Marine Spirotetronates: Biosynthetic Edifices That Inspire Drug Discovery
Abstract
:1. Introduction
2. Spirotetronates: Derivation, Classifications, and Biological Relevance
3. Class I Marine Spirotetronates
3.1. Biosynthesis
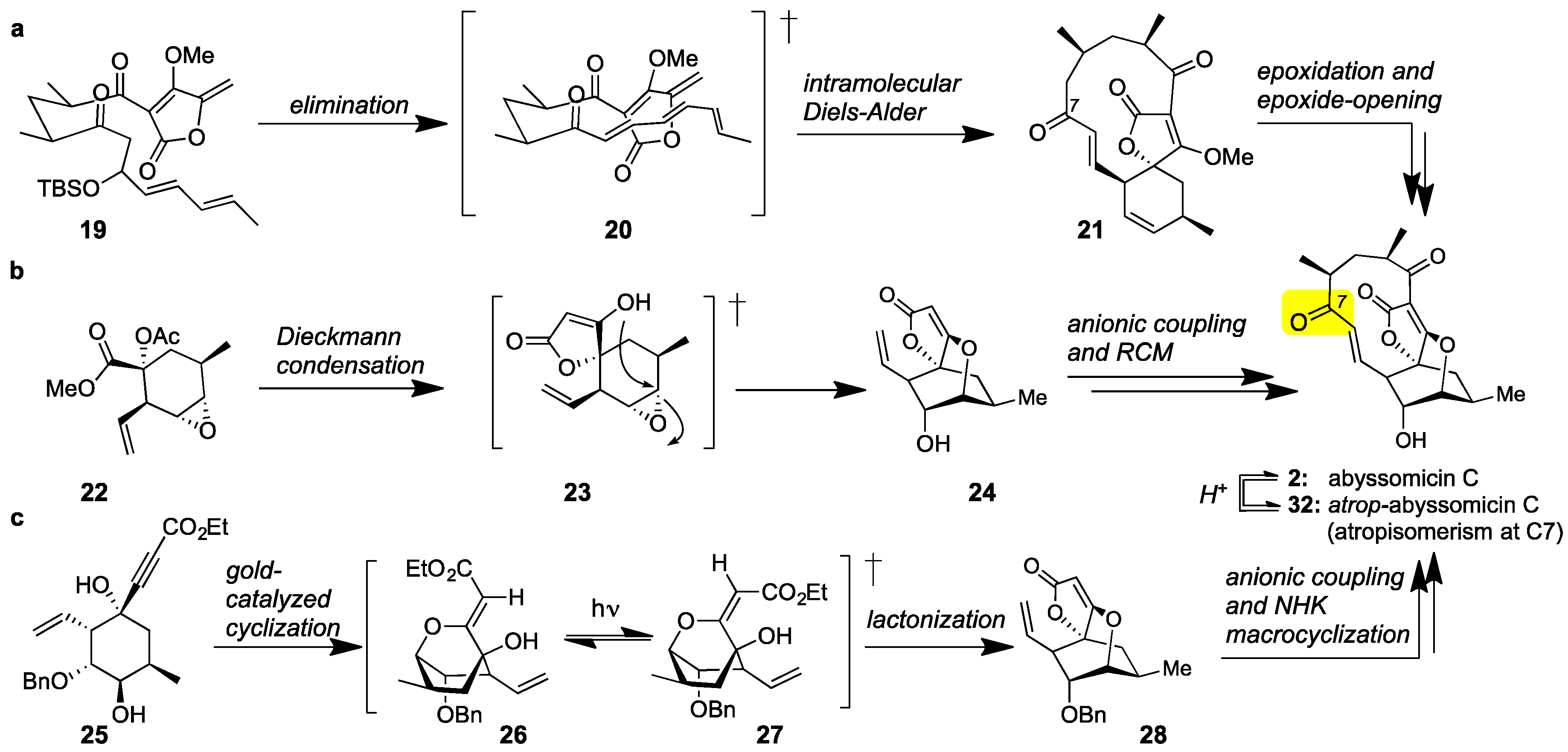
3.2. Synthesis
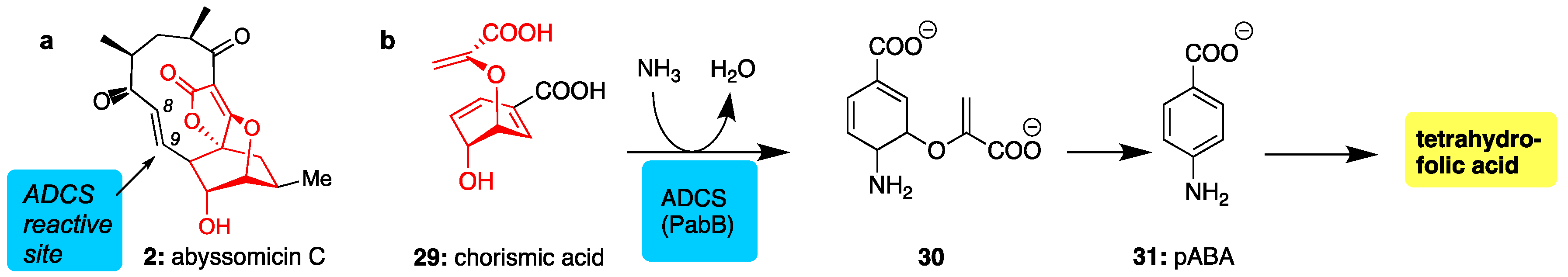
3.3. Biological Relevance
4. Class II Marine Spirotetronates
4.1. Biosynthesis
4.2. Synthesis
4.3. Biological Relevance
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Suttle, C.A. Viruses in the sea. Nature 2005, 437, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Wommack, K.E.; Colwell, R.R. Virioplankton: Viruses in aquatic ecosystems. Microbiol. Mol. Biol. Rev. 2000, 64, 69–114. [Google Scholar] [CrossRef]
- Maranger, R.; Bird, D.F. Viral Abundance in Aquatic Systems: A Comparison between Marine and Fresh Waters. Mar. Ecol. Prog. Ser. 1995, 121, 217–226. [Google Scholar] [CrossRef]
- Fuhrman, J.A. Marine viruses and their biogeochemical and ecological effects. Nature 1999, 399, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Denef, V.J.; Banfield, J.F. In situ evolutionary rate measurements show ecological success of recently emerged bacterial hybrids. Science 2012, 336, 462–466. [Google Scholar] [CrossRef]
- Arp, J.; Götze, S.; Mukherji, R.; Mattern, D.J.; García-Altares, M.; Klapper, M.; Brock, D.A.; Brakhage, A.A.; Strassmann, J.E.; Queller, D.C.; et al. Synergistic activity of cosecreted natural products from amoebae-associated bacteria. Proc. Natl. Acad. Sci. USA 2018, 115, 3758–3763. [Google Scholar] [CrossRef]
- Fischbach, M.A.; Walsh, C.T. Antibiotics for emerging pathogens. Science 2009, 325, 1089–1093. [Google Scholar] [CrossRef]
- Rossiter, S.E.; Fletcher, M.H.; Wuest, W.M. Natural Products as Platforms to Overcome Antibiotic Resistance. Chem. Rev. 2017, 117, 12415–12474. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, J.; Hu, G.; Yu, J.; Zhu, X.; Lin, Y.; Chen, S.; Yuan, J.; Hu, Y.; Chen, J.; et al. Statistical Research on the Bioactivity of New Marine Natural Products Discovered during the 28 Years from 1985 to 2012. Mar. Drugs 2015, 13, 202–221. [Google Scholar] [CrossRef] [Green Version]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [Green Version]
- Challinor, V.L.; Bode, H.B. Bioactive natural products from novel microbial sources. Ann. N. Y. Acad. Sci. 2015, 1354, 82–97. [Google Scholar] [CrossRef]
- Foster, W.; Raoult, A. Early descriptions of antibiosis. J. R. Coll. Gen. Pract. 1974, 24, 889–894. [Google Scholar] [PubMed]
- Donowitz, G.R.; Mandell, G.L. Beta-Lactam Antibiotics. N. Engl. J. Med. 1988, 419–426. [Google Scholar]
- Hawkey, P.M. The growing burden of antimicrobial resistance. J. Antimicrob. Chemother. 2008, 62, i1–i9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C. Molecular mechanisms that confer antibacterial drug resistance. Nature 2000, 406, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Hampton, T. Report reveals scope of US antibiotic resistance threat. JAMA J. Am. Med. Assoc. 2013, 310, 1661–1663. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.C.; Fowler, T.; Watson, J.; Livermore, D.M.; Walker, D. Annual Report of the Chief Medical Officer: Infection and the rise of antimicrobial resistance. Lancet 2013, 381, 1606–1609. [Google Scholar] [CrossRef]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad Bugs, No Drugs: No ESKAPE! An Update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Luqmani, Y.A. Mechanisms of Drug Resistance in Cancer Chemotherapy. Med. Princ. Pract. 2005, 14, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-P.; Hsieh, C.-H.; Wu, Y.-S. The Emergence of Drug Transporter-Mediated Multidrug Resistance to Cancer Chemotherapy. Mol. Pharm. 2011, 8, 1996–2011. [Google Scholar] [CrossRef]
- Gulder, T.A.M.; Moore, B.S. Salinosporamide natural products: Potent 20 S proteasome inhibitors as promising cancer chemotherapeutics. Angew. Chem. Int. Ed. Engl. 2010, 49, 9346–9367. [Google Scholar] [CrossRef]
- Malve, H. Exploring the ocean for new drug developments: Marine pharmacology. J. Pharm. Bioallied Sci. 2016, 8, 83–91. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Chen, J.S.; Edmonds, D.J.; Estrada, A.A. Recent Advances in the Chemistry and Biology of Naturally Occurring Antibiotics. Angew. Chem. Int. Ed. 2009, 48, 660–719. [Google Scholar] [CrossRef] [Green Version]
- Donia, M.; Hamann, M.T. Marine natural products and their potential applications as anti-infective agents. Lancet Infect. Dis. 2003, 3, 338–348. [Google Scholar] [CrossRef]
- Patridge, E.; Gareiss, P.; Kinch, M.S.; Hoyer, D. An analysis of FDA-approved drugs: Natural products and their derivatives. Drug Discov. Today 2016, 21, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Shen, B. A New Golden Age of Natural Products Drug Discovery. Cell 2015, 163, 1297–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altmann, K.-H. Drugs from the Oceans: Marine Natural Products as Leads for Drug Discovery. Chim. Int. J. Chem. 2017, 71, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, C. Marine Natural Products in Medicinal Chemistry. ACS Med. Chem. Lett. 2018, 9, 959–961. [Google Scholar] [CrossRef]
- Chaugule, S.R.; Indap, M.M.; Chiplunkar, S.V. Marine Natural Products: New Avenue in Treatment of Osteoporosis. Front. Mar. Sci. 2017, 4, 384. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Marine natural products and related compounds in clinical and advanced preclinical trials. J. Nat. Prod. 2004, 67, 1216–1238. [Google Scholar] [CrossRef] [PubMed]
- Montaser, R.; Luesch, H. Marine natural products: A new wave of drugs? Future Med. Chem. 2011, 3, 1475–1489. [Google Scholar] [CrossRef] [PubMed]
- Ortholand, J.-Y.; Ganesan, A. Natural products and combinatorial chemistry: Back to the future. Curr. Opin. Chem. Biol. 2004, 8, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.D. Unlocking the potential of natural products in drug discovery. Microb. Biotechnol. 2019, 12, 55–57. [Google Scholar] [CrossRef]
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2009, 8, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Gerwick, W.H.; Moore, B.S. Lessons from the Past and Charting the Future of Marine Natural Products Drug Discovery and Chemical Biology. Chem. Biol. 2012, 19, 85–98. [Google Scholar] [CrossRef] [Green Version]
- Keller-Schierlein, W.; Muntwyler, R.; Pache, W.; Zähner, H. Stoffwechselprodukte von Mikroorganismen 73. Mitteilung [1] Chlorothricin und Des-chlorothoricin. Helv. Chim. Acta 1969, 52, 127–142. [Google Scholar] [CrossRef]
- Lacoske, M.H.; Theodorakis, E.A. Spirotetronate Polyketides as Leads in Drug Discovery. J. Nat. Prod. 2015, 78, 562–575. [Google Scholar] [CrossRef]
- Vieweg, L.; Reichau, S.; Schobert, R.; Leadlay, P.F.; Süssmuth, R.D. Recent advances in the field of bioactive tetronates. Nat. Prod. Rep. 2014, 31, 1554–1584. [Google Scholar] [CrossRef] [Green Version]
- Gui, C.; Zhang, S.; Zhu, X.; Ding, W.; Huang, H.; Gu, Y.-C.; Duan, Y.; Ju, J. Antimicrobial Spirotetronate Metabolites from Marine-Derived Micromonospora harpali SCSIO GJ089. J. Nat. Prod. 2017, 80, 1594–1603. [Google Scholar] [CrossRef]
- Pache, W.; Chapman, D. Interaction of antibiotics with membranes: Chlorothricin. Biochim. Biophys. Acta Biomembr. 1972, 255, 348–357. [Google Scholar] [CrossRef]
- Kang, M.; Jones, B.D.; Mandel, A.L.; Hammons, J.C.; DiPasquale, A.G.; Rheingold, A.L.; La Clair, J.J.; Burkart, M.D. Isolation, Structure Elucidation, and Antitumor Activity of Spirohexenolides A and B. J. Org. Chem. 2009, 74, 9054–9061. [Google Scholar] [CrossRef]
- Nakashima, T.; Miura, M.; Hara, M. Tetrocarcin A inhibits mitochondrial functions of Bcl-2 and suppresses its anti-apoptotic activity. Cancer Res. 2000, 60, 1229–1235. [Google Scholar]
- León, B.; Navarro, G.; Dickey, B.J.; Stepan, G.; Tsai, A.; Jones, G.S.; Morales, M.E.; Barnes, T.; Ahmadyar, S.; Tsiang, M.; et al. Abyssomicin 2 Reactivates Latent HIV-1 by a PKC- and HDAC-Independent Mechanism. Org. Lett. 2015, 17, 262–265. [Google Scholar] [CrossRef] [Green Version]
- Sadaka, C.; Ellsworth, E.; Hansen, P.R.; Ewin, R.; Damborg, P.; Watts, J. Review on abyssomicins: Inhibitors of the chorismate pathway and folate biosynthesis. Molecules 2018, 23, E1371. [Google Scholar] [CrossRef]
- Bister, B.; Bischoff, D.; Ströbele, M.; Riedlinger, J.; Reicke, A.; Wolter, F.; Bull, A.T.; Zähner, H.; Fiedler, H.-P.; Süssmuth, R.D. Abyssomicin C—A Polycyclic Antibiotic from a MarineVerrucosispora Strain as an Inhibitor of thep-Aminobenzoic Acid/Tetrahydrofolate Biosynthesis Pathway. Angew. Chem. Int. Ed. 2004, 43, 2574–2576. [Google Scholar] [CrossRef]
- Chen, H.; Du, L. Iterative polyketide biosynthesis by modular polyketide synthases in bacteria. Appl. Microbiol. Biotechnol. 2016, 100, 541–557. [Google Scholar] [CrossRef]
- Pfeifer, B.A.; Khosla, C. Biosynthesis of polyketides in heterologous hosts. Microbiol. Mol. Biol. Rev. 2001, 65, 106–118. [Google Scholar] [CrossRef]
- Sun, Y.; Hong, H.; Gillies, F.; Spencer, J.B.; Leadlay, P.F. Glyceryl-S-Acyl Carrier Protein as an Intermediate in the Biosynthesis of Tetronate Antibiotics. ChemBioChem 2008, 9, 150–156. [Google Scholar] [CrossRef]
- Liu, Y.; Samborskyy, M.; Kanchanabanca, C.; Sun, Y.; Hong, H.; Tao, W.; Deng, Z.; Hahn, F.; Leadlay, P.F. Unusual Acetylation-Elimination in the Formation of Tetronate Antibiotics. Angew. Chem. Int. Ed. 2013, 52, 5785–5788. [Google Scholar]
- Byrne, M.J.; Lees, N.R.; Han, L.-C.; van der Kamp, M.W.; Mulholland, A.J.; Stach, J.E.M.; Willis, C.L.; Race, P.R. The Catalytic Mechanism of a Natural Diels–Alderase Revealed in Molecular Detail. J. Am. Chem. Soc. 2016, 138, 6095–6098. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ding, W.; Yao, Z.; Tu, J.; Wang, L.; Huang, H.; Li, S.; Ju, J. AbmV Catalyzes Tandem Ether Installation and Hydroxylation during Neoabyssomicin/Abyssomicin Biosynthesis. Org. Lett. 2018, 20, 4854–4857. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, Q.; Qin, F.; Sun, C.; Liang, H.; Wei, X.; Wong, N.-K.; Ye, L.; Zhang, Y.; Shao, M.; et al. Neoabyssomicins A–C, polycyclic macrolactones from the deep-sea derived Streptomyces koyangensis SCSIO 5802. Tetrahedron 2017, 73, 5366–5372. [Google Scholar] [CrossRef]
- Tu, J.; Li, S.; Chen, J.; Song, Y.; Fu, S.; Ju, J.; Li, Q. Characterization and heterologous expression of the neoabyssomicin/abyssomicin biosynthetic gene cluster from Streptomyces koyangensis SCSIO 5802. Microb. Cell Fact. 2018, 17, 28. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.D.; La Clair, J.J.; Moore, C.E.; Rheingold, A.L.; Burkart, M.D. Convergent Route to the Spirohexenolide Macrocycle. Org. Lett. 2010, 12, 4516–4519. [Google Scholar] [CrossRef]
- Niu, D.; Hoye, T.R. A Concise Total Synthesis of (±)- and (−)-Okilactomycin D. Org. Lett. 2012, 14, 828–831. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.B.; Basu, K.; Bosanac, T. Total synthesis of (−)-okilactomycin. J. Am. Chem. Soc. 2007, 129, 14872–14874. [Google Scholar] [CrossRef]
- Tenenbaum, J.M.; Morris, W.J.; Custar, D.W.; Scheidt, K.A. Synthesis of (−)-Okilactomycin by a Prins-Type Fragment-Assembly Strategy. Angew. Chem. Int. Ed. 2011, 50, 5892–5895. [Google Scholar] [CrossRef]
- Zapf, C.W.; Harrison, B.A.; Drahl, C.; Sorensen, E.J. A Diels-Alder Macrocyclization Enables an Efficient Asymmetric Synthesis of the Antibacterial Natural Product Abyssomicin C. Angew. Chem. 2005, 117, 6691–6695. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Harrison, S.T. Total Synthesis of Abyssomicin C, Atrop-abyssomicin C, and Abyssomicin D: Implications for Natural Origins of Atrop-abyssomicin C. J. Am. Chem. Soc. 2007, 129, 429–440. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Harrison, S.T. Total Synthesis of Abyssomicin C and atrop-Abyssomicin C. Angew. Chem. Int. Ed. 2006, 45, 3256–3260. [Google Scholar] [CrossRef]
- Nicolaou, K.; Harrison, S.; Chen, J. Discoveries from the Abyss: The Abyssomicins and Their Total Synthesis. Synthesis 2009, 2009, 33–42. [Google Scholar] [CrossRef]
- Roush, W.R.; Limberakis, C.; Kunz, R.K.; Barda, D.A. Diastereoselective Synthesis of the endo- and exo-Spirotetronate Subunits of the Quartromicins. The First Enantioselective Diels−Alder Reaction of an Acyclic (Z)-1,3-Diene. Org. Lett. 2002, 4, 1543–1546. [Google Scholar] [CrossRef] [PubMed]
- Bihelovic, F.; Saicic, R.N. Total Synthesis of (−)-atrop-Abyssomicin C. Angew. Chem. Int. Ed. 2012, 51, 5687–5691. [Google Scholar] [CrossRef]
- Mézailles, N.; Ricard, L.; Gagosz, F. Phosphine Gold(I) Bis-(trifluoromethanesulfonyl)imidate Complexes as New Highly Efficient and Air-Stable Catalysts for the Cycloisomerization of Enynes. Org. Lett. 2005, 7, 4133–4136. [Google Scholar] [CrossRef]
- Matovic, R.; Bihelovic, F.; Gruden-Pavlovic, M.; Saicic, R.N. Total synthesis and biological evaluation of atrop-O-benzyl-desmethylabyssomicin C. Org. Biomol. Chem. 2014, 12, 7682–7685. [Google Scholar] [CrossRef]
- Blancquaert, D.; Storozhenko, S.; Loizeau, K.; De Steur, H.; De Brouwer, V.; Viaene, J.; Ravanel, S.; Rébeillé, F.; Lambert, W.; Van Der Straeten, D. Folates and Folic Acid: From Fundamental Research Toward Sustainable Health. CRC Crit. Rev. Plant Sci. 2010, 29, 14–35. [Google Scholar] [CrossRef]
- Rebeille, F.; Ravanel, S.; Jabrin, S.; Douce, R.; Storozhenko, S.; Van Der Straeten, D. Folates in plants: Biosynthesis, distribution, and enhancement. Physiol. Plant. 2006, 126, 330–342. [Google Scholar] [CrossRef]
- Keller, S.; Schadt, H.S.; Ortel, I.; Süssmuth, R.D. Action of atrop-Abyssomicin C as an Inhibitor of 4-Amino-4-deoxychorismate Synthase PabB. Angew. Chem. Int. Ed. 2007, 46, 8284–8286. [Google Scholar] [CrossRef]
- Riedlinger, J.; Reicke, A.; Zähner, H.; Krismer, B.; Bull, A.T.; Maldonado, L.A.; Ward, A.C.; Goodfellow, M.; Bister, B.; Bischoff, D.; et al. Abyssomicins, inhibitors of the para-aminobenzoic acid pathway produced by the marine Verrucosispora strain AB-18-032. J. Antibiot. 2004, 57, 271–279. [Google Scholar] [CrossRef]
- Wang, Q.; Song, F.; Xiao, X.; Huang, P.; Li, L.; Monte, A.; Abdel-Mageed, W.M.; Wang, J.; Guo, H.; He, W.; et al. Abyssomicins from the South China Sea Deep-Sea Sediment Verrucosispora sp.: Natural Thioether Michael Addition Adducts as Antitubercular Prodrugs. Angew. Chem. Int. Ed. 2013, 52, 1231–1234. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.-Y.; Tian, Z.-H.; Shao, L.; Qu, X.-D.; Zhao, Q.-F.; Tang, J.; Tang, G.-L.; Liu, W. Genetic Characterization of the Chlorothricin Gene Cluster as a Model for Spirotetronate Antibiotic Biosynthesis. Chem. Biol. 2006, 13, 575–585. [Google Scholar] [CrossRef] [Green Version]
- Fang, J.; Zhang, Y.; Huang, L.; Jia, X.; Zhang, Q.; Zhang, X.; Tang, G.; Liu, W. Cloning and characterization of the tetrocarcin A gene cluster from Micromonospora chalcea NRRL 11289 reveals a highly conserved strategy for tetronate biosynthesis in spirotetronate antibiotics. J. Bacteriol. 2008, 190, 6014–6025. [Google Scholar] [CrossRef]
- Zhang, H.; White-Phillip, J.A.; Melançon, C.E.; Kwon, H.J.; Yu, W.L.; Liu, H.W. Elucidation of the kijanimicin gene cluster: Insights into the biosynthesis of spirotetronate antibiotics and nitrosugars. J. Am. Chem. Soc. 2007, 129, 14670–14683. [Google Scholar] [CrossRef] [PubMed]
- Chijiwa, S.; Park, H.-R.; Furihata, K.; Ogata, M.; Endo, T.; Kuzuyama, T.; Hayakawa, Y.; Shin-ya, K. Biosynthetic studies of versipelostatin, a novel 17-membered α-tetronic acid involved macrocyclic compound isolated from Streptomyces versipellis. Tetrahedron Lett. 2003, 44, 5897–5900. [Google Scholar] [CrossRef]
- Hashimoto, T.; Hashimoto, J.; Teruya, K.; Hirano, T.; Shin-ya, K.; Ikeda, H.; Liu, H.; Nishiyama, M.; Kuzuyama, T. Biosynthesis of Versipelostatin: Identification of an Enzyme-Catalyzed [4+2]-Cycloaddition Required for Macrocyclization of Spirotetronate-Containing Polyketides. J. Am. Chem. Soc. 2015, 137, 572–575. [Google Scholar] [CrossRef]
- Hirayama, N.; Kasai, M.; Shirahata, K.; Ohashi, Y.; Sasada, Y. The structure of tetronolide, the aglycone of antitumor antibiotic tetrocarcin. Tetrahedron Lett. 1980, 21, 2559–2560. [Google Scholar] [CrossRef]
- Roush, W.R.; Sciotti, R.J. Enantioselective Total Synthesis of (−)-Chlorothricolide via the Tandem Inter- and Intramolecular Diels−Alder Reaction of a Hexaenoate Intermediate. J. Am. Chem. Soc. 1998, 120, 7411–7419. [Google Scholar] [CrossRef]
- Takeda, K.; Kawanishi, E.; Nakamura, H.; Yoshii, E. Total synthesis of tetronolide, the aglycon of tetrocarcins. Tetrahedron Lett. 1991, 32, 4925–4928. [Google Scholar] [CrossRef]
- Roush, W.R.; Reilly, M.L.; Koyama, K.; Brown, B.B. A Formal Total Synthesis of (+)-Tetronolide, the Aglycon of the Tetrocarcins: Enantio- and Diastereoselective Syntheses of the Octahydronaphthalene (Bottom-Half) and Spirotetronate (Top-Half) Fragments. J. Org. Chem. 1997, 62, 8708–8721. [Google Scholar] [CrossRef]
- Boeckman, R.K.; Shao, P.; Wrobleski, S.T.; Boehmler, D.J.; Heintzelman, G.R.; Barbosa, A.J. Toward the development of a general chiral auxiliary. A total synthesis of (+)-tetronolide via a tandem ketene-trapping [4 + 2] cycloaddition strategy. J. Am. Chem. Soc. 2006, 128, 10572–10588. [Google Scholar] [CrossRef]
- Lacoske, M.H.; Xu, J.; Mansour, N.; Gao, C.; Theodorakis, E.A. Synthetic strategies toward the decalin motif of maklamicin and related spirotetronates. Org. Chem. Front. 2015, 2, 388–393. [Google Scholar] [CrossRef]
- Trullinger, T.K.; Qi, J.; Roush, W.R. Studies on the synthesis of quartromicins A3and D3: Synthesis of the vertical and horizontal bis-spirotetronate fragments. J. Org. Chem. 2006, 71, 6915–6922. [Google Scholar] [CrossRef]
- Kaneko, M.; Nakashima, T.; Uosaki, Y.; Hara, M.; Ikeda, S.; Kanda, Y. Synthesis of tetrocarcin derivatives with specific inhibitory activity towards Bcl-2 functions. Bioorg. Med. Chem. Lett. 2001, 11, 887–890. [Google Scholar] [CrossRef]
- Tamaok, T.; Tomita, F.; Obi, Y.; Kawamura, F.; Saito, H. Mechanism of Action of Tetrocarcin A. Agric. Biol. Chem. 1983, 47, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Tinhofer, I.; Anether, G.; Senfter, M.; Pfaller, K.; Bernhard, D.; Hara, M.; Greil, R. Stressful death of T-ALL tumor cells after treatment with the anti-tumor agent Tetrocarcin-A. FASEB J. 2002, 16, 1295–1297. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.M.; Cory, S. The Bcl-2 protein family: Arbiters of cell survival. Science 1998, 281, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, X.; Bhalla, K.; Kim, C.N.; Ibrado, A.M.; Cai, J.; Peng, T.I.; Jones, D.P.; Wang, X. Prevention of apoptosis by Bcl-2: Release of cytochrome c from mitochondria blocked. Science 1997, 275, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Hockenbery, D.; Nuñez, G.; Milliman, C.; Schreiber, R.D.; Korsmeyer, S.J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature 1990, 348, 334–336. [Google Scholar] [CrossRef]
- Hockenbery, D.M.; Oltvai, Z.N.; Yin, X.-M.; Milliman, C.L.; Korsmeyer, S.J. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell 1993, 75, 241–251. [Google Scholar] [CrossRef]
- Oltval, Z.N.; Milliman, C.L.; Korsmeyer, S.J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programed cell death. Cell 1993, 74, 609–619. [Google Scholar] [CrossRef]
- Nakajima, H.; Sakaguchi, K.; Fujiwara, I.; Mizuta, M.; Tsuruga, M.; Magae, J.; Mizuta, N. Apoptosis and inactivation of the PI3-kinase pathway by tetrocarcin A in breast cancers. Biochem. Biophys. Res. Commun. 2007, 356, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Vellanki, S.H.; Cruz, R.G.B.; Jahns, H.; Hudson, L.; Sette, G.; Eramo, A.; Hopkins, A.M. Natural compound Tetrocarcin-A downregulates Junctional Adhesion Molecule-A in conjunction with HER2 and inhibitor of apoptosis proteins and inhibits tumor cell growth. Cancer Lett. 2019, 440–441, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Ebnet, K. Junctional Adhesion Molecules (JAMs): Cell Adhesion Receptors With Pleiotropic Functions in Cell Physiology and Development. Physiol. Rev. 2017, 97, 1529–1554. [Google Scholar] [CrossRef]
- Cruz, P.G.; Fribley, A.M.; Miller, J.R.; Larsen, M.J.; Schultz, P.J.; Jacob, R.T.; Tamayo-Castillo, G.; Kaufman, R.J.; Sherman, D.H. Novel Lobophorins Inhibit Oral Cancer Cell Growth and Induce Atf4—and Chop—Dependent Cell Death in Murine Fibroblasts. ACS Med. Chem. Lett. 2015, 6, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Li, S.; Chen, Y.; Tian, X.; Zhang, H.; Zhang, G.; Zhang, W.; Yang, X.; Zhang, S.; Ju, J.; et al. Lobophorins E and F, new spirotetronate antibiotics from a South China Sea-derived Streptomyces sp. SCSIO 01127. J. Antibiot. 2011, 64, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Bradner, W.T.; Claridge, C.A.; Huftalen, J.B. Antitumor activity of kijanimicin. J. Antibiot. 1983, 36, 1078–1079. [Google Scholar] [CrossRef]
- Braña, A.; Sarmiento-Vizcaíno, A.; Osset, M.; Pérez-Victoria, I.; Martín, J.; de Pedro, N.; de la Cruz, M.; Díaz, C.; Vicente, F.; Reyes, F.; et al. Lobophorin K, a New Natural Product with Cytotoxic Activity Produced by Streptomyces sp. M-207 Associated with the Deep-Sea Coral Lophelia pertusa. Mar. Drugs 2017, 15, 144. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Hu, Y.; Wang, Q.; Zhou, H.; Wang, Y.; Gan, M. Tetrocarcins N and O, glycosidic spirotetronates from a marine-derived Micromonospora sp. identified by PCR-based screening. RSC Adv. 2016, 6, 91773–91778. [Google Scholar] [CrossRef]
- Gong, T.; Zhen, X.; Li, X.-L.; Chen, J.-J.; Chen, T.-J.; Yang, J.-L.; Zhu, P.; Gong, T.; Zhen, X.; Li, X.-L.; et al. Tetrocarcin Q, a New Spirotetronate with a Unique Glycosyl Group from a Marine-Derived Actinomycete Micromonospora carbonacea LS276. Mar. Drugs 2018, 16, 74. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, J.L.; Sanchez, L.M.; Koo, B.-M.; Doherty, J.S.; Rajendram, M.; Huang, K.C.; Gross, C.A.; Linington, R.G. Marine Mammal Microbiota Yields Novel Antibiotic with Potent Activity Against Clostridium difficile. ACS Infect. Dis. 2018, 4, 59–67. [Google Scholar] [CrossRef]
- Pérez-Bonilla, M.; Oves-Costales, D.; de la Cruz, M.; Kokkini, M.; Martín, J.; Vicente, F.; Genilloud, O.; Reyes, F. Phocoenamicins B and C, New Antibacterial Spirotetronates Isolated from a Marine Micromonospora sp. Mar. Drugs 2018, 16, 95. [Google Scholar] [CrossRef]
- Kasanah, N.; Hamann, M.T. Development of antibiotics and the future of marine microorganisms to stem the tide of antibiotic resistance. Curr. Opin. Investig. Drugs 2004, 5, 827–837. [Google Scholar] [PubMed]
- O’Connell, K.M.G.; Hodgkinson, J.T.; Sore, H.F.; Welch, M.; Salmond, G.P.C.; Spring, D.R. Combating Multidrug-Resistant Bacteria: Current Strategies for the Discovery of Novel Antibacterials. Angew. Chem. Int. Ed. 2013, 52, 10706–10733. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.W.; Davids, M.S.; Pagel, J.M.; Kahl, B.S.; Puvvada, S.D.; Gerecitano, J.F.; Kipps, T.J.; Anderson, M.A.; Brown, J.R.; Gressick, L.; et al. Targeting BCL2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2016, 374, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Stilgenbauer, S.; Eichhorst, B.; Schetelig, J.; Coutre, S.; Seymour, J.F.; Munir, T.; Puvvada, S.D.; Wendtner, C.-M.; Roberts, A.W.; Jurczak, W.; et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: A multicentre, open-label, phase 2 study. Lancet Oncol. 2016, 17, 768–778. [Google Scholar] [CrossRef]
- Cang, S.; Iragavarapu, C.; Savooji, J.; Song, Y.; Liu, D. ABT-199 (venetoclax) and BCL-2 inhibitors in clinical development. J. Hematol. Oncol. 2015, 8, 129. [Google Scholar] [CrossRef] [Green Version]
- Davids, M.S.; Roberts, A.W.; Seymour, J.F.; Pagel, J.M.; Kahl, B.S.; Wierda, W.G.; Puvvada, S.; Kipps, T.J.; Anderson, M.A.; Salem, A.H.; et al. Phase I First-in-Human Study of Venetoclax in Patients With Relapsed or Refractory Non-Hodgkin Lymphoma. J. Clin. Oncol. 2017, 35, 826–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konopleva, M.; Pollyea, D.A.; Potluri, J.; Chyla, B.; Hogdal, L.; Busman, T.; McKeegan, E.; Salem, A.H.; Zhu, M.; Ricker, J.L.; et al. Efficacy and Biological Correlates of Response in a Phase II Study of Venetoclax Monotherapy in Patients with Acute Myelogenous Leukemia. Cancer Discov. 2016, 6, 1106–1117. [Google Scholar] [CrossRef]
- Wender, P.A.; Hardman, C.T.; Ho, S.; Jeffreys, M.S.; Maclaren, J.K.; Quiroz, R.V.; Ryckbosch, S.M.; Shimizu, A.J.; Sloane, J.L.; Stevens, M.C. Scalable synthesis of bryostatin 1 and analogs, adjuvant leads against latent HIV. Science 2017, 358, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Villaume, M.T.; Sella, E.; Saul, G.; Borzilleri, R.M.; Fargnoli, J.; Johnston, K.A.; Zhang, H.; Fereshteh, M.P.; Dhar, T.G.M.; Baran, P.S. Antroquinonol A: Scalable Synthesis and Preclinical Biology of a Phase 2 Drug Candidate. ACS Cent. Sci. 2016, 2, 27–31. [Google Scholar] [CrossRef]
- Seiple, I.B.; Zhang, Z.; Jakubec, P.; Langlois-Mercier, A.; Wright, P.M.; Hog, D.T.; Yabu, K.; Allu, S.R.; Fukuzaki, T.; Carlsen, P.N.; et al. A platform for the discovery of new macrolide antibiotics. Nature 2016, 533, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Newhouse, T.; Baran, P.S.; Hoffmann, R.W. The economies of synthesis. Chem. Soc. Rev. 2009, 38, 3010. [Google Scholar] [CrossRef] [PubMed]
- Wender, P.A.; Verma, V.A.; Paxton, T.J.; Pillow, T.H. Function-Oriented Synthesis, Step Economy, and Drug Design. Acc. Chem. Res. 2008, 41, 40–49. [Google Scholar] [CrossRef]
- Vong, B.G.; Li, H.; Theodorakis, E.A.; Tisdale, E.J.; Chowdhury, C. Regioselective Synthesis of the Bridged Tricyclic Core of Garcinia Natural Products via Intramolecular Aryl Acrylate Cycloadditions. Org. Lett. 2002, 4, 909–912. [Google Scholar]
- Saitman, A.; Rulliere, P.; Sullivan, S.D.E.; Theodorakis, E.A. Total Synthesis of Norcembrenolide B and Scabrolide D. Org. Lett. 2011, 13, 5854–5857. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Rivas, F.; Fischer, D.; González, M.A.; Theodorakis, E.A. Stereoselective synthesis of the ABC ring system of norzoanthamine. Org. Lett. 2004, 6, 941–944. [Google Scholar] [CrossRef]
- Ling, T.; Poupon, E.; Rueden, E.J.; Theodorakis, E.A. Synthesis of (−)-ilimaquinone via a radical decarboxylation and quinone addition reaction. Org. Lett. 2002, 4, 819–822. [Google Scholar] [CrossRef]
- Chantarasriwong, O.; Cho, W.C.; Batova, A.; Chavasiri, W.; Moore, C.; Rheingold, A.L.; Theodorakis, E.A. Evaluation of the pharmacophoric motif of the caged Garcinia xanthones. Org. Biomol. Chem. 2009, 7, 4886–4894. [Google Scholar] [CrossRef]
- Walsh, C.T. A chemocentric view of the natural product inventory. Nat. Chem. Biol. 2015, 11, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Ridley, C.P.; Lee, H.Y.; Khosla, C. Evolution of polyketide synthases in bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 4595–4600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischbach, M.A.; Walsh, C.T. Assembly-Line Enzymology for Polyketide and Nonribosomal Peptide Antibiotics: Logic, Machinery, and Mechanisms. Chem. Rev. 2006, 106, 3468–3496. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Zhang, W. Engineering modular polyketide synthases for production of biofuels and industrial chemicals. Curr. Opin. Biotechnol. 2018, 50, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, M.A.; Clardy, J. One pathway, many products. Nat. Chem. Biol. 2007, 3, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Clardy, J.; Walsh, C. Lessons from natural molecules. Nature 2004, 432, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Fenical, W.; Jensen, P.R. Developing a new resource for drug discovery: Marine actinomycete bacteria. Nat. Chem. Biol. 2006, 2, 666–673. [Google Scholar] [CrossRef]
- Salvador-Reyes, L.A.; Luesch, H. Biological targets and mechanisms of action of natural products from marine cyanobacteria. Nat. Prod. Rep. 2015, 32, 478–503. [Google Scholar] [CrossRef]
- Gould, K. Antibiotics: From prehistory to the present day. J. Antimicrob. Chemother. 2016, 71, 572–575. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Rigol, S. A brief history of antibiotics and select advances in their synthesis. J. Antibiot. 2018, 71, 153–184. [Google Scholar] [CrossRef]
- Dhakal, D.; Pokhrel, A.R.; Shrestha, B.; Sohng, J.K. Marine rare actinobacteria: Isolation, characterization, and strategies for harnessing bioactive compounds. Front. Microbiol. 2017, 8, 1106. [Google Scholar] [CrossRef] [PubMed]
- Clardy, J.; Fischbach, M.A.; Currie, C.R. The natural history of antibiotics. Curr. Biol. 2009, 19, R437–R441. [Google Scholar] [CrossRef] [PubMed] [Green Version]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braddock, A.A.; Theodorakis, E.A. Marine Spirotetronates: Biosynthetic Edifices That Inspire Drug Discovery. Mar. Drugs 2019, 17, 232. https://doi.org/10.3390/md17040232
Braddock AA, Theodorakis EA. Marine Spirotetronates: Biosynthetic Edifices That Inspire Drug Discovery. Marine Drugs. 2019; 17(4):232. https://doi.org/10.3390/md17040232
Chicago/Turabian StyleBraddock, Alexander A., and Emmanuel A. Theodorakis. 2019. "Marine Spirotetronates: Biosynthetic Edifices That Inspire Drug Discovery" Marine Drugs 17, no. 4: 232. https://doi.org/10.3390/md17040232




