Dual BACE1 and Cholinesterase Inhibitory Effects of Phlorotannins from Ecklonia cava—An In Vitro and in Silico Study
Abstract
:1. Introduction
2. Results
2.1. In Vitro Inhibitory Study of Phlorotannins on BACE1 and AChE
2.2. Kinetic Study of BACE1 and AChE Inhibition
2.3. In Silico Docking Study of the Inhibition of BACE1 and AChE by Phlorotannins
3. Discussion
4. Materials and Methods
4.1. General
4.2. Enzyme inhibition Studies
4.3. Enzyme Kinetic Study
4.4. Molecular Docking Study
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Selkoe, D. Alzheimer’s disease results from the cerebral accumulation and cytotoxicity of amyloid beta-protein. J. Alzheimers Dis. 2001, 3, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Götz, J.; Schild, A.; Hoerndli, F.; Pennanen, L. Amyloid-induced neurofibrillary tangle formation in Alzheimer’s disease: Insight from transgenic mouse and tissue-culture models. Int. J. Dev. Neurosci. 2004, 7, 453–465. [Google Scholar]
- Lin, G.; Koelsch, S.; Dashti, A. Human aspartic protease memapsin 2 cleaves the β-secretase site of Aβ-amyloid precursor protein. Proc. Natl. Acad. Sci. USA 2000, 97, 1456–1460. [Google Scholar] [CrossRef] [PubMed]
- Bittner, T.; Fuhrmann, M.; Burgold, S.; Jung, C.; Volbracht, C.; Steiner, H.; Mitteregger, G.; Kretzschmar, H.; Haass, C.; Herms, J. Gamma-secretase inhibition reduces spine density in vivo via an amyloid precursor protein-dependent pathway. J. Neurosci. 2009, 29, 10405–10409. [Google Scholar] [CrossRef] [PubMed]
- De Strooper, B. Lessons from a failed γ-secretase Alzheimer trial. Cell 2014, 159, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Peters, F.; Salihoglu, H.; Rodrigues, E.; Herzog, E.; Blume, T.; Filser, S.; Dorostkar, M.; Shimshek, D.; Brose, N.; Neumann, U.; et al. BACE1 inhibition more effectively suppresses initiation than progression of β-amyloid pathology. Acta Neuropathol. 2018, 135, 695–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neumann, U.; Rueeger, H.; Machauer, R.; Veenstra, S.; Lueoend, R.; Tintelnot-Blomley, M.; Laue, G.; Beltz, K.; Vogg, B.; Schmid, P.; et al. A novel BACE inhibitor NB-360 shows a superior pharmacological profile and robust reduction of amyloid-β and neuroinflammation in APP transgenic mice. Mol. Neurodegenr. 2015, 10, 44–58. [Google Scholar] [CrossRef]
- Thakker, D.; Sankaranarayanan, S.; Weatherspoon, M.; Harrison, J.; Pierdomenico, M.; Heisel, J.; Pierdomenico, M.; Heisel, J.; Thompson, L.; Haskell, R.; et al. Centrally delivered BACE1 inhibitor activates microglia and reverses amyloid pathology and cognitive deficit in aged Tg2576 mice. J. Neurosci. 2015, 35, 6931–6936. [Google Scholar] [CrossRef]
- Kennedy, M.; Stamford, A.; Chen, X.; Cox, K.; Cumming, J.; Dockendorf, M.; Egan, M.; Ereshefsky, L.; Hodgson, R.; Hyde, L.; et al. The BACE1 inhibitor verubecestat (MK-8931) reduces CNS β-amyloid in animal models and in Alzheimer’s disease patients. Sci. Transl. Med. 2016, 8, 363ra150. [Google Scholar] [CrossRef]
- Cheret, C.; Willem, M.; Fricker, F.; Wende, H.; Wulf-Goldenberg, A.; Tahirovic, S.; Nave, K.; Saftig, P.; Haass, C.; Garratt, A.; et al. Bace1 and Neuregulin-1 cooperate to control formation and maintenance of muscle spindles. EMBO J. 2013, 32, 2015–2028. [Google Scholar] [CrossRef] [Green Version]
- Filser, S.; Ovsepian, S.; Masana, M.; Blazquez-Llorca, L.; Brandt Elvang, A.; Volbracht, C.; Müller, M.; Jung, C.; Herms, J. Pharmacological inhibition of BACE1 impairs synaptic plasticity and cognitive functions. Biol. Psychiatry. 2015, 77, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Xiang, X.; Filser, S.; Marinković, P.; Dorostkar, M.; Crux, S.; Neumann, U.; Shimshek, D.; Rammes, G.; Haass, C.; et al. Beta-site amyloid precursor protein cleaving enzyme 1 inhibition impairs synaptic plasticity via seizure protein 6. Biol. Psychiatry 2018, 83, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Kisialiou, A.; Lamonaca, P.; Moroni, R.; Prinzi, G.; Fini, M. New drugs from marine organisms in alzheimer’s disease. Mar. Drugs 2016, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Rees, T.; Hammond, P.; Soreq, H.; Younkin, S.; Brimijoin, S. Acetylcholinesterase promotes beta-amyloid plaques in cerebral cortex. Neurobiol. Aging 2003, 24, 777–787. [Google Scholar] [CrossRef]
- Inestrosa, N.; Dinamarca, M.; Alvarez, A. Amyloid–cholinesterase interactions. FEBS J. 2008, 275, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Dinamarca, M.; Sagal, J.; Quintanilla, R.; Godoy, J.; Arrázola, M.; Inestrosa, N. Amyloid-beta-Acetylcholinesterase complexes potentiate neurodegenerative changes induced by the Abeta peptide. Implications for the pathogenesis of Alzheimer’s disease. Mol. Neurodegener. 2010, 5, 4–19. [Google Scholar] [CrossRef] [PubMed]
- Prado-Prado, F.; García, I. Review of theoretical studies for prediction of neurodegenerative inhibitors. Mini Rev. Med. Chem. 2012, 12, 452–466. [Google Scholar] [CrossRef]
- Folch, J.; Petrov, D.; Ettcheto, M.; Abad, S.; Sánchez-López, E.; García, M.; Olloquequi, J.; Beas-Zarate, C.; Auladell, C.; Camins, A. Current research therapeutic strategies for Alzheimer’s disease treatment. Neural Plast. 2016, 2016, 8501693. [Google Scholar] [CrossRef]
- Kang, I.; Jeon, Y.; Yin, X.; Nam, J.; You, S.; Hong, M.; Jang, B.; Kim, M. Butanol extract of Ecklonia cava prevents production and aggregation of beta-amyloid, and reduces beta-amyloid mediated neuronal death. Food Chem. Toxicol. 2011, 49, 2252–2259. [Google Scholar] [CrossRef]
- Velmurugan, B.; Rathinasamy, B.; Lohanathan, B.; Thiyagarajan, V.; Weng, C. Neuroprotective Role of Phytochemicals. Molecules 2018, 23, 2485. [Google Scholar] [CrossRef]
- Kang, M.-C.; Kang, N.; Ko, S.-C.; Kim, Y.-B.; Jeon, Y.-J. Anti-obesity effects of seaweeds of Jeju Island on the differentiation of 3T3-L1 preadipocytes and obese mice fed a high-fat diet. Food Chem. Toxicol. 2016, 90 (Suppl. C), 36–44. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Ko, S.-C.; Kang, M.-C.; Lee, D.; Jeon, Y.-J. Octaphlorethol A, a marine algae product, exhibits antidiabetic effects in type 2 diabetic mice by activating AMP-activated protein kinase and upregulating the expression of glucose transporter 4. Food Chem. Toxicol. 2016, 91 (Suppl. C), 58–64. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.; Fernando, I.; Samarakoon, K.; Lakmal, H.; Kim, E.-A.; Kwon, O.; Dilshara, M.; Lee, J.-B.; Jeon, Y.-J. Anti-inflammatory and anti-cancer activities of sterol rich fraction of cultured marine microalga Nannochloropsis oculata. Algae 2016, 31, 277–287. [Google Scholar] [CrossRef]
- Ko, S.-C.; Jung, W.-K.; Lee, S.-H.; Lee, D.; Jeon, Y.-J. Antihypertensive effect of an enzymatic hydrolysate from Styela clava flesh tissue in type 2 diabetic patients with hypertension. Nutr. Res. Pract. 2017, 11, 396–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Lee, W.; Oh, J.; Cui, Y.; Ryu, B.; Jeon, Y.-J. Protective effect of sulfated polysaccharides from celluclast-assisted extract of Hizikia fusiforme against ultraviolet-B-induced skin damage by regulating NF-κB, AP-1, and MAPKs signaling pathways in vitro in human dermal fibroblasts. Mar. Drugs 2018, 16, 239. [Google Scholar] [CrossRef] [PubMed]
- Wijesekara, I.; Yoon, N.; Kim, S. Phlorotannins from Ecklonia cava (Phaeophyceae): Biological activities and potential health benefits. Biofactors 2010, 36, 408–414. [Google Scholar] [CrossRef]
- Shibata, T.; Kawaguchi, S.; Hama, Y.; Inagaki, M.; Yamaguchi, K.; Nakamura, T. Local and chemical distribution of phlorotannins in brown algae. J. Appl. Phycol. 2004, 16, 291–296. [Google Scholar] [CrossRef]
- Ahn, G.; Kim, K.; Cha, S.; Song, C.; Lee, J.; Heo, M.; Yeo, I.; Lee, N.; Jee, Y.; Kim, J.; et al. Antioxidant activities of phlorotannins purified from Ecklonia cava on free radical scavenging using ESR and H2O2-mediated DNA damage. Eur. Food Res. Technol. 2007, 226, 71–79. [Google Scholar] [CrossRef]
- Lee, S.; Li, Y.; Karadeniz, F.; Kim, M.; Kim, S. a–Glycosidase and a–amylase inhibitory activities of phloroglucinal derivatives from edible marine brown alga, Ecklonia cava. J. Sci. Food Agric. 2009, 89, 1552–1558. [Google Scholar] [CrossRef]
- Athukorala, Y.; Jeon, Y.J. Screening for Angiotensin 1-converting enzyme inhibitory activity of Ecklonia cava. J. Food Sci. Nutr. 2005, 10, 134–139. [Google Scholar] [CrossRef]
- Artan, M.; Li, Y.; Karadeniz, F.; Lee, S.; Kim, M.; Kim, S. Anti-HIV-1 activity of phloroglucinol derivative, 6,6′ -bieckol, from Ecklonia cava. Bioorg. Med. Chem. 2008, 16, 7921–7926. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Kang, K.; Piao, M.; Ko, D.; Wang, Z.; Lee, I.; Kim, B.; Jeong, I.; Shin, T.; Park, J.; et al. Eckol protects V79-4 lung fibroblast cells against t-ray radiation-induced apoptosis via the scavenging of reactive oxygen species and inhibiting of the c-Jun NH2-terminal kinase pathway. Eur. J. Pharmacol. 2008, 591, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Kang, I.; Jang, B.; In, S.; Choi, B.; Kim, M.; Kim, M. Phlorotannin-rich Ecklonia cava reduces the production of beta-amyloid by modulating alpha- and gamma-secretase expression and activity. Neurotoxicology 2013, 34, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Youn, K.; Kim, D.; Ahn, M.-R.; Yoon, E.; Kim, O.-Y.; Jun, M. Anti-Neuroinflammatory Property of Phlorotannins from Ecklonia cava on Aβ25-35-Induced Damage in PC12 Cells. Mar. Durgs 2019, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Hedstrom, L. Serine protease mechanism and specificity. Chem. Rev. 2002, 102, 4501–4524. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Jannat, S.; Jung, H.; Choi, R.; Roy, A.; Choi, J. Anti-Alzheimer’s disease potential of coumarins from Angelica decursiva and Artemisia capillaris and structure-activity analysis. Asian Pac. J. Trop. Med. 2016, 9, 103–111. [Google Scholar] [CrossRef]
- Lee, S.; Youn, K.; Lim, G.; Lee, J.; Jun, M. In Silico Docking and In Vitro Approaches towards BACE1 and Cholinesterases Inhibitory Effect of Citrus Flavanones. Molecules 2018, 23, 1509. [Google Scholar] [CrossRef]
- Nguyen, V.; Zhao, B.; Seong, S.; Kim, J.; Woo, M.; Choi, J.; Min, B. Inhibitory effects of serratene-type triterpenoids from Lycopodium complanatum on cholinesterases and β-secretase 1. Chem. Biol. Interact. 2017, 274, 150–157. [Google Scholar] [CrossRef]
- Jung, H.; Karki, S.; Kim, J.; Choi, J. BACE1 and cholinesterase inhibitory activities of Nelumbo nucifera embryos. Arch. Pharm. Res. 2015, 38, 1178–1187. [Google Scholar] [CrossRef]
- Jung, H.; Oh, S.; Choi, J. Molecular docking studies of phlorotannins from Eisenia bicyclis with BACE1 inhibitory activity. Bioorg. Med. Chem. Lett. 2010, 20, 3211–3215. [Google Scholar] [CrossRef]
- Ahn, M.-J.; Yoon, K.-D.; Min, S.-Y.; Lee, J.; Kim, J.; Kim, T.; Kim, S.; Kim, N.-K.; Huh, H.; Kim, J. Inhibition of HIV-1 Reverse Transcriptase and Protease by Phlorotannins from the Brown Alga Ecklonia cava. Biol. Pharm. Bull. 2004, 27, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Fujimoto, K.; Nagayama, K.; Yamaguchi, K.; Nakamura, T. Inhibitory activity of brown algal phlorotannins against hyaluronidase. Int. J. Food Sci. Technol. 2002, 37, 703–709. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Cha, S.; Ko, J.; Kang, M.; Kim, D.; Heo, S.; Kim, J.; Heu, M.; Kim, Y.; Jung, W.; et al. Neuroprotective effects of phlorotannins isolated from a brown alga, Ecklonia cava, against H2O2-induced oxidative stress in murine hippocampal HT22 cells. Environ. Toxicol. Pharmacol. 2012, 34, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Myung, C.; Shin, H.; Bao, H.; Yeo, S.; Lee, B.; Kang, J. Improvement of memory by dieckol and phlorofucofuroeckol in ethanol-treated mice: Possible involvement of the inhibition of acetylcholinesterase. Arch. Pharm. Res. 2005, 28, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, K.; Iwamura, Y.; Shibata, T.; Hirayama, I.; Nakamura, T. Bactericidal activity of phlorotannins from the brown alga Ecklonia kurome. J. Antimicrob. Chemoth. 2002, 50, 889–893. [Google Scholar] [CrossRef]
- Turck, D.; Bresson, J.-L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.; Mangelsdorf, I.; McArdle, H.; Naska, A.; et al. Safety of Ecklonia cava phlorotannins as a novel food pursuant to Regulation (EC) No 258/97. EFSA J. 2017, 15, 5003–5018. [Google Scholar]
- Abourashed, E. Bioavailability of Plant-Derived Antioxidants. Antioxidants 2013, 2, 309–325. [Google Scholar] [CrossRef] [Green Version]
- Rengasamy, K.; Kulkarni, M.; Stirk, W.; Staden, J. Advances in algal drug research with emphasis on enzyme inhibitors. Biotechnol. Adv. 2014, 32, 1364–1381. [Google Scholar] [CrossRef]
- Kwak, J.; Yang, Z.; Yoon, B.; He, Y.; Uhm, S.; Shin, H.; Lee, B.; Yoo, Y.; Lee, K.; Han, S.; et al. Blood-brain barrier-permeable fluorone-labeled dieckols acting as neuronal ER stress signaling inhibitors. Biomaterials 2015, 61, 52–60. [Google Scholar] [CrossRef]





| Compounds | IC50 (mean ± SD, µM) a | Ki value (µM) d | Inhibition Mode e | |||
|---|---|---|---|---|---|---|
| BACE1 | AChE | BACE1 | AChE | BACE1 | AChE | |
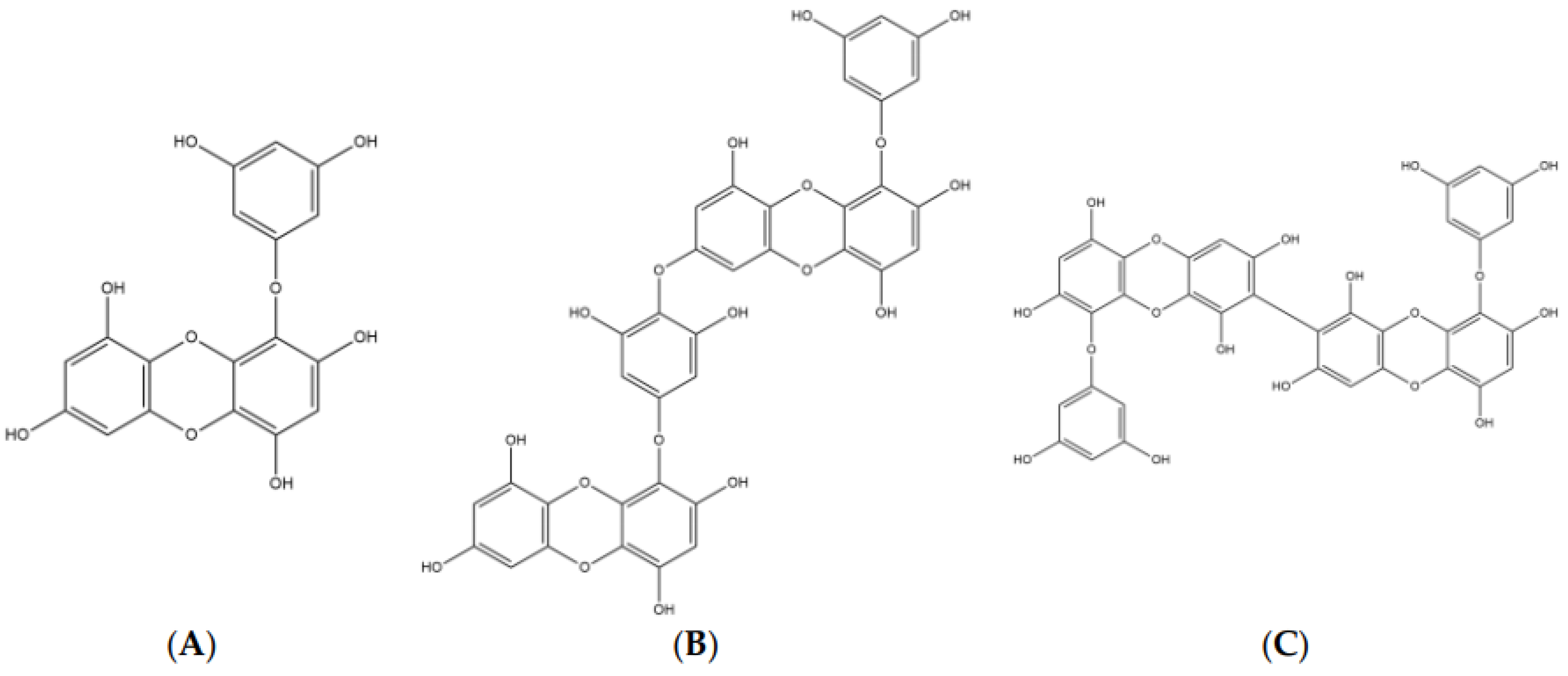
| Eckol | 7.67 ± 0.71 | 10.03 ± 0.94 | 31.2 | 37.3 | Non-competitive | Competitive |
| Dieckol | 2.34 ± 0.10 | 5.69 ± 0.42 | 20.1 | 12.3 | Non-competitive | Competitive |
| 8,8′-Bieckol | 1.62 ± 0.14 | 4.59 ± 0.32 | 13.9 | 11.4 | Non-competitive | Competitive |
| Resveratrol b | 14.89 ± 0.54 | - | - | - | - | - |
| Galantamine c | - | 0.99 ± 0.07 | - | - | - | - |
| Compounds (μM) | TACE (α-Secretase) | Trypsin | Chymotrypsin | Elastase | |
|---|---|---|---|---|---|
| Eckol | 50 | 19.29 ± 1.52 | 3.59 ± 0.57 | 1.48 ± 0.19 | 6.06 ± 0.13 |
| 100 | 10.60 ± 0.53 | 4.73 ± 0.25 | 2.65 ± 0.06 | 4.37 ± 0.27 | |
| Dieckol | 50 | 16.90 ± 1.01 | 6.44 ± 0.76 | 4.39 ± 0.44 | 6.70 ± 0.85 |
| 100 | 18.33 ± 0.41 | −16.64 ± 1.40 | 1.43 ± 0.02 | 6.20 ± 0.14 | |
| 8,8′-Bieckol | 50 | 11.07 ± 0.53 | 2.98 ± 0.26 | 0.86 ± 0.02 | 5.26 ± 0.43 |
| 100 | 3.57 ± 0.05 | −23.05 ± 0.32 | 0.33 ± 0.01 | 7.52 ± 0.24 | |
| Ligand | Lowest Energy (Kcal/mol) | No. of H-Bonds | H-Bonds Interaction Residues | Bond Distance (Å) |
|---|---|---|---|---|
| Eckol | −8.8 | 2 | GLY34 SER36 | 3.277 3.239 |
| Dieckol | −10.1 | 3 | TRP76 THR232 LYS321 | 2.960 3.149 3.488 |
| 8,8′-Bieckol | −9.0 | 4 | LYS107 GLY230 THR231 SER325 | 3.120 2.773 3.098 2.887 |
| Compounds | Lowest Energy (Kcal/mol) | No. of H-Bonds | H-Bonds Interaction Residues | Bond Distance (Å) |
|---|---|---|---|---|
| Eckol | −8.8 | 5 | THR83 TRP86 TYR124 SER125 | 2.855 2.712 3.134 2.883 & 3.313 |
| Dieckol | −9.5 | 4 | ASN233 THR238 ARG296 HIS405 | 3.399 2.837 3.344 3.181 |
| 8,8′-Bieckol | −9.2 | 1 | ARG296 | 3.151 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Jun, M. Dual BACE1 and Cholinesterase Inhibitory Effects of Phlorotannins from Ecklonia cava—An In Vitro and in Silico Study. Mar. Drugs 2019, 17, 91. https://doi.org/10.3390/md17020091
Lee J, Jun M. Dual BACE1 and Cholinesterase Inhibitory Effects of Phlorotannins from Ecklonia cava—An In Vitro and in Silico Study. Marine Drugs. 2019; 17(2):91. https://doi.org/10.3390/md17020091
Chicago/Turabian StyleLee, Jinhyuk, and Mira Jun. 2019. "Dual BACE1 and Cholinesterase Inhibitory Effects of Phlorotannins from Ecklonia cava—An In Vitro and in Silico Study" Marine Drugs 17, no. 2: 91. https://doi.org/10.3390/md17020091