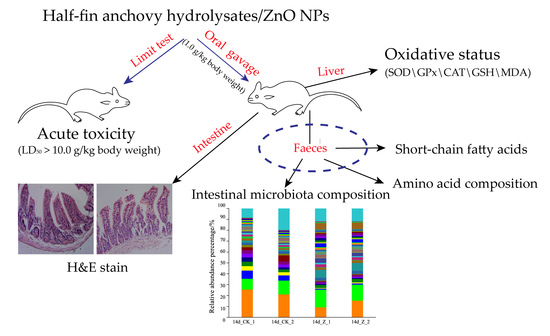
Nanocomposite of Half-Fin Anchovy Hydrolysates/Zinc Oxide Nanoparticles Exhibits Actual Non-Toxicity and Regulates Intestinal Microbiota, Short-Chain Fatty Acids Production and Oxidative Status in Mice
Abstract
:1. Introduction
2. Results
2.1. Acute Oral Toxicity of HAHp(3.0)/ZnO NPs
2.2. Histopathology
2.3. Effect of HAHp(3.0)/ZnO NPs on the Alteration of Intestinal Microbiota Composition
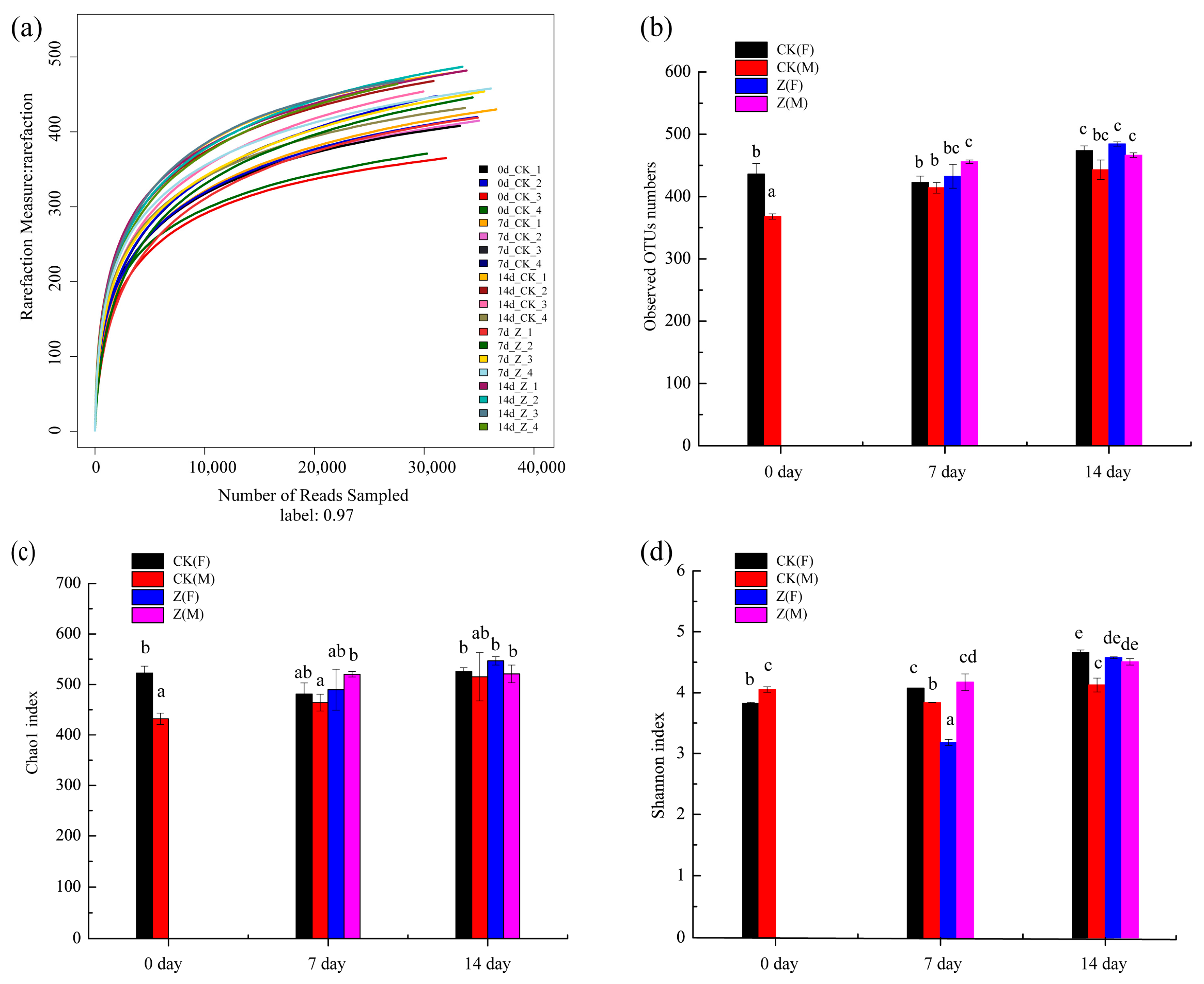
2.3.1. Species Abundance and Diversity
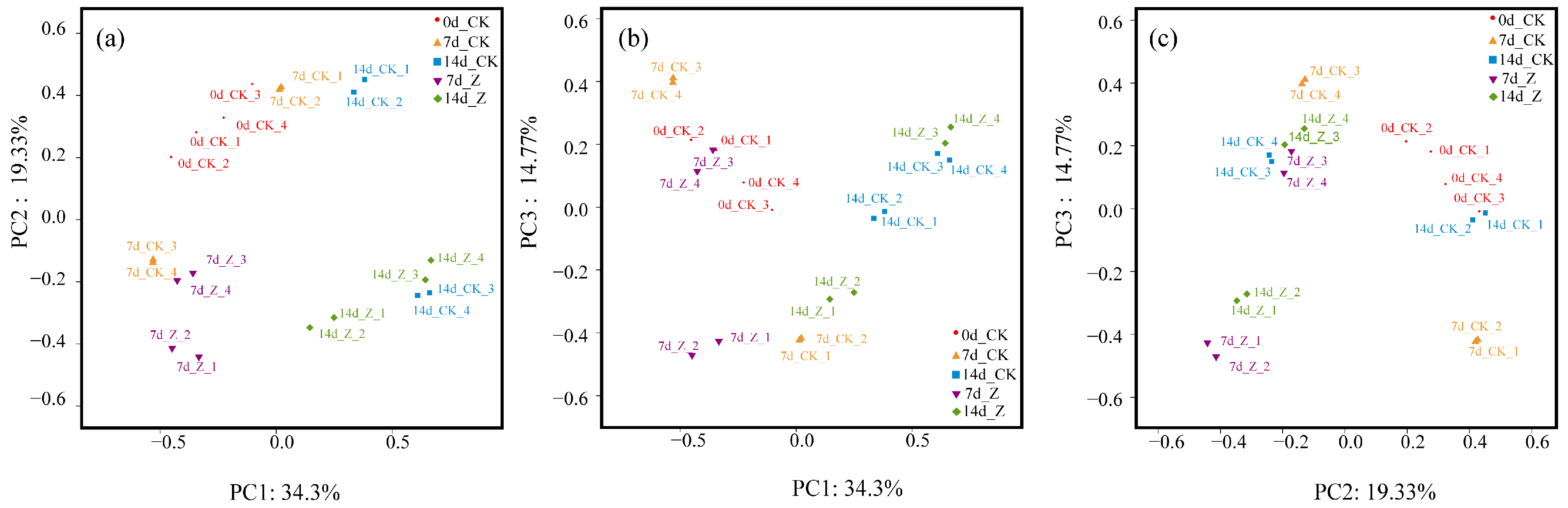
2.3.2. Principal Coordinates Analysis (PCoA)
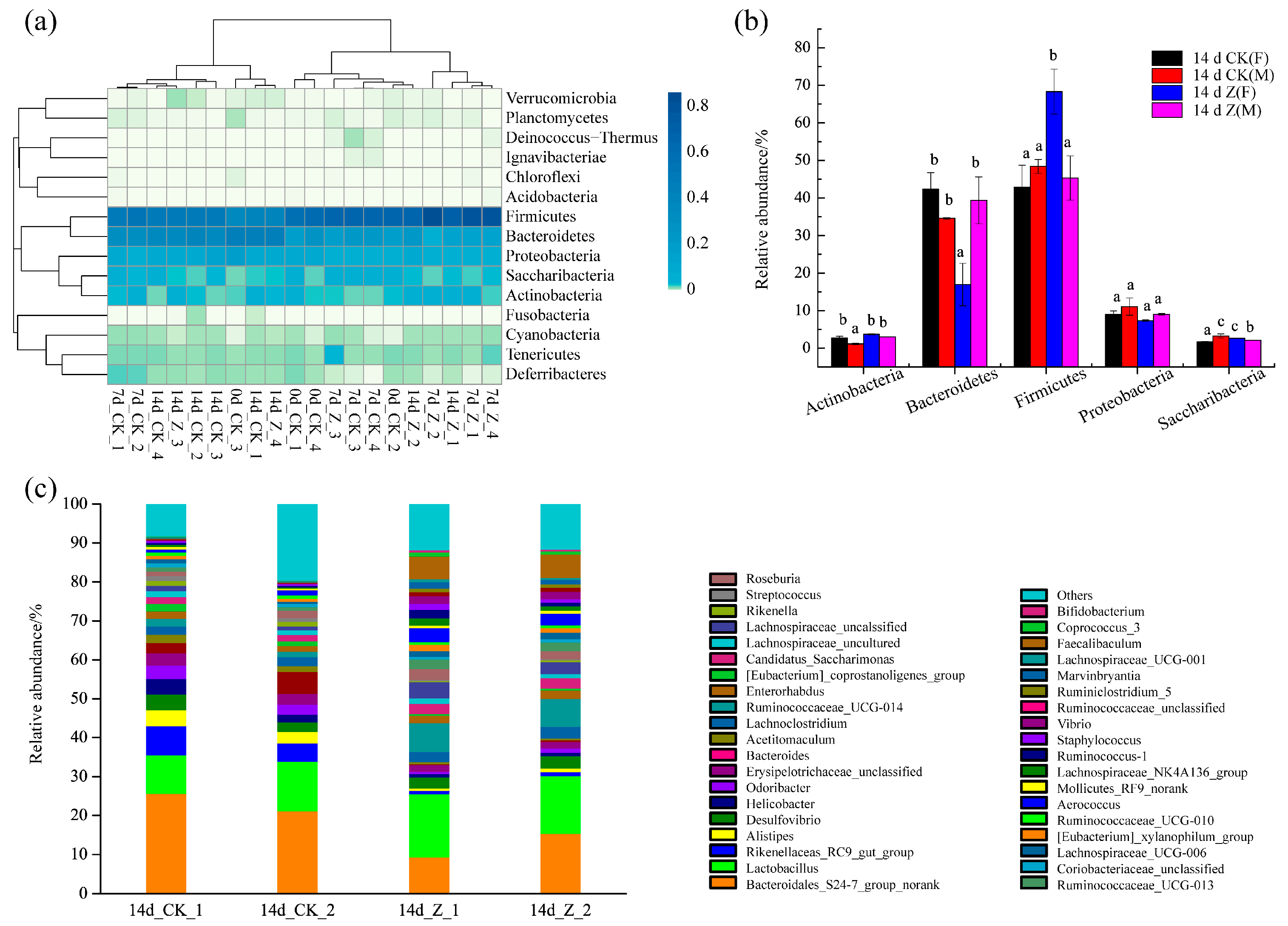
2.3.3. Composition of the Intestinal Microbiota at the Phylum and Genus Levels
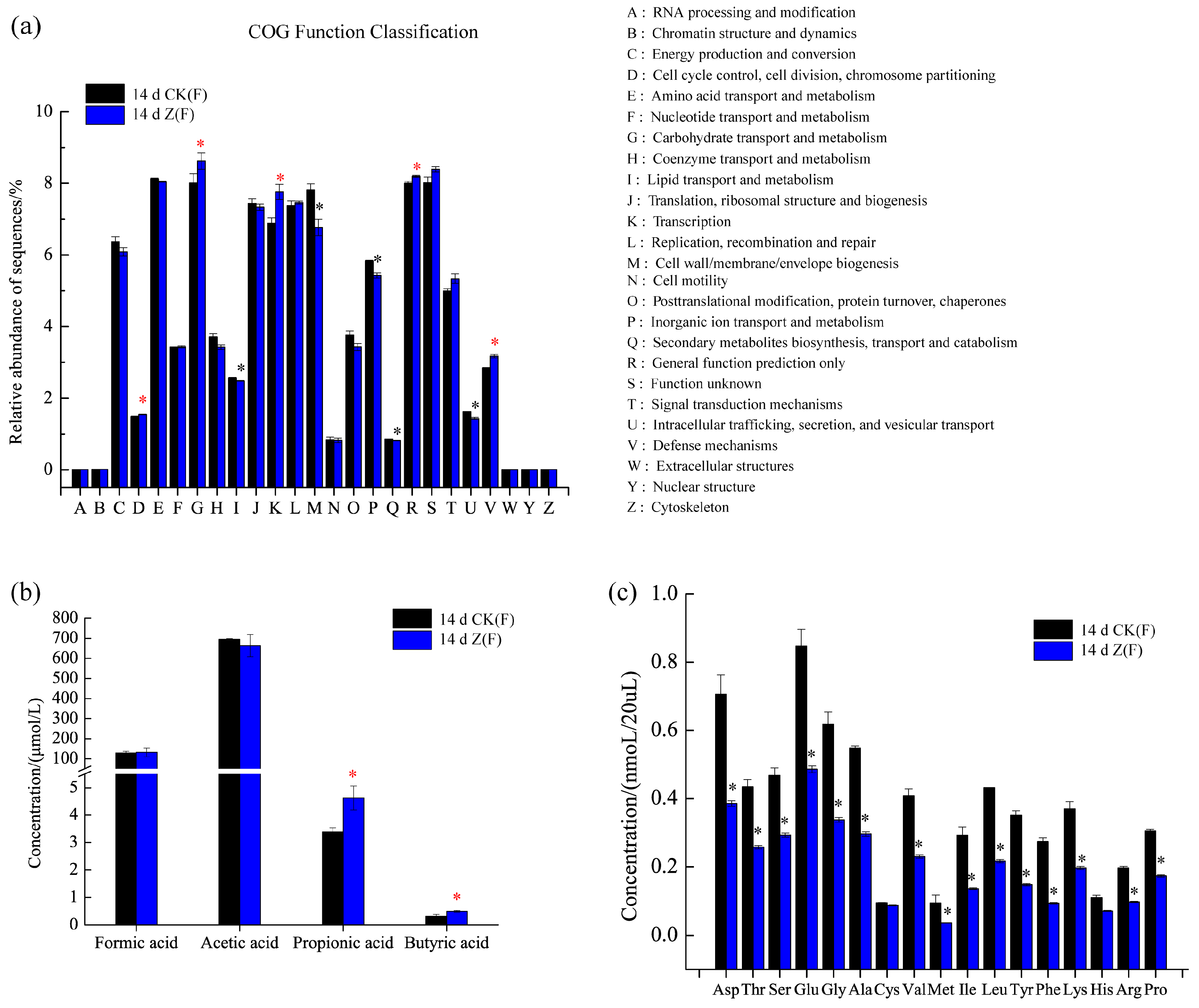
2.4. Function Prediction, SCFAs and Amino Acid Concentrations in Feces
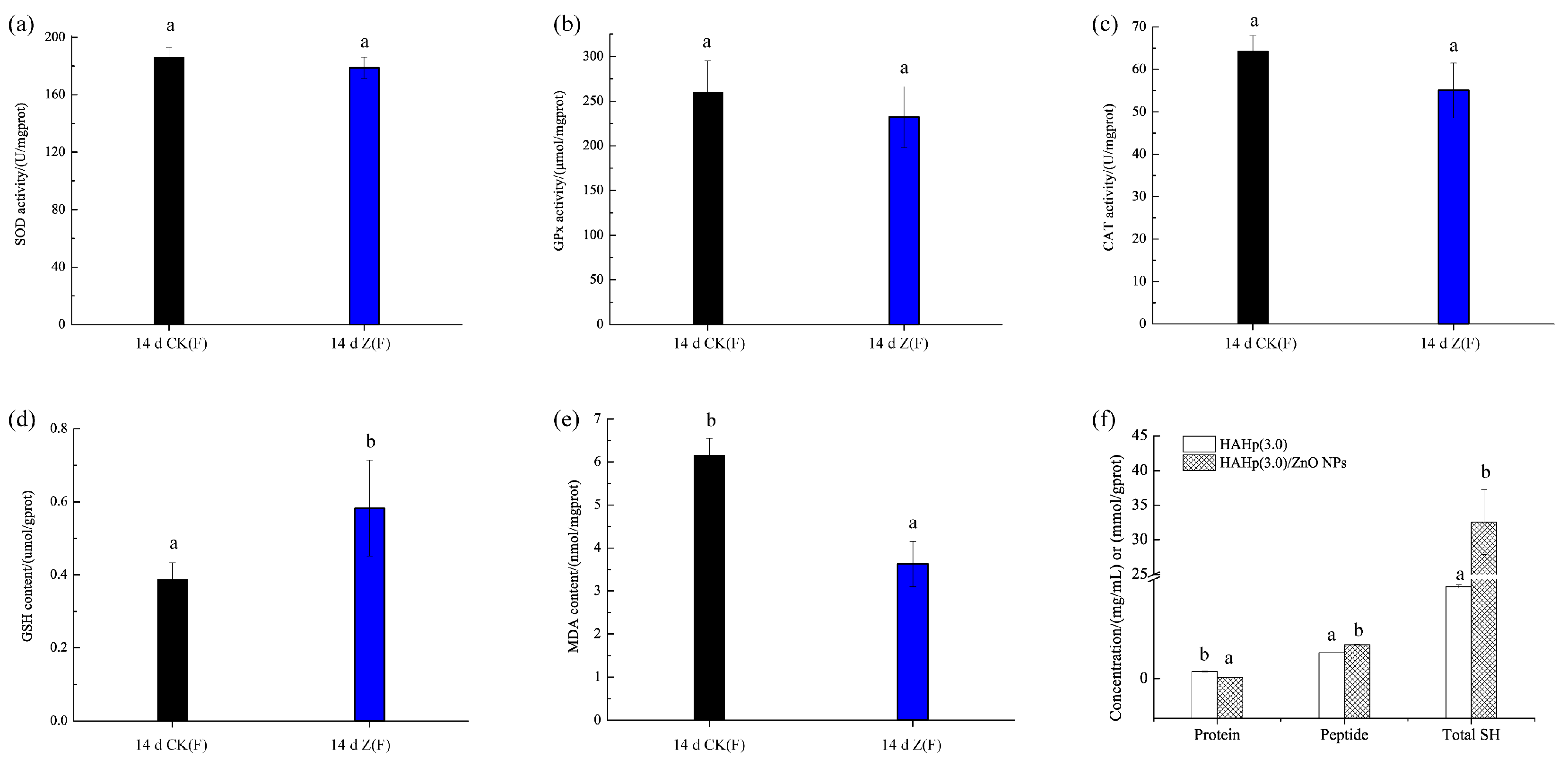
2.5. Changes of Liver Oxidative Status
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of the Nanocomposite of HAHp and ZnO NPs
4.3. Experimental Animals
4.4. Acute Oral Toxicity Study
4.5. Continuous Oral Administration of HAHp(3.0)/ZnO NPs in Mice
4.6. Histopathology
4.7. DNA Isolation and Polymerase Chain Reaction (PCR) Amplification and Sequencing
4.8. Bioinformatics Analysis
4.9. Determination of SCFAs in Feces
4.10. Determination of Amino Acid in Feces
4.11. Determination of Oxidative Status
4.12. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jin, T.; Sun, D.; Su, J.Y.; Zhang, H.; Sue, H.J. Antimicrobial efficacy of zinc oxide quantum dots against Listeria monocytogenes, Salmonella, Enteritidis, and Escherichia coli O157:H7. J. Food Sci. 2009, 1, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Rajendra, R.; Balakumar, C.; Ahammed, H.; Jayakumar, S.; Vaideki, K.; Rajesh, E. Use of zinc oxide nano particles for production of antimicrobial textiles. Int. J. Eng. Sci. Technol. 2010, 2, 202–208. [Google Scholar] [CrossRef]
- Bajpai, K.S.; Chand, N.; Chaurasia, V. Nano zinc oxide-loaded calcium alginate films with potential antibacterial properties. Food Bioprocess Technol. 2012, 5, 1871–1881. [Google Scholar] [CrossRef]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Feng, W.Y.; Wang, T.C.; Jia, G.; Wang, M.; Shi, J.W.; Zhang, F.; Zhao, Y.L.; Chai, Z.F. Acute toxicity of nano- and micro-scale zinc powder in healthy adult mice. Toxicol. Lett. 2006, 161, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Sow, L.C.; Peh, Y.R.; Pekerti, B.N.; Fu, C.; Bansal, N.; Yang, H. Nanostructural analysis and textural modification of tilapia fish gelatin affected by gellan and calcium chloride addition. LWT Food Sci. Technol. 2017, 85, 137–145. [Google Scholar] [CrossRef]
- Docter, D.; Westmeier, D.; Markiewicz, M.; Stolte, S.; Knauer, S.K.; Stauber, R.H. The nanoparticle biomolecule corona: Lessons learned—Challenge accepted? Chem. Soc. Rev. 2015, 44, 6094–6121. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.Y.; Yang, Y.H.; Li, X.L.; Lai, H.Q.; Huang, Y.Y.; He, L.Z.; Zheng, W.J.; Chen, T.F. RGD peptide-conjugated selenium nanoparticles: Antiangiogenesis by suppressing VEGF-VEGFR2-ERK/AKT pathway. Nanomedicine 2016, 12, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Keleştemur, S.; Altunbek, M.; Culha, M. Influence of EDC/NHS coupling chemistry on stability and cytotoxicity of ZnO nanoparticles modified with proteins. Appl. Surf. Sci. 2017, 403, 455–463. [Google Scholar] [CrossRef]
- Yu, X.; Ang, H.C.; Yang, H.; Zheng, C.; Zhang, Y. Low temperature cleanup combined with magnetic nanoparticle extraction to determine pyrethroids residue in vegetables oils. Food Control 2017, 74, 112–120. [Google Scholar] [CrossRef]
- Song, R.; Shi, Q.Q.; Abdrabboh, G.A.A.; Wei, R.B. Characterization and antibacterial activity of the nanocomposite of half-fin anchovy (Setipinna taty) hydrolysates/zinc oxide nanoparticles. Process Biochem. 2017, 62, 223–230. [Google Scholar] [CrossRef]
- Bäumler, A.J.; Sperandio, V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 2016, 535, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Rajoka, M.S.R.; Shi, J.; Mehwish, H.M.; Zhu, J.; Li, Q.; Shao, D.; Huang, Q.; Yang, H. Interaction between diet composition and gut microbiota and its impact on gastrointestinal tract health. Food Sci. Hum. Wellness 2017, 3, 121–130. [Google Scholar] [CrossRef]
- Arias, M.; Cobo, M.; Jaime-Sánchez, P.; Pastor, J.; Marijuan, P.; Pardo, J.; Rezusta, A.; Campo, R.D. Gut microbiota and systemic inflammation changes after bread consumption: The ingredients and the processing influence. J. Funct. Foods 2017, 32, 98–105. [Google Scholar] [CrossRef]
- Guida, S.; Venema, K. Gut microbiota and obesity: Involvement of the adipose tissue. J. Funct. Foods 2015, 14, 407–423. [Google Scholar] [CrossRef]
- Feng, X.; Ng, V.K.; Mikš-Krajnik, M.; Yang, H. Effects of fish gelatin and tea polyphenol coating on the spoilage and degradation of myofibril in fish fillet during cold storage. Food Bioprocess Technol. 2017, 10, 89–102. [Google Scholar] [CrossRef]
- Yasmin, A.; Butt, M.S.; Afzaal, M.; Baak, M.; Nadeem, M.T.; Shahid, M.Z. Prebiotics, gut microbiota and metabolic risks: Unveiling the relationship. J. Funct. Foods 2015, 17, 189–201. [Google Scholar] [CrossRef]
- Qin, J.J.; Li, Y.R.; Cai, Z.M.; Li, S.H.; Zhu, J.F.; Zhang, F.; Liang, S.S.; Zhang, W.W.; Guan, Y.L.; Shen, D.Q.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; DiBaise, J.K.; Zuccolo, A.; Kudrna, D.; Braidotti, M.; Yu, Y.; Parameswaran, P.; Crowell, M.D.; Wing, R.; Rittmann, B.E.; et al. Human gut microbiota in obesity and after gastric bypass. Proc. Natl. Acad. Sci. USA 2009, 106, 2365–2370. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Yang, F.; Li, A.; Prifti, E.; Chen, Y.; Shao, L.; Guo, J.; Le Chatelier, E.; Yao, J.; Wu, L.; et al. Alterations of the human gut microbiome in liver cirrhosis. Nature 2014, 513, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Ussher, J.R.; Lopaschuk, G.D.; Arduini, A. Gut microbiota metabolism of L-carnitine and cardiovascular risk. Atherosclerosis 2013, 231, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Serban, D.E. Gastrointestinal cancers: Influence of gut microbiota, probiotics and prebiotics. Cancer Lett. 2014, 345, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Fraher, M.H.; O’Toole, P.W.; Quigley, E.M. Techniques used to characterize the gut microbiota: A guide for the clinician. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Brugère, J.F.; Mihajlovski, A.; Missaoui, M.; Peyret, P. Tools for stools: The challenge of assessing human intestinal microbiota using molecular diagnostics. Expert Rev. Mol. Diagn. 2009, 9, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Finegold, S.M.; Dowd, S.E.; Gontcharova, V.; Liu, C.; Henley, K.E.; Wolcott, R.D.; Youn, E.; Summanen, P.H.; Granpeesheh, D.; Dixon, D.; et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe 2010, 16, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Serino, M.; Chabo, C.; Burcelin, R. Intestinal microbi OMICS to define health and disease in human and mice. Curr. Pharm. Biotechnol. 2012, 13, 746–758. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermudez-Humaran, L.G.; Gratadoux, J.J.; Blugeon, S.; Bridonneau, C.; Furet, J.P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Louis, P.; Flint, H.J. Cultivable bacterial diversity from the human colon. Lett. Appl. Microbiol. 2007, 44, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Bervoets, L.; Van Hoorenbeeck, K.; Kortleven, I.; Van Noten, C.; Hens, N.; Vael, C.; Goossens, H.; Desager, K.N.; Vankerckhoven, V. Differences in gut microbiota composition between obese and lean children: A cross-sectional study. Gut Pathog. 2013, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Shen, D.Q.; Fang, Z.W.; Jie, Z.Y.; Qiu, X.M.; Zhang, C.F.; Chen, Y.L.; Ji, L.N. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS ONE 2013, 8, e71108. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Wei, R.B.; Zhang, B.; Wang, D.F. Optimization of the antibacterial activity of half-fin anchovy (Setipinna taty) hydrolysates. Food Bioprocess Technol. 2012, 5, 1979–1989. [Google Scholar] [CrossRef]
- Song, R.; Wei, R.B.; Ruan, G.Q.; Luo, H.Y. Isolation and identification of antioxidative peptides from peptic hydroly sates of half-fin anchovy (Setipinna taty). LWT Food Sci. Technol. 2015, 60, 221–229. [Google Scholar] [CrossRef]
- Song, R.; Wei, R.B.; Zhang, B.; Yang, Z.S.; Wang, D.F. Antioxidant and antiproliferative activities of heated sterilized pepsin hydrolysate derived from half-fin anchovy (Setipinna taty). Mar. Drugs 2011, 9, 1142–1156. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.J.; Li, Q.Z.; Yin, Y.Q.; Wang, J.H.; Sun, S.X.; Yang, F.; Li, L.R.; Wang, J.; Wang, Q.; Li, Y.S. Composition of intestinal microflora associated with Yang-deficiency. J. Tradit. Chin. Med. Sci. 2017, 4, 254–260. [Google Scholar] [CrossRef]
- Byerley, L.O.; Samuelson, D.; Blanchard, I.V.E.; Luo, M.; Lorenzen, B.N.; Banks, S.; Ponder, M.A.; Welsh, D.A.; Taylor, C.M. Changes in the gut microbial communities following addition of walnuts to the diet. J. Nutr. Biochem. 2017, 48, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, W.H.; Haberer, P.; Snel, J.; Schillinger, U.; Huis in’t, V.; Jos, H.J. Overview of gut flora and probiotics. Int. J. Food Microbiol. 1998, 41, 85–101. [Google Scholar] [CrossRef]
- Jayarambabu, N.; Rao, K.V.; Rajendar, V. Biogenic synthesis, characterization, acute oral toxicity studies of synthesized Ag and ZnO nanoparticles using aqueous extract of Lawsonia inermis. Mater. Lett. 2018, 211, 43–47. [Google Scholar] [CrossRef]
- Deplancke, B.; Gaskins, H.R. Microbial modulation of innate defense: Gobletcells and the intestinal mucus layer. Am. J. Clin. Nutr. 2001, 73, 1131S–1141S. [Google Scholar]
- Morais, M.B. Signs and symptoms associated with digestive tract development. J. Pediatr. 2016, 92, S46–S56. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.G.; Wen, C.; Wang, L.C.; Wang, T.; Zhou, Y.M. Effects of zinc-bearing clinoptilolite on growth performance, cecal microflora and intestinal mucosal function of broiler chickens. Anim. Feed Sci. Technol. 2014, 189, 98–106. [Google Scholar] [CrossRef]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed]
- Martinez, I.; Lattimer, J.M.; Hubach, K.L.; Case, J.A.; Yang, J.; Weber, C.G.; Louk, J.A.; Rose, D.J.; Kyureghian, G.; Peterson, D.A.; et al. Gut microbiome composition is linked to whole grain-induced immunological improvements. ISME J. 2013, 7, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Li, K.K.; Tian, P.J.; Wang, S.D.; Lei, P.; Qu, L.; Huang, J.P.; Shan, Y.J.; Li, B.L. Targeting gut microbiota: Lactobacillus alleviated type 2 diabetes via inhibiting LPS secretion and activating GPR43 pathway. J. Funct. Foods 2017, 38, 561–570. [Google Scholar] [CrossRef]
- Yu, A.Q.; Li, L. The potential role of probiotics in cancer prevention and treatment. Nutr. Cancer 2016, 68, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.J.; Amorim, M.; Marques, C.; Calhau, C.; Pintado, M. Effects of whey peptide extract on the growth of probiotics and gut microbiota. J. Funct. Foods 2016, 21, 507–516. [Google Scholar] [CrossRef]
- Bedford, A.; Gong, J. Implications of butyrate and its derivatives for gut health and animal production. Anim. Nutr. J. 2017. [Google Scholar] [CrossRef]
- Saulnier, D.M.; Riehle, K.; Mistretta, T.A.; Diaz, M.A.; Mandal, D.; Raza, S.; Weidler, E.M.; Qin, X.; Coarfa, C.; Milosavljevic, A.; et al. Gastrointestinal microbiome signatures of pediatric patients with irritable bowel syndrome. Gastroenterology 2011, 141, 1782–1791. [Google Scholar] [CrossRef] [PubMed]
- Marcobal, A.; Barboza, M.; Froehlich, J.W.; Block, D.E.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Consumption of human milk oligosaccharides by gut-related microbes. J. Agric. Food Chem. 2010, 58, 5334–5340. [Google Scholar] [CrossRef] [PubMed]
- Huërou-Luron, I.L.; Blat, S.; Boudry, G. Breast- v. formula-feeding: Impacts on the digestive tract and immediate and long-term health effects. Nutr. Res. Rev. 2010, 23, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Kitada, Y.; Shimomura, Y.; Naito, Y. Bifidobacterium animalis subsp. Lactis LKM512 reduces levels of intestinal trimethylamine produced by intestinal microbiota in healthy volunteers: A double-blind, placebo-controlled study. J. Funct. Foods 2017, 36, 94–101. [Google Scholar] [CrossRef]
- Tanaka, M.; Nakayama, J. Development of the gut microbiota in infancy and its impact on health in later life. Allergol. Int. 2017, 66, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Mitsuoka, T. Development of functional foods. Biosci. Microbiota Food Health 2014, 33, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Dodd, C.E.R.; Aldsworth, T.; Stein, R.A.; Cliver, D.O.; Riemann, H.P. Chapter 11—Vibrio. In Foodborne Diseases, 3rd ed.; Jones, J.L., Ed.; Academic Press: Dordrecht, The Netherlands; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 243–252. [Google Scholar]
- Liu, N.N.; Zhang, S.S.; Zhang, W.W.; Li, C.H. Vibrio sp. 33 a potential bacterial antagonist of Vibrio splendidus pathogenic to sea cucumber (Apostichopus japonicus). Aquaculture 2017, 470, 68–73. [Google Scholar] [CrossRef]
- How PICRUSt Works. Available online: http://picrust.github.io/picrust/tutorials/algorithm_description.html (accessed on 1 April 2017).
- Delgado-Andrade, C.; Cueva, S.P.; Peinado, M.J.; Rufián-Henares, J.A.; Navarro, M.P.; Rubio, L.A. Modifications in bacterial groups and short chain fatty acid production in the gut of healthy adult rats after long-term consumption of dietary Maillard reaction products. Food Res. Int. 2017, 100, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Kenzie, C.M.; Vuillermin, P.J.; Goverse, G.; Vinuesa, C.G.; Mebius, R.E.; Macia, L.; Mackay, C.R. Dietary fiber and bacterial SCFA enhance oral tolerance and protect against food allergy through diverse cellular pathways. Cell Rep. 2016, 15, 2809–2824. [Google Scholar] [CrossRef] [PubMed]
- Anhê, F.F.; Varin, T.V.; Le Barz, M.; Desjardins, Y.; Levy, E.; Roy, D.; Marette, A. Gut microbiota dysbiosis in obesity-linked metabolic diseases and prebiotic potential of polyphenol-rich extracts. Curr. Obes. Rep. 2015, 4, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Carretta, M.D.; Hidalgo, A.I.; Burgos, J.; Opazo, L.; Castro, L.; Hidalgo, M.A.; Figueroa, C.D.; Taubert, A.; Hermosilla, C.; Burgos, R.A. Butyric acid stimulates bovine neutrophil functions and potentiates the effect of platelet activating factor. Immunopathology 2016, 176, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Wang, B.H.; Wu, J.F.; Jiang, X.Y.; Tang, H.R.; Nielsen, O.H. Modulation of gut microbiota in pathological states. Engineering 2017, 3, 83–89. [Google Scholar] [CrossRef]
- Zhuang, Y.; Ma, Q.; Guo, Y.; Sun, L. Protective effects of rambutan (Nephelium lappaceum) peel phenolics on H2O2-induced oxidative damages in HepG2 cells and d-galactose-induced aging mice. Food Chem. Toxicol. 2017, 108, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Roca, B.; Delgado-Andrade, C.; Navarro, M.P.; Seiquer, I. Effects of Maillard reaction products from glucose–lysine model systems on oxidative stress markers and against oxidative induction by hydrogen peroxide in Caco-2 cells. J. Food Nutr. Res. 2011, 50, 237–248. [Google Scholar]
- Yan, H.; Harding, J.J. Glycation-induced inactivation and loss of antigenicity of catalase and superoxide dismutase. Biochem. J. 1997, 328, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Shahid, F.; Farooqui, Z.; Rizwan, S.; Abidi, S.; Parwez, I.; Khan, F. Oral administration of Nigella sativa oil ameliorates the effect of cisplatin on brush border membrane enzymes, carbohydrate metabolism and antioxidant system in rat intestine. Exp. Toxicol. Pathol. 2017, 69, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Claus, S.P.; Guillou, H.; Ellero-Simatos, S. The gut microbiota: A major player in the toxicity of environmental pollutants? NPJ Biofilms Microbiomes 2016, 2, 16003. [Google Scholar] [CrossRef] [PubMed]
- Frémont, M.; Coomans, D.; Massart, S.; De Meirleir, K. High-throughput 16S rRNA gene sequencing reveals alterations of intestinal microbiota in myalgic encephalomyelitis/chronic fatigue syndrome patients. Anaerobe 2013, 22, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.V.; Macpherson, A.J. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat. Rev. Immunol. 2010, 10, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.N.; Kovacs-Nolan, J.; Jiang, B.; Tsao, R.; Mine, Y. Peptides derived from eggshell membrane improve antioxidant enzyme activity and glutathione synthesis against oxidative damage in Caco-2 cells. J. Funct. Foods 2014, 11, 571–580. [Google Scholar] [CrossRef]
- Togashi, T.; Yokoo, N.; Umetsu, M.; Ohara, S.; Naka, T.; Takami, S.; Abe, H.; Kumagai, I.; Adschiri, T. Material-binding peptide application-ZnO crystal structure control by means of a ZnO-binding peptide. J. Biosci. Bioeng. 2011, 111, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Valdovinos-Flores, C.; Gonsebatt, M.E. The role of amino acid transporters in GSH synthesis in the blood–brain barrier and central nervous system. Neurochem. Int. 2012, 61, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Kalaras, M.D.; Richie, J.P.; Calcagnotto, A.; Beelman, R.B. Mushrooms: A rich source of the antioxidants ergothioneine and glutathione. Food Chem. 2017, 233, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, E.M.; Fargier-Lagrange, M.; Mulvihill, D.M.; O’Mahony, J.A. Characterisation of heat-induced protein aggregation in whey protein isolate and the influence of aggregation on the availability of amino groups as measured by the ortho-phthaldialdehyde (OPA) and trinitrobenzenesulfonic acid (TNBS) methods. Food Chem. 2017, 229, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Asarat, M.; Vasiljevic, T.; Ravikumar, T.; Apostolopoulos, V.; Donkor, O. Extraction and purification of short-chain fatty acids from fermented reconstituted skim milk supplemented with inulin. Food Anal. Methods 2016, 9, 3069–3079. [Google Scholar] [CrossRef]
- Song, R.; Zhang, K.Q.; Wei, R.B. In vitro antioxidative activities of squid (Ommastrephes bartrami) viscera autolysates and identification of active peptides. Process Biochem. 2016, 51, 1674–1682. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]

| Group | Body Weight/g | Liver Coefficient/(g/kg) | Thymus Coefficient/(mg/10 g) | Spleen Coefficient/(mg/10 g) | |
|---|---|---|---|---|---|
| 0 Day | 14 Day | ||||
| CK | F: 19.79 ± 1.93 a | 21.10 ± 1.32 a | F: 46.41 ± 3.70 a | F: 33.34 ± 5.85 a | F: 40.49 ± 5.57 b |
| M: 22.13 ± 1.35 b | 23.93 ± 1.82 b | M: 48.33 ± 3.14 a | M: 26.01 ± 1.14 a | M: 30.44 ± 6.77 a | |
| HAHp(3.0)/ZnO NPs | F: 19.86 ± 0.83 a | 20.87 ± 1.24 a | F: 45.29 ± 5.56 a | F: 31.67 ± 7.64 a | F: 40.32 ± 6.38 b |
| M: 21.83 ± 1.59 b | 22.54 ± 2.31 ab | M: 48.29 ± 3.42 a | M: 25.50 ± 9.27 a | M: 30.28 ± 2.39 a | |
| Genus | Taxon | 14 d CK(F) | 14 d Z(F) | p |
|---|---|---|---|---|
| Bacteroidales_S24-7_group_norank | p__Bacteroidetes; c__Bacteroidia; o__Bacteroidales; f__Bacteroidales_S24-7_group; g__norank | 23.41 | 12.40 | * |
| Lactobacillus | p__Firmicutes; c__Bacilli; o__Lactobacillales; f__Lactobacillaceae; g__Lactobacillus; s__Lactobacillus_murinus | 11.34 | 15.51 | * |
| Rikenellaceae_RC9_gut_group | p__Bacteroidetes; c__Bacteroidia; o__Bacteroidales; f__Rikenellaceae; g__Rikenellaceae_RC9_gut_group; s__unidentified | 6.07 | 0.94 | * |
| Alistipes | p__Bacteroidetes; c__Bacteroidia; o__Bacteroidales; f__Rikenellaceae; g__Alistipes; s__uncultured_bacterium | 3.54 | 0.75 | * |
| Desulfovibrio | p__Proteobacteria; c__Deltaproteobacteria; o__Desulfovibrionales; f__Desulfovibrionaceae; g__Desulfovibrio; s__uncultured_bacterium | 3.29 | 3.02 | - |
| Helicobacter | p__Proteobacteria; c__Epsilonproteobacteria; o__Campylobacterales; f__Helicobacteraceae; g__Helicobacter; s__Helicobacter_ganmani | 2.94 | 0.87 | - |
| Odoribacter | p__Bacteroidetes; c__Bacteroidia; o__Bacteroidales; f__Porphyromonadaceae; g__Odoribacter; s__unidentified | 3.02 | 0.86 | * |
| Erysipelotrichaceae_unclassified | p__Firmicutes; c__Erysipelotrichia; o__Erysipelotrichales; f__Erysipelotrichaceae | 3.00 | 1.68 | * |
| Bacteroides | p__Bacteroidetes; c__Bacteroidia; o__Bacteroidales; f__Bacteroidaceae; g__Bacteroides | 4.11 | 0.31 | * |
| Acetitomaculum | p__Firmicutes; c__Clostridia; o__Clostridiales; f__Lachnospiraceae; g__Acetitomaculum; s__uncultured_bacterium | 1.81 | 0.51 | - |
| Lachnoclostridium | p__Firmicutes; c__Clostridia; o__Clostridiales; f__Lachnospiraceae; g__Lachnoclostridium | 2.19 | 2.85 | - |
| Ruminococcaceae_UCG-014 | p__Firmicutes; c__Clostridia; o__Clostridiales; f__Ruminococcaceae; g__Ruminococcaceae_UCG-014; s__unidentified | 1.71 | 7.26 | * |
| Enterorhabdus | p__Actinobacteria; c__Actinobacteria; o__Coriobacteriales; f__Coriobacteriaceae; g__Enterorhabdus; s__uncultured_bacterium | 1.67 | 2.03 | - |
| [Eubacterium]_coprostanoligenes_group | p__Firmicutes; c__Clostridia; o__Clostridiales; f__Ruminococcaceae; g__[Eubacterium]_coprostanoligenes_group | 1.58 | 0.45 | - |
| Candidatus_Saccharimonas | p__Saccharibacteria; c__Unknown_Class; o__Unknown_Order; f__Unknown_Family; g__Candidatus_Saccharimonas; s__uncultured_bacterium | 1.70 | 2.69 | * |
| Lachnospiraceae_uncultured | p__Firmicutes; c__Clostridia; o__Clostridiales; f__Lachnospiraceae; g__Lachnoclostridium | 1.34 | 1.15 | - |
| Lachnospiraceae_unclassified | p__Firmicutes; c__Clostridia; o__Clostridiales; f__Lachnospiraceae; g__Lachnoclostridium | 1.19 | 3.62 | - |
| Rikenella | p__Bacteroidetes; c__Bacteroidia; o__Bacteroidales; f__Rikenellaceae; g__Rikenella; s__Rikenella_microfusus_DSM_15922 | 1.28 | 0.38 | - |
| Streptococcus | p__Firmicutes; c__Bacilli; o__Lactobacillales; f__Streptococcaceae; g__Streptococcus; s__Streptococcus_acidominimus | 1.04 | 0.30 | * |
| Roseburia | p__Firmicutes; c__Clostridia; o__Clostridiales; f__Lachnospiraceae; g__Roseburia | 1.54 | 2.40 | - |
| Ruminococcaceae_UCG-013 | p__Firmicutes; c__Clostridia; o__Clostridiales; f__Ruminococcaceae; g__Ruminococcaceae_UCG-013 | 1.01 | 2.44 | * |
| Coriobacteriaceae_unclassified | p__Actinobacteria; c__Actinobacteria; o__Coriobacteriales; f__Coriobacteriaceae | 0.88 | 0.73 | - |
| Lachnospiraceae_UCG-006 | p__Firmicutes; c__Clostridia; o__Clostridiales; f__Lachnospiraceae; g__Lachnospiraceae_UCG-006; s__uncultured_bacterium | 0.81 | 1.52 | - |
| [Eubacterium]_xylanophilum_group | p__Firmicutes; c__Clostridia; o__Clostridiales; f__Lachnospiraceae; g__[Eubacterium]_xylanophilum_group; s__uncultured_bacterium | 0.85 | 1.43 | - |
| Ruminococcaceae_UCG-010 | p__Firmicutes; c__Clostridia; o__Clostridiales; f__Ruminococcaceae; g__Ruminococcaceae_UCG-010; s__unidentified | 0.84 | 0.69 | - |
| Aerococcus | p__Firmicutes; c__Bacilli; o__Lactobacillales; f__Aerococcaceae; g__Aerococcus | 1.07 | 3.29 | * |
| Mollicutes_RF9_norank | p__Tenericutes; c__Mollicutes; o__Mollicutes_RF9; f__norank; g__norank | 0.47 | 0.69 | - |
| Lachnospiraceae_NK4A136_group | p__Firmicutes; c__Clostridia; o__Clostridiales; f__Lachnospiraceae; g__Lachnospiraceae_NK4A136_group | 0.47 | 1.54 | - |
| Ruminococcus_1 | p__Firmicutes; c__Clostridia; o__Clostridiales; f__Ruminococcaceae; g__Ruminococcus_1; s__unidentified | 0.45 | 1.54 | - |
| Staphylococcus | p__Firmicutes; c__Bacilli; o__Bacillales; f__Staphylococcaceae; g__Staphylococcus | 0.43 | 1.20 | * |
| Vibrio | p__Proteobacteria; c__Gammaproteobacteria; o__Vibrionales; f__Vibrionaceae; g__Vibrio | 0.22 | 2.02 | * |
| Ruminococcaceae_unclassified | p__Firmicutes; c__Clostridia; o__Clostridiales; f__Ruminococcaceae | 0.19 | 0.96 | * |
| Ruminiclostridium_5 | p__Firmicutes; c__Clostridia; o__Clostridiales; f__Ruminococcaceae; g__Ruminiclostridium_5 | 0.16 | 0.89 | * |
| Marvinbryantia | p__Firmicutes; c__Clostridia; o__Clostridiales; f__Lachnospiraceae; g__Marvinbryantia | 0.15 | 1.45 | * |
| Lachnospiraceae_UCG-001 | p__Firmicutes; c__Clostridia; o__Clostridiales; f__Lachnospiraceae; g__Lachnospiraceae_UCG-001; s__unidentified | 0.13 | 0.62 | - |
| Faecalibaculum | p__Firmicutes; c__Erysipelotrichia; o__Erysipelotrichales; f__Erysipelotrichaceae; g__Faecalibaculum; s__uncultured_bacterium | 0.13 | 5.95 | * |
| Coprococcus_3 | p__Firmicutes; c__Clostridia; o__Clostridiales; f__Lachnospiraceae; g__Coprococcus_3 | 0.07 | 0.87 | * |
| Bifidobacterium | p__Actinobacteria; c__Actinobacteria; o__Bifidobacteriales; f__Bifidobacteriaceae; g__Bifidobacterium | 0.01 | 0.53 | * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, R.; Yao, J.; Shi, Q.; Wei, R. Nanocomposite of Half-Fin Anchovy Hydrolysates/Zinc Oxide Nanoparticles Exhibits Actual Non-Toxicity and Regulates Intestinal Microbiota, Short-Chain Fatty Acids Production and Oxidative Status in Mice. Mar. Drugs 2018, 16, 23. https://doi.org/10.3390/md16010023
Song R, Yao J, Shi Q, Wei R. Nanocomposite of Half-Fin Anchovy Hydrolysates/Zinc Oxide Nanoparticles Exhibits Actual Non-Toxicity and Regulates Intestinal Microbiota, Short-Chain Fatty Acids Production and Oxidative Status in Mice. Marine Drugs. 2018; 16(1):23. https://doi.org/10.3390/md16010023
Chicago/Turabian StyleSong, Ru, Jianbin Yao, Qingqing Shi, and Rongbian Wei. 2018. "Nanocomposite of Half-Fin Anchovy Hydrolysates/Zinc Oxide Nanoparticles Exhibits Actual Non-Toxicity and Regulates Intestinal Microbiota, Short-Chain Fatty Acids Production and Oxidative Status in Mice" Marine Drugs 16, no. 1: 23. https://doi.org/10.3390/md16010023
APA StyleSong, R., Yao, J., Shi, Q., & Wei, R. (2018). Nanocomposite of Half-Fin Anchovy Hydrolysates/Zinc Oxide Nanoparticles Exhibits Actual Non-Toxicity and Regulates Intestinal Microbiota, Short-Chain Fatty Acids Production and Oxidative Status in Mice. Marine Drugs, 16(1), 23. https://doi.org/10.3390/md16010023