Zhang, W.J.; Li, K.; Yamori, T.; Dan, S.G.; et al. In vitro antitumor activity of stellettin B, a triterpene from marine sponge Jaspis stellifera, on human glioblastoma cancer SF295 cells. Mar. Drugs 2014, 12, 4200–4213. [Google Scholar] [CrossRef] [PubMed]
- Trisciuoglio, D.; Uranchimeg, B.; Cardellina, J.H.; Meragelman, T.L.; Matsunaga, S.; Fusetani, N.; Del Bufalo, D.; Shoemaker, R.H.; Melillo, G. Induction of apoptosis in human cancer cells by candidaspongiolide, a novel sponge polyketide. J. Natl. Cancer Inst. 2008, 100, 1233–1246. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Cerella, C.; Eifes, S.; Chateauvieux, S.; Morceau, F.; Jaspars, M.; Dicato, M.; Diederich, M. Heteronemin, a spongean sesterterpene, inhibits TNF alpha-induced NF-kappa B activation through proteasome inhibition and induces apoptotic cell death. Biochem. Pharm. 2010, 79, 610–622. [Google Scholar] [CrossRef]
- Wu, J.C.; Wang, C.T.; Hung, H.C.; Wu, W.J.; Wu, D.C.; Chang, M.C.; Sung, P.J.; Chou, Y.W.; Wen, Z.H.; Tai, M.H. Heteronemin is a novel c-Met/STAT3 inhibitor against advanced prostate cancer cells. Prostate 2016, 76, 1469–1483. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.H.; Kuo, S.M.; Wu, Y.J.; Su, J.H. Improvement and enhancement of antibladder carcinoma cell effects of heteronemin by the nanosized hyaluronan aggregation. Int. J. Nanomed. 2016, 11, 1237–1251. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Bae, S.J.; Kim, N.D.; Jung, J.H.; Choi, Y.H. Induction of apoptosis by dideoxypetrosynol A, a polyacetylene from the sponge Petrosia sp., in human skin melanoma cells. Int. J. Mol. Med. 2004, 14, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, K.; Yamagishi, T.; Ichihara, T.; Nakaike, S.; Iguchi, K.; Yamada, Y.; Fukumoto, H.; Yoneda, T.; Samata, K.; Ikeya, H.; et al. Mechanism of action of aragusterol a (YTA0040), a potent anti-tumor marine steroid targeting the G(1) phase of the cell cycle. Int. J. Cancer 2000, 88, 810–819. [Google Scholar] [CrossRef]
- Bae, S.Y.; Song, J.; Shin, Y.; Kim, W.K.; Oh, J.; Choi, T.J.; Jeong, E.J.; Park, S.H.; Jang, E.J.; Kang, J.I.; et al. Anti-proliferative effect of (19Z)-halichondramide from the sponge Chondrosia corticata via G2/M cell cycle arrest and suppression of mTOR signaling in human lung cancer cells. Cancer Res. 2014, 74. [Google Scholar] [CrossRef]
- Aoki, S.; Kong, D.; Matsui, K.; Kobayashi, M. Smenospongine, a spongean sesquiterpene aminoquinone, induces erythroid differentiation in k562 cells. Anti-Cancer Drug 2004, 15, 363–369. [Google Scholar] [CrossRef]
- Aoki, S.; Kong, D.X.; Matsui, K.; Kobayashi, M. Erythroid differentiation in K562 chronic myelogenous cells induced by crambescidin 800, a pentacyclic guanidine alkaloid. Anticancer Res. 2004, 24, 2325–2330. [Google Scholar] [PubMed]
- Fung, S.Y.; Sofiyev, V.; Schneiderman, J.; Hirschfeld, A.F.; Victor, R.E.; Woods, K.; Piotrowski, J.S.; Deshpande, R.; Li, S.C.; de Voogd, N.J.; et al. Unbiased screening of marine sponge extracts for antiinflammatory agents combined with chemical genomics identifies girolline as an inhibitor of protein synthesis. ACS Chem. Biol. 2014, 9, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Kim, S.K. Matrix metalloproteinase inhibitors (MMPIs) from marine natural products: The current situation and future prospects. Mar. Drugs 2009, 7, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Kim, G.D.; Jeon, J.E.; Shin, J.; Lee, S.K. Antimetastatic effect of halichondramide, a trisoxazole macrolide from the marine sponge Chondrosia corticata, on human prostate cancer cells via modulation of epithelial-to-mesenchymal transition. Mar. Drugs 2013, 11, 2472–2485. [Google Scholar] [CrossRef] [PubMed]
- Robert, J. Comparative study of tumorigenesis and tumor immunity in invertebrates and nonmammalian vertebrates. Dev. Comp. Immunol. 2010, 34, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Monahan-Earley, R.; Dvorak, A.M.; Aird, W.C. Evolutionary origins of the blood vascular system and endothelium. J. Thromb. Haemost. 2013, 11, 46–66. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.; Blumbach, B.; Müller, I.M. Evolution of the innate and adaptive immune systems: Relationships between potential immune molecules in the lowest metazoan phylum (Porifera) and those in vertebrates. Transplantation 1999, 68, 1215–1227. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ćetković, H.; Halasz, M.; Herak Bosnar, M. Sponges: A Reservoir of Genes Implicated in Human Cancer. Mar. Drugs 2018, 16, 20. https://doi.org/10.3390/md16010020
Ćetković H, Halasz M, Herak Bosnar M. Sponges: A Reservoir of Genes Implicated in Human Cancer. Marine Drugs. 2018; 16(1):20. https://doi.org/10.3390/md16010020
Chicago/Turabian StyleĆetković, Helena, Mirna Halasz, and Maja Herak Bosnar. 2018. "Sponges: A Reservoir of Genes Implicated in Human Cancer" Marine Drugs 16, no. 1: 20. https://doi.org/10.3390/md16010020



