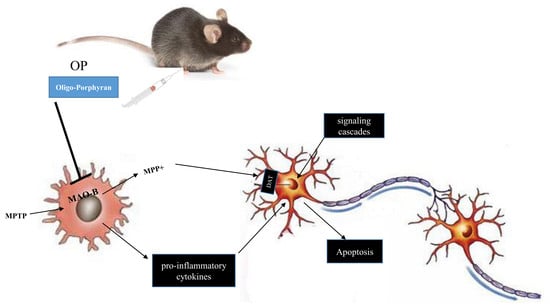
Oligo-Porphyran Ameliorates Neurobehavioral Deficits in Parkinsonian Mice by Regulating the PI3K/Akt/Bcl-2 Pathway
Abstract
:1. Introduction
2. Results
2.1. Analysis of OP
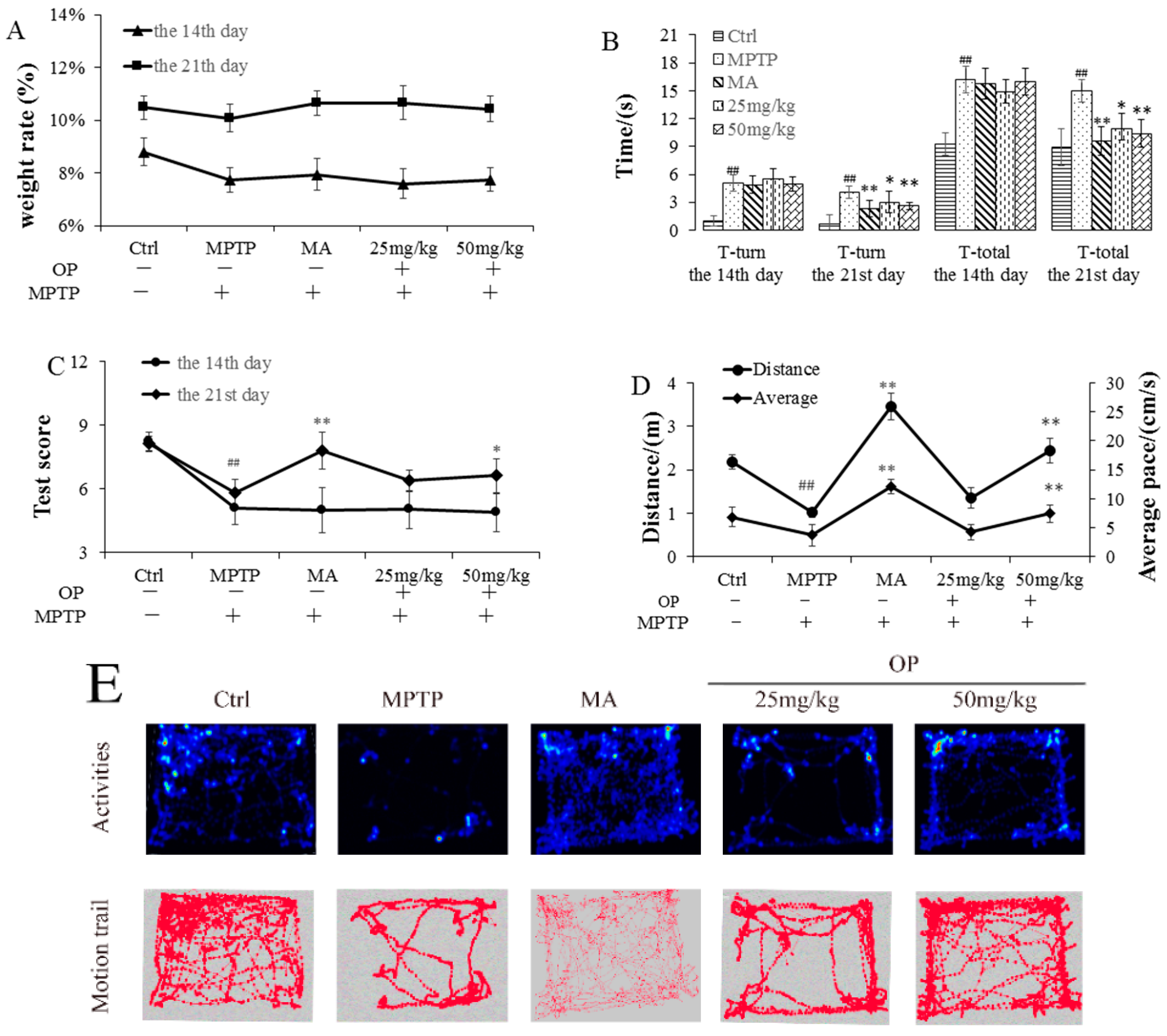
2.2. Effects on Behavioral Patterns
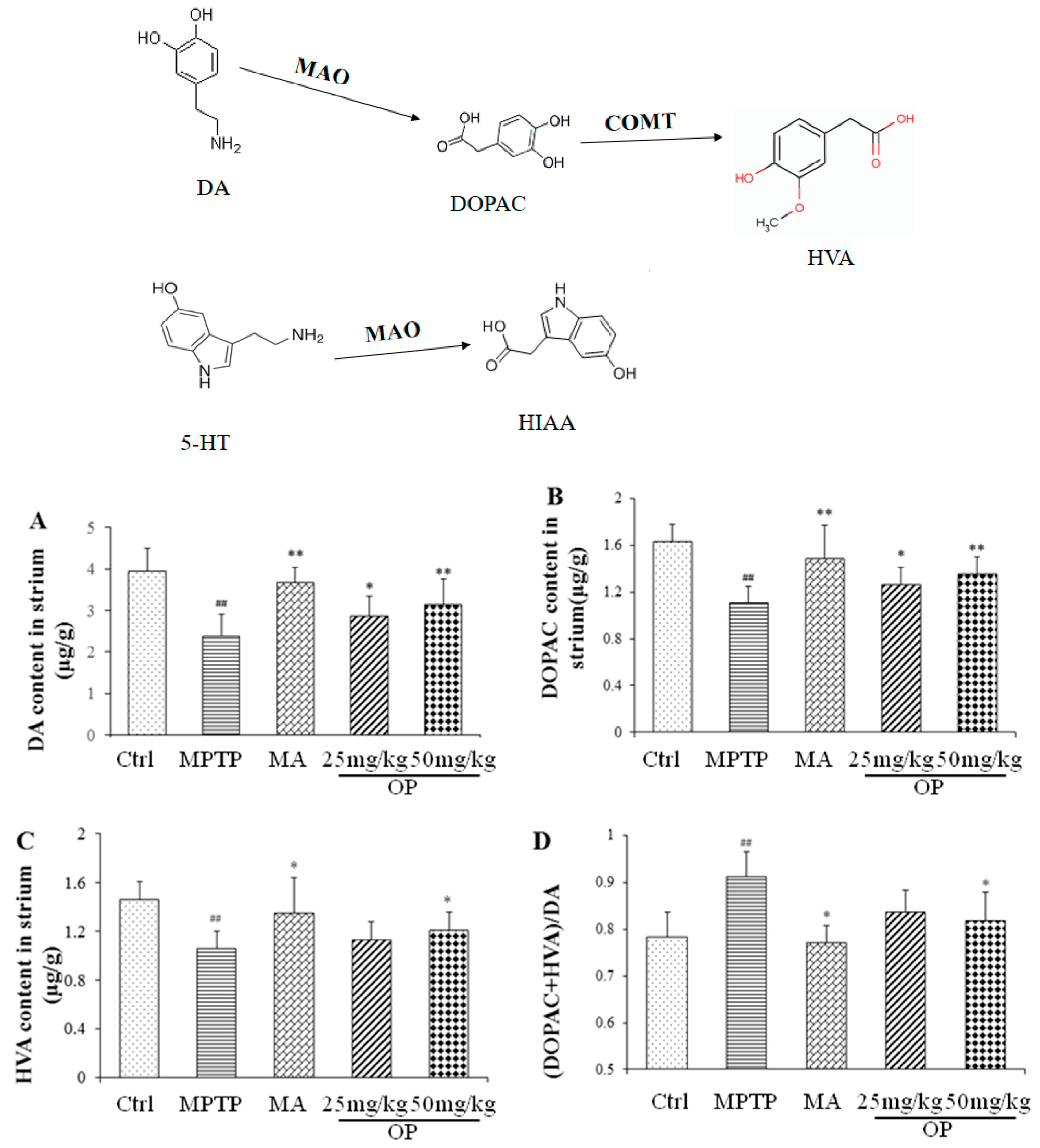
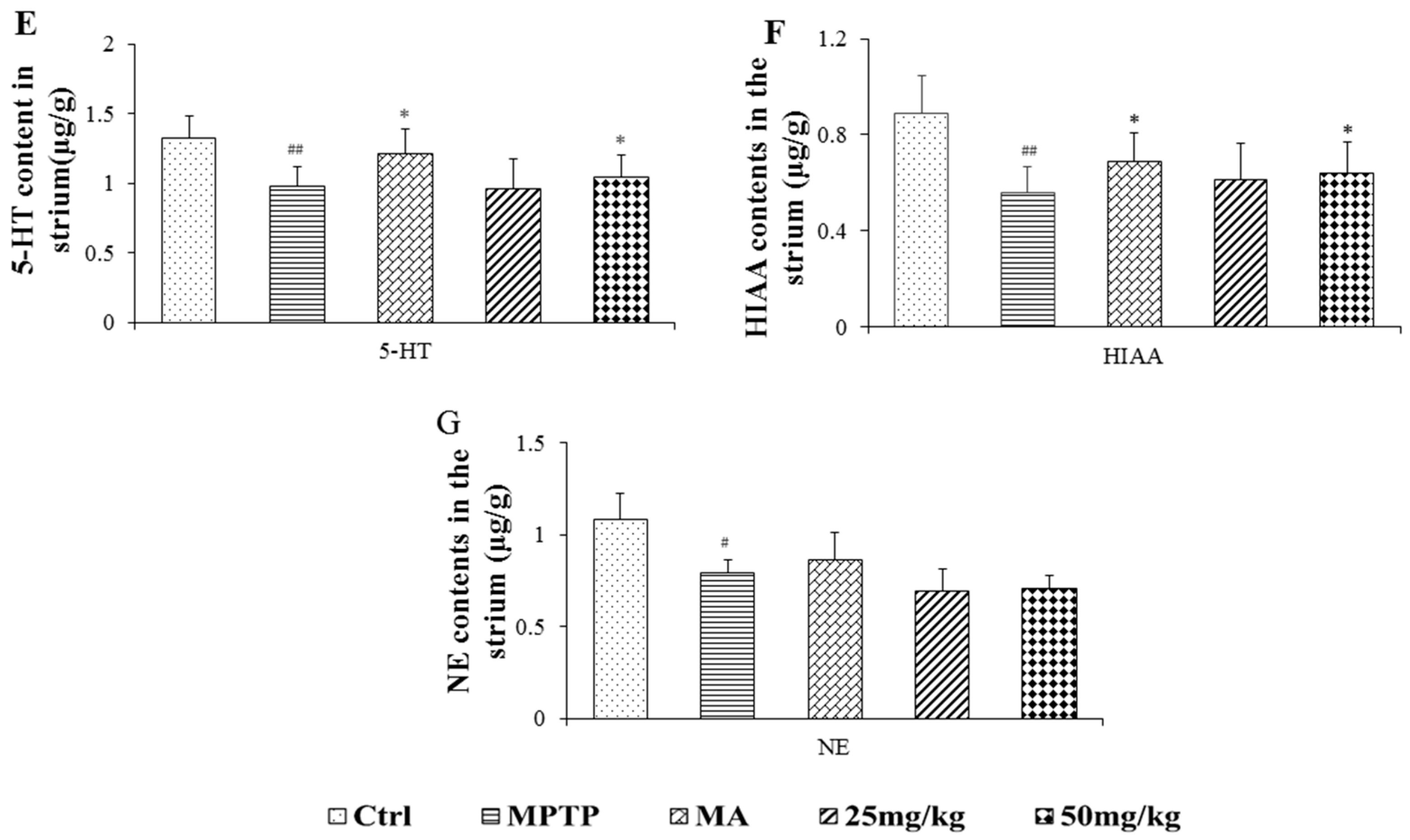
2.3. Effects on Quantification of DA, Norepinephrine, 5-Hydroxytryptamine, and Their Metabolites
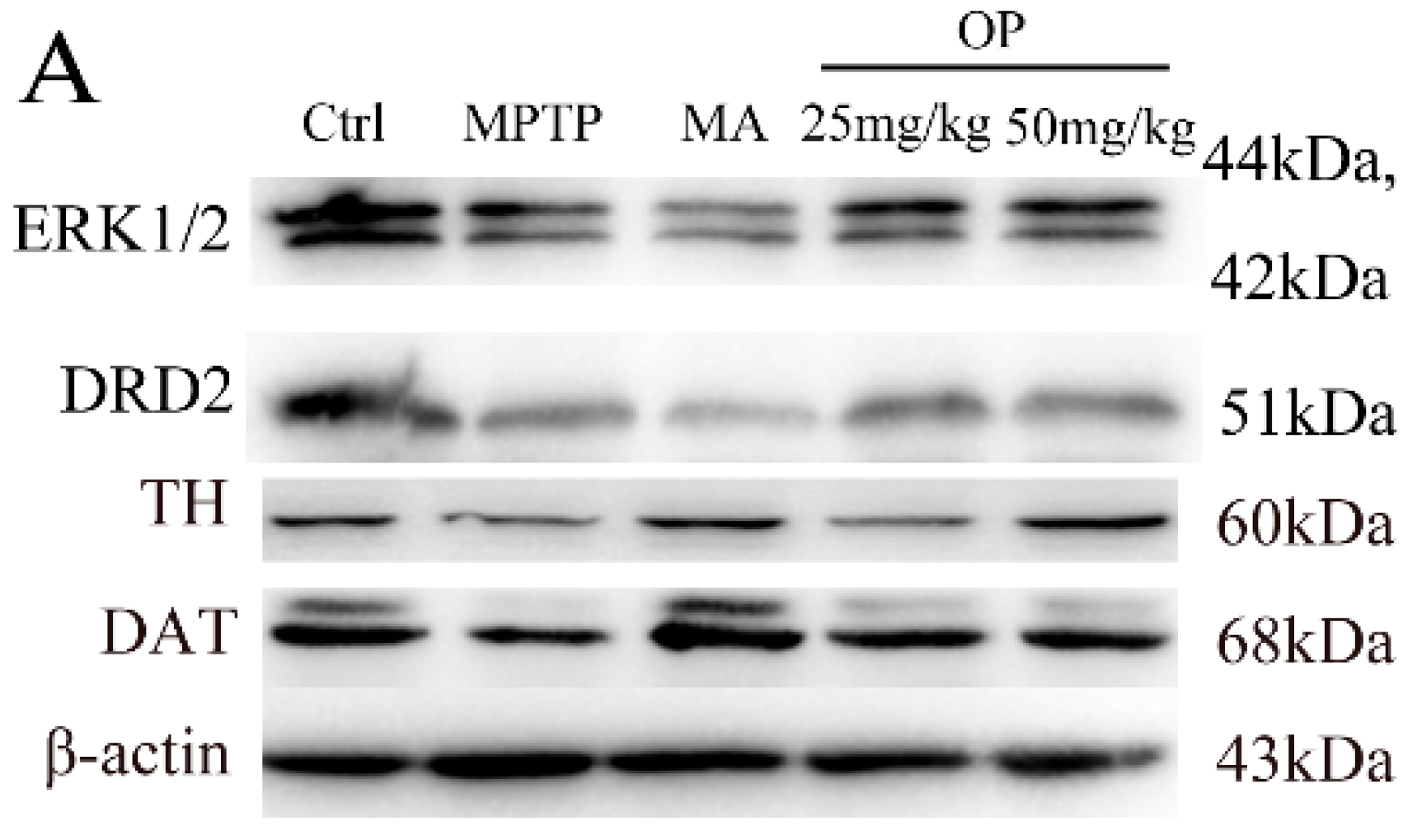
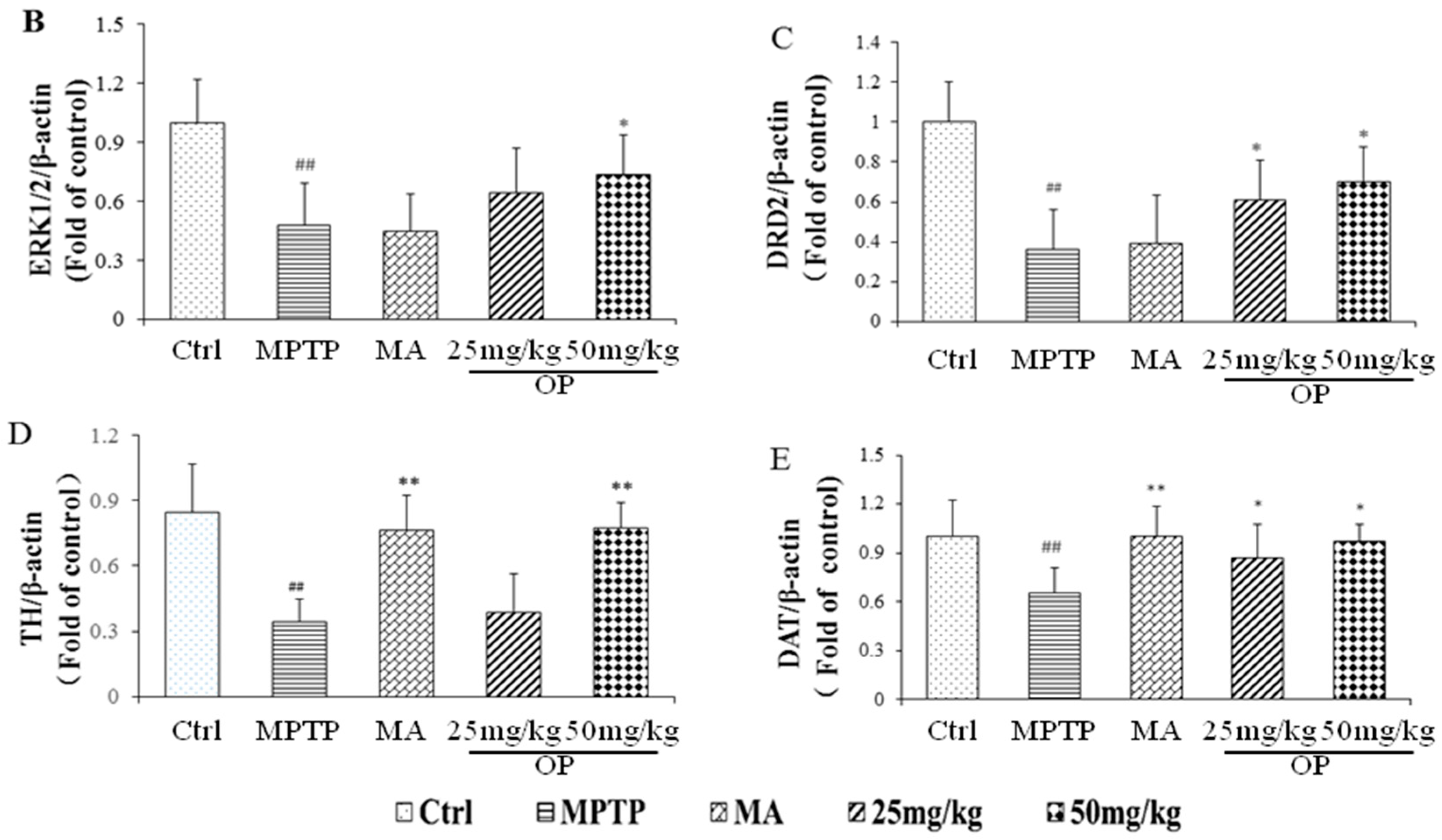
2.4. Effects on Expression of Extracellular Signal-Regulated Protein Kinases 1 and 2, Dopamine Receptor D2, Dopamine Receptor D2, and Dopamine Transporter
2.5. Effects on PI3K, Akt, and GSK 3β Expression
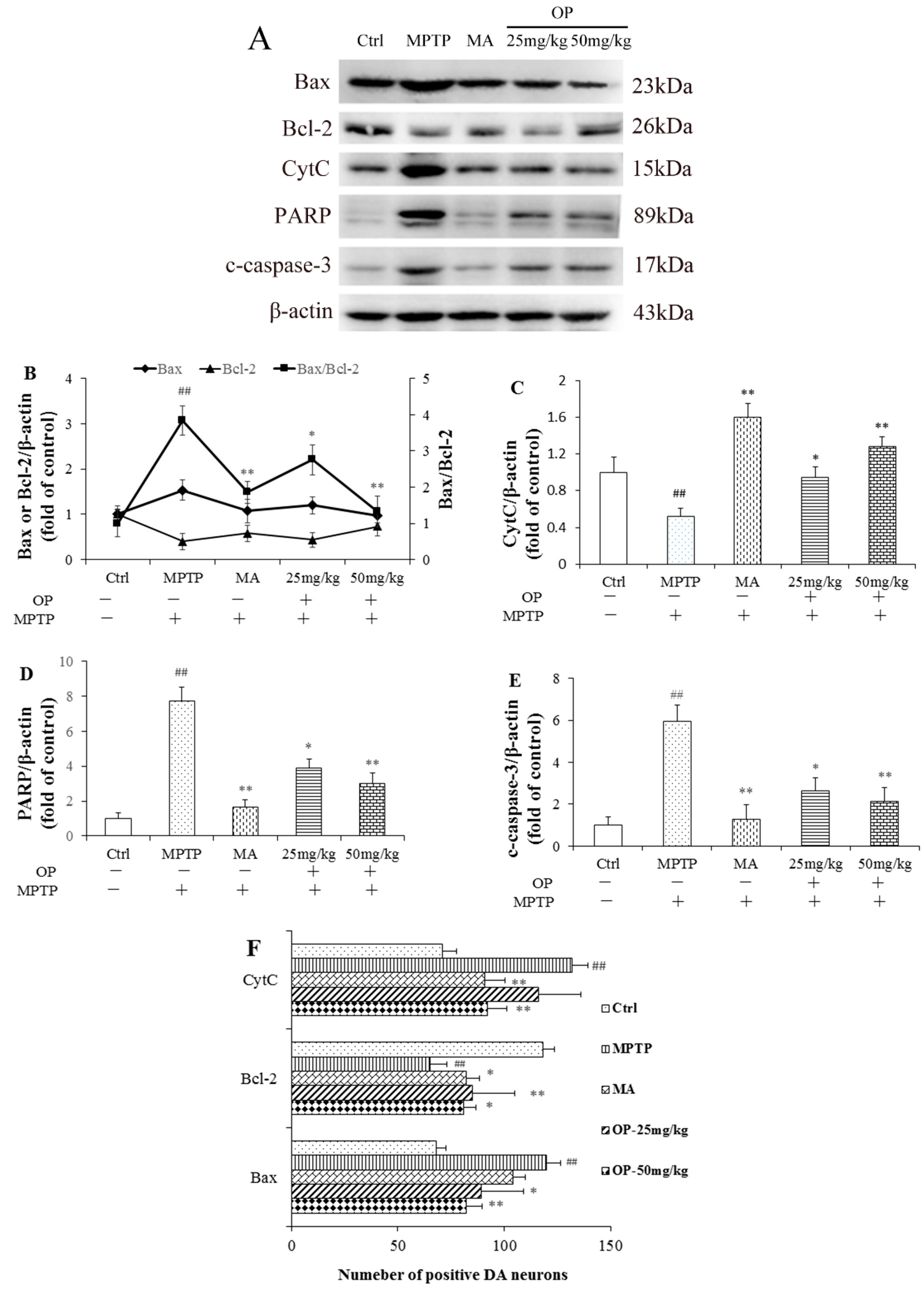
2.6. Effects on Bax/Bcl-2, CytC, Poly ADP Ribose Polymerase, and Cleaved Caspase-3 Levels
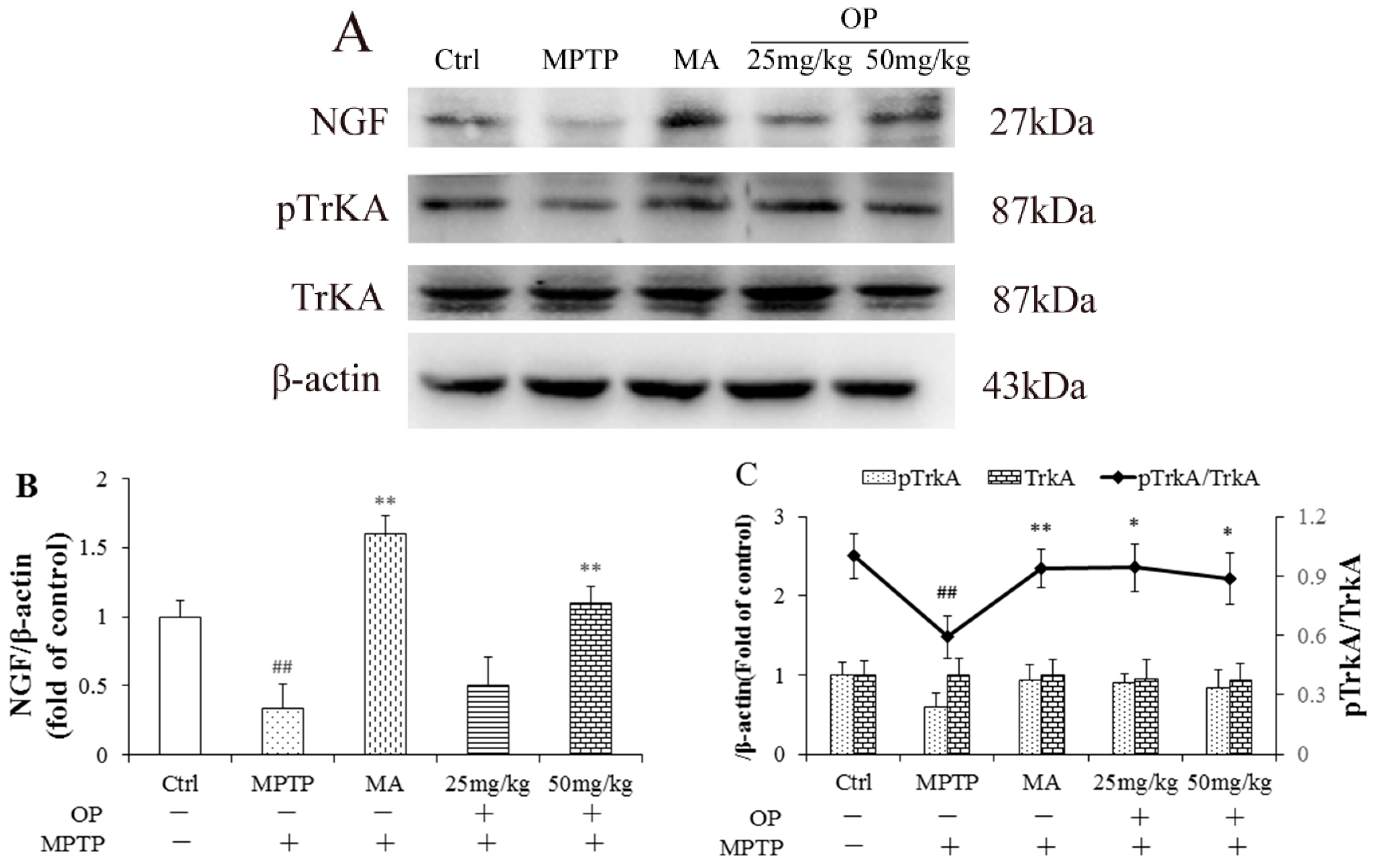
2.7. Effects on Nerve Growth Factor and Tropomyosin Receptor Kinase A Levels
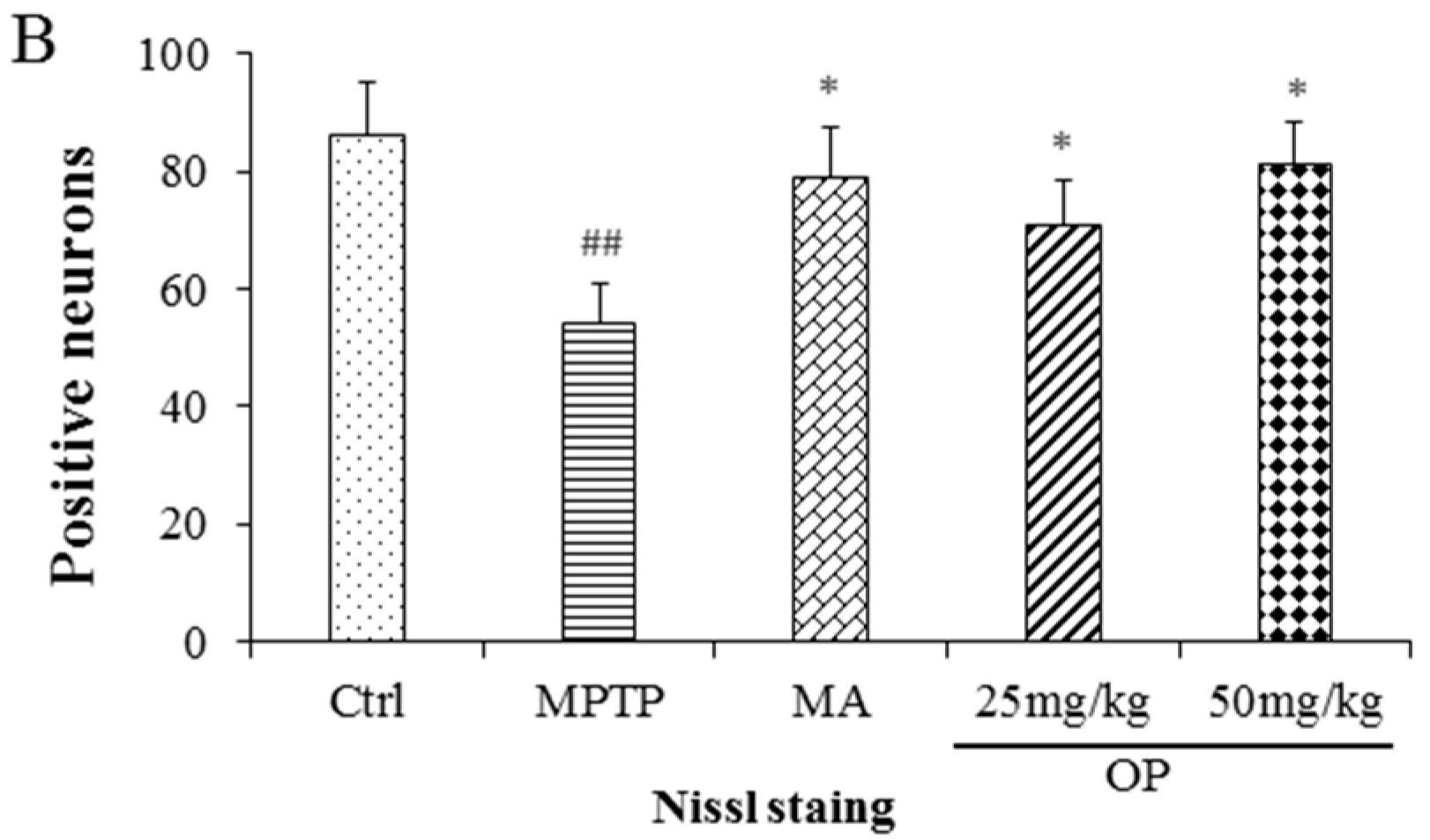
2.8. Effects on Dopaminergic Neuronal Loss
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Preparation and Analysis of OP
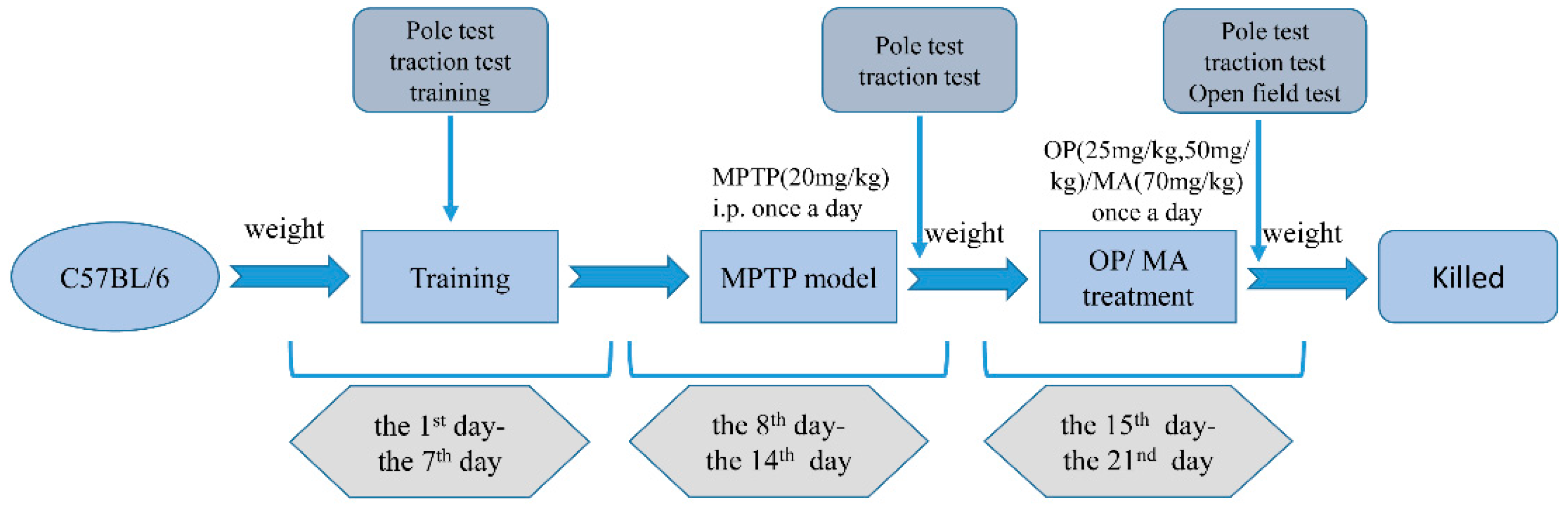
4.3. Animal Treatment
4.4. Behavioral Evaluation
4.4.1. Open Field Test
4.4.2. Pole Test
4.4.3. Traction Test
4.5. Collection of Brain Tissue
4.6. HPLC Analysis of DA, 5-HT, NE, and Their Metabolites
4.7. Western Blotting Analysis
4.8. Immunohistochemistry
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zheng, M.; Liu, C.; Fan, Y.; Yan, P.; Shi, D.; Zhang, Y. Neuroprotection by Paeoniflorin in the MPTP mouse model of Parkinson’s disease. Neuropharmacology 2017, 116, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Olanow, C.W.; Schapira, A.H. Therapeutic prospects for Parkinson disease. Ann. Neurol. 2013, 74, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Shulman, J.M.; De Jager, P.L.; Feany, M.B. Parkinson’s disease: Genetics and pathogenesis. Annu. Rev. Pathol. 2011, 6, 193–222. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Oroz, M.C.; Jahanshahi, M.; Krack, P.; Litvan, I.; Macias, R.; Bezard, E.; Obeso, J.A. Initial clinical manifestations of Parkinson’s disease: Features and pathophysiological mechanisms. Lancet Neurol. 2009, 8, 1128–1139. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, L.; Chen, L.; Hao, D.; Chen, J. Neuroprotection by tetrahydroxystilbene glucoside in the MPTP mouse model of Parkinson’s disease. Toxicol. Lett. 2013, 222, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, H.; Zhang, X.; Li, X.; Geng, L.; Zhang, H.; Zhang, Q. Sulfated Hetero-Polysaccharides Protect SH-SY5Y Cells from H2O2-Induced Apoptosis by Affecting the PI3K/Akt Signaling Pathway. Mar. Drugs 2017, 15, 110. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.Y.; Kim, D.Y. Treadmill exercise improves motor and memory functions in cerebral palsy rats through activation of PI3K-Akt pathway. J. Exerc. Rehabil. 2017, 13, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Qin, L.; Huang, F.; Wang, X.; Yang, L.; Shi, H.; Wu, H.; Zhang, B.; Chen, Z.; Wu, X. Amentoflavone protects dopaminergic neurons in MPTP-induced Parkinson’s disease model mice through PI3K/Akt and ERK signaling pathways. Toxicol. Appl. Pharmacol. 2017, 319, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Scuruchi, M.; D’Ascola, A.; Avenoso, A.; Campana, S.; Abusamra, Y.A.; Spina, E.; Calatroni, A.; Campo, G.M.; Campo, S. 6-Mer Hyaluronan Oligosaccharides Modulate Neuroinflammation and alpha-Synuclein Expression in Neuron-Like SH-SY5Y Cells. J. Cell. Biochem. 2016, 117, 2835–2843. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Wang, W.; Wang, S.; Zhang, L.; Guo, Y. An Overview of the Protective Effects of Chitosan and Acetylated Chitosan Oligosaccharides against Neuronal Disorders. Mar. Drugs 2017, 15, 89. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.-W.; Du, X.-G.; Zhang, X.; Wang, X.; Hu, D.-Y.; Meng, T.; Chen, Y.-L.; Geng, M.-Y.; Shen, J.-K. Synthesis and bioassay of β-(1,4)-d-mannans as potential agents against Alzheimer’s disease. Acta Pharmacol. Sin. 2013, 34, 1585–1591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Qi, H.; Zhao, T.; Deslandes, E.; Ismaeli, N.M.; Molloy, F.; Critchley, A.T. Chemical characteristics of a polysaccharide from Porphyra capensis (Rhodophyta). Carbohydr. Res. 2005, 340, 2447–2450. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hou, Y.; Duan, D.; Zhang, Q. The Structure and Nephroprotective Activity of Oligo-Porphyran on Glycerol-Induced Acute Renal Failure in Rats. Mar. Drugs 2017, 15, 135. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wang, S.; Yao, C.; Xu, Z.; Xu, X. Hypolipidemic effect of porphyran extracted from Pyropia yezoensis in ICR mice with high fatty diet. J. Appl. Phycol. 2015, 28, 1315–1322. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Xiao, L.; Liu, C.; Qi, H.; Zhang, Z. In vivo antihyperlipidemic and antioxidant activity of porphyran in hyperlipidemic mice. Carbohydr. Polym. 2017, 174, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Isaka, S.; Cho, K.; Nakazono, S.; Abu, R.; Ueno, M.; Kim, D.; Oda, T. Antioxidant and anti-inflammatory activities of porphyran isolated from discolored nori (Porphyra yezoensis). Int. J. Biol. Macromol. 2015, 74, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.K.; Seth, B.; Agarwal, S.; Yadav, A.; Karmakar, M.; Gupta, S.K.; Choubey, V.; Sharma, A.; Chaturvedi, R.K. Ethosuximide Induces Hippocampal Neurogenesis and Reverses Cognitive Deficits in an Amyloid-beta Toxin-induced Alzheimer Rat Model via the Phosphatidylinositol 3-Kinase (PI3K)/Akt/Wnt/beta-Catenin Pathway. J. Biol. Chem. 2015, 290, 28540–28558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, G.; Wu, Y.; Sha, H.; Zhang, P.; Jia, J. BDNF promotes EGF-induced proliferation and migration of human fetal neural stem/progenitor cells via the PI3K/Akt pathway. Molecules 2011, 16, 10146–10156. [Google Scholar] [CrossRef] [PubMed]
- Fournier, N.M.; Lee, B.; Banasr, M.; Elsayed, M.; Duman, R.S. Vascular endothelial growth factor regulates adult hippocampal cell proliferation through MEK/ERK- and PI3K/Akt-dependent signaling. Neuropharmacology 2012, 63, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Maiti, P.; Gregg, L.C.; McDonald, M.P. MPTP-induced executive dysfunction is associated with altered prefrontal serotonergic function. Behav. Brain Res. 2016, 298, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Zare, K.; Eidi, A.; Roghani, M.; Rohani, A.H. The neuroprotective potential of sinapic acid in the 6-hydroxydopamine-induced hemi-parkinsonian rat. Metab. Brain Dis. 2015, 30, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Y.; Liu, J.-Y.; Yang, C.-B.; Malampati, S.; Huang, Y.-Y.; Li, M.-X.; Li, M.; Song, J.-X. Neuroprotective Natural Products for the Treatment of Parkinson’s Disease by Targeting the Autophagy-Lysosome Pathway: A Systematic Review. Phytother. Res. 2017, 31, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Brown, A.; Fisher, D.; Wu, Y.; Warren, J.; Cui, X. Tissue Specific Expression of Cre in Rat Tyrosine Hydroxylase and Dopamine Active Transporter-Positive Neurons. PLoS ONE 2016, 11, e0149379. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Liu, F. A peptide disrupting the D2R-DAT interaction protects against dopamine neurotoxicity. Exp. Neurol. 2017, 295, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Jiang, B.-H.; Yang, P.-H.; Cao, Z.; Shi, X.; Lin, M.C.M.; He, M.-L.; Kung, H.-F. Phosphatidylinositol 3-Kinase Signaling Is Involved in Neurogenesis during Xenopus Embryonic Development. J. Biol. Chem. 2004, 279, 28509–28514. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.; Zhang, L.X.; Sun, X.Y.; Ding, J.H.; Lu, M.; Hu, G. Caspase-1 Deficiency Alleviates Dopaminergic Neuronal Death via Inhibiting Caspase-7/AIF Pathway in MPTP/p Mouse Model of Parkinson’s Disease. Mol. Neurobiol. 2017, 54, 4292–4302. [Google Scholar] [CrossRef] [PubMed]
- Tatton, W.G.; Chalmers-Redman, R.; Brown, D.; Tatton, N. Apoptosis in Parkinson’s disease: Signals for neuronal degradation. Ann. Neurol. 2003, 53, S61–S72. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zu, G.; Zhang, X.; Wang, X.; Li, S.; Gong, X.; Liang, Z.; Zhao, J. Neuroprotective effects of ginsenoside Rg1 through the Wnt/beta-catenin signaling pathway in both in vivo and in vitro models of Parkinson’s disease. Neuropharmacology 2016, 101, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Li, L.; Holscher, C. Neuroprotective effects of (Val8)GLP-1-Glu-PAL in the MPTP Parkinson’s disease mouse model. Behav. Brain Res. 2015, 293, 107–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, Y.; Wang, J.; Simerly, T.; Jin, W.; Zhang, H.; Zhang, Q. Hydrogen peroxide released from Pyropia yezoensis induced by oligo-porphyrans: Mechanisms and effect. J. Appl. Phycol. 2014, 27, 1639–1649. [Google Scholar] [CrossRef]
- Hu, X.; Song, Q.; Li, X.; Li, D.; Zhang, Q.; Meng, W.; Zhao, Q. Neuroprotective effects of Kukoamine A on neurotoxin-induced Parkinson’s model through apoptosis inhibition and autophagy enhancement. Neuropharmacology 2017, 117, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Dekker, A.D.; De Deyn, P.P.; Rots, M.G. Epigenetics: The neglected key to minimize learning and memory deficits in Down syndrome. Neurosci. Biobehav. Rev. 2014, 45, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, D.; Vermeiren, Y.; Aerts, T.; De Deyn, P.P. Novel and sensitive reversed-phase high-pressure liquid chromatography method with electrochemical detection for the simultaneous and fast determination of eight biogenic amines and metabolites in human brain tissue. J. Chromatogr. A 2014, 1353, 28–39. [Google Scholar] [CrossRef] [PubMed]


| Peak | Structure |
|---|---|
| 421.07, z = 1 | [Gal2-SO3H-H]− |
| 583.12, z = 1 | [Gal3-SO3H-H]− |
| 412.06, z = 2 | [Gal4-(SO3H)2-2H]2− |
| 493.09, z = 2 | [Gal5-(SO3H)2-2H]2− |
| 409.06, z = 3 | [Gal6-(SO3H)3-3H]3− |
| 463.08, z = 3 | [Gal7-(SO3H)3-3H]3− |
| 517.09, z = 3 | [Gal8-(SO3H)3-3H]3− |
| Antibody | Host | Application | Source | Dilutions |
|---|---|---|---|---|
| NGF | Rabbit | WB | Affinity Biosciences | 1:1000 |
| TH | Rabbit | WB | Affinity Biosciences | 1:1000 |
| DRD2 | Rabbit | WB | Affinity Biosciences | 1:1000 |
| PARP | Rabbit | WB | Affinity Biosciences | 1:1000 |
| ERK1/2 | Rabbit | WB | Affinity Biosciences | 1:1000 |
| pTrkA | Rabbit | WB/IHC | Affinity Biosciences | 1:1000 |
| TrkA | Rabbit | WB | Affinity Biosciences | 1:1000 |
| pAkt | Rabbit | WB/IHC | Affinity Biosciences | 1:1000/1:200 |
| Akt | Rabbit | WB/IHC | Affinity Biosciences | 1:1000 |
| pGSK 3β | Rabbit | WB/IHC | Affinity Biosciences | 1:1000 |
| GSK 3β | Rabbit | WB/IHC | Affinity Biosciences | 1:1000 |
| pPI3K | Rabbit | WB/IHC | Affinity Biosciences | 1:1000/1:200 |
| PI3K | Rabbit | WB/IHC | Affinity Biosciences | 1:1000 |
| Bax | Rabbit | WB/IHC | Affinity Biosciences | 1:1000/1:200 |
| Bcl-2 | Mouse | WB/IHC | Affinity Biosciences | 1:1000/1:200 |
| c-caspase-3 | Rabbit | WB | Abcam | 1:1000 |
| CytC | Rabbit | WB/IHC | Affinity Biosciences | 1:1000/1:200 |
| β-actin | Rabbit | WB | Affinity Biosciences | 1:2000 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Geng, L.; Zhang, J.; Wang, J.; Zhang, Q.; Duan, D.; Zhang, Q. Oligo-Porphyran Ameliorates Neurobehavioral Deficits in Parkinsonian Mice by Regulating the PI3K/Akt/Bcl-2 Pathway. Mar. Drugs 2018, 16, 82. https://doi.org/10.3390/md16030082
Liu Y, Geng L, Zhang J, Wang J, Zhang Q, Duan D, Zhang Q. Oligo-Porphyran Ameliorates Neurobehavioral Deficits in Parkinsonian Mice by Regulating the PI3K/Akt/Bcl-2 Pathway. Marine Drugs. 2018; 16(3):82. https://doi.org/10.3390/md16030082
Chicago/Turabian StyleLiu, Yingjuan, Lihua Geng, Jingjing Zhang, Jing Wang, Qi Zhang, Delin Duan, and Quanbin Zhang. 2018. "Oligo-Porphyran Ameliorates Neurobehavioral Deficits in Parkinsonian Mice by Regulating the PI3K/Akt/Bcl-2 Pathway" Marine Drugs 16, no. 3: 82. https://doi.org/10.3390/md16030082
APA StyleLiu, Y., Geng, L., Zhang, J., Wang, J., Zhang, Q., Duan, D., & Zhang, Q. (2018). Oligo-Porphyran Ameliorates Neurobehavioral Deficits in Parkinsonian Mice by Regulating the PI3K/Akt/Bcl-2 Pathway. Marine Drugs, 16(3), 82. https://doi.org/10.3390/md16030082